Introduction
Malignant gliomas, particularly glioblastomas
(GBMs), are the most common primary tumors of the central nervous
system (1). According to World
Health Organization (WHO) classification, malignant gliomas mainly
include WHO grade II, grade III and GBM (WHO-grade IV) (2). High-grade glioma, including grade III
and GBM, is the most malignant type and has the worst prognosis,
requiring significant lesion resection combined with radiotherapy
or/and temozolomide (TMZ) chemotherapy (3). Patients with GBM have a mean survival
time of only 14.4 months (1), which
is likely due to chemoradiotherapy resistance. Therefore, new
therapeutic targets that increase sensitivity to chemoradiotherapy
are urgently needed. Evidence suggests that therapeutic effects can
be altered by gene alterations. Gliomas exhibiting isocitrate
dehydrogenase 1/2 (IDH1/2) mutations are associated with a
relatively good prognosis compared with wild-type (4). Studies have found that the expression
of mutant IDH1 causes the glioma hypermethylator phenotype, which
may serve a critical role in glioma treatment (5,6).
Oligodendrogliomas exhibiting loss of chromosome 1p/19q respond
favorably to combined procarbazine, lomustine and vincristine
treatment (7–9). Researchers have demonstrated that the
loss of chromosome 1p/19q is associated with the downregulation of
tumor suppressor genes or the mutation of specific genes, including
CIC and NOTCH1 (10,11). O6-methylguanine-DNA
methyltransferase (MGMT) promoter methylation has been used to
guide glioblastoma treatment decisions (12). Resistance to TMZ therapy is thought
to arise when the methylated DNA bases are repaired by MGMT, or
when the mismatch repair (MMR) pathway functions deficiently
(12). Patients with high-grade
glioma exhibiting promoter hypermethylation of the MGMT gene, which
silences its expression, have been shown to respond well to TMZ
therapy (1,12). These results suggest that gene
alterations serve important roles in the regulation of
chemoradiotherapy sensitivity in gliomas.
Receptor tyrosine kinases (RTKs), a family of
cell-surface receptors, constitute important signaling pathways in
tumor development (13). RTKs are
key regulators of critical cellular processes, including
proliferation and differentiation, cell survival, metabolism and
migration, and cell cycle control (13–15). In
the human genome, a total of 58 RTKs have been identified (16,17). All
RTKs have an extracellular ligand-binding domain, a single
transmembrane helix and a cytoplasmic region that has protein
tyrosine kinase activity (13).
Agonist binding to the extracellular domain evokes dimerization,
and leads to autophosphorylation of the tyrosine kinase domain in a
‘trans’ orientation, which serves as a site of assembly of protein
complexes and stimulates multiple signaling pathways (13,16).
RTKs are of widespread interest as drug targets in numerous types
of cancer, including epidermal growth factor receptor (EGFR) in
metastatic non-small-cell lung cancer and vascular endothelial
growth factor in metastatic colorectal carcinoma (14). A number of diseases, including glioma
and lung cancer, result from genetic changes or abnormalities that
alter the activity, abundance, cellular distribution and/or
regulation of RTKs (10,17). It has been reported that alterations
of RTKs, including mutation, expression changes or rearrangement
can result in drug resistance. EGFR mutation serves a critical role
in resistance to gefitinib in lung cancer with anaplastic lymphoma
kinase gene rearrangement (18). It
has also been found that RTK activation may increase sensitivity to
chemotherapy. High levels of hepatocyte receptor tyrosine kinase
(HGFR/Met) signaling pathway activation by hepatocyte growth factor
(HGF) can increase sensitivity to the Met inhibitor crizotinib in
patients with glioma (19).
Therefore, it is hypothesized that the expression of RTKs may
influence TMZ chemotherapy in patients with high-grade glioma.
In the present study, gene alterations before and
after chemoradiotherapy were compared in terms of various Cancer
Genome Atlas subtypes, including IDH1/2 mutation, EGFR variant III
(EGFRvIII) mutation, tumor protein P53 (TP53) mutation, phosphatase
and tensin homolog (PTEN) mutation, MGMT promoter methylation and
telomerase reverse transcriptase (TERT) mutation, as well as the
expression profile of 58 RTKs. The results demonstrated that, when
stimulated by growth factors, the activation of insulin-like growth
factor 1 receptor (IGF1R) signaling increased tumor cell
sensitivity to TMZ, and TMZ was able to influence IGF1R expression.
Survival analysis demonstrated that high expression of IGF1R
predicts longer overall survival in patients treated with TMZ.
