Introduction
The best therapeutic modality for clinical stages I,
II, and appropriate stage IIIA non-small-cell lung cancer (NSCLC)
is complete surgical resection (1,2).
However, despite improvements in diagnostic and therapeutic
approaches, only 60% of patients survive 5 years after surgery
(1).
To improve the survival rates, the first trial of
adjuvant chemotherapy was performed decades ago (1). Subsequently, randomized trials reported
the benefit of platinum-based adjuvant chemotherapy (PBAC) in
patients with pathologic stages II and III NSCLC with lymph node
involvement, and subset analyses suggested a benefit in patients
with large IB tumors (3–5). However, the 5 year overall survival
(OS) advantage for patients who underwent adjuvant chemotherapy was
reported to be a modest 5.4% (6).
Furthermore, PBAC often causes adverse events. Several randomized
trials of adjuvant chemotherapy for NSCLC have reported that almost
30% of patients experienced grade III or IV toxicity and there were
0.8–2.0% of adjuvant chemotherapy-related deaths (7). Thus, its significant toxicity may limit
its use.
On the basis of the above, it is very important to
adapt adjuvant chemotherapy and to identify useful factors
predicting its efficacy.
Mutations in the epidermal growth factor receptor
(EGFR), most of which have been detected in lung
adenocarcinoma, are predictors of response to EGFR tyrosine kinase
inhibitors (TKIs), which have proven efficacy in the treatment of
advanced stage NSCLC (8,9).
Although EGFR mutations are prognostic
factors in only unresectable advanced NSCLC, there are few reports
of their utility as prognostic factors in resectable NSCLC
(10). In particular, there are no
studies in the context of predictive factors for PBAC effects.
EGFR mutations are categorized into two
groups, common mutations and minor mutations. Common mutations
comprise 85% of all EGFR mutations and include two subtypes,
in-frame deletions in exon19 (19 del) and the point mutation L858R
in exon21 (21L858R) (11). Those two
mutations are expected to have different biological features
(11,12).
Here, we investigated EGFR mutations as
prognostic factors in postoperative EGFR mutation-positive
(MT) patients treated with PBAC and whether the above two common
mutations are associated with differential responses to PBAC.
Materials and methods
Patient selection and study
design
A total of 720 consecutive patients with
adenocarcinoma underwent pulmonary resection with no evidence of
residual cancer either macroscopically or microscopically between
January 2009 and December 2013 at Tokyo Medical University
Hospital. Of these, 171 patients in pathological stages II and III
(per the 7th Edition of the TNM Classification for Lung and Pleural
Tumors of the Union for International Cancer Control) were enrolled
(13). We excluded 45 patients
because they had received preoperative chemotherapy, radiotherapy,
or both; because their tumors had a mutation in EGFR exon20
(an indicator of resistance to EGFR-TKIs), mutations in the
echinoderm microtubule-associated protein-like 4-anaplastic
lymphoma kinase fusion gene, or other minor mutations; or because
the presence of EGFR mutations could not be analyzed. We
also excluded 10 patients who had received adjuvant chemotherapy
other than platinum-based regimens such as uracil-tegafur or
tegafur-gimeracil-oteracil and six patients who received only the
best supportive care including palliative radiotherapy for
controlling pain from bone metastasis as lung cancer recurrence and
no treatments such as radiotherapy, chemotherapy, or surgical
treatment after disease recurrence. We enrolled the remaining 110
patients in the study. The study followed a retrospective, single
institutional design and investigated EGFR mutation subtype
in patients who had undergone surgical resection of adenocarcinoma.
This study was approved by the ethics committee of the Tokyo
Medical University; the approval number is 2016-167.
Analysis of patients
We reviewed the medical records of patients to
assess the following clinicopathological information: Age; sex;
smoking history; surgical procedures; tumor differentiation; blood
vessel invasion; lymphatic permeation; visceral pleural invasion;
EGFR mutation status; PBAC regimen; OS defined as the time
elapsed from the date of surgery to the date of death; and
disease-free survival (DFS) defined as the time elapsed from the
date of pulmonary resection to the date of initial recurrence or
death.
We divided the patients into a mutation (MT) group
and a wild-type (WT) group based on their EGFR status and
analyzed their prognosis.
After surgical resection, the patients were examined
at 3 month intervals for 3 years, then at 6 month intervals for the
next 2 years, and thereafter at 1 year intervals. The systemic
evaluations of patients included physical examinations, chest
roentgenograms, chest and abdominal computed tomography (CT) scans,
and tumor marker measurement. Brain magnetic resonance imaging and
bone scintigraphy or positron emission tomography/CT scanning was
performed every year.
EGFR mutation analysis
All of the surgical specimens collected were fixed
in 10% formalin and embedded in paraffin. Representative sections
were routinely stained with hematoxylin and eosin. Experienced
pathologists reviewed the samples to confirm that the sections
contained carcinoma cells. EGFR mutation analysis of the
histology specimens was screened using the direct sequencing method
until October 2009. Direct sequencing method has a detection
sensitivity of around 25%, while the cycleave polymerase chain
reaction method is around 1 to 5%. So we use cycleave polymerase
chain reaction method (after November 2009), as previously
described (14,15).
Statistical analysis
OS and DFS were estimated using the Kaplan-Meier
method. Differences in survival rates were determined using
log-rank analysis. Hazard ratios and their 95% confidence intervals
were calculated using the Cox proportional hazards model to
determine independent predictors of the OS and DFS. Significance
differences among the categorized groups were compared using
Chi-square tests. P<0.05 was considered to indicate a
statistically significant difference. Statistical analyses were
performed using the software package SPSS (version 24.0; SPSS,
Inc.).
Results
Patient demographics
The median follow-up period for all of the patients
was 35.9 months (range, 0.27–88.5 months). Characteristics of the
patients are summarized in Table I.
PBAC was carried out in 62 patients (56.4%). The most common
regimen of the PBAC comprised cisplatin and vinorelbine (41 of 62;
66.1%), and other regimens were as described in Table I. Forty-eight patients (of 110
patients; 44%) did not received adjuvant chemotherapy. The most
common reason was the patient's comorbidity such as renal
dysfunction, etc. The second most common reason was the patient's
preference. Another one was elder and poor performance status.
 | Table I.Patient characteristics (n=110). |
Table I.
Patient characteristics (n=110).
| Variables | Value (%) |
|---|
| Sex |
|
| Men | 64 (58) |
|
Women | 46 (42) |
| Age (years) |
|
| Median
age | 66 |
|
Range | 40–88 |
| Smoking habits |
|
|
Ever-smoker/unknown | 51 (46) |
| Never
smoker | 59 (54) |
| Surgical
procedure |
|
|
Lobectomy | 109 (99) |
|
Segmentectomy | 1 (1) |
| Pathological
stage |
|
| II | 52 (47) |
|
III | 58 (53) |
| Vascular
invasion |
|
|
Positive | 86 (78) |
| Lymphatic
permeation |
|
|
Positive | 98 (89) |
| Viscreral visceral
pleural invasion |
|
|
Positive | 47 (43) |
| Tumor
differentiation |
|
|
Poor | 31 (28) |
|
Well/Moderate | 79 (72) |
| Adjuvant
chemotherapy |
|
|
PBAC | 62 (56) |
|
None | 48 (44) |
| Tumor
recurrence |
|
|
Recurrence | 63 (57) |
|
Non-recurrence | 47 (43) |
| EGFR mutation
status |
|
| Mutant;
Exon 19/21 | 50 (45); 21 (19)/29
(26) |
|
Wild-type | 60 (55) |
| PBAC regimen |
|
|
Cisplatin + Vinorelbine | 41 (37) |
|
Cisplatin + Gemcitabine | 1 (1) |
|
Cisplatin +
tegafur/gimeracil/oteracil | 1 (1) |
|
Cisplatin + Docetaxel | 7 (6) |
|
Cisplatin + Pemetrexed | 1 (1) |
|
Carboplatin + Gemcitabine | 8 (7) |
|
Carboplatin + Paclitaxel | 3 (3) |
|
Observation | 48 (44) |
Among the 110 patients, 63 (57.2%) patients had
confirmed recurrence. EGFR mutations were detected in 50
patients (45.6%), and the most common EGFR mutation was
L858R (29 of 50; 58.0%). The second most common mutation was 19 del
(21 of 50; 40.2%).
The differences between patients in the PBAC group
and those in the observation group are shown in Table II. Statistically significant
differences in age were found between the two groups (P<0.001).
In the PBAC group, five patients (of 62 patients; 8%) were over 75
years old. In the observation group, 17 patients (of 48 patients;
35%) were over 75 years old. There were no statistically
significant differences in the other clinicopathological factors
between the groups.
 | Table II.Patient characteristics based on PBAC
status. |
Table II.
Patient characteristics based on PBAC
status.
|
| PBAC status, n
(%) |
|
|---|
|
|
|
|
|---|
| Variables | PBAC (n=62) | None (n=48) | P-value |
|---|
| Sex |
|
| 0.112 |
|
Men | 32 (29) | 32 (29) |
|
|
Women | 30 (27) | 16 (15) |
|
| Age, years |
|
| <0.001 |
|
<75 | 57 (52) | 31 (28) |
|
|
≥75 | 5 (1) | 17 (15) |
|
| Smoking habits |
|
| 0.774 |
|
Ever-smoker/unknown | 34 (31) | 25 (23) |
|
| Never
smoker | 28 (25) | 23 (21) |
|
| Surgical
procedure |
|
| 0.377 |
|
Lobectomy | 61 (55) | 48 (44) |
|
|
Segmentectomy | 1 (1) | 0 |
|
| Pathological
stage |
|
| 0.097 |
| II | 25 (23) | 27 (25) |
|
|
III | 37 (34) | 21 (19) |
|
| Vascular
invasion |
|
| 0.477 |
|
Present | 50 (45) | 36 (33) |
|
|
Absent | 12 (11) | 12 (11) |
|
| Lymphatic
permeation |
|
| 0.088 |
|
Present | 58 (53) | 40 (36) |
|
|
Absent | 4 (3) | 8 (8) |
|
| Viscreralvisceral
pleural |
|
| 0.333 |
| invasion |
|
Present | 24 (22) | 23 (21) |
|
|
Absent | 38 (34) | 25 (23) |
|
| Tumor
differentiation |
|
| 0.840 |
|
Poor | 17 (15) | 14 (13) |
|
|
Well/Moderate | 45 (41) | 34 (31) |
|
| Tumor
recurrence |
|
| 0.562 |
|
Recurrence | 37 (34) | 26 (23) |
|
|
Non-recurrence | 25 (23) | 22 (20) |
|
| EGFR
mutation status |
|
| 0.752 |
|
Mutant | 29 (26) | 21 (19) |
|
|
Wild-type | 33 (30) | 27 (25) |
|
Prognostic outcomes
Survival time was investigated on the basis of
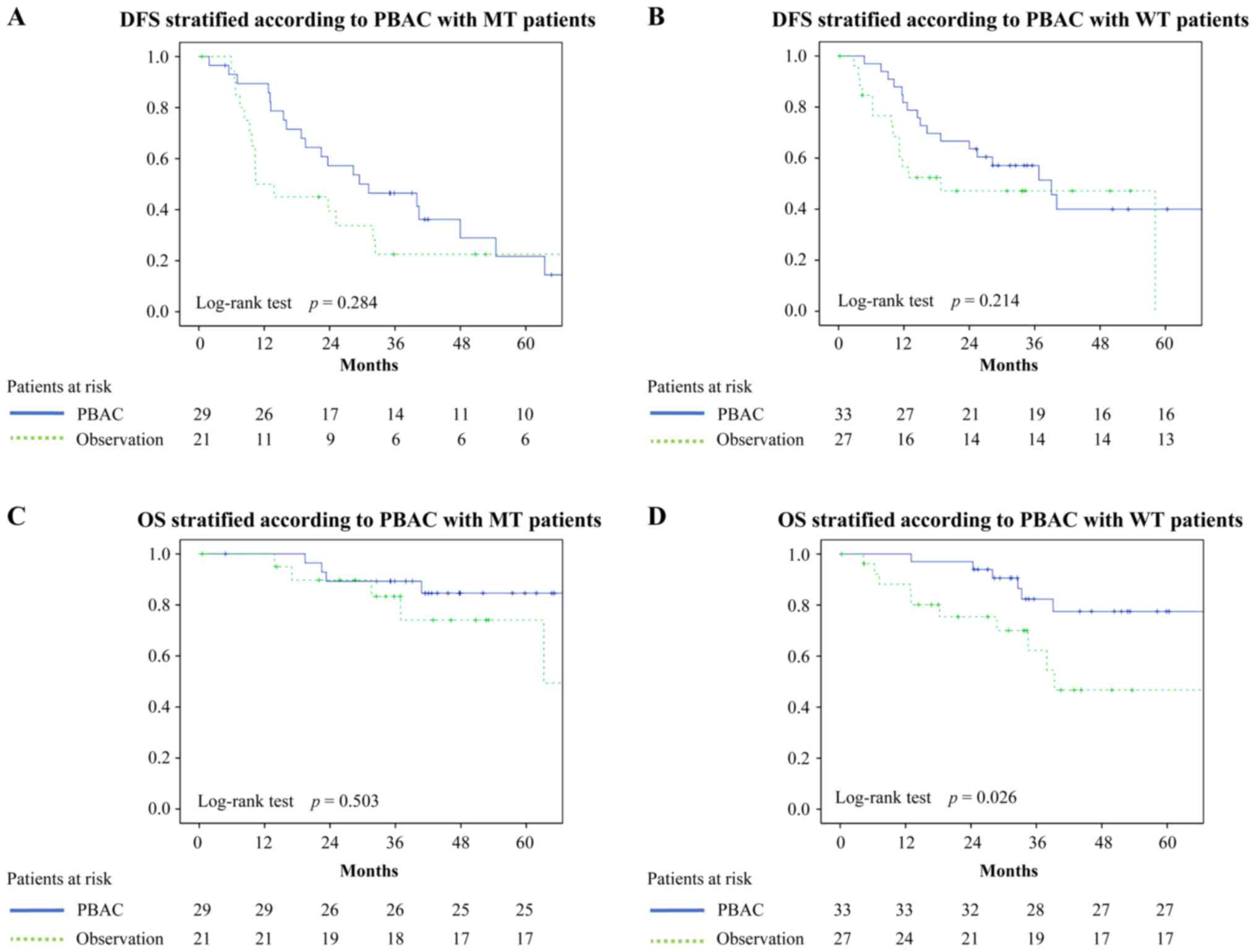
EGFR mutation status and PBAC status. DFS curves stratified
on the basis of EGFR and PBAC status are shown in Fig. 1A and B. The 3 year DFS rates in the
groups were as follows: MT with PBAC, 46.5%; MT without PBAC,
22.5%; WT with PBAC, 57.1%; and WT without PBAC, 47.1%; there were
no statistically significant differences among them.
The OS curves stratified on the basis of EGFR
and PBAC status are shown in Fig. 1C and
D. The 3 year OS rates in the groups were as follows: MT with
PBAC, 89.3%; MT without PBAC, 83.3%; WT with PBAC, 82.3%; WT
without PBAC, 62.2%; Fig. 1D shows
statistically significant differences in OS of WT patients with
PBAC and that of WT patients without PBAC (P=0.026). There were no
statistically significant differences between MT patients with PBAC
and MT patients without PBAC (P=0.503).
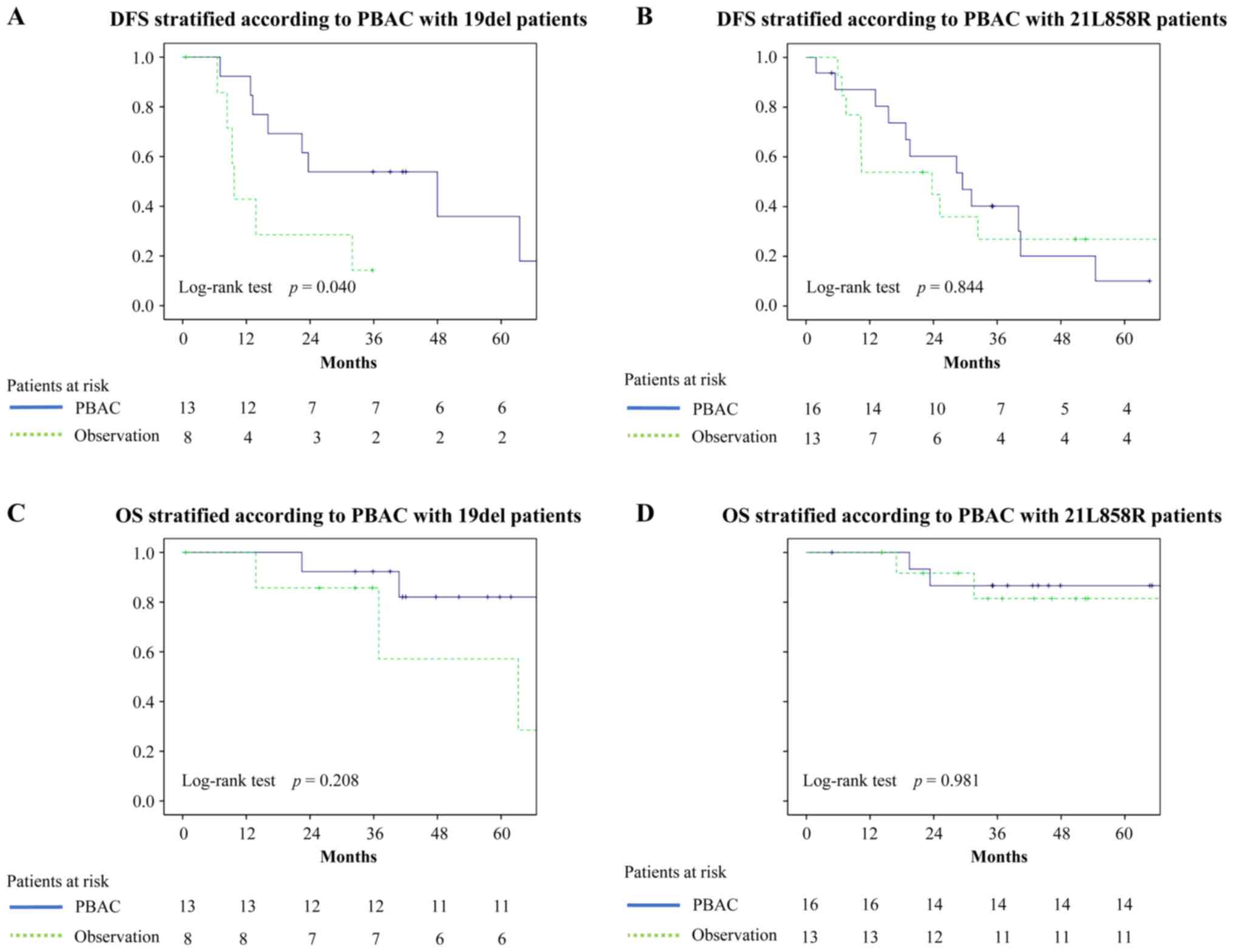
We also examined OS and DFS of the patients with
mutants stratified per mutational subtypes (Fig. 2). The 3 year DFS of the patients in
the stratified groups was as follows: 19 del with PBAC, 53.8%; 19
del without PBAC, 14.3%; 21L858R with PBAC, 40.2%; and 21L858R
without PBAC, 26.9%.
The 3 year OS rates of the above groups of patients
were as follows: 19 del with PBAC, 92.3%; 19 del without PBAC,
85.7%; 21L858R with PBAC, 86.7%; and 21L858R without PBAC, 81.5%.
Statistical analysis of patients with 19 del revealed that there
were statistically significant differences in their DFS rates,
dependent on PBAC being carried out (P=0.040). On the other hand,
there were no statistically significant differences in their OS
rates (P=0.208). Statistical analysis of patients with 21L858R
found no statistically significant differences in OS (P=0.981) or
DFS (P=0.844).
There were no statistical difference about DFS in
patients with 19 del according to drugs combination of PBAC
(P=0.99).
Risk factors for poor prognosis
The potential risk factors for DFS and OS were
analyzed using univariate survival analysis based on mutational
status (Table III). In MT
patients, univariate survival analysis showed that blood vessel
invasion was the only risk factor associated with DFS (HR, 2.781;
CI, 1.140–6.783; P=0.025). On the other hand, there were no
significant risk factors associated with OS. In WT patients, the
potential risk factors associated with OS were visceral pleural
invasion (HR, 3.326; CI, 1.202–9.207; P=0.021) and PBAC (HR, 3.005;
CI, 1.088–8.300; P=0.034). Analysis of DFS in WT patients using
univariate survival analysis showed that smoking was the only
associated risk factor (HR, 2.174; CI, 1.046–4.518; P=0.038).
Visceral pleural invasion was risk factor for OS in univariate
analysis, but not in multivariate analysis. It might be because of
small sample sizes.
 | Table III.Univariate and Multivariate analysis
of disease-free survival and overall survival. |
Table III.
Univariate and Multivariate analysis
of disease-free survival and overall survival.
| A, All
patients |
|---|
|
|---|
|
| Overall
survival | Disease-free
survival |
|---|
|
|
|
|
|---|
|
|
| MVA |
| MVA |
|---|
|
|
|
|
|
|
|---|
| Variable | UVA P-value | Hazard ratio | 95%CI | P-value | UVA P-value | Hazard ratio | 95%CI | P-value |
|---|
| Sex | 0.471 | – | – | – | 0.233 | – | – | – |
| Age | 0.118 | – | – | – | 0.130 | – | – | – |
| Smoking habits | 0.093 | – | – | – | 0.032 | 1.695 | 1.047–2.746 | 0.032 |
| Pathological
stage | 0.186 | – | – | – | 0.216 | – | – | – |
| Vascular
invasion | 0.034 | 4.872 | 1.073–22.122 | 0.04 | 0.084 | – | – | – |
| Lymphatic
premeation | 0.403 | – | – | – | 0.854 | – | – | – |
| Viscreral pleural
invasion | 0.021 | 2.000 | 0.915–4.373 | NS (0.082) | 0.036 | 1.681 | 1.038–2.723 | 0.035 |
| Tumor
differentiation | 0.020 | 2.260 | 1.040–4.911 | 0.039 | 0.709 | – | – | – |
| Adjuvant
chemotherapy | 0.030 | 2.519 | 1.143–5.550 | 0.022 | 0.073 | – | – | – |
|
| B, Patients with
EGFR wild-type |
|
|
| Overall
survival | Disease-free
survival |
|
|
|
|
|
|
| MVA |
| MVA |
|
|
|
|
|
|
|
Variable | UVA
P-value | Hazard
ratio | 95%CI | P-value | UVA
P-value | Hazard
ratio | 95%CI | P-value |
|
| Sex | 0.987 | – | – | – | 0.319 | – | – | – |
| Age | 0.489 | – | – | – | 0.920 | – | – | – |
| Smoking habits | 0.227 | – | – | – | 0.038 | – | – | – |
| Pathological
stage | 0.433 | – | – | – | 0.872 | – | – | – |
| Vascular
invasion | 0.125 | – | – | – | 0.583 | – | – | – |
| Lymphatic
premeation | 0.526 | – | – | – | 0.500 | – | – | – |
| Viscreral pleural
invasion | 0.021 | 3.242 | 1.173–8.958 | 0.023 | 0.050 | – | – | – |
| Tumor
differentiation | 0.085 | – | – | – | 0.290 | – | – | – |
| Adjuvant
chemotherapy | 0.034 | 2.928 | 1.059–8.091 | 0.038 | 0.218 | – | – | – |
|
| B, Patients with
EGFR mutant |
|
|
| Overall
survival | Disease-free
survival |
|
|
|
|
|
|
| MVA |
| MVA |
|
|
|
|
|
|
|
Variable | UVA
P-value | Hazard
ratio | 95%CI | P-value | UVA
P-value | Hazard
ratio | 95%CI | P-value |
|
| Sex | 0.127 | – | – | – | 0.925 | – | – | – |
| Age | 0.068 | – | – | – | 0.073 | – | – | – |
| Smoking habits | 0.186 | – | – | – | 0.767 | – | – | – |
| Pathological
stage | 0.118 | – | – | – | 0.136 | – | – | – |
| Vascular
invasion | 0.172 | – | – | – | 0.025 | – | – | – |
| Lymphatic
premeation | 0.759 | – | – | – | 0.444 | – | – | – |
| Viscreral pleural
invasion | 0.558 | – | – | – | 0.433 | – | – | – |
| Tumor
differentiation | 0.286 | – | – | – | 0.944 | – | – | – |
| Adjuvant
chemotherapy | 0.505 | – | – | – | 0.287 | – | – | – |
| Mutational
status | 0.343 | – | – | – | 0.955 | – | – | – |
Discussion
We performed the present study to clarify whether
EGFR mutation status can be considered a prognostic factor
predicting the effect of PBAC and to elucidate whether there is a
difference in the effect of adjuvant chemotherapy based on
mutational subtype.
We found that EGFR mutation status was not
predictive of OS or DFS benefit from PBAC in lung adenocarcinoma;
however, in WT patients, PBAC was a significant prognostic factor
for OS. This indicates that EGFR mutation status might be
considered as a predicting factor for poor effect of PBAC and MT
patients might benefit less from adjuvant chemotherapy than WT
patients would. In general, EGFR-TKI as first-line treatment for
advanced NSCLC prolongs progression-free survival and increases the
objective response rate compared with platinum doublet chemotherapy