Introduction
Cancer, a major global public health problem, has
been the first or second leading cause of mortality in the majority
of countries thus far throughout the 21st century, and the
incidence and mortality rates are rapidly increasing worldwide
(1). Despite significant advances in
the diagnosis and treatment of cancer, tumor metastasis is a major
barrier to increasing life expectancy and favorable clinical
outcomes, as >90% of cases of cancer-associated mortality occur
as a result of metastatic disease (2,3).
In cancer, epithelial-to-mesenchymal transition
(EMT) is associated with the metastatic process, tumor stemness and
resistance to therapy (4). EMT is a
cellular process in which cells lose their epithelial
characteristics, acquire mesenchymal features and secrete matrix
metalloproteinases (MMPs), which enable them to adopt more
efficient motile and invasive properties. The common molecular
markers of EMT include the loss of cell-cell adhesion mediated by
downregulation of E-cadherin, and increased expression of
mesenchymal markers (such as N-cadherin and vimentin), MMPs and
transcription factors, including Twist, Slug and Snail (5). Increasing evidence has demonstrated
that EMT can be induced by several growth factors, such as
epidermal growth factor (EGF), the transforming growth factor (TGF)
superfamily, vascular endothelial growth factor and fibroblast
growth factor, produced by tumor-associated stroma (6–9). EGF
activates the intrinsic protein-tyrosine kinase activity of its
receptor EGFR on the cell surface and initiates signal
transduction, such as the PI3K/Akt, Janus kinase and
mitogen-activated protein kinase (MAPK)/ERK signaling pathways,
which have been reported to serve important roles in the EMT
process by regulating the expression of EMT biomarkers (10,11).
TGF-β1 is one of the most potent inducers of EMT via activation of
the classical Smad2/3 signaling pathway, or other downstream
pathways (non-Smad signaling pathways), including the PI3K/Akt, ERK
and c-Jun N-terminal kinase signaling pathways (12,13).
Therefore, altering the EGF- and TGF-β1-mediated signaling pathways
may be an efficient strategy to prevent EMT progression.
Tanshinone IIA (Tan IIA;
C19H18O3), a phenanthrenequinone,
is one of the major lipophilic components extracted from the
medicinal herb Salvia miltiorrhiza Bunge (14). Over the past few decades, Tan IIA has
been proven to possess potential protective effects against cardiac
fibrosis, atherosclerosis, and cardiovascular and endocrine system
diseases (15–18). The anticancer effects and underlying
molecular mechanisms of Tan IIA have also been studied extensively
in a number of different cancer cell types in vitro and
tumor types in vivo (19).
For example, studies have reported that Tan IIA causes apoptosis in
a number of different types of cancer, including esophageal, colon,
breast, lung and liver cancer (20–24). In
addition, Tan IIA has been revealed to inhibit yes-associated
protein 1 transcriptional activity, thereby inhibiting its effects
on cervical carcinoma stem cell migration and invasion (25). Tan IIA has also been demonstrated to
inhibit EMT in human bladder cancer cells via the STAT3-chemokine
(C-C motif) ligand 2 signaling pathway (26). Tan IIA inhibits the migration and
invasion of HNE-1NPC nasopharyngeal carcinoma cells through
inhibition of MMP-2 and MMP-9 (27).
However, the effects of Tan IIA on EGF- and TGF-β1-induced EMT
processes and signaling molecules have not yet been
investigated.
As the molecular interactions between PI3K/Akt and
ERK signaling are prevalent in EGF- and TGF-β1-treated cancer
cells, these interactions have significant roles in the initiation
of EMT (28). Therefore, the present
study aimed to investigate whether Tan IIA inhibits EMT, migration
and invasion in EGF- and TGF-β1-treated HepG2 cells by deactivating
these two signaling pathways, which, to the best of our knowledge,
has not yet been reported. The present study could provide a novel
insight into the anticancer molecular mechanisms of Tan IIA.
Materials and methods
Cell lines and reagents
The human liver cancer HepG2 cell line was purchased
from the Cell Bank of the Institute of Biochemistry and Cell
Biology, Chinese Academy of Sciences. The cells were grown in
high-glucose DMEM supplemented with 10% FBS and 1% glutamine
penicillin-streptomycin solution (all from Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in a 5% CO2 incubator. Tan IIA
with a purity of >98% was purchased from the National Institutes
for Food and Drug Control. Human recombinant EGF and TGF-β1 were
purchased from PeproTech, Inc. MTT, LY294002 and U0126 were
purchased from Sigma-Aldrich; Merck KGaA.
Cell viability assay
HepG2 cells were seeded in 96-well plates
(5×103 cells/well) overnight in an incubator and treated
with Tan IIA (0, 0.25, 0.5, 1, 2, 4 and 8 µM) for 24, 48 and 72 h
at 37°C. A total of 20 µl 5 mg/ml MTT was added to each well, and
the cells were incubated at 37°C for an additional 4 h in an
incubator, the formazan was dissolved with 100 µl of DMSO. A
microplate reader (Bio-Rad Laboratories, Inc.) was used to analyze
the absorbance at a wavelength of 490 nm.
Morphology observations
HepG2 cells were seeded in 6-well plates
(1×105 cells/well) overnight at 37°C, and treated with
EGF (2.5, 5, 10 and 20 ng/ml) for 48 h. Cell morphology images were
captured using a light microscope (magnification ×200, Olympus
Corporation).
Colony formation assay
HepG2 cells were seeded in 6-well plates
(1×103 cells/well) overnight, then co-treated with EGF
(20 ng/ml)/TGF-β1 (10 ng/ml) and Tan IIA (0.5, 1 and 2 µM) at 37°C
for 2 weeks. The cells were washed with PBS, fixed with 4%
paraformaldehyde for 10 min at room temperature, and stained with
0.1% crystal violet for 20 min at room temperature. Colonies were
imaged and counted using Image-Pro Plus software (version 6.0;
National Institutes of Health).
Wound healing assay
HepG2 cells were seeded in a 6-well plate
(3×105 cells/well) in 6-well plates and grown to 100%
confluence. A wound was carefully made with a 10-µl pipette tip in
the middle of the well, and the wells were washed with PBS. The
cells were then incubated with fresh serum-free medium containing
EGF (20 ng/ml)/TGF-β1 (10 ng/ml) with or without Tan IIA (0.5, 1.0
and 2.0 µM) for 24 h at 37°C, and images of cell migration were
acquired at 0 and 24 h using an inverted light microscope (Olympus
Corporation).
Transwell invasion assay
HepG2 cells (3×104 cells/well) in medium
with 1% FBS containing Tan IIA (0.5, 1 and 2 µM) were cultured in
the Matrigel-coated upper chamber of an 8-µm Transwell (Invitrogen;
Thermo Fisher Scientific, Inc.), and 15% FBS medium containing EGF
(20 ng/ml)/TGF-β1 (10 ng/ml) or not was added into the lower
chamber. After 48 h of culture at 37°C in a 5% CO2
incubator, non-invasive cells on the upper side of the membrane
were scraped with cotton swabs, and the cells on the lower surfaces
were fixed with 4% paraformaldehyde for 10 min at room temperature
and stained with 0.1% crystal violet for 20 min at room
temperature. The invasive cells were imaged under a light
microscope, and the crystal violet was dissolved in DMSO. The
absorbance of the invasive cells was analyzed at a wavelength of
600 nm using a microplate reader (Bio-Rad Laboratories, Inc.) to
indicate the invasion rates.
