Introduction
Lung cancer is one of the most common malignancies
in the world, including small cell lung cancer and non-small cell
lung cancer (NSCLC). NSCLC includes squamous cell carcinoma,
adenocarcinoma, and large cell carcinoma. Compared with small cell
carcinoma, NSCLC cells grow slowly and have a relatively slow
diffusion and metastasis (1). In the
past 50 years, many countries have reported a significant increase
in incidence and mortality of lung cancer. However, the cause of
lung cancer is still unclear to a certain extent. It has been shown
that long-term heavy smoking is closely related to the occurrence
of lung cancer (2). Moreover, 80–85%
of lung cancer is NSCLC. Postoperative recurrence of NSCLC is the
most prominent feature of treatment (3). Poor prognosis is also an important
feature in patients with advanced NSCLC (4). Therefore, early detection, diagnosis
and treatment are the key to improving the cure rate of NSCLC.
Different functions of microRNAs (miRNAs) have been
identified in human cancers, including NSCLC. For example, miR-598
suppressed migration and invasion by inhibiting
epithelial-mesenchymal transition (EMT) and Derlin-1 in NSCLC
(5). miR-550a-3p promoted
proliferation and metastasis of NSCLC cells by downregulating TIMP2
(6). Recently, miR-133a, a type of
non-coding RNA, was demonstrated to function as gene expression
regulators involved in tumorigenesis of human cancers. For example,
the inhibitory effect of miR-133a was identified in gallbladder
carcinoma (7). Moreover, it was
reported that the low expression of miR-133a was associated with
adverse clinical outcomes in patients with esophageal squamous cell
cancer (8). In addition, the low
expression of miR-133a was found to predict poor prognosis in NSCLC
patients (9). Moreover, previous
studies have shown that miR-133a exerts its effects by regulating
the expression of target genes, such as USP39 (10). However, the regulatory mechanism of
miR-133a remains unclear in NSCLC. Therefore, the role and target
gene of miR-133a was explored in NSCLC.
As a prominent member of Src family tyrosinekinases
(SFKs), YES proto-oncogene 1 (YES1) is a key regulator for tumor
growth (11). It was reported that
the important role of YES1 is also involved in the development of
human cancers. Inhibition of YES1 restrained the migration,
invasion and proliferation of pancreatic cancer cells (12). Moreover, YES1 was identified as a
central mediator of cell growth in malignant mesothelioma (13). Additionally, YES1 has been found to
play an important role in the nuclear translocation of epidermal
growth factor receptor (14). Seki
et al (15) proposed that
YES1 functioned as a proto-oncogene in primary human gastric
cancer. In NSCLC, the function of YES1 and the interaction with
miR-133a are ambiguous.
This study focused on measuring the abnormal
expression of miR-133a and analyzing the prognostic relevance of
miR-133a in NSCLC. In addition, the effect of miR-133a on the
proliferation of NSCLC cells was also investigated. Furthermore,
the relationship between miR-133a and YES1 was verified. This study
will help us better understand the molecular mechanism of miR-133a
in NSCLC.
Materials and methods
Clinical tissues
Fifty-two human NSCLC tissues and adjacent normal
lung tissues (from patients with lung adenocarcinoma) were obtained
from The Affiliated Zhuzhou Hospital of Xiangya Medical College CSU
(Zhuzhou, China). NSCLC patients provided informed consent and
received no other treatment except surgery. Each sample was
confirmed by histopathological evaluation using hematoxylin and
eosin staining. Clinical data were recorded at the time of
resection and patients were prospectively followed-up to ascertain
vital status. The tissues were frozen in liquid nitrogen and then
stored at −80°C in a refrigerator. This study was approved by the
Institutional Ethics Committee of The Affiliated Zhuzhou Hospital
of Xiangya Medical College CSU.
Cell line culture
Normal human bronchial epithelial cell line BEAS-2B
(ATCC® CRL-9609™) and H1299 (ATCC®
CRL-5803™), A549 (ATCC® CCL-185™) NSCLC cell lines were
obtained from ATCC (Manassas). The cells were then seeded in
RPMI-1640 medium containing 10% fetal bovine serum (FBS) and
incubated at 37°C in an atmosphere with 5% CO2 for 24 h.
In addition, 293 cells (BNCC100530, BNCC) were cultured in DMEM
medium supplemented with 10% fetal bovine serum (FBS), 100 U/ml
penicillin, and 100 mg/ml streptomycin (Invitrogen; Thermo Fisher
Scientifc, Inc.) and incubated at 37°C in an atmosphere with 5%
CO2 for 24 h.
Cell transfection
miR-133a mimics (forward,
5′-UUUGGUCCCCUUCAACCAGCUG-3′ and reverse,
5′-GCUGGUUGAAGGGGACCAAAUU-3′) or inhibitor
(5′-CAGCUGGUUGAAGGGGACCAAA-3′) and the negative control (miR-NC
forward, 5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′) were obtained from GenePharma
(Shanghai, China). YES1 siRNA (5′-ACTCAGGAATCGGCTCTGGAA-3′) and
scramble siRNA (si-NC, 5′-GACCTGTACGCCAACACAGTG-3′) were also
obtained from GenePharma. They were then transferred to A549 and
H1299 cells using Lipofectamine 2000 (Invitrogen), respectively,
according to the manufacturer's protocols.
Plasmid constructs
For the overexpression of YES1, the primers used for
amplification were: YES1 forward, 5′-GCGGGTACCATGGGCTGCATTAAAA-3′
and reverse, 5′-GGCCTCGAGTTATAAATTTTCTCCTGGCT-3′. The YES1 cDNA
products were cloned into the mammalian expression pcDNA3 vector
(Invitrogen) at sites ‘KpnI and XhoI’ (Takara). Next,
the vector was transfected into A549 and H1299 cells with miR-133a
mimics using Lipofectamine 2000 (Invitrogen) for 48 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA in NSCLC tissues and cell lines was
extracted using TRIzol reagent (Invitrogen). cDNA was synthesized
using the PrimeScript™ RT Reagent kit (DRR037A; Takara). We
performed RT-qPCR using SYBR-Premix Ex Taq™ II (DRR041A; Takara) on
the ABI 7300 HT Sequence Detection System (Biosystems). The cycling
conditions were: 95°C for 15 sec, then 40 cycles at the following
conditions: 94°C for 15 sec, 56°C for 15 sec, 66°C for 30 sec. The
normalization of miRNA and mRNA was U6 and GAPDH, respectively. U6
was used for the normalization of miR-133a. GAPDH was used for the
normalization of YES1. The expression was calculated using the
2−∆∆cq method (16). All
RT-qPCRs were run in triplicate. The specific primer pairs used
were: miR-133a forward, 5′-TGCTTTGCTAGAGCTGGTAAAATG-3′ and reverse,
5′-AGCTACAGCTGGTTGAAGGG-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and
reverse, 5′-AACGCTTCACGAATTTGCGT-3′; YES1 forward,
5′-TCCAGAACTTTTTCACTTCAGTC-3′ and reverse,
5′-TCTACATTTTCCTCTCTGTTCATC-3′; GAPDH forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′.
CCK-8 assay
CCK-8 assay was performed to measure cell
proliferation based on the manufacturer's instructions. Next,
5×104 NSCLC cells were incubated in 96-well plates for
0, 24, 48 and 72 h at 37°C. They were placed in an incubator
containing 5% CO2 at 37°C. Subsequently, 10 µl CCK-8
reagents (Dojindo) were added to incubate the cells for 2 h.
Finally, the cells were detected using a microplate reader
(Molecular Devices) at an absorbance of 450 nm. All experiments
were run in triplicate.
Bioinformatics analysis
Bioinformatics prediction TargetScan version 7.1
online software (www.targetscan.org) was used to predict the potential
targets of miR-133a, according to the manufacturer's instructions.
Briefly, human was selected as the target species and miR-133a was
inserted as the investigated miRNA. miR-133a was predicted to be
able to directly bind to the seeding sequences of the 3′-UTR of
YES1.
Dual luciferase assay
Luciferase reporter vectors were constructed by
amplifying the 3′-UTR of wild-type or mutant YES1 and subcloning
them downstream of the luciferase gene in the pcDNA3.1 luciferase
vector (Promega). Then, 50 ng of the above vectors containing
firefly luciferase together with 25 ng of miR-133a mimics were
transfected into human embryonic kidney 293 cells (ATCC®
CRL-3216™). The high transfection efficiency of 293 cells
contributes to show clearer changes in luciferase activity to
verify the relationship between miR-133a and YES1. 293 cells only
with 50 ng of pcDNA3.1 luciferase vector were used as the control
(Blank). Subsequently, the luciferase activity was measured through
dual luciferase assay system (Promega). All experiments were run in
triplicate.
Statistical analysis
Data were shown as mean ± SD. The data were analyzed
using SPSS 19.0 or Graphpad Prism 6 (GraphPad Software, Inc.). The
association between miR-133a and clinicopathological
characteristics in NSCLC patients was calculated by χ2
test. Pearson's correlation analysis was performed to examine the
correlation between the miR-133a and YES1 expression in NSCLC
tissues. Differences between groups were calculated by Tukey's
one-way ANOVA. Survival curves were detected by Kaplan-Meier
analysis and Cox analysis. Log-rank test was used to compare
survival differences. Significant difference was defined at
P<0.05.
Results
miR-133a is downregulated in
NSCLC
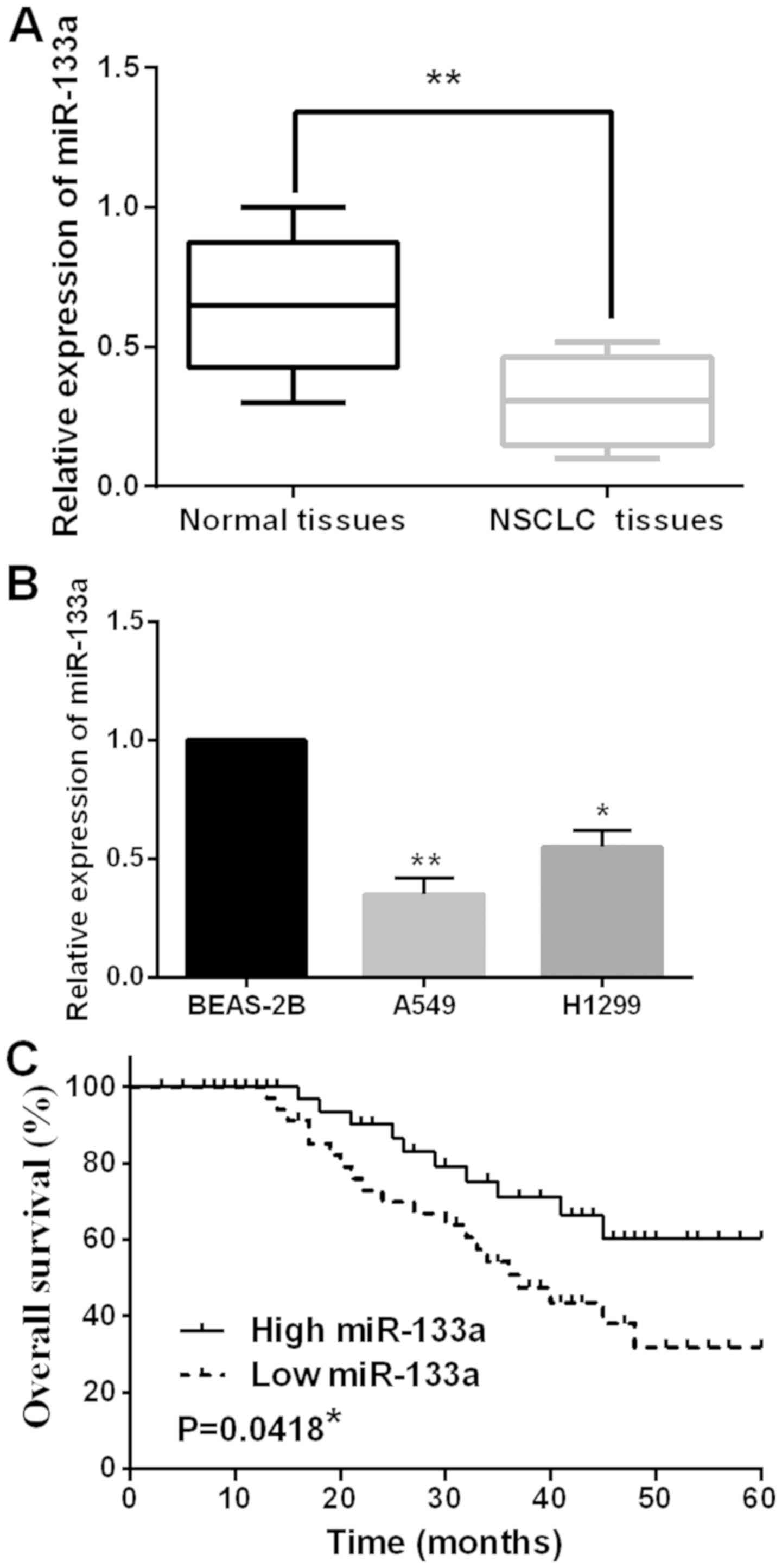
First, the expression levels of miR-133a in 52 cases
of NSCLC tissues were examined. The median relative expression
level of miR-133a in these tissues was taken as a cut-off point to
separate low and high expression of miR-133a. We found that the
aberrant expression of miR-133a was associated with lymph node
metastasis and tumor stage (Table
I). Furthermore, the expression of miR-133a was reduced in
NSCLC tissues compared to normal tissues (Fig. 1A). Then, miR-133a expression was
observed in H1299, A549 and BEAS-2B cell lines. Similarly,
downregulation of miR-133a was also detected in H1299 and A549 cell
lines compared to BEAS-2B cells (Fig.
1B). In addition, low miR-133a expression was found to have a
shorter overall survival rate in NSCLC patients (P=0.0418, Fig. 1C). These results suggest that
miR-133a may be involved in tumorigenesis of NSCLC.
 | Table I.Association between miR-133a
expression and their clinicopathological characteristics in NSCLC
patients. |
Table I.
Association between miR-133a
expression and their clinicopathological characteristics in NSCLC
patients.
|
|
| miR-133a |
|
| YES1 |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Characteristics | Cases | High | Low | P-value | Cases | High | Low | P-value |
|---|
| Age (years) |
|
|
| 0.256 |
|
|
| 0.325 |
| ≥60 | 30 | 12 | 18 |
| 31 | 22 | 9 |
|
|
<60 | 22 | 5 | 17 |
| 21 | 16 | 5 |
|
| Sex |
|
|
| 0.235 |
|
|
| 0.301 |
| Male | 32 | 9 | 23 |
| 33 | 24 | 9 |
|
|
Female | 20 | 8 | 12 |
| 19 | 14 | 5 |
|
| Tumor size (mm) |
|
|
| 0.082 |
|
|
| 0.073 |
| ≤3 | 35 | 9 | 26 |
| 36 | 28 | 8 |
|
|
>3 | 17 | 8 | 9 |
| 16 | 10 | 6 |
|
| Lymph nodes
metastasis |
|
|
| 0.048a |
|
|
| 0.032a |
|
Yes | 12 | 5 | 7 |
| 11 | 5 | 6 |
|
| No | 40 | 12 | 28 |
| 41 | 33 | 8 |
|
| Tumor stage |
|
|
| 0.045a |
|
|
| 0.033a |
|
I–II | 41 | 11 | 30 |
| 39 | 31 | 8 |
|
|
III–IV | 11 | 6 | 5 |
| 13 | 7 | 6 |
|
Overexpression of miR-133a inhibits
cell proliferation in NSCLC
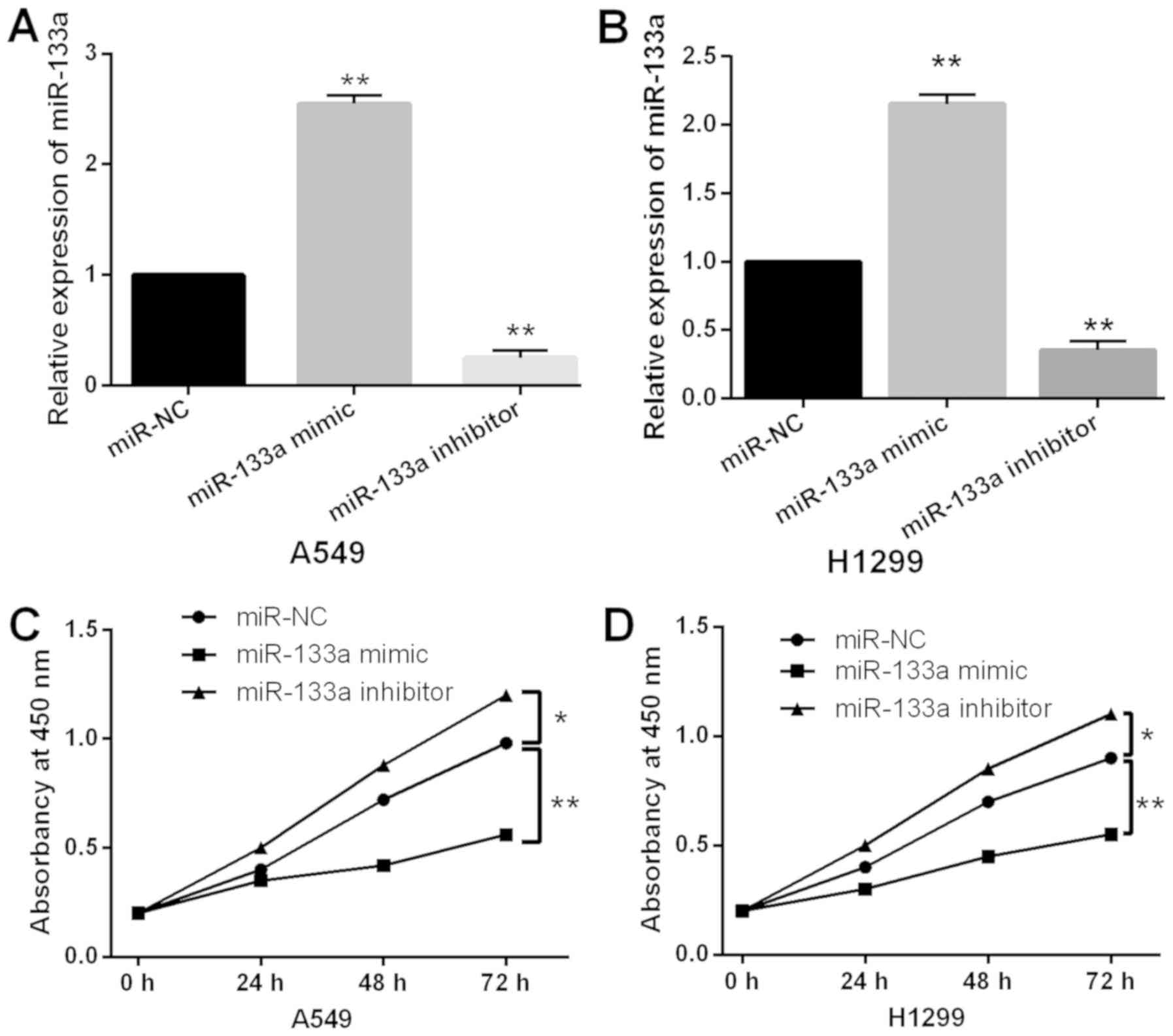
To explore the role of miR-133a in NSCLC, A549 and
H1299 cells with miR-133a mimics or inhibitor was prepared to
perform gain-loss functional experiment. First, we found that
miR-133a expression was increased by miR-133a mimics and decreased
by miR-133a inhibitor in A549 and H1299 cells (Fig. 2A and B). Next, the overexpression of
miR-133a was found to inhibit cell proliferation in A549 cells.
Additionally, downregulation of miR-133a promoted A549 cell
proliferation (Fig. 2C).
Consistently, the same effect of miR-133a on cell proliferation was
also identified in H1299 cells (Fig.
2D). These findings revealed that miR-133a regulated
tumorigenesis of NSCLC by restraining cell proliferation.
YES1 is a direct target gene of
miR-133a
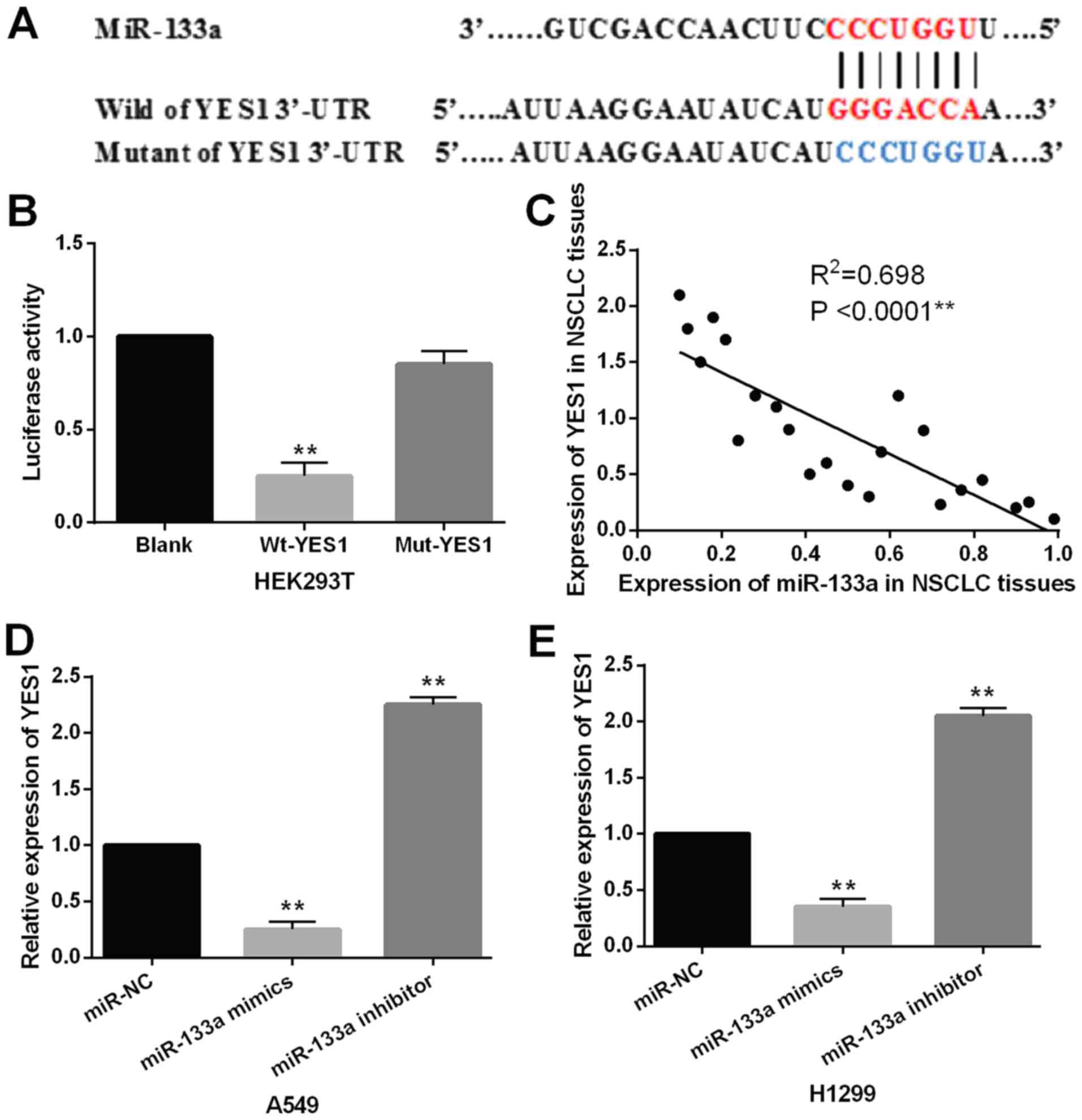
YES1 was selected as a target gene of miR-133a.
TargetScan database (http://www.targetscan.org/) shows that miR-133a has a
binding site with the 3′-UTR of YES1 (Fig. 3A). Luciferase reporter was preformed
to confirm this prediction in 293 cells. We found that miR-133a
mimics significantly reduced the luciferase activity of Wt-YES1.
However, the luciferase activity of Mut-YES1 was not affected by
miR-133a mimics (Fig. 3B). Then, a
negative correlation between YES1 and miR-133a was identified in
NSCLC tissues (P<0.0001, R2=0.698; Fig. 3C). Next, the expression level of YES1
was examined in A549 and H1299 cells with miR-133a mimics or
inhibitor. The results showed that miR-133a mimics reduced YES1
expression, while miR-133a inhibitor enhanced YES1 expression in
A549 and H1299 cells (Fig. 3D and
E). Thus, YES1 was confirmed as a direct target of miR-133a and
inversely regulated miR-133a expression in NSCLC.
Downregulation of YES1 inhibits
proliferation of NSCLC cells
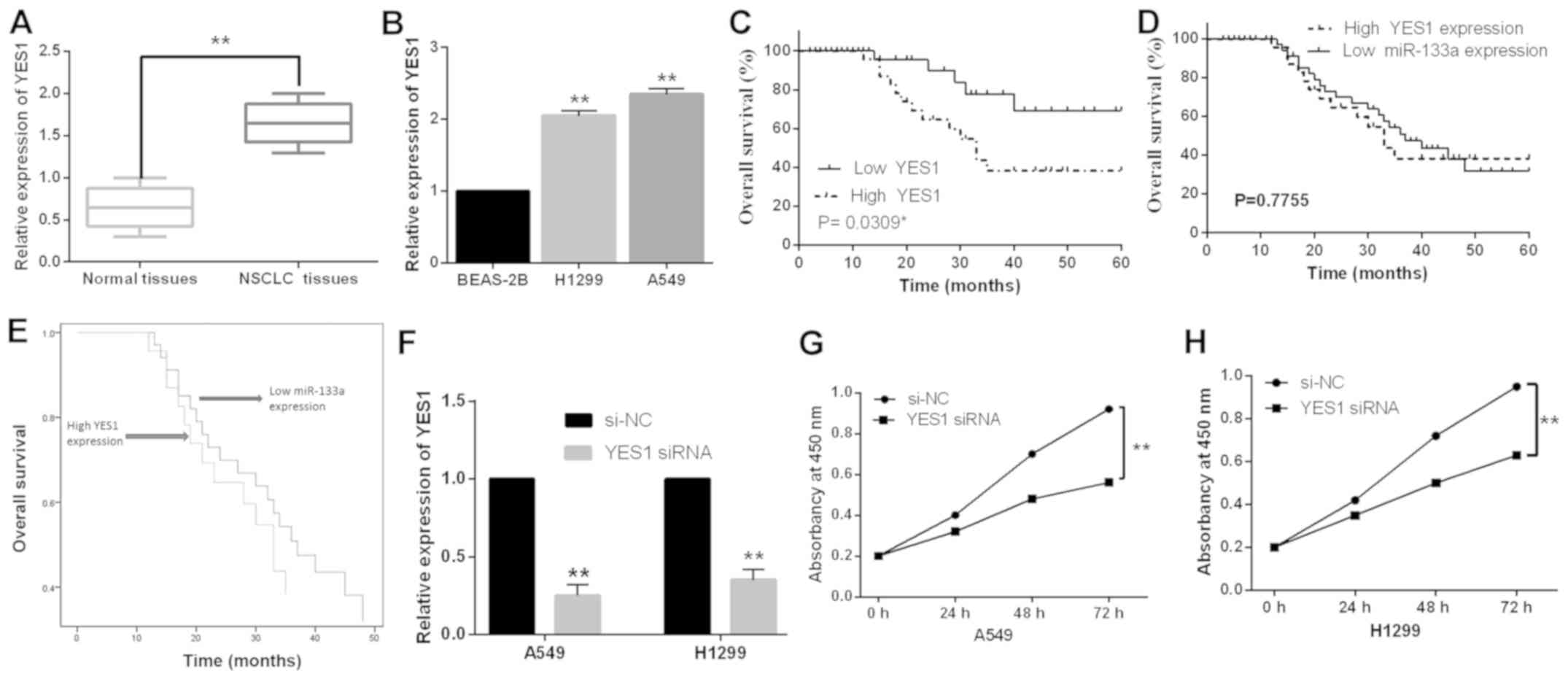
Next, the expression levels of YES1 were detected in
52 cases of NSCLC tissues. The median relative expression level of
YES1 in these tissues was taken as a cut-off point to distinguish
the low or high YES1 expression. The abnormal expression of YES1
was found to be correlated with lymph node metastasis and tumor
stage (Table I). In addition, the
expression of YES1 was increased in NSCLC tissues compared to
normal tissues (Fig. 4A). Similarly,
upregulation of YES1 was also observed in H1299 and A549 cell lines
compared to BEAS-2B cells (Fig. 4B).
Furthermore, in NSCLC patients high YES1 expression was found to
predict poor prognosis (P=0.0309, Fig.
4C). Next, the unfavorable patterns (low miR-133a expression
and high YES expression) were combined in the Kaplan-Meier analysis
and Cox analysis. We found that there was no significant difference
in overall survival rate between low miR-133a expression and high
YES1 expression (P>0.05, Fig. 4D and
E). To explore the effect of YES1 on cell proliferation in
NSCLC, YES1 siRNA was transfected into H1299 and A549 cells.
RT-qPCR showed that the expression of YES1 was significantly
reduced by YES1 siRNA in A549 and H1299 cells compared with the
control (Fig. 4F). Functionally,
knockdown of YES1 was found to inhibit cell proliferation in A549
and H1299 cells (Fig. 4G and H).
These results suggested that YES1 promoted cell proliferation and
predicted prognosis in NSCLC.
Upregulation of YES1 abolishes the
inhibitory effect of miR-133a in NSCLC
To verify the interaction between miR-133a and YES1
in NSCLC, YES1 overexpression vector was transfected into A549 and
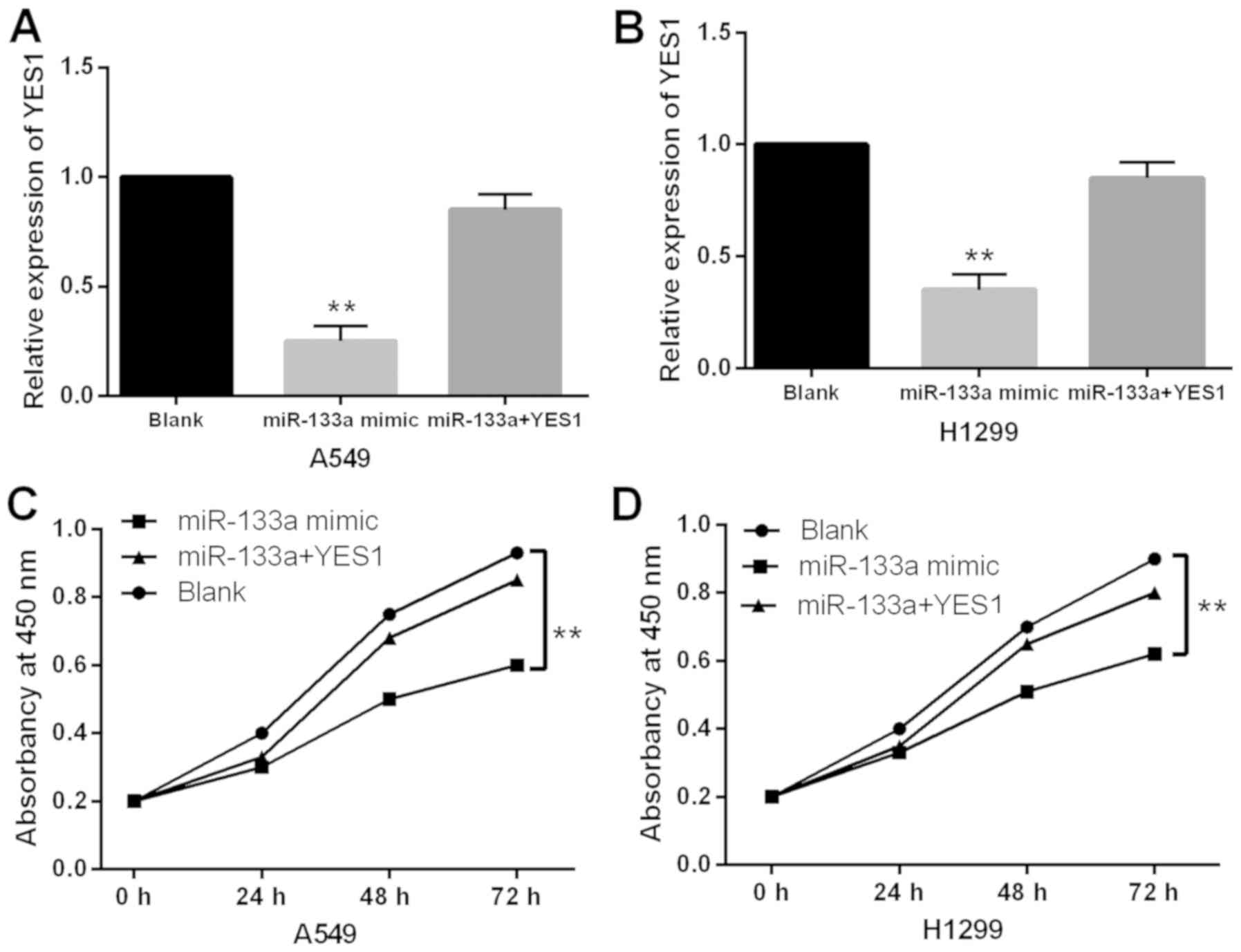
H1299 cells containing miR-133a mimics. First, it was found that
miR-133a mimics-induced decrease in YES1 expression was restored by
YES1 overexpression vector in A549 and H1299 cells (Fig. 5A and 5B). Functionally, the
inhibitory effect of miR-133a on cell proliferation was weakened by
the upregulation of YES1 in A549 and H1299 cells (Fig. 5C and D). Collectively, upregulation
of YES1 abolished the inhibitory effect of miR-133a in NSCLC.
Discussion
An increasing number of miRNAs has been proposed to
be involved in the pathogenesis of human cancer, including NSCLC
(17,18). Among them, the downregulation of
miR-133a was identified in several human cancers, including breast
cancer (19) and colorectal cancer
(20). In this study, miR-133a was
also downregulated in NSCLC. Furthermore, this downregulation was
found to be correlated with adverse lymph node metastasis and tumor
stage in NSCLC patients, which has not been reported in previous
studies. We also found that low miR-133a expression was closely
correlated with shorter overall survival in NSCLC patients.
Consistent with our results, miR-133a was also reported to predict
poor prognosis in patients with hepatocellular carcinoma (21). In addition, the overexpression of
miR-133a was identified to restrain NSCLC cell proliferation in our
study. Previous studies have demonstrated the inhibitory effect of
miR-133a on cell proliferation in gastric cancer (22) and laryngeal carcinoma (23). These findings indicate that miR-133a
may promote the tumor growth of NSCLC. However, the correlation
between miR-133a expression and tumor size in NSCLC patients were
not found in the present study. We speculate that this difference
may be due to the small number of samples we collected. The number
of samples will be expanded to further confirm our results.
In addition, it is well-known that miRNAs exhibit
their effect through regulating expression of target genes
(24). Moreover, miR-133a was also
identified to regulate tumorigenesis by mediating target genes,
such as SENP1 (25) and USP39
(26). To further explain the
regulatory mechanism of miR-133a in NSCLC, we also investigated its
downstream target in this study. Here, YES1 was confirmed as direct
target gene of miR-133a, which has not been found in previous
studies. Furthermore, we found that miR-133a restrained
proliferation of NSCLC cells by downregulating YES1.
The role of YES1 was also explored in the current
study. Upregulation of YES1 was detected in NSCLC, which was
correlated with worse clinical outcomes and prognosis in NSCLC
patients. Moreover, knockdown of YES1 was found to suppress NSCLC
cell proliferation. Similar to our results, it has been reported
that YES1 promoted lung cancer growth and progression (27). In the present study, upregulation of
YES1 was found to abolish the inhibitory effect of miR-133a on cell
proliferation in NSCLC. Similar interaction between YES1 and other
miRNAs was also reported in previous studies, such as miR-140-5p
(28). All these findings suggest
that miR-133a serves as an inhibitor for NSCLC development by
suppressing YES1 expression. However, the role of miR-133a in
vivo and the effect of miR-133a on NSCLC cell metastasis have
not yet been investigated.
In conclusion, downregulation of miR-133a was
detected in NSCLC and was correlated with poor clinical outcomes
and prognosis in NSCLC patients. Importantly, miR-133a inhibited
proliferation of NSCLC cells via targeting YES1. Our findings show
potential to provide a new approach to the diagnosis and treatment
of NSCLC.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
YS contributed to the study design, data analysis
and drafted the manuscript. FC was involved in data acquisition and
revision of the manuscript. YL contributed to the study design,
data analysis, and revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Ethics
Committee of The Affiliated Zhuzhou Hospital of Xiangya Medical
College CSU (Zhuzhou, China). Signed informed consents were
obtained from the patients or their guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ramalingam SS, Owonikoko TK and Khuri FR:
Lung cancer: New biological insights and recent therapeutic
advances. CA Cancer J Clin. 61:91–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sharma A, O'Connor R, Celestino P, Killion
S, Griswold-Krupski L and Bansal-Travers M: Focus groups and
in-depth interviews to guide the development of lung cancer
screening informational materials. J Cancer Educ. Apr 20–2018.(Epub
ahead of print). doi: 10.1007/s13187-018-1362-4.
|
|
3
|
Pao W and Girard N: New driver mutations
in non-small-cell lung cancer. Lancet Oncol. 12:175–180. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tang Z, Li J, Shen Q, Feng J, Liu H, Wang
W, Xu L, Shi G, Ye X, Ge M, et al: Contribution of upregulated
dipeptidyl peptidase 9 (DPP9) in promoting tumorigenicity,
metastasis and the prediction of poor prognosis in non-small cell
lung cancer (NSCLC). Int J Cancer. 140:1620–1632. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang F, Wei K, Qin Z, Liu W, Shao C, Wang
C, Ma L, Xie M, Shu Y and Shen H: miR-598 suppresses invasion and
migration by negative regulation of Derlin-1 and
epithelial-mesenchymal transition in non-small cell lung cancer.
Cell Physiol Biochem. 47:245–256. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang JZ, Bian L, Hou JG and Wang HY:
miR-550a-3p promotes non-small cell lung cancer cell proliferation
and metastasis through down-regulating TIMP2. Eur Rev Med Pharmacol
Sci. 22:4156–4165. 2018.PubMed/NCBI
|
|
7
|
Huang Y, Wu Y, Dong J, Han D, Yang S and
Jiang L: MicroRNA-133a-3p exerts inhibitory effects on gallbladder
carcinoma via targeting RBPJ. Am J Cancer Res. 6:2448–2462.
2016.PubMed/NCBI
|
|
8
|
Gao SH, Liu J, Zhang HJ, Zhao N and Zhang
J: Low miR-133a expression is a predictor of outcome in patients
with esophageal squamous cell cancer. Eur Rev Med Pharmacol Sci.
20:3788–3792. 2016.PubMed/NCBI
|
|
9
|
Wang Y, Li J, Chen H, Mo Y, Ye H, Luo Y,
Guo K, Mai Z, Zhang Y, Chen B, et al: Down-regulation of miR-133a
as a poor prognosticator in non-small cell lung cancer. Gene.
591:333–337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai J, Liu T, Huang P, Yan W, Guo C, Xiong
L and Liu A: USP39, a direct target of microRNA-133a, promotes
progression of pancreatic cancer via the AKT pathway. Biochem
Biophys Res Commun. 486:184–190. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yeung CL, Ngo VN, Grohar PJ, Arnaldez FI,
Asante A, Wan X, Khan J, Hewitt SM, Khanna C, Staudt LM, et al:
Loss-of-function screen in rhabdomyosarcoma identifies CRKL-YES as
a critical signal for tumor growth. Oncogene. 32:5429–5438. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Je DW, O YM, Ji YG, Cho Y and Lee DH: The
inhibition of SRC family kinase suppresses pancreatic cancer cell
proliferation, migration, and invasion. Pancreas. 43:768–776. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sato A, Sekine M, Virgona N, Ota M and
Yano T: Yes is a central mediator of cell growth in malignant
mesothelioma cells. Oncol Rep. 28:1889–1893. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iida M, Brand TM, Campbell DA, Li C and
Wheeler DL: Yes and Lyn play a role in nuclear translocation of the
epidermal growth factor receptor. Oncogene. 32:759–767. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Seki T, Fujii G, Mori S, Tamaoki N and
Shibuya M: Amplification of c-yes-1 proto-oncogene in a primary
human gastric cancer. Jpn J Cancer Res. 76:907–910. 1985.PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang B, Sun L, Li J and Jiang R: miR-577
suppresses cell proliferation and epithelial-mesenchymal transition
by regulating the WNT2B mediated Wnt/β-catenin pathway in non-small
cell lung cancer. Mol Med Rep. 18:2753–2761. 2018.PubMed/NCBI
|
|
18
|
Han L, Chen W, Xia Y, Song Y, Zhao Z,
Cheng H and Jiang T: miR-101 inhibits the proliferation and
metastasis of lung cancer by targeting zinc finger E-box binding
homeobox 1. Am J Transl Res. 10:1172–1183. 2018.PubMed/NCBI
|
|
19
|
Sui Y, Zhang X, Yang H, Wei W and Wang M:
MicroRNA-133a acts as a tumour suppressor in breast cancer through
targeting LASP1. Oncol Rep. 39:473–482. 2018.PubMed/NCBI
|
|
20
|
Li W, Chen A, Xiong L, Chen T, Tao F, Lu
Y, He Q, Zhao L, Ou R and Xu Y: miR-133a acts as a tumor suppressor
in colorectal cancer by targeting eIF4A1. Tumour Biol.
39:10104283176983892017.PubMed/NCBI
|
|
21
|
Sun L, Guo Z, Sun J, Li J, Dong Z, Chen J,
Kan Q and Yu Z: miR-133a acts as an anti-oncogene in hepatocellular
carcinoma by inhibiting FOSL2 through TGF-beta/Smad3 signaling
pathway. Biomed Pharmacother. 107:168–176. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li C, Li X, Gao S, Li C and Ma L:
MicroRNA-133a inhibits proliferation of gastric cancer cells by
downregulating ERBB2 expression. Oncol Res. 25:1169–1176. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li H, Wang Y and Li YZ: MicroRNA-133a
suppresses the proliferation, migration, and invasion of laryngeal
carcinoma cells by targeting CD47. Tumour Biol. 37:16103–16113.
2016. View Article : Google Scholar
|
|
24
|
Farazi TA, Juranek SA and Tuschl T: The
growing catalog of small RNAs and their association with distinct
Argonaute/Piwi family members. Development. 135:1201–1214. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou GQ, Han F, Shi ZL, Yu L, Li XF, Yu C,
Shen CL, Wan DW, Zhu XG, Li R, et al: miR-133a-3p targets
SUMO-specific protease 1 to inhibit cell proliferation and cell
cycle progress in colorectal cancer. Oncol Res. 26:795–800. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dong X, Su H, Jiang F, Li H, Shi G and Fan
L: miR-133a, directly targeted USP39, suppresses cell proliferation
and predicts prognosis of gastric cancer. Oncol Lett. 15:8311–8318.
2018.PubMed/NCBI
|
|
27
|
Garmendia I, Pajares MJ, Hermida-Prado F,
Ajona D, Bértolo C, Sainz C, Lavín A, Remírez AB, Valencia K,
Moreno H, et al: YES1 drives lung cancer growth and progression and
predicts sensitivity to dasatinib. Am J Respir Crit Care Med. Jun
5–2019.(Epub ahead of print). doi: 10.1164/rccm.201807-1292OC.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fang Z, Yin S, Sun R, Zhang S, Fu M, Wu Y,
Zhang T, Khaliq J and Li Y: miR-140-5p suppresses the
proliferation, migration and invasion of gastric cancer by
regulating YES1. Mol Cancer. 16:1392017. View Article : Google Scholar : PubMed/NCBI
|