Introduction
Lung cancer is a highly prevalent malignancy and its
incidence is increasing in numerous countries (1). In the United States, lung cancer was
the second most prevalent cancer type in both men and women in 2018
(2). Moreover, lung cancer is
responsible for 26 and 25% of cancer-related mortalities in men and
women, respectively (2). Even
following active treatment (such as surgical resection, chemo- and
radiation therapy), <50% patients with lung cancer at Stage I
(AJCC), and <5% of patients at Stage IV live longer than five
years following initial diagnosis (3). In effect, the overall survival rates of
lung cancer patients have not significantly improved during the
past several decades, primarily due to the fact that early
diagnosis is uncommon, and that the majority of patients with lung
cancer are diagnosed at advanced disease stages (4,5).
The p53 pathway is a well-characterized
tumor-suppressive pathway implicated in a multitude of cancer types
(6). It inhibits tumor progression
via the regulation of various cancer cell properties, such as tumor
cell proliferation, or the enhancement of tumor cell apoptosis
(7). This pathway is frequently
inactivated in cancer cells (8). The
involvement of p53 in cancer development is known to be regulated
by specific long non-coding (lnc)RNAs (>200 nucleotides)
(9). This family of RNAs is not
protein coding, but participates in numerous cellular processes by
regulating gene expression (10).
Prostate cancer associated transcript 19 (PCAT19) is an oncogenic
lncRNA involved in the progression of prostate cancer (11). Moreover, it has been discovered that
PCAT19 can also promote the development of laryngeal carcinoma
(12). The present study was
performed to investigate the role of PCAT19 in the progression of
non-small cell lung cancer (NSCLC), a major subtype of lung cancer,
using both specimens from NSCLC patients and an NSCLC cell
line.
Materials and methods
Patient selection and follow-up
Jilin Province Tumor Hospital (Changchun, China)
admitted a total of 155 patients with NSCLC between August 2011 and
December 2013. For the present study, 66 cases (40 men and 26
women; mean age, 46- 68 years; age range, 57.7± 8.9 years) were
selected according to the following inclusion criteria: i) The
patients were newly diagnosed; and ii) agreed to a 5-year
follow-up. The exclusion criteria were: i) Patients with recurrent
NSCLC; ii) patients diagnosed with other diseases; and iii)
patients that had received any other therapy <3 months before
surgery. The 66 patients comprised 12, 14, 16 and 24 cases of
stages I–IV cancer (AJCC), respectively. Follow-up for the 66
patients occurred 5 years after the date of admission, and patient
survival conditions were monitored and recorded. Follow-up of
patients that succumbed to unrelated diseases or accidents was
excluded. All patients were informed of the experimental protocol
and written informed consent was obtained from every participant.
The present study was approved by the Ethics Committee of Jilin
Province Tumor Hospital.
Tissues and NSCLC cells
NSCLC and adjacent normal tissue samples (<3 cm
from the tumor) were collected from each patient during diagnosis,
via a histopathological biopsy. The tissue samples were
independently confirmed by ≥3 pathologists and the tissue weight
ranged between 0.015 and 0.018 g. The H1993 human NSCLC cell line,
obtained from the American Tissue Culture Collection, was used in
the present study. Cells were cultured at 37°C and 5%
CO2, in RPMI-1640 medium (Sigma-Aldrich; Merck KGaA)
containing 10% fetal bovine serum (FBS; Sigma-Aldrich; Merck
KGaA).
Transient transfection
pcDNA3 vectors expresssing PCAT19 and p53, and empty
pcDNA3 vectors were purchased from Sangon Biotech Co., Ltd. PCAT19
small interfering (si)RNA (5′-GAUCCUUAGGUUCUCAGAAAC-3′) and
negative control (NC) siRNA (5′-CGCUUACCCAAAUGCAUUGGC-3′) were
purchased from Shanghai GenePharma Co., Ltd. H1993 cells were
harvested at a confluence of 70–80%, and 1×105 cells
were transfected with 10 nM PCAT19- and p53-expressing pcDNA3
vectors, (10 nM empty pcDNA3 vector was used as the NC) 40 nM
PCAT19 siRNA and 40 nM NC siRNA, using Lipofectamine 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Untransfected cells
were used as the control (C). Subsequent experiments were performed
using cells collected at 24 h post-transfection.
Reverse transcription-quantitative
(RT-q)PCR
TRIzol® reagent (Thermo Fisher
Scientific, Inc.) was used to extract the total RNA from patient
tissue samples (1 ml TRIzol®/0.015 g tissue) and H1993
cells (1 ml TRIzol®/1×105 cells). All RNA
samples were quantified and DNase I digestion was performed (1 h at
37°C). The SensiFAST™ cDNA Synthesis Kit (Bioline, Meridian
Bioscience Inc.) was used to reverse-transcribe RNA into cDNA
according to the manufacturer's instructions, and the
SYBR® Green Master Mix (Bio-Rad Laboratories, Inc.) was
used to prepare the PCR reaction mixtures according to the
manufacturer's instructions. 18S rRNA was selected as the
endogenous control and the expression of PCAT19 and p53 mRNA were
detected. The primer sequences were as follows: PCAT19 forward,
5′-TCAGAACAGGGAACCATTGG-3′ and reverse, 5′-CAAGAAGATTCTTATCAGCT-3′;
p53 forward, 5′-GCCCAACAACACCAGCTCCT-3′ and reverse,
5′-CCTGGGCATCCTTGAGTTCCT-3′; and human 18S rRNA forward,
5′-CTACCACATCCAAGGAAGCA-3′ and reverse,
5′-TTTTTCGTCACTACCTCCCCG-3′. The thermocycling conditions were:
95°C for 30 sec, 40 cycles of 95°C for 10 sec followed by 55°C for
40 sec. The mRNA levels were quantified using the 2−ΔΔCq
method and normalized to the internal reference gene (13).
Cell proliferation assay
H1993 cells were harvested at 24 h
post-transfection, and 3×104 cells were resuspended in 1
ml Eagle's Minimum Essential Medium (Sigma-Aldrich; Merck KGaA)
containing 10% FBS (Sigma-Aldrich; Merck KGaA)) to produce a
single-cell suspension. Cell suspensions were added into a 96-well
cell culture plate at 0.1 ml per well. Triplicate wells were set
for each cell transfection group, and the cells were incubated at
37°C and 5% CO2 for 24, 48, 72 or 96 h. Cell Counting
Kit-8 solution (10 µl; Sigma-Aldrich; Merck KGaA) was added into
the wells 4 h before the end of each time point according to the
manufacturer's instructions. Following the addition of 10 µl DMSO,
the optical density values were measured at 450 nm using a
microplate reader.
Western blotting
PCAT19 cells were harvested at 24 h
post-transfection and 2×105 cells per sample were
treated with 1 ml RIPA solution (Thermo Fisher Scientific, Inc.) to
extract the total protein. The samples were quantified using a
bicinchoninic acid assay. After denaturation, the protein samples
were separated via SDS-PAGE on a 10% gel and transferred onto PVDF
membranes, which were subsequently blocked with 5% non-fat milk for
20 min at room temperature. The membranes were then incubated with
rabbit polyclonal primary antibodies against p53 (cat. no.
ab131442; 1:800) and the endogenous control GAPDH (cat. no. ab9485;
1:800; both Abcam) overnight at 4°C. Incubation with an IgG-horse
radish peroxidase secondary antibody (cat. no. MBS435036; 1:1,000;
MyBioSource, Inc.) was used to further probe the membranes at 25°C
for 2 h. Bands were visualized using ECL reagents (Sigma-Aldrich;
Merck KGaA) and processed using ImageJ software (version 1.46;
National Institutes of Health).
Statistical analysis
All experiments were performed in triplicate and
mean values were calculated to perform data comparisons. Data were
expressed as the means ± standard deviation. Differences in gene
expression between normal and NSCLC tissues were analyzed using a
paired Student's t-test. Differences in cell proliferation or gene
expression among different cell groups were analyzed using ANOVA
(one-way) followed by Tukey's post-hoc test. Linear regression was
used to conduct correlation analysis. Based on Youden's index, the
66 cases were grouped into low- and high-PCAT19 expression level
groups, with 30 and 36 patients in each group, respectively.
Kaplan-Meier analysis and the log-rank test were used to construct
and compare survival curves. P<0.05 was considered to indicate a
statistically significant difference.
Results
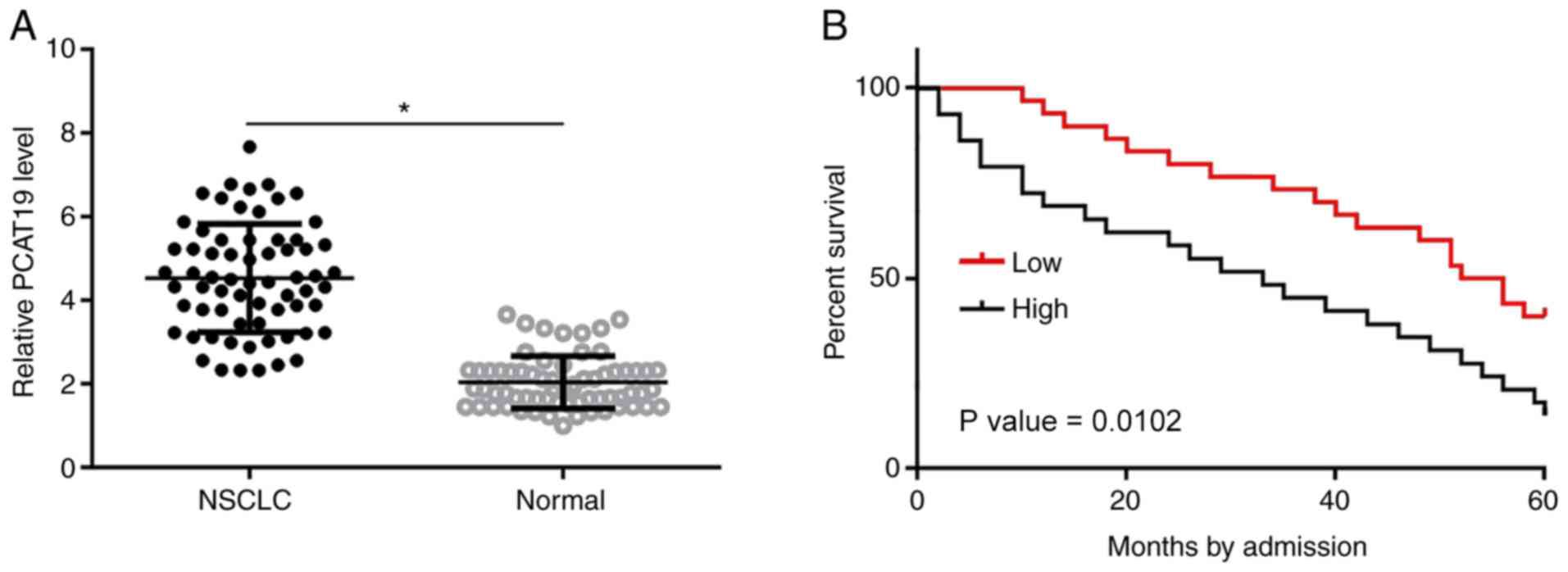
PCAT19 is upregulated in NSCLC tissues
and correlates with poor survival time in patients with NSCLC
PCAT19 expression levels in NSCLC and paratumor
tissues collected from patients with NSCLC (n=66) were determined
using RT-qPCR. Differences in expression levels between these two
groups were compared using the paired Student's t-test. The results
indicated that the expression level of PCAT19 was significantly
higher in 59/66 NSCLC tissues compared with the paratumor tissues
(P<0.05; Fig. 1A). Survival
curves were plotted for high- and low-PCAT19 expression groups. It
was found that the overall survival rate of the high-expression
level group was significantly lower than that of the low-expression
level group (P<0.05; Fig. 1B).
Expression levels of PCAT19 increased slightly in patients at more
advanced clinical stages, but the changes were not statistically
significant (data not shown).
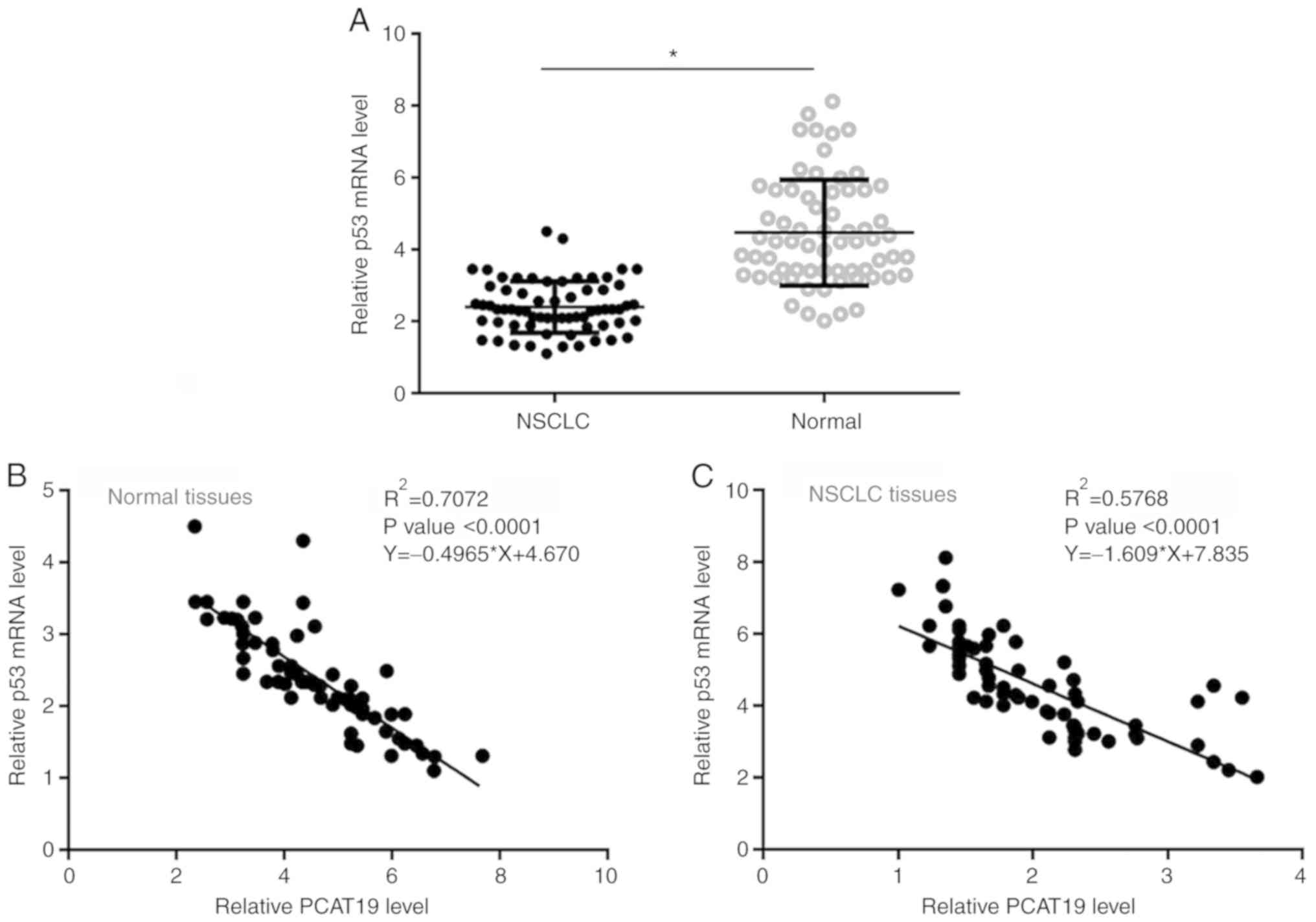
p53 expression is negatively
correlated with that of PCAT19
Expression levels of p53 in the paratumor and tumor
tissues of NSCLC patients (n=66) were also quantified using
RT-qPCR. The data between the two tissue types were compared using
the paired Student's t-test. Expression levels of p53 mRNA were
significantly lower in NSCLC tissues compared with normal tissues
(P<0.05; Fig. 2A). The
correlation between p53 and PCAT19 expression levels was analyzed
using linear regression. It was found that the expression levels of
p53 and PCAT19 exhibited a significant negative correlation in
normal (Fig. 2B) and NSCLC tissues
(Fig. 2C).
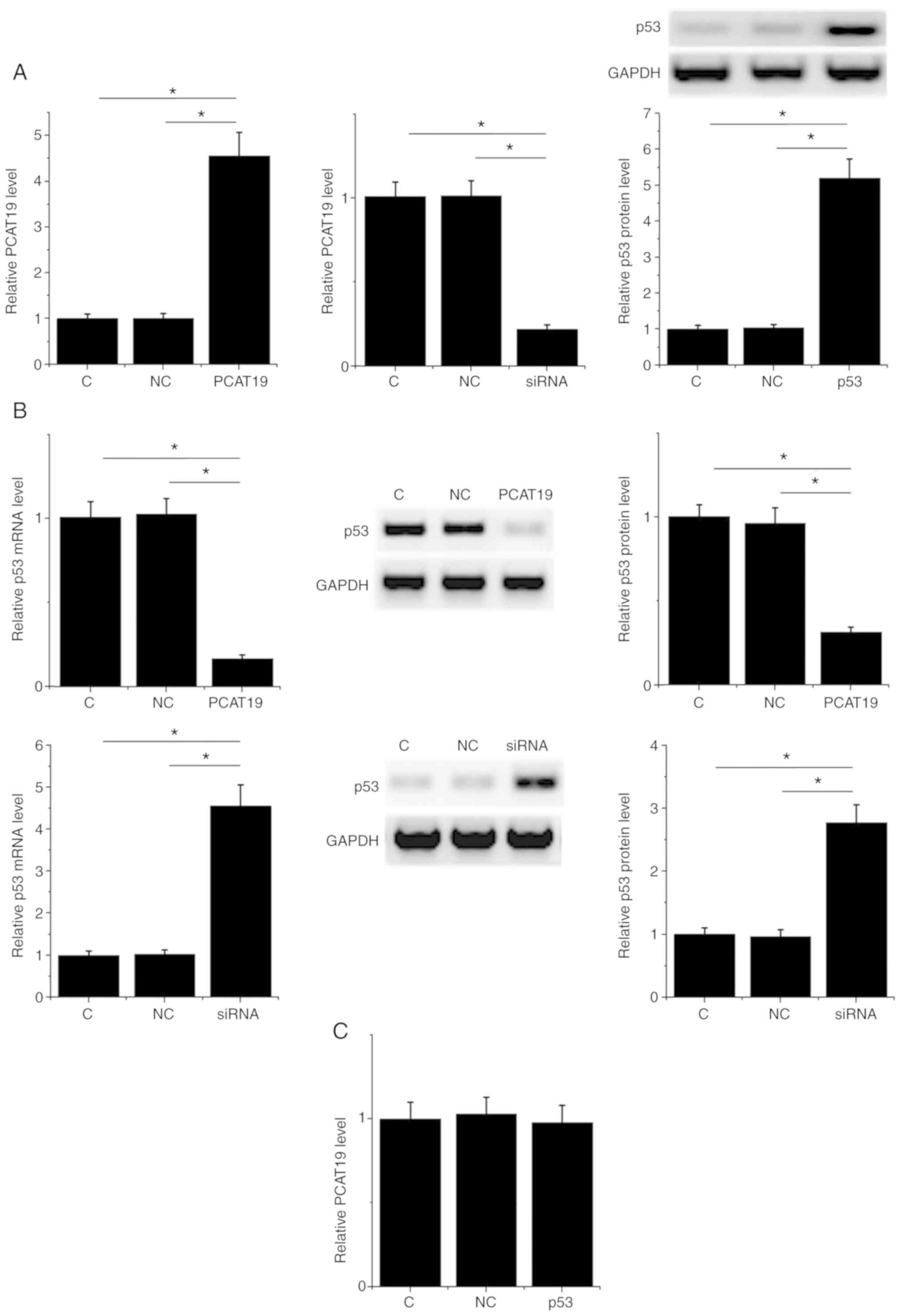
PCAT19 negatively regulates p53
expression in H1993 cells
PCAT19 and p53 expression vectors, and PCAT19-siRNA,
were used to transfect H1993 cells. PCAT19 and p53 expression
levels were significantly altered at 24 h post-transfection
compared with the control (non-transfected cells) and negative
control groups (cells transfected with an empty vector or NC siRNA)
(P<0.05; Fig. 3A). Moreover, in
the two control groups, PCAT19-silencing resulted in the
upregulation, whilst PCAT19 overexpression led to the
downregulation of p53 at both the mRNA and protein levels
(P<0.05; Fig. 3B). However,
expression of PCAT19 in H1993 cells was not significantly affected
by the transfection of the p53 expression vector (Fig. 3C).
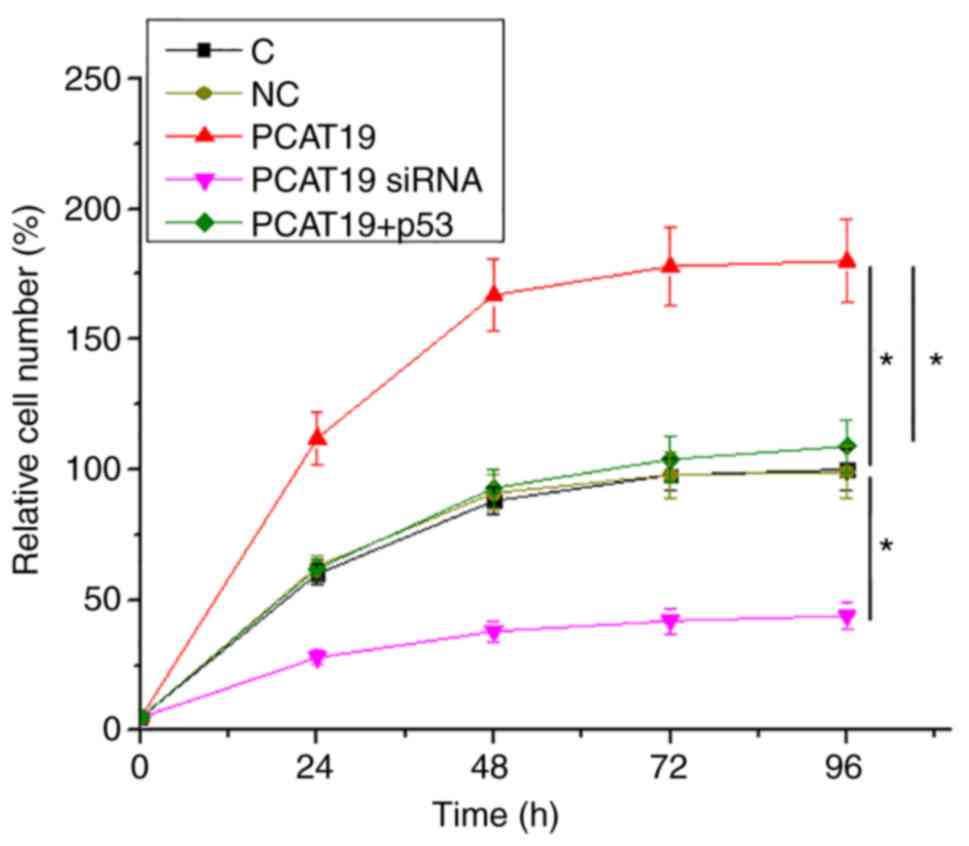
PCAT19 regulates H1993 cell
proliferation through p53
Cell proliferation data were compared among groups
by performing one-way ANOVA followed by Tukey's post-hoc test. It
was observed that compared to the two controls, PCAT19-silencing
led to a decrease, whilst PCAT19 overexpression led to an increase
in the proliferation rate of NSCLC cells. Furthermore, p53
overexpression attenuated the effects of PCAT19 overexpression on
cellular proliferation (P<0.05;Fig.
4).
Discussion
In the present study, the functionality and clinical
value of PCAT19 for use in the treatment of NSCLC was evaluated. It
was discovered that PCAT19 expression was upregulated in NSCLC
tissues, and may promote cancer cell proliferation by negatively
regulating the expression level of p53. In addition, high
expression levels of PCAT19 predicted poorer overall survival times
in NSCLC patients compared with patients with low expression
levels.
The same lncRNAs are likely to play similar roles in
different types of cancer. For example, HOX transcript antisense
RNA (HOTAIR) is upregulated in most, if not all types of cancer,
and the overexpression of HOTAIR promotes cancer cell invasion and
migration, and suppresses apoptosis (14). However, the pathogenesis of different
types of cancer is different. Therefore, it is not reasonable to
use the function of a specific lncRNA in one type of cancer to
speculate or predict its role in another. For example, lncRNA
taurine-upregulated gene 1 (TUG1) is upregulated in a number of
malignancies, and promotes these cancers in similar ways, such as
initiating cell invasion and proliferation (15). However, in a recent study, Li et
al (16) reported that TUG1
promoted cancer cell apoptosis in glioma, indicating its
tumor-suppressive function in this disease. The oncogenic role of
PCAT19 has been published in prostate cancer and laryngeal
carcinoma (11,12). To the best of our knowledge, the
present study is the first to report the upregulated expression of
PCAT19 in NSCLC. The positive regulation of cancer cell
proliferation by PCAT19 was also revealed. Therefore, PCAT19 is
likely to be an oncogenic lncRNA in NSCLC.
Early diagnosis of NSCLC is uncommon and
problematic, primarily because of non-specific and late-developing
symptoms, but also due to a lack of reliable biomarkers (17). To improve survival rates in patients
with NSCLC, it is critical to improve diagnosis, and more
accurately understand disease progression and the risk of
mortality. The present study indicates that patients with high
expression levels of PCAT19 were significantly more likely to
exhibit low survival rates. Therefore, measurement of pre-treatment
levels of PCAT19 may help to improve the prognosis of patients with
NSCLC.
p53 signaling in cancer biology can be regulated by
certain specific lncRNAs (18). In
the current study, it was determined that PCAT19 is a negative
regulator of p53 and influences the proliferation of NSCLC cells.
Moreover, PCAT19 has previously been found to regulate micro RNA
(miR)-182, which exhibits crosstalk with p53 (19). Therefore, miR-182 may be a mediator
of the interaction between PCAT19 and p53.
In conclusion, the present study demonstrates that
PCAT19 is upregulated in NSCLC tissues and may promote the
proliferation of NSCLC cells via the downregulation of p53.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ and LZ designed the study. XZ, QW, YX, BW, CJ, LW
and HS performed the experiments. HZ, ZW, QZ and SS analyzed the
data. LZ drafted the manuscript. All authors approved the final
version of the manuscript.
Ethics approval and consent to
participate
All patients were informed of the experimental
protocol and written informed consent was obtained from every
participant. The present study was approved by the Jilin Province
Tumor Hospital Ethics Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Postmus PE, Kerr KM, Oudkerk M, Senan S,
Waller DA, Vansteenkiste J, Escriu C and Peters S; ESMO Guidelines
Committee, : Early and locally advanced non-small-cell lung cancer
(NSCLC): ESMO clinical practice guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 28 (Suppl_4):iv1–iv21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Labuschagne CF, Zani F and Vousden KH:
Control of metabolism by p53-cancer and beyond. Biochim Biophys
Acta Rev Cancer. 1870:32–42. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Joerger AC and Fersht AR: The p53 pathway:
Origins, inactivation in cancer, and emerging therapeutic
approaches. Annu Rev Biochem. 85:375–404. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huarte M: The emerging role of lncRNAs in
cancer. Nat Med. 21:1253–1261. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Engreitz JM, Haines JE, Perez EM, Munson
G, Chen J, Kane M, McDonel PE, Guttman M and Lander ES: Local
regulation of gene expression by lncRNA promoters, transcription
and splicing. Nature. 539:452–455. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hua JT, Ahmed M, Guo H, Zhang Y, Chen S,
Soares F, Lu J, Zhou S, Wang M, Li H, et al: Risk SNP-mediated
promoter-enhancer switching drives prostate cancer through lncRNA
PCAT19. Cell. 174:564–575.e18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu S, Guo J and Zhang W: lncRNA PCAT19
promotes the proliferation of laryngocarcinoma cells via modulation
of the miR-182/PDK4 axis. J Cell Biochem. 120:12810–12821. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu Y, Zhang L, Wang Y, Li H, Ren X, Wei F,
Yu W, Wang X, Zhang L, Yu J and Hao X: Long noncoding RNA HOTAIR
involvement in cancer. Tumour Biol. 35:9531–9538. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Z, Shen J, Chan MT and Wu WK: TUG1: A
pivotal oncogenic long non-coding RNA of human cancers. Cell
Prolif. 49:471–475. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J, Zhang M, An G and Ma Q: LncRNA TUG1
acts as a tumor suppressor in human glioma by promoting cell
apoptosis. Exp Biol Med (Maywood). 241:644–649. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Canadian Task Force on Preventive Health
Care, . Recommendations on screening for lung cancer. CMAJ.
188:425–432. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei GH and Wang X: lncRNA MEG3 inhibit
proliferation and metastasis of gastric cancer via p53 signaling
pathway. Eur Rev Med Pharmacol Sci. 21:3850–3856. 2017.PubMed/NCBI
|
|
19
|
Handa H, Hashimoto A, Hashimoto S, Sugino
H, Oikawa T and Sabe H: Epithelial-specific histone modification of
the miR-96/182 locus targeting AMAP1 mRNA predisposes p53 to
suppress cell invasion in epithelial cells. Cell Commun Signal.
16:942018. View Article : Google Scholar : PubMed/NCBI
|