Materials and methods
Data collection
The characteristics of patients were collected from
the Chinese Glioma Genome Atlas (CGGA; http://www.cgga.org.cn). Samples that were used for
RNA sequencing, classical marker detection and survival analysis
were from patients who underwent surgical resection between January
2005 and December 2015. The information about radiotherapy and
chemotherapy treatment in patients with recurrent glioma was as
follows: 22 patients received radiotherapy and 27 patients received
chemotherapy. Patients were eligible for the study if their
diagnosis was established histologically by two neuropathologists,
according to the 2007 WHO classification guidelines (2). Only samples composed of ≥80% tumor
cells were selected for analysis. The total number of patients
included in the analysis was 182. This study was approved by the
Ethics Committee of Beijing Tiantan Hospital (Beijing, China), and
written informed consent was obtained from each patient.
Cell lines
The human GBM cell lines, H4 and LN-229, were
obtained from the Institute of Biochemistry and Cell Biology,
Chinese Academy of Sciences (Shanghai, China). The H4 cell line is
derived from an astrocytoma patient who was originally diagnosed
with a neuroglioma, and the LN-229 cell line is derived from a
patient with glioblastoma (https://www.atcc.org/). Cells were maintained in DMEM
(Gibco; Thermo Fisher Scientific, Inc.) containing 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 50 U/ml
penicillin G and 250 µg/ml streptomycin in a humidified atmosphere
containing 5% CO2 at 37°C (20). Cells with or without stimulation for
30 min with fibroblast growth factor (FGF; PeproTech; 10 ng/ml) or
IGF1 (PeproTech, 100 ng/ml) were then treated with TMZ at 37°C for
1–5 days (50 µM; Sigma-Aldrich; Merck KGaA) (21,22).
Western blot analysis
Whole-cell lysates were prepared using RIPA buffer
(Cell Signaling Technology, Inc.). The protein determination used
Pierce Bicinchoninic Acid Protein Assay kit (Thermo Fisher
Scientific, Inc.). Equal amounts of total protein (30 µg) were
separated by 10% SDS/PAGE, transferred to a PVDF membrane (EMD
Millipore) and detected using an ECL Western Blotting Detection
System (Bio-Rad Laboratories, Inc.). 5% skim milk was used to block
the membrane at room temperature for 1 h. Primary rabbit polyclonal
antibodies against IGF1R (ProteinTech Group, Inc.; cat. no.
10297-1-AP; dilution, 1:200) were used at 4°C overnight. An
antibody against GAPDH (ProteinTech Group, Inc.; cat. no.
60004-1-Ig; dilution, 1:5,000) was used as the loading control.
Goat anti-rabbit IgG-HRP and goat anti-mouse IgG-HRP (OriGene
Technologies, Inc.; cat. nos. TA130024 and TA130005; dilution,
1:5,000) secondary antibodies were used at room temperature for 1 h
(20). The software used for
densitometry was ImageJ version 1.46 (National Institutes of
Health).
MTT assay
H4 and LN-229 cells (2,000–3,000) were seeded in
96-well plates, and after 24 h, the cells were stimulated with or
without FGF or IGF1 for 30 min before TMZ treatment. Dimethyl
sulfoxide was used to dissolve the purple formazan. MTT activity
was measured in triplicate on days 1, 2, 3, 4 and 5. Proliferation
curves were constructed by plotting the average of the triplicate
values calculated by optical density measurements at a wavelength
of 570 nm by a 96-well plate reader (23).
Statistical analysis
Differentially expressed genes were detected by
unpaired Student's t-test using SPSS version 13.0 (SPSS, Inc.,
Chicago, IL, USA). Differential genes between primary high-grade
gliomas and recurrent gliomas after chemoradiotherapy were
identified by Student's t-test. Kaplan-Meier survival analysis was
used to estimate survival distribution. The log-rank test was
applied to assess the statistical significance between the
stratified survival groups using GraphPad Prism version 4.0
statistical software (GraphPad Software, Inc.). KEGG pathway
analysis was performed using DAVID (http://david.abcc.ncifcrf.gov/). Cut-off points for
different variables were set according to the median value. A
two-sided P-value <0.05 was regarded to indicate a statistically
significant difference.
Results
Alterations in the distribution of
established biomarkers
Numerous reliable molecular markers, such as TP53
and IDH1 mutations, are accepted as frequent alterations in glioma
development (4–6). In the present study, whether these
markers are altered in glioma following chemoradiotherapy was
investigated. A total of 182 high-grade glioma samples (47 primary
grade III, 88 primary GBM, 25 recurrent grade III and 22 recurrent
GBM samples) were collected with information regarding PTEN
mutation status, IDH1/2 status, TP53 mutation status, MGMT promoter
methylation status, EGFRvIII mutation status, TERT mutation status
and TCGA subtype, from the CGGA dataset and are presented in
Table I. It was observed that tumor
recurrence was more common in older patients. There was a decline
in the EGFRvIII, TP53, PTEN and TERT mutation rates from primary
high-grade gliomas to recurrent gliomas after chemoradiotherapy.
However, the frequency of IDH1/2 mutations and MGMT promoter
methylation was increased in gliomas following chemoradiotherapy,
which may explain the insensitivity of recurrent gliomas to TMZ
chemotherapy (Table I).
 | Table I.Established biomarkers in initial and
recurrent high grade astrocytomas. |
Table I.
Established biomarkers in initial and
recurrent high grade astrocytomas.
|
| Cases, % (n/N) |
|---|
|
|
|
|---|
| Variables | Grade III | Grade IV | Recurrent Grade
III | Recurrent Grade
IV |
|---|
| Sex |
|
|
|
|
| Male | 63.8 (30/47) | 62.5 (55/88) | 60.0 (15/25) | 63.6 (14/22) |
|
Female | 36.2 (17/47) | 37.5 (33/88) | 40.0 (10/25) | 36.4 (8/22) |
| Age (years) |
|
|
|
|
|
≤42 | 44.7 (21/47) | 27.3 (24/88) | 72.0 (18/25) | 36.4 (8/22) |
|
>42 | 55.3 (26/47) | 72.7 (64/88) | 32.0 (7/25) | 63.6 (14/22) |
| Cancer Genome Atlas
subtype |
|
|
|
|
|
Neural | 19.1 (9/47) | 14.8 (13/88) | 24.0 (6/25) | 9.1 (2/22) |
|
Proneural | 38.3 (18/47) | 8.0 (7/88) | 48.0 (12/25) | 22.7 (5/22) |
|
Classical | 29.8 (14/47) | 36.4 (32/88) | 12.0 (3/25) | 22.7 (5/22) |
|
Mesenchymal | 12.8 (6/47) | 40.9 (36/88) | 16.0 (4/25) | 45.5 (10/22) |
| IDH1/2 |
|
|
|
|
|
Mutation | 40.9 (18/44) | 11.7 (9/77) | 70.8 (17/24) | 31.8 (7/22) |
|
Wild-type | 59.1 (26/44) | 88.3 (68/77) | 29.2 (7/24) | 68.2 (15/22) |
| EGFRvIII |
|
|
|
|
|
Mutation | 10.6 (5/47) | 14.8 (13/88) | 4.0 (1/25) | 4.5 (1/22) |
|
Wild-type | 89.4 (42/47) | 85.2 (75/88) | 96.0 (24/25) | 95.5 (21/22) |
| TP53 |
|
|
|
|
|
Mutation | 17.6 (3/17) | 24.4 (11/45) | 7.7 (1/13) | 16.7 (2/12) |
|
Wild-type | 82.4 (14/17) | 75.4 (34/45) | 92.3 (12/13) | 83.3 (10/12) |
| PTEN |
|
|
|
|
|
Mutation | 26.7 (4/15) | 20.9 (9/43) | 0 (0/12) | 18.2 (2/11) |
|
Wild-type | 73.3 (11/15) | 79.1 (34/43) | 100 (12/12) | 81.8 (9/11) |
| MGMT |
|
|
|
|
|
Methylated | 58.3 (21/36) | 40.0 (34/85) | 62.5 (15/24) | 44.4 (8/18) |
|
Unmethylated | 41.7 (15/36) | 60.0 (51/85) | 37.5 (9/24) | 55.6 (10/18) |
| TERT |
|
|
|
|
|
Mutation | 52.6 (20/38) | 34.9 (22/63) | 17.6 (3/17) | 31.6 (6/19) |
|
Wild-type | 47.4 (18/38) | 65.1 (41/63) | 82.4 (14/17) | 68.4 (13/19) |
Dysregulation of RTK kinase family
members in high-grade gliomas after chemoradiotherapy
RTKs play important roles in glioma development, and
alterations of RTKs can influence the results of chemoradiotherapy.
In the present study, the RNA sequencing information of 135 primary
high-grade gliomas and 47 recurrent high-grade gliomas, following
standardized radiotherapy and ≥1 cycle of TMZ chemotherapy, was
analyzed for the expression of 58 RTKs. The expression of 6 RTKs,
including IGF1R, macrophage stimulating 1 receptor, Fms-related
tyrosine kinase (FLT)4, FLT3, fibroblast growth factor receptor 1
(FGFR1) and apoptosis-associated tyrosine kinase (AATK), was
demonstrated to be significantly altered after chemoradiotherapy.
The expression of these RTKs, with the exception of FLT4, was
increased in recurrent gliomas after chemotherapy (Table II).
 | Table II.Significantly changed receptor
tyrosine kinases between initial and recurrent gliomas. |
Table II.
Significantly changed receptor
tyrosine kinases between initial and recurrent gliomas.
| Gene | Full name | Location | Fold-change | P-value |
|---|
| IGF1R | Insulin-like growth
factor 1 receptor | 15q26.3 | 2.0 | 0.01 |
| MST1R | Macrophage
stimulating 1 receptor | 3p21.3 | 1.8 | 0.01 |
| FLT4 | Fms-related
tyrosine kinase 4 | 5q35.3 | 1.5 | 0.02 |
| FLT3 | Fms-related
tyrosine kinase 3 | 13q12 | 0.7 | 0.02 |
| FGFR1 | Fibroblast growth
factor receptor 1 | 8p11.23-p11.22 | 1.4 | 0.05 |
| AATK |
Apoptosis-associated tyrosine kinase | 17q25.3 | 1.4 | 0.05 |
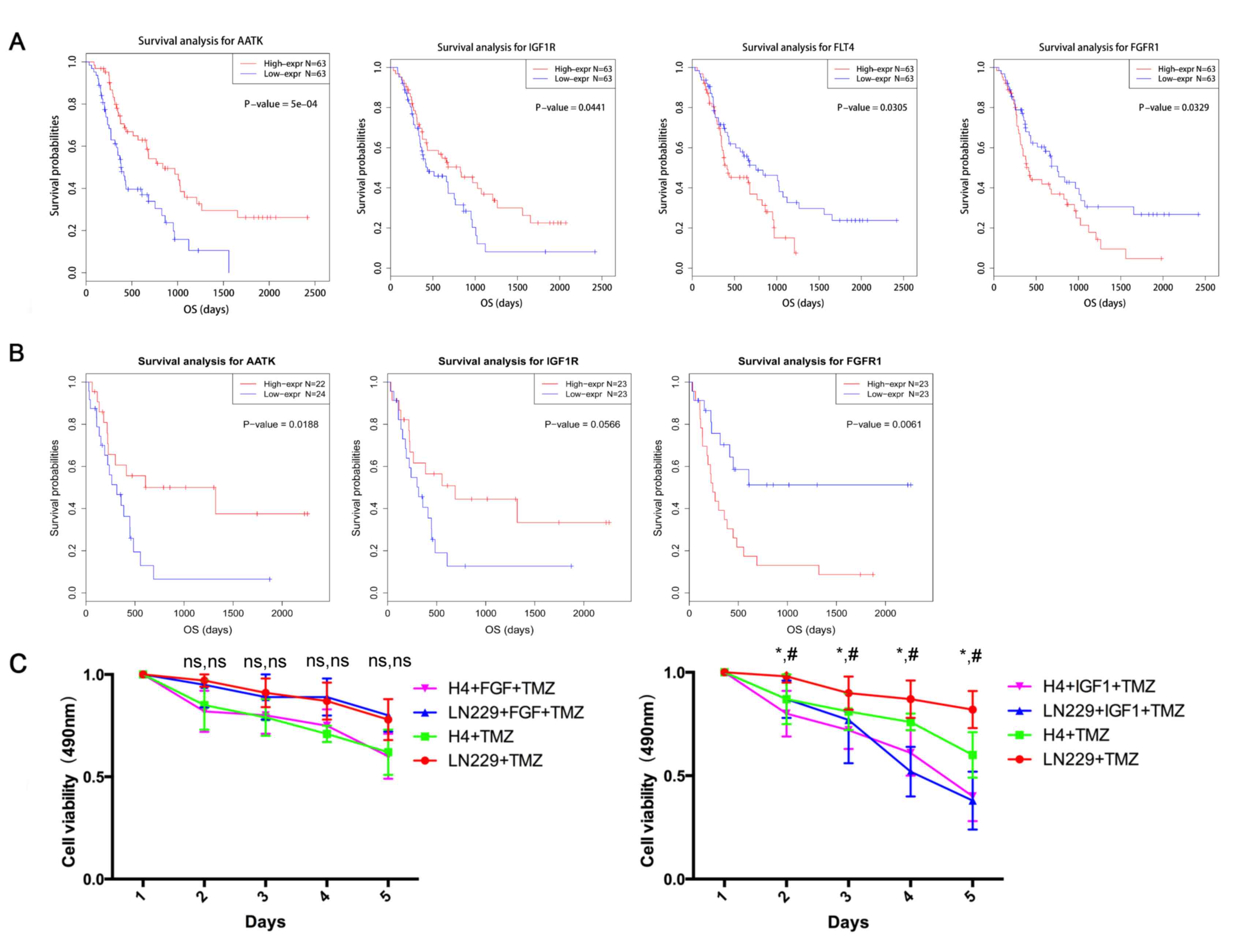
RTKs are associated with prognosis and
tumor cell viability
Among the collected samples, 126 primary high-grade
glioma cases and 46 recurrent high-grade glioma cases had
accessible survival information. Survival analysis indicated that
high expression of IGF1R and AATK, and low expression of FLT4 and
FGFR1 predicted longer overall survival time in primary glioma
(Fig. 1A). In recurrent gliomas,
high expression of AATK and IGF1R, and low expression of FGFR1 were
associated with shorter overall survival time (Fig. 1B). Previous reports have indicated
that the high expression of IGF1R is an independent prognostic
factor in tumors, including glioma and breast cancer (24–27).
Clinical reports have also suggested that high activation of the
Met (HGFR) signaling pathway by HGF can increase
crizotinib-sensitivity in lung cancer (22,24,25).
Therefore, the RTK signaling pathway may influence treatment
effectiveness. In the present study, FGF and IGF were used to
stimulate tumor cells before TMZ treatment, and it was demonstrated
that IGF stimulation assisted TMZ-induced tumor-cell death, whereas
FGF did not induce sensitization to TMZ chemotherapy (Fig. 1C).
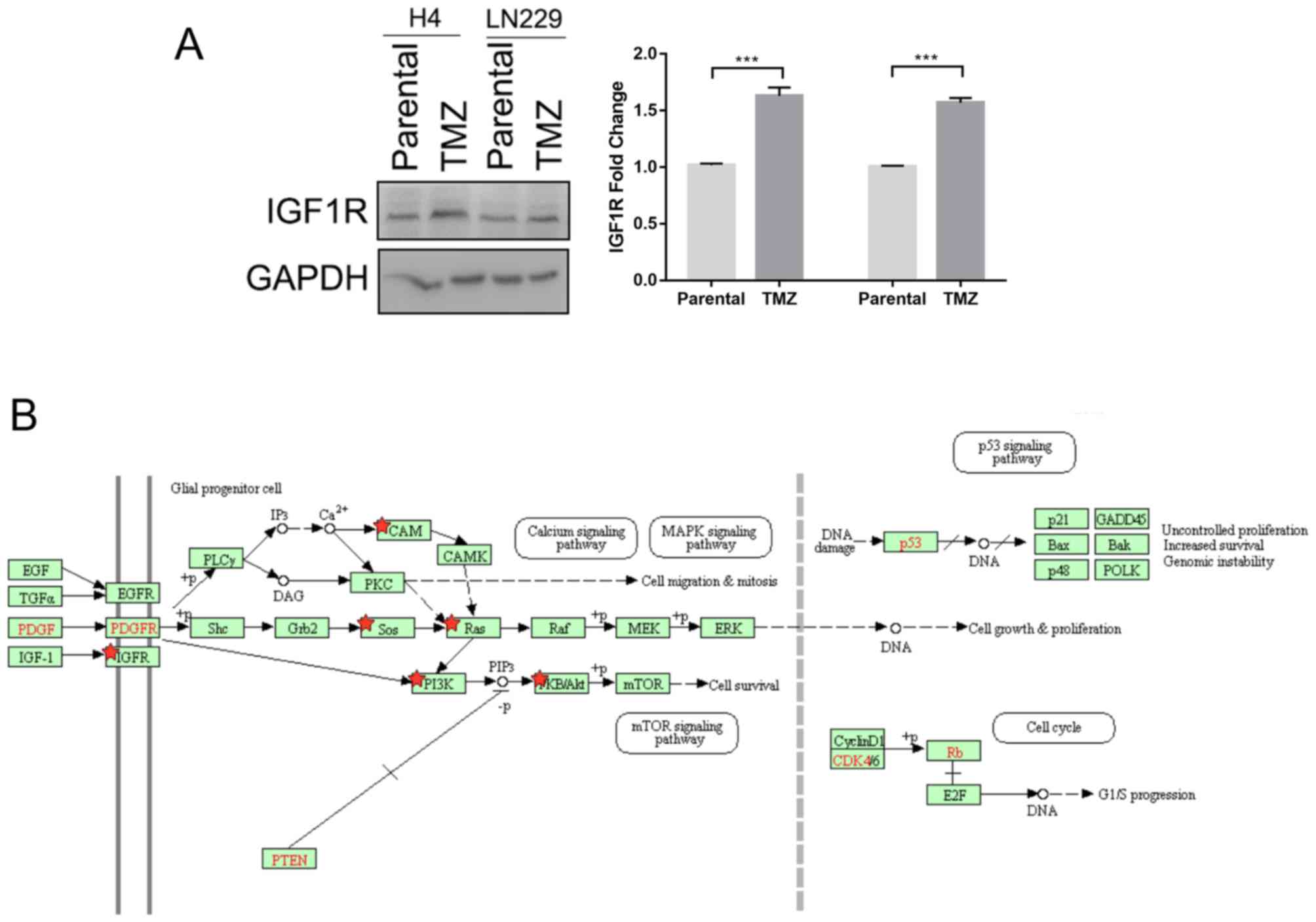
TMZ increases IGF1R expression and
activates IGF1R signaling
RNA sequencing data revealed that the expression of
IGF1R was increased in recurrent gliomas following treatment,
indicative of activation of the IGF1R signal pathway (Table II). This was confirmed in the
present study, in which the expression level of IGF1R was
demonstrated to be increased in glioma cells after TMZ treatment
(Fig. 2A). Differential genes
between primary high-grade gliomas and recurrent gliomas after
chemoradiotherapy were identified, and used to perform pathway
analysis. The results indicated that the IGF1R signaling pathway
was activated by TMZ treatment. The PDGFR signaling pathway was
also demonstrated to be activated. These pathways regulate the cell
cycle and promote cell growth, which may result in tumor-cell
sensitivity to chemoradiotherapy (Fig.
2B).
Discussion
High-grade gliomas, particularly GBM, are the most
common type of primary brain tumor, and have a high mortality rate
(1). Surgical resection followed by
adjuvant chemoradiotherapy is the standard mode of treatment;
however, its effectiveness is limited (3). The biological effects of radiation are
largely the result of DNA damage, which can induce cell death
(16). However, cells attempt to
repair the DNA injury induced by radiation via several pathways
(16). Key genes affecting these
radiation-repair pathways include ATM, which is associated with
ataxia telangiectasia; Ku, which is involved in the repair of
double-strand DNA breaks; and XRCC2 (16). TMZ, an oral alkylating agent, is
currently a first-line treatment for patients with high-grade
glioma (1,3). However, TMZ results in DNA methylation
and the failure of MMR (12). A
number of genes, including MGMT, can cause TMZ-resistance in cancer
cells by repairing TMZ-induced DNA methylation (12). In the present study, the high
frequency of IDH1/2 and MGMT promoter methylation in recurrent
gliomas indicated that gliomas exhibiting these markers were more
likely to recur or to be resistant to chemoradiotherapy.
The RTK family regulates tumor development and
treatment response and previous studies have found that alterations
of RTK function can increase or reduce therapeutic resistance
(13–15). A number of studies focused on IGF1R
have identified that IGF1R expression is associated with numerous
types of tumors, including glioma (26–29).
IGF-1R has also been confirmed as a direct target gene of numerous
microRNAs that are associated with glioma proliferation and
invasion (27,30). The IGF1R pathway has been reported to
be associated with treatment resistance in glioma (29). In the present study, it was indicated
that high expression levels of IGF1R increased sensitivity to
chemoradiotherapy in high-grade gliomas. IGF1R serves a critical
role in transformation events, particularly during
epithelial-mesenchymal transition; it is highly overexpressed in
malignant tissues and plays important roles in tumorigenesis,
metastasis and resistance in numerous types of human tumors
(22,24,25).
However, the prognostic relevance of IGF1R has long been a matter
of controversy. High expression levels of IGF-1R have been
associated with an increased risk of disease and poor survival
rates in patients with tumors in certain studies, while other
studies have demonstrated that high IGF-1R expression may predict a
longer survival time and moderate sensitivity to anti-cancer drugs
in other types of human tumors (25–28).
Liang et al (24) reported
that IGF-1 treatment affected the viability of classical Hodgkin
lymphoma (cHL) cell lines, and increased the phosphorylation of
IGF1R, Akt and ERK, while IGF-1R expression in Hodgkin and
Reed-Sternberg cells predicted a favorable outcome, despite the
oncogenic effect of IGF-1R in cHL cell lines. Mountzios et
al (25) demonstrated that
aberrant expression of components of the IGF1R pathway is
associated with relatively good clinical outcomes in patients with
luminal A and B, node-positive early breast cancer, and suggested
that hormone-receptor positive, HER2-negative tumors may explain,
at least in part, the prognostic role of the IGFR pathway. Another
large-sample study found that IGF1R mRNA expression was a
prognostic marker in the entire cohort and in the luminal subtype
groups (22,24,25). In
the present study, a high IGFR1 mRNA expression level was indicated
to be also a prognostic factor for the survival of patients with
high-grade glioma. Tumor cells stimulated by IGF1 recombinant
cytokine exhibited increased sensitivity to TMZ treatment.
Increased activation of IGF1R was also evident following
radiotherapy and TMZ chemotherapy. IGF1R protein expression was
increased in glioma cells treated with TMZ. These data suggest that
IGF1R signaling may contribute to the response of tumor cells to
chemoradiotherapy. The prognostic value of IGF1R may be dependent
on its expression level in patients with gliomas.
A limitation of the present study is that although
the use of paired tumor samples to compare the biological changes
after treatment would have been the optimal approach, this was not
possible. The reason is that during the course of treatment, the
majority of patients either refused another surgery following
recurrence, or underwent surgery elsewhere. Therefore, the
collection of paired samples for analysis was limited. Further
investigation of the downstream effectors of IGF1R is required. The
differences in the expression of these genes in the dataset before
and after chemoradiotherapy should be analyzed and the results
further verified by cell experiments. In addition, the knockdown of
key genes should be conducted to determine their effect on
sensitivity to TMZ, as this may assist in selecting the optimal
treatment for glioma.
In conclusion, the present study demonstrated that
the expression levels of some RTKs are significantly altered after
chemoradiotherapy in patients with glioma. These RTKs may serve
important roles in the regulation of therapeutic sensitivity and
the results of the present study may provide a basis for future
research. High IGF-1R expression in patients with glioma predicts a
favorable outcome, and may be included in future clinical risk
stratification following validation by future large prospective
studies.
Acknowledgements
Not applicable.
Funding
The present study was supported by funding from The
National Nature Science Foundation of China (grant no.
81502495).
Availability of data and materials
The datasets generated and analyzed during the
present study are available in the CGGA repository (http://www.cgga.org.cn).
Authors' contributions
YL and KW designed the experiments. KW, RH, CW and
ZZ analyzed the data and contributed analytical tools. KW, GL and
HH performed the experiments. RH and KW wrote the paper.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Beijing Tiantan Hospital (Beijing, China), and written informed
consent was obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jiang T, Mao Y, Ma W, Mao Q, You Y, Yang
X, Jiang C, Kang C, Li X, Chen L, et al: CGCG clinical practice
guidelines for the management of adult diffuse gliomas. Cancer
Lett. 375:263–273. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stupp R, Brada M, van den Bent MJ, Tonn JC
and Pentheroudakis G; ESMO Guidelines Working Group, : High-grade
glioma: ESMO clinical practice guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 25 (Suppl):iii93–iii101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cancer Genome Atlas Research Network, ;
Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR, Cooper LA,
Rheinbay E, Miller CR, Vitucci M, et al: Comprehensive, integrative
genomic analysis of diffuse lower-grade gliomas. New Engl J Med.
372:2481–2498. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mock A, Geisenberger C, Orlik C, Warta R,
Schwager C, Jungk C, Dutruel C, Geiselhart L, Weichenhan D,
Zucknick M, et al: LOC283731 promoter hypermethylation
prognosticates survival after radiochemotherapy in IDH1 wild-type
glioblastoma patients. Int J Cancer. 139:424–432. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Turcan S, Rohle D, Goenka A, Walsh LA,
Fang F, Yilmaz E, Campos C, Fabius AW, Lu C, Ward PS, et al: IDH1
mutation is sufficient to establish the glioma hypermethylator
phenotype. Nature. 483:479–483. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cairncross G, Wang M, Shaw E, Jenkins R,
Brachman D, Buckner J, Fink K, Souhami L, Laperriere N, Curran W
and Mehta M: Phase III trial of chemoradiotherapy for anaplastic
oligodendroglioma: Long-term results of RTOG 9402. J Clin Oncol.
31:337–343. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Intergroup Radiation Therapy Oncology
Group Trial 1942, ; Cairncross G, Berkey B, Shaw E, Jenkins R,
Scheithauer B, Brachman D, Buckner J, Fink K, Souhami L, et al:
Phase III trial of chemotherapy plus radiotherapy compared with
radiotherapy alone for pure and mixed anaplastic oligodendroglioma:
Intergroup radiation therapy oncology group trial 9402. J Clin
Oncol. 24:2707–2714. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kaloshi G, Benouaich-Amiel A, Diakite F,
Taillibert S, Lejeune J, Laigle-Donadey F, Renard MA, Iraqi W,
Idbaih A, Paris S, et al: Temozolomide for low-grade gliomas:
predictive impact of 1p/19q loss on response and outcome.
Neurology. 68:1831–1836. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bettegowda C, Agrawal N, Jiao Y, Sausen M,
Wood LD, Hruban RH, Rodriguez FJ, Cahill DP, McLendon R, Riggins G,
et al: Mutations in CIC and FUBP1 contribute to human
oligodendroglioma. Science. 333:1453–1455. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yip S, Butterfield YS, Morozova O,
Chittaranjan S, Blough MD, An J, Birol I, Chesnelong C, Chiu R,
Chuah E, et al: Concurrent CIC mutations, IDH mutations, and 1p/19q
loss distinguish oligodendrogliomas from other cancers. J Pathol.
226:7–16. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hegi ME, Diserens AC, Gorlia T, Hamou MF,
de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani
L, et al: MGMT gene silencing and benefit from temozolomide in
glioblastoma. N Engl J Med. 352:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schlessinger J: Cell signaling by receptor
tyrosine kinases. Cell. 103:211–225. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Regad T: Targeting RTK signaling pathways
in cancer. Cancers(Basel). 7:1758–1784. 2015.PubMed/NCBI
|
|
15
|
Sundaram MV: RTK/Ras/MAPK signaling.
WormBook. 1–19. 2006.PubMed/NCBI
|
|
16
|
Blume-Jensen P and Hunter T: Oncogenic
kinase signalling. Nature. 411:355–365. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ou SH, Soo RA, Kubo A, Kawaguchi T and Ahn
MJ: Will the requirement by the US FDA to simultaneously Co-develop
companion diagnostics (CDx) delay the approval of receptor tyrosine
kinase inhibitors for RTK-rearranged (ROS1-, RET-, AXL-, PDGFR-α-,
NTRK1-) non-small cell lung cancer globally? Front Oncol. 4:582014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hata AN, Niederst MJ, Archibald HL,
Gomez-Caraballo M, Siddiqui FM, Mulvey HE, Maruvka YE, Ji F, Bhang
HE, Krishnamurthy Radhakrishna V, et al: Tumor cells can follow
distinct evolutionary paths to become resistant to epidermal growth
factor receptor inhibition. Nat Med. 22:262–269. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie Q, Bradley R, Kang L, Koeman J,
Ascierto ML, Worschech A, De Giorgi V, Wang E, Kefene L, Su Y, et
al: Hepatocyte growth factor (HGF) autocrine activation predicts
sensitivity to MET inhibition in glioblastoma. Proc Natl Acad Sci
USA. 109:570–575. 2016. View Article : Google Scholar
|
|
20
|
Liu Y, Hu H, Wang K, Zhang C, Wang Y, Yao
K, Yang P, Han L, Kang C, Zhang W and Jiang T: Multidimensional
analysis of gene expression reveals TGFB1I1-induced EMT contributes
to malignant progression of astrocytomas. Oncotarget.
5:12593–12606. 2014.PubMed/NCBI
|
|
21
|
Cartland SP, Genner SW, Zahoor A and
Kavurma MM: Comparative evaluation of TRAIL, FGF-2 and
VEGF-A-induced angiogenesis in vitro and in vivo. Int J Mol Sci.
17(pii): E20252016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gong Y, Yao E, Shen R, Goel A, Arcila M,
Teruya-Feldstein J, Zakowski MF, Frankel S, Peifer M, Thomas RK, et
al: High expression levels of total IGF-1R and sensitivity of NSCLC
cells in vitro to an anti-IGF-1R antibody (R1507). PLoS One.
4:e72732009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou Y, Li S, Li J, Wang D and Li Q:
Effect of microRNA-135a on cell proliferation, migration, invasion,
apoptosis and tumor angiogenesis through the IGF-1/PI3K/Akt
signaling pathway in non-small cell lung cancer. Cell Physiol
Biochem. 42:1431–1446. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liang Z, Diepstra A, Xu C, van Imhoff G,
Plattel W, Van Den Berg A and Visser L: Insulin-like growth factor
1 receptor is a prognostic factor in classical Hodgkin lymphoma.
PLoS One. 9:e874742014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mountzios G, Aivazi D, Kostopoulos I,
Kourea HP, Kouvatseas G, Timotheadou E, Zebekakis P, Efstratiou I,
Gogas H, Vamvouka C, et al: Differential expression of the
insulin-like growth factor receptor among early breast cancer
subtypes. PLoS One. 9:e914072014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Almiron Bonnin DA, Ran C, Havrda MC, Liu
H, Hitoshi Y, Zhang Z, Cheng C, Ung M and Israel MA:
Insulin-mediated signaling facilitates resistance to PDGFR
inhibition in proneural hPDGFB-driven gliomas. Mol Cancer Ther.
16:705–716. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang H, Tang C, Na M, Ma W, Jiang Z, Gu Y,
Ma G, Ge H, Shen H and Lin Z: miR-422a inhibits glioma
proliferation and invasion by targeting IGF1 and IGF1R. Oncol Res.
25:187–194. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arun S, Vanisree AJ and Ravisankar S:
Connexin 30 downregulates Insulin-like growth factor receptor-1,
abolishes Erk and potentiates effects of an IGF-R inhibitor in a
glioma cell line. Brain Res. 1643:80–90. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma Y, Tang N, Thompson RC, Mobley BC,
Clark SW, Sarkaria JN and Wang J: InsR/IGF1R pathway mediates
resistance to EGFR inhibitors in glioblastoma. Clin Cancer Res.
22:1767–1776. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang J, Wang W, Fang D, Jin X, Ding L and
Sun X: MicroRNA-186 targets IGF-1R and exerts tumor-suppressing
functions in glioma. Mol Med Rep. 16:7821–7828. 2017. View Article : Google Scholar : PubMed/NCBI
|