Immunofluorescence analysis
Cells (2×104 cells/well) in 4-well
chamber slides were co-treated with EGF (20 ng/ml)/TGF- β1 (10
ng/ml) and Tan IIA (2 µM) for 48 h at 37°C. Immunofluo-rescence
analysis was performed as previously described (7). After the cells were incubated overnight
at 4°C with anti-E-cadherin (cat. no. 3195) and anti-vimentin (cat.
no. 5741) antibodies (1:200 dilution; Cell Signaling Technology,
Inc.), the cells were incubated with the corresponding secondary
antibodies conjugated to fluorescein isothiocyanate (cat. no. 4412;
1:1,000; Cell Signaling Technology, Inc.) for 1 h at room
temperature, and the nuclei were stained with DAPI (Beyotime
Institute of Biotechnology) for 15 min at room temperature. Images
were acquired using a Zeiss 880 laser confocal microscope (Zeiss
AG).
Western blot analysis
Cells were seeded in 6-well culture plates
(3×105 cells/well) and were untreated or co-treated with
EGF (20 ng/ml)/TGF-β1 (10 ng/ml) and Tan IIA (2 µM)/LY294002 (20
µM)/U0126 (20 µM) for 48 h at 37°C. The total protein was extracted
using RIPA buffer mixed with 1% PMSF (Beyotime Institute of
Biotechnology), and the protein concentration was analyzed using a
bicinchoninic acid protein assay kit (Nanjing KeyGen Biotech Co.,
Ltd.), as previously described (29). Equal amounts of total protein (30 µg)
samples were separated by SDS-PAGE (8–12% gel) and transferred onto
PVDF membranes (EMD Millipore). The membranes were then blocked in
5% skimmed milk for 3 h at room temperature and further incubated
overnight at 4°C with primary antibodies against GAPDH (cat. no.
5174), E-cadherin (cat. no. 3195), N-cadherin (cat. no. 13116),
MMP-2 (cat. no. 87809), Snail (cat. no. 3879), vimentin (cat. no.
5741), Akt (cat. no. 4691), phosphorylated (p)-Akt (cat. no. 4060),
ERK1/2 (cat. no. 4695) and p-ERK1/2 (cat. no. 4376) (1:1,000
dilution; Cell Signaling Technology, Inc.). This was followed by
incubation with the appropriate goat anti-rabbit IgG secondary
antibodies (cat. no. AS014; 1:3,000; ABclonal Biotechnology Co.,
Ltd.) for 1 h at room temperature. The protein bands were
visualized using an ECL reagent (EMD Millipore) and analyzed using
the Tanon 5200 image acquisition system (Tanon Science and
Technology Co., Ltd.), as previously described (30). The band intensity of western blotting
was measured by densitometry using Image-Pro Plus J software
(version 6.0; National Institutes of Health).
Statistical analysis
Data were statistically analyzed using SPSS software
(version 23.0; IBM Corp.). Values are presented as the mean ±
standard deviation using GraphPad Prism software (version 6.0;
GraphPad Software, Inc.). One-way ANOVA followed by Duncan's
post-hoc test was used for comparisons between multiple groups,
while unpaired Student's t-test was used for comparisons between
two groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Establishment of the EGF-induced EMT
model in HepG2 cells
Previous studies have demonstrated that HepG2 cells
treated with 10 ng/ml TGF-β1 for 48 h exhibit fibroblast-like cell
morphology, and a clearly induced EMT phenotype with significantly
altered expression of EMT markers (7,31). The
present study first established a model of EGF-induced EMT in HepG2
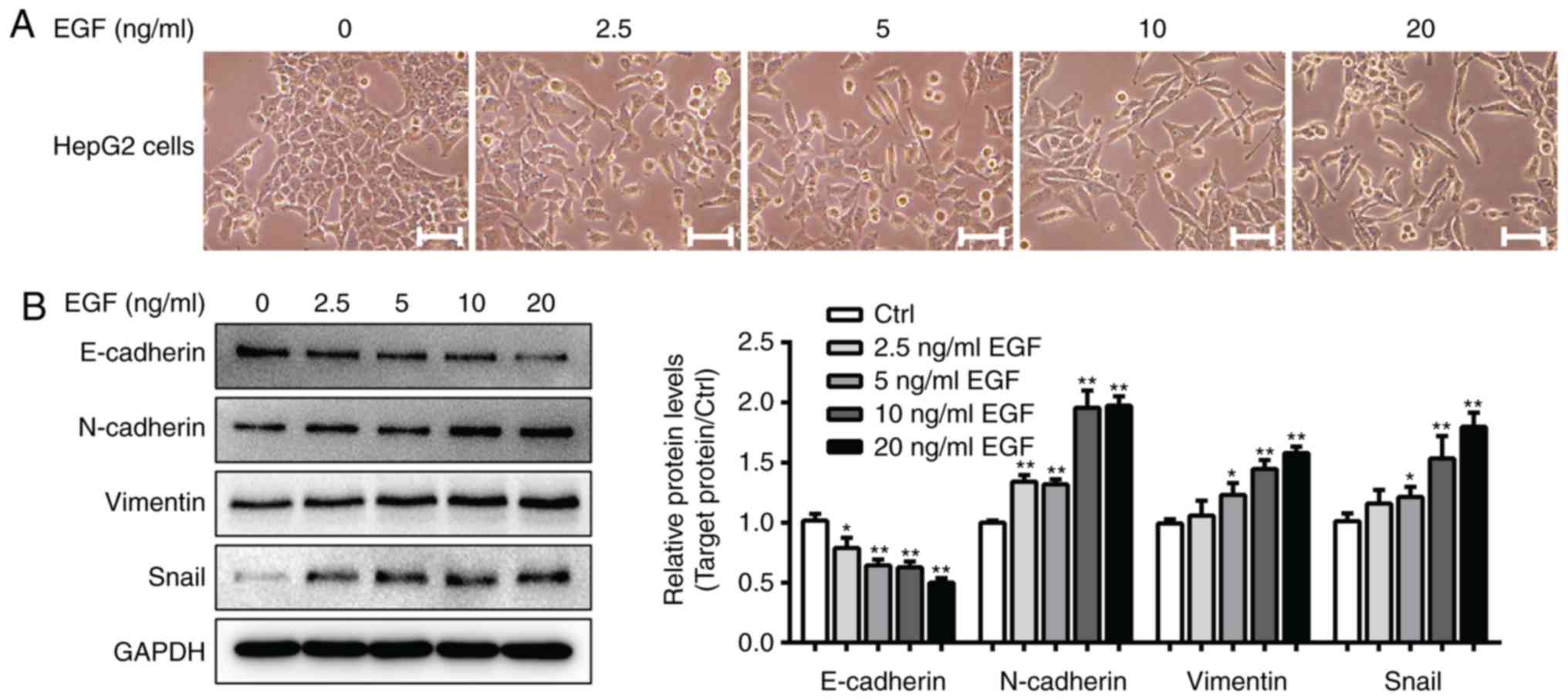
cells. As presented in Fig. 1A,
control HepG2 cells grew in clusters and exhibited a classical
cobblestone epithelial morphology and well-organized cell-cell
associations, whereas HepG2 cells treated with EGF (5–20 ng/ml)
exhibited an EMT-like cell morphology. The cells had a spindle-like
mesenchymal morphology and were separated from one another.
Furthermore, western blot analysis demonstrated that 20 ng/ml EGF
treatment significantly decreased E-cadherin expression and
increased N-cadherin, vimentin and Snail expression levels in
EGF-treated cells compared with in cells from the control group
(P<0.05; Fig. 1B). As a result,
20 ng/ml EGF and 10 ng/ml TGF-β1 were used to induce EMT in the
subsequent experiments.
Tan IIA inhibits the viability and
colony formation of HepG2 cells
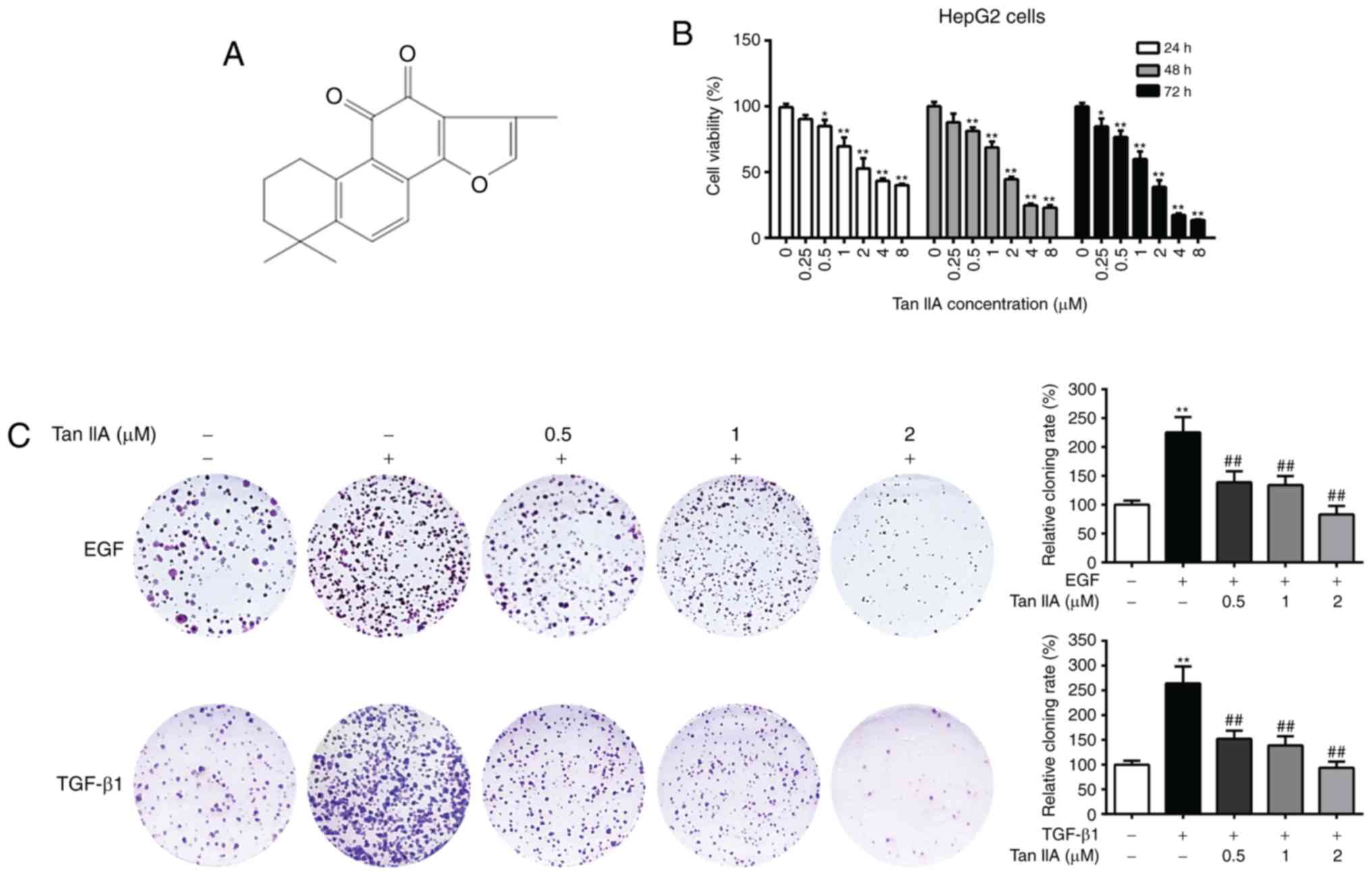
The chemical structure of Tan IIA is presented in
Fig. 2A. The cytotoxic effects of
Tan IIA on HepG2 cells were evaluated by MTT assay. As presented in
Fig. 2B, Tan IIA significantly
suppressed HepG2 cell viability in a dose-dependent manner, and
higher concentrations of Tan IIA (≥2 µM) time-dependently inhibited
cell viability. The IC50 values of Tan IIA at 24, 48 and
72 h were 2.28, 1.62 and 1.13 µM, respectively. It has been
reported that treatment with EGF and TGF-β1 promotes the malignant
proliferation of cancer cells (32).
In order to investigate the effects of Tan IIA on the EGF- and
TGF-β1-mediated clonogenic potential of HepG2 cells, a colony
formation assay was performed. The results demonstrated that EGF-
and TGF-β1-treated HepG2 cells had significantly increased colony
numbers compared with the untreated cells, while Tan IIA (0.5, 1
and 2 µM) treatment dose-dependently inhibited the clonogenic
ability of EGF- and TGF-β1-treated HepG2 cells (P<0.05; Fig. 2C). These results indicated that Tan
IIA exerted a strong inhibitory effect on the proliferation of
HepG2 cells.
Tan IIA decreases the EGF- and
TGF-β1-induced migration and invasion of HepG2 cells
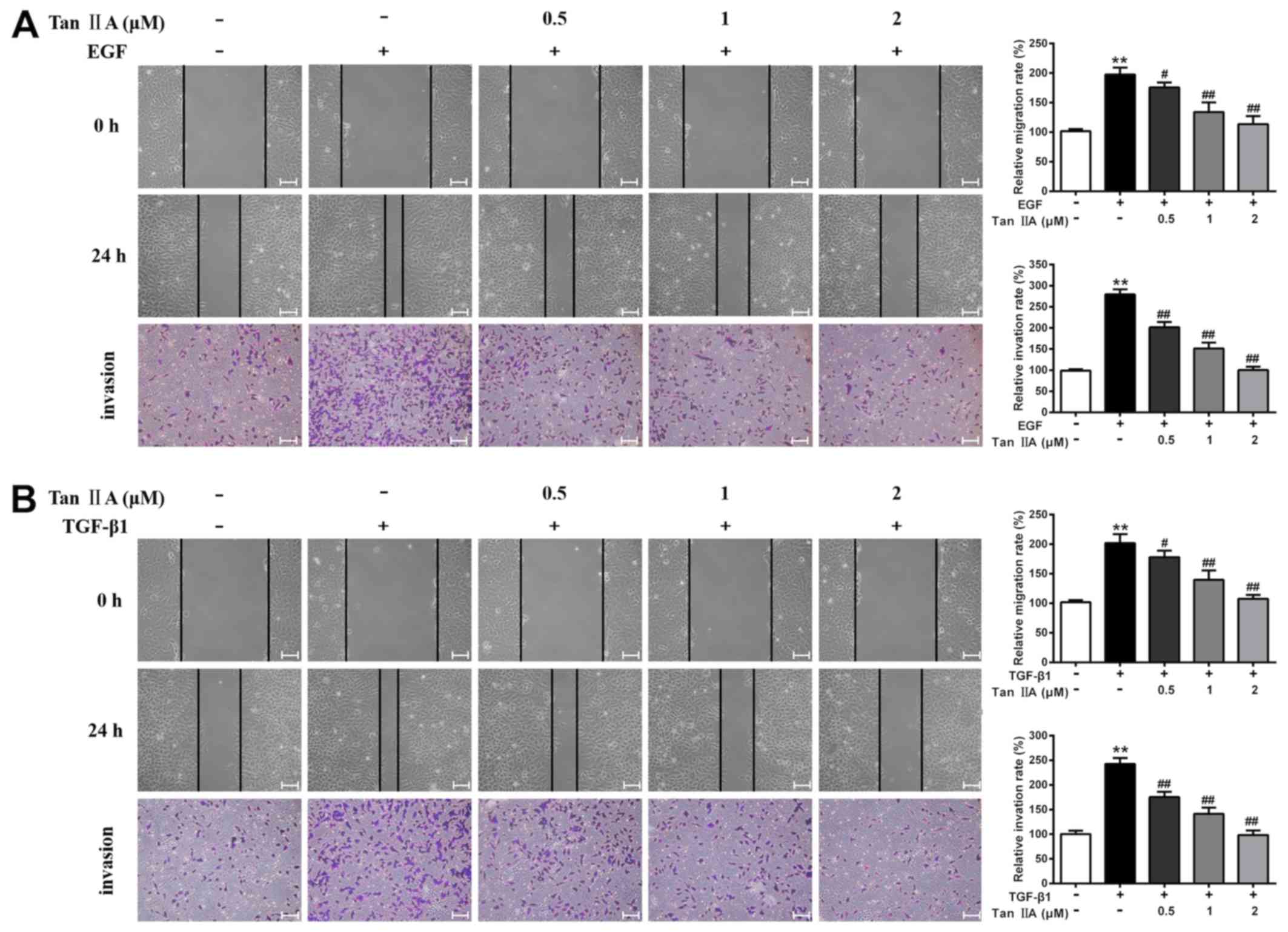
Accumulating evidence has suggested that EGF and
TGF-β1 can significantly enhance the motility of cancer cells
(33). The present study
investigated the effects of Tan IIA on the migratory and invasive
abilities of EGF- and TGF-β1-treated HepG2 cells using wound
healing and Transwell assays. The results revealed that the
migratory and invasive potential of EGF- and TGF-β1-treated HepG2
cells was markedly higher than that of control cells, whereas Tan
IIA (0.5, 1 and 2 µM) significantly inhibited EGF- and
TGF-β1-enhanced cell migration and invasion in a dose-dependent
manner (P<0.05; Fig. 3). In
particular, treatment with 2 µM Tan IIA led to the most significant
inhibition of EGF- and TGF-β1-induced proliferation, migration and
invasion of HepG2 cells. Therefore, 2 µM Tan IIA was used for the
subsequent experiments.
Tan IIA inhibits EGF- and
TGF-β1-mediated induction of EMT biomarkers in HepG2 cells
To ascertain whether Tan IIA can inhibit EGF- and
TGF-β1-induced EMT, the expression levels of the epithelial marker
E-cadherin and the mesenchymal marker vimentin were analyzed in
HepG2 cells by immunofluorescence staining. The results revealed
that the expression levels of E-cadherin were markedly decreased in
EGF- and TGF-β1-treated HepG2 cells, whereas vimentin expression
was significantly elevated (Fig.
4A). Compared with in the two model groups, 2 µM Tan IIA
markedly increased the expression levels of E-cadherin and
decreased vimentin expression. In addition, the present study
further analyzed the expression of EMT-associated biomarkers in
HepG2 cells via western blotting. The results indicated that, in
HepG2 cells, EGF- and TGF-β1-induced downregulation of E-cadherin
expression, and upregulation of N-cadherin, vimentin, Snail and
MMP-2 expression, was reversed by Tan IIA (Fig. 4B). These results confirmed that Tan
IIA could effectively reverse EGF- and TGF-β1-induced EMT in HepG2
cells.
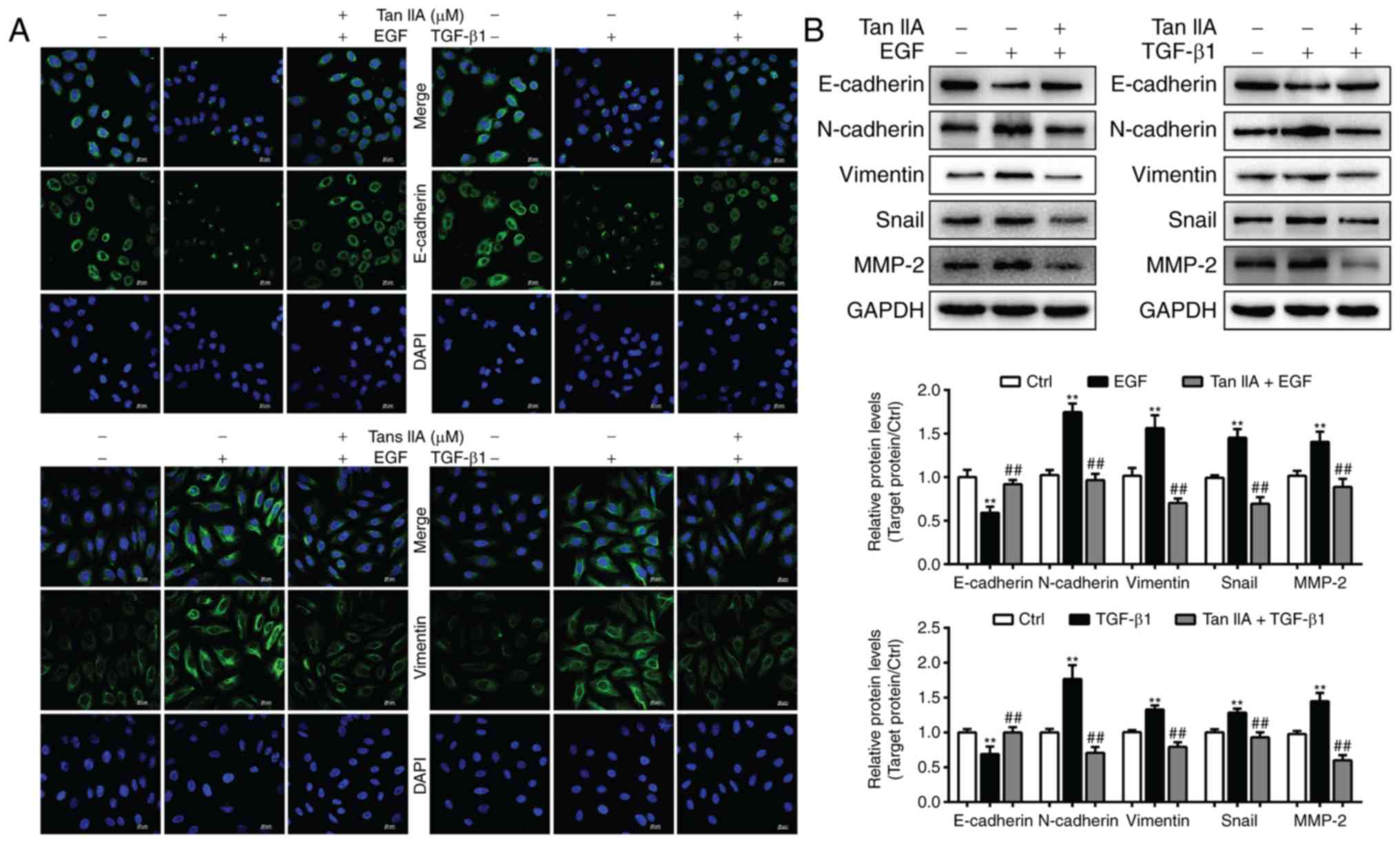 | Figure 4.Tan IIA reverses EGF- and
TGF-β1-induced EMT in HepG2 cells. (A) E-cadherin and vimentin
expression in HepG2 cells was determined by confocal microscopy.
Scale bars, 20 µm. (B) Expression of E-cadherin, MMP-2, N-cadherin,
vimentin and Snail in HepG2 cells that were untreated or treated
with 20 ng/ml EGF, 10 ng/ml TGF-β1 and 2 µM Tan IIA were analyzed
via western blotting. **P<0.01 vs. the control group;
##P<0.01 vs. the EGF or TGF-β1 group. EGF, epidermal
growth factor; EMT, epithelial-mesenchymal transition; MMP, matrix
metalloproteinase; Tan IIA, tanshinone IIA; TGF, transforming
growth factor. |
Tan IIA inhibits EGF- and
TGF-β1-induced EMT in HepG2 cells by regulating the PI3K/Akt/ERK
signaling pathway
In order to investigate the potential molecular
mechanism underlying the inhibitory effect of Tan IIA on the EGF-
and TGF-β1-induced EMT process in HepG2 cells, the expression
levels of proteins in the PI3K/Akt/ERK pathway were investigated in
cells treated with EGF and TGF-β1 using western blot analysis. As
presented in Fig. 5A, treatment with
20 ng/ml EGF and 10 ng/ml TGF-β1 for 48 h strongly induced the
phosphorylation of Akt and ERK1/2 (two downstream signaling
molecules of PI3K), whereas total Akt and ERK1/2 expression levels
were unchanged. However, Tan IIA treatment significantly decreased
the upregulated p-Akt and p-ERK1/2 expression levels induced by EGF
and TGF-β1.
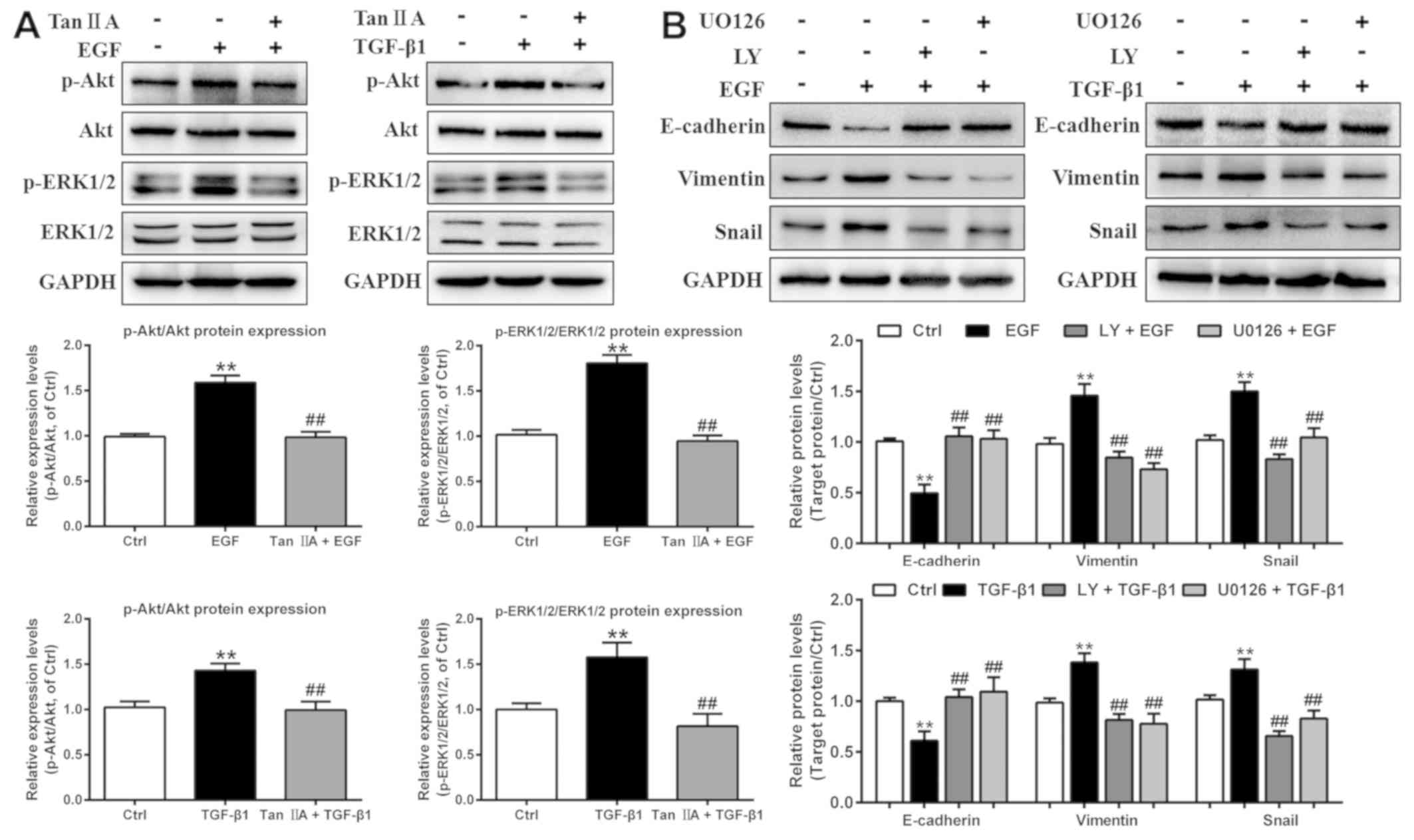 | Figure 5.Tan IIA inhibits EMT by deactivating
the PI3K/Akt/ERK signaling pathway in EGF- and TGF-β1-treated HepG2
cells. (A) Phosphorylation and expression of Akt and ERK1/2 in
HepG2 cells that were untreated or treated with 20 ng/ml EGF, 10
ng/ml TGF-β1 and 2 µM Tan IIA were analyzed by western blotting.
(B) Protein expression levels of E-cadherin, vimentin and Snail in
HepG2 cells that were untreated or treated with 20 ng/ml EGF, 10
ng/ml TGF-β1, 20 µM LY, and 20 µM U0126 were analyzed by western
blotting. **P<0.01 vs. the control group; ##P<0.01
vs. the EGF or TGF-β1 group. EGF, epidermal growth factor; EMT,
epithelial-mesenchymal transition; LY, LY294002; p-,
phosphorylated; Tan IIA, tanshinone IIA; TGF, transforming growth
factor. |
To further investigate the function of the
PI3K/Akt/ERK signaling pathway in EGF- and TGF-β1-induced EMT in
HepG2 cells, these cells were treated with the PI3K-specific
inhibitor LY294002 and the MEK-specific inhibitor U0126. Compared
with the groups treated with EGF or TGF-β1 alone, LY294002 and
U0126 notably inhibited the protein expression levels of vimentin
and Snail, and increased the protein expression levels of
E-cadherin in EGF- and TGF-β1-treated HepG2 cells, consistent with
the results of Tan IIA treatment (Fig.
5B). Overall, the results from the present study suggested that
Tan IIA suppressed EGF- and TGF-β1-induced EMT via inhibition of
the PI3K/Akt/ERK signaling pathways in HepG2 cells.
Discussion
EMT is a reversible cellular process that promotes
tumor stemness, metastasis and resistance to therapy; the secretion
of EGF or TGF-β1 by the tumor is associated with EMT progression
(34,35). Previous studies have confirmed that
TGF-β1 induces EMT in HepG2 cells (7,31);
however, the association between EGF and HepG2 cells has rarely
been reported. The present study first established an EMT model of
HepG2 cells induced by EGF. The results demonstrated that in
response to EGF, HepG2 cells lost their epithelial morphology and
adopted a mesenchymal-like spindle-shaped morphology. The
expression levels of two mesenchymal markers (N-cadherin and
vimentin) and a transcriptional factor (Snail) were increased,
whereas the expression levels of an epithelial marker (E-cadherin)
were decreased. These results indicated that treatment of HepG2
cells with 20 ng/ml EGF for 48 h successfully promoted EMT.
Tan IIA, which is an effective therapeutic agent
with multi-targeted actions, has been used to treat numerous human
diseases in China (36). Analysis of
the cancer cell death mechanism associated with Tan IIA has been
provided by numerous studies. Notably, Tan IIA has been reported to
significantly increase apoptotic cell death rate and decrease HepG2
cell-based tumor growth in nude mice by inhibiting CYP2J2 activity
(37). Tan IIA may also cause
apoptosis of HepG2 cells by inducing cell cycle arrest at the
sub-G1 stages and by affecting the
microRNA30b-p53-PTPN11/SHP2 signaling pathway (38). Additional reports have demonstrated
that Tan IIA suppresses the invasive and migratory abilities of
astrocytoma cells via the Notch-1 pathway (39), and inhibits TGF-β-induced EMT by
inhibiting the Smad signaling pathway and transcription factors
(40). However, the mechanism of
action underlying Tan IIA in EGF- and TGF-β1-induced EMT in HepG2
cells remains unclear. The present study used HepG2 cells as a
reliable model of EMT induced by EGF and TGF-β1 to investigate
whether Tan IIA has antimetastatic effects in vitro. The
results revealed that Tan IIA significantly inhibited the
clonogenic ability of EGF- and TGF-β1-treated HepG2 cells in a
dose-dependent manner. Furthermore, Tan IIA clearly inhibited the
EGF- and TGF-β1-enhanced migration and invasion of HepG2 cells, as
demonstrated using wound healing and Transwell assays.
Immunofluorescence staining and western blotting revealed that Tan
IIA rescued alterations in EMT-associated biomarker proteins
(MMP-2, E-cadherin, N-cadherin, vimentin and Snail) induced by EGF
and TGF-β1 in HepG2 cells, indicating that Tan IIA reversed EMT
mediated by EGF and TGF-β1.
EGF binds to EGFR on the cell surface, which
triggers classical EGF/EGFR signaling pathways resulting in
upregulation of ERK1/2 and AKT phosphorylation (41). For example, EGF treatment induces EMT
in pancreatic cancer cells via the integrin/EGFR-ERK/MAPK signaling
pathway (42). Activated p-ERK1/2
and p-AKT mediate the process of EMT in human breast cancer
MDA-MB-231 cells (43). Continuous
EGF treatment has been demonstrated to upregulate Snail and Twist
expression to induce EMT in prostate cancer PC-3 cells via the
EGFR/PI3K/Akt/ERK signaling pathway (44). Despite the well-known role of the
TGF-β1-induced Smad-dependent pathway in the EMT process, non-Smad
signaling pathways mediated by TGF-β1 have also been demonstrated
to serve a crucial role in cancer progression (7,45). In
addition, the protein expression levels of p-ERK have been reported
to be notably upregulated in TGF-β1-induced U-87 glioblastoma cells
(46). The PI3K/Akt/mTOR signaling
pathway activated by TGF-β1 is able to induce EMT in PANC-1, AsPC-1
(47) and HCT116 cells (48), consistent with the role of EGF.
Therefore, it was hypothesized that deactivating the PI3K/Akt/ERK
signaling pathway may reverse EMT in EGF- and TGF-β1-treated HepG2
cells. The present study demonstrated that treatment with Tan IIA
significantly decreased EGF- and TGF-β1-mediated Akt and ERK
phosphorylation in HepG2 cells. Furthermore, LY294002, a PI3K/Akt
inhibitor, and U0126, an ERK inhibitor, increased the expression
levels of E-cadherin, and inhibited the expression levels of
vimentin and Snail in HepG2 cells treated with EGF and TGF-β1.
Overall, the results from the present study indicated that Tan IIA
may have an opposing role in EGF- and TGF-β1-induced EMT by
inhibiting the activation of Akt and ERK phosphorylation. It has
been reported that the Smad2/3 pathway also serves a predominant
role in EMT in various types of cancer; however, the exact role of
Smad proteins in TGF-β-induced EMT remains controversial and
depends on an array of cofactors (49).
A limitation of the present study was that the EGF-
and TGF-β1-induced EMT models used only involved the human liver
cancer cell line HepG2. The value of this research could increase
by performing all experiments in at least an additional
authenticated liver cancer cell line. In addition, cell cycle
analysis would demonstrate whether Tan IIA exerts any impact on
cell proliferation in EGF- and TGF-β1-induced cell models, which
would be worth investigating in further detail in future studies.
Given that the anticancer activity of Tan IIA has attracted
interest in the past decade, it is possible that Tan IIA also
directly affects Smad signaling, and may have anti-EMT effects on
other liver cancer cell lines treated with EGF or TGF-β1, as well
as in animal models of metastasis in vivo. In addition, the
investigation of Tan IIA alone on the proliferation and migration,
as well as on the EMT markers of liver cancer cells, compared with
the EGF- and TGF-β1-treated cells may also vastly increase the
value of the research, which should be investigated in future
studies.
In conclusion, the present study demonstrated the
potential anti-EMT effects of Tan IIA in EGF- and TGF-β1-induced
cell models. To the best of our knowledge, the present study is the
first to suggest that Tan IIA efficiently targets the PI3K/Akt/ERK
signaling pathway to suppress EGF- and TGF-β1-induced migration,
invasion and EMT in HepG2 cells. Furthermore, these results
suggested that Tan IIA could be developed as a potential
therapeutic agent for the treatment of metastatic dissemination of
cancer cells.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Special
Foundation of High-Level University Construction for Valuable
Chinese Herbal Medicines in China (grant no. E4640117112018).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
GW and SX conceived and designed the experiments. LZ
coordinated the experiments and contributed to the interpretation
of the data and manuscript writing. WL and HZ performed the
immunofluorescence and western blotting assays. XC performed the
cell experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mittal V: Epithelial mesenchymal
transition in tumor metastasis. Annu Rev Pathol. 13:395–412. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mehlen P and Puisieux A: Metastasis: A
question of life or death. Nat Rev Cancer. 6:449–458. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pastushenko I, Brisebarre A, Sifrim A,
Fioramonti M, Revenco T, Boumahdi S, Van Keymeulen A, Brown D,
Moers V, Lemaire S, et al: Identification of the tumour transition
states occurring during EMT. Nature. 556:463–468. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Puisieux A, Brabletz T and Caramel J:
Oncogenic roles of EMT-inducing transcription factors. Nat Cell
Biol. 16:488–494. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Said NA and Williams ED: Growth factors in
induction of epithelial-mesenchymal transition and metastasis.
Cells Tissues Organs. 193:85–97. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xing S, Yu W, Zhang X, Luo Y, Lei Z, Huang
D, Lin J, Huang Y, Huang S, Nong F, et al: Isoviolanthin extracted
from dendrobium officinale reverses TGF-β1-mediated
epithelial-mesenchymal transition in hepatocellular carcinoma cells
via deactivating the TGF-β/Smad and PI3K/Akt/mTOR signaling
pathways. Int J Mol Sci. 19(pii): E15562018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fantozzi A, Gruber DC, Pisarsky L, Heck C,
Kunita A, Yilmaz M, Meyer-Schaller N, Cornille K, Hopfer U,
Bentires-Alj M and Christofori G: VEGF-mediated angiogenesis links
EMT-induced cancer stemness to tumor initiation. Cancer Res.
74:1566–1575. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mori S, Kodaira M, Ito A, Okazaki M,
Kawaguchi N, Hamada Y, Takada Y and Matsuura N: Enhanced expression
of integrin αvβ3 induced by TGF-β Is required for the enhancing
effect of fibroblast growth factor 1 (FGF1) in TGF-β-induced
epithelial-mesenchymal transition (EMT) in mammary epithelial
cells. PLoS One. 10:e01374862015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grassi ML, Palma CS, Thome CH, Lanfredi
GP, Poersch A and Faca VM: Proteomic analysis of ovarian cancer
cells during epithelial-mesenchymal transition (EMT) induced by
epidermal growth factor (EGF) reveals mechanisms of cell cycle
control. J Proteomics. 151:2–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stewart TA, Azimi I, Brooks AJ, Thompson
EW, Roberts-Thomson SJ and Monteith GR: Janus kinases and Src
family kinases in the regulation of EGF-induced vimentin expression
in MDA-MB-468 breast cancer cells. Int J Biochem Cell Biol.
76:64–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miyazono K: Transforming growth
factor-beta signaling in epithelial-mesenchymal transition and
progression of cancer. Proc Jpn Acad Ser B Phys Biol Sci.
85:314–323. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ren W, Zhang Y, Zhang L, Lin Q, Zhang J
and Xu G: Overexpression of collagen type V α1 chain in human
breast invasive ductal carcinoma is mediated by TGF-β1. Int J
Oncol. Mar 15–2018.(Epub ahead of print). View Article : Google Scholar
|
|
14
|
Li ZM, Xu SW and Liu PQ: Salvia
miltiorrhiza Burge (Danshen): A golden herbal medicine in
cardiovascular therapeutics. Acta Pharmacol Sin. 39:802–824. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang L, Zhu J, Zheng M, Zou R, Zhou Y and
Zhu M: Tanshinone IIA protects against subclinical
lipopolysaccharide induced cardiac fibrosis in mice through
inhibition of NADPH oxidase. Int Immunopharmacol. 60:59–63. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen W, Tang F, Xie B, Chen S, Huang H and
Liu P: Amelioration of atherosclerosis by tanshinone IIA in
hyperlipidemic rabbits through attenuation of oxidative stress. Eur
J Pharmacol. 674:359–364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao S, Liu Z, Li H, Little PJ, Liu P and
Xu S: Cardiovascular actions and therapeutic potential of
tanshinone IIA. Atherosclerosis. 220:3–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu BL, Li J, Zhou J, Li WW and Wu HF:
Tanshinone IIA decreases the levels of inflammation induced by
Aβ1–42 in brain tissues of Alzheimer's disease model rats.
Neuroreport. 27:883–893. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao YF, Wang SF, Li X, Zhang YL and Qiao
YJ: The anticancer mechanism investigation of Tanshinone
IIA by pharmacological clustering in protein network.
BMC Syst Biol. 12:902018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu YQ, Wang BY, Wu F, An YK and Zhou XQ:
Influence of tanshinone IIA on the apoptosis of human esophageal
Ec-109 cells. Nat Prod Commun. 11:17–19. 2016.PubMed/NCBI
|
|
21
|
He L and Gu K: Tanshinone IIA regulates
colorectal cancer apoptosis via attenuation of Parkin-mediated
mitophagy by suppressing AMPK/Skp2 pathways. Mol Med Rep.
18:1692–1703. 2018.PubMed/NCBI
|
|
22
|
Su CC, Chien SY, Kuo SJ, Chen YL, Cheng CY
and Chen DR: Tanshinone IIA inhibits human breast cancer MDA-MB-231
cells by decreasing LC3-II, Erb-B2 and NF-κBp65. Mol Med Rep.
5:1019–1022. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim EO, Kang SE, Im CR, Lee JH, Ahn KS,
Yang WM, Um JY, Lee SG and Yun M: Tanshinone IIA induces TRAIL
sensitization of human lung cancer cells through selective ER
stress induction. Int J Oncol. 48:2205–2212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin CY, Chang TW, Hsieh WH, Hung MC, Lin
IH, Lai SC and Tzeng YJ: Simultaneous induction of apoptosis and
necroptosis by Tanshinone IIA in human hepatocellular carcinoma
HepG2 cells. Cell Death Discov. 2:160652016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qin J, Shi H, Xu Y, Zhao F and Wang Q:
Tanshinone IIA inhibits cervix carcinoma stem cells migration and
invasion via inhibiting YAP transcriptional activity. Biomed
Pharmacother. 105:758–765. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang SY, Chang SF, Liao KF and Chiu SC:
Tanshinone IIA inhibits epithelial-mesenchymal transition in
bladder cancer cells via modulation of STAT3-CCL2 signaling. Int J
Mol Sci. 18(pii): E16162017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou M, Zhou G, Hu S and Zhang L:
Tanshinone IIA suppress the proliferation of HNE-1 nasopharyngeal
carcinoma an in vitro study. Saudi J Biol Sci. 25:267–272. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fu H, He Y, Qi L, Chen L, Luo Y, Chen L,
Li Y, Zhang N and Guo H: cPLA2α activates PI3K/AKT and inhibits
Smad2/3 during epithelial-mesenchymal transition of hepatocellular
carcinoma cells. Cancer Lett. 403:260–270. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xing S, Zhang X, Ke H, Lin J, Huang Y and
Wei G: Physicochemical properties of polysaccharides from
Dendrobium officinale by fractional precipitation and their
preliminary antioxidant and anti-HepG2 cells activities in vitro.
Chem Cent J. 12:1002018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheng Z, Wei W, Wu Z, Wang J, Ding X,
Sheng Y, Han Y and Wu Q: ARPC2 promotes breast cancer proliferation
and metastasis. Oncol Rep. 41:3189–3200. 2019.PubMed/NCBI
|
|
31
|
Deng G, Zeng S, Ma J, Zhang Y, Qu Y, Han
Y, Yin L, Cai C, Guo C and Shen H: The anti-tumor activities of
Neferine on cell invasion and oxaliplatin sensitivity regulated by
EMT via Snail signaling in hepatocellular carcinoma. Sci Rep.
7:416162017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gore J, Deitz SL, Wilson JL and Korc M:
Abstract 969: TGF-beta cross-talks with the EGF receptor family to
promote proliferation of pancreatic cancer cells with dysfunctional
RB. Cancer Res. 74:9692014.
|
|
33
|
Xu ZH, Jiang YY, Steed H, Davidge S and Fu
YX: TGFβ and EGF synergistically induce a more invasive phenotype
of epithelial ovarian cancer cells. Biochem Biophys Res Commun.
401:376–381. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wendt MK, Allington TM and Schiemann WP:
Mechanisms of the epithelial-mesenchymal transition by TGF-beta.
Future Oncol. 5:1145–1168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lu ZM, Ghosh S, Wang ZY and Hunter T:
Downregulation of caveolin-1 function by EGF leads to the loss of
E-cadherin, increased transcriptional activity of beta-catenin, and
enhanced tumor cell invasion. Cancer Cell. 4:499–515. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu S and Liu P: Tanshinone II-A: New
perspectives for old remedies. Expert Opin Ther Pat. 23:149–153.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jeon YJ, Kim JS, Hwang GH, Wu Z, Han HJ,
Park SH, Chang W, Kim LK, Lee YM, Liu KH and Lee MY: Inhibition of
cytochrome P450 2J2 by tanshinone IIA induces apoptotic cell death
in hepatocellular carcinoma HepG2 cells. Eur J Pharmacol.
764:480–488. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ren X, Wang C, Xie B, Hu L, Chai H, Ding
L, Tang L, Xia Y and Dou X: Tanshinone IIA induced cell death via
miR30b-p53-PTPN11/SHP2 signaling pathway in human hepatocellular
carcinoma cells. Eur J Pharmacol. 796:233–241. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dong W, Zhang Y, Chen X and Jia Y:
High-dose tanshinone iia suppresses migration and proliferation
while promoting apoptosis of astrocytoma cells via Notch-1 pathway.
Neurochem Res. 43:1855–1861. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Duan H, Ma L, Liu H, Zhang Y, Zhang Z, Yan
X and Li X: Tanshinone IIA attenuates epithelial-mesenchymal
transition to inhibit the tracheal narrowing. J Surg Res.
206:252–262. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pan M, Schinke H, Luxenburger E, Kranz G,
Shakhtour J, Libl D, Huang Y, Gaber A, Pavšič M, Lenarčič B, et al:
EpCAM ectodomain EpEX is a ligand of EGFR that counteracts
EGF-mediated epithelial-mesenchymal transition through modulation
of phospho-ERK1/2 in head and neck cancers. PLoS Biol.
16:e20066242018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sheng W, Chen C, Dong M, Wang G, Zhou J,
Song H, Li Y, Zhang J and Ding S: Calreticulin promotes EGF-induced
EMT in pancreatic cancer cells via Integrin/EGFR-ERK/MAPK signaling
pathway. Cell Death Dis. 8:e31472017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bhat FA, Sharmila G, Balakrishnan S,
Arunkumar R, Elumalai P, Suganya S, Raja Singh P, Srinivasan N and
Arunakaran J: Quercetin reverses EGF-induced epithelial to
mesenchymal transition and invasiveness in prostate cancer (PC-3)
cell line via EGFR/PI3K/Akt pathway. J Nutr Biochem. 25:1132–1139.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tsai PC, Fu YS, Chang LS and Lin SR:
Taiwan cobra cardiotoxin III suppresses EGF/EGFR-mediated
epithelial-to-mesenchymal transition and invasion of human breast
cancer MDA-MB-231 cells. Toxicon. 111:108–120. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu Z, Kuang W, Zhou Q and Zhang Y: TGF-β1
secreted by M2 phenotype macrophages enhances the stemness and
migration of glioma cells via the SMAD2/3 signalling pathway. Int J
Mol Med. 42:3395–3403. 2018.PubMed/NCBI
|
|
46
|
Ouanouki A, Lamy S and Annabi B:
Anthocyanidins inhibit epithelial-mesenchymal transition through a
TGFβ/Smad2 signaling pathway in glioblastoma cells. Mol Carcinog.
56:1088–1099. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pei Z, Fu W and Wang G: A natural product
toosendanin inhibits epithelial-mesenchymal transition and tumor
growth in pancreatic cancer via deactivating Akt/mTOR signaling.
Biochem Biophys Res Commun. 493:455–460. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Han S, Bui NT, Ho MT, Kim YM, Cho M and
Shin DB: Dexamethasone inhibits TGF-β1-induced cell migration by
regulating the ERK and AKT pathways in human colon cancer cells Via
CYR61. Cancer Res Treat. 48:1141–1153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fuxe J, Vincent T and Garcia de Herreros
A: Transcriptional crosstalk between TGF-β and stem cell pathways
in tumor cell invasion: Role of EMT promoting Smad complexes. Cell
Cycle. 9:2363–2374. 2010. View Article : Google Scholar : PubMed/NCBI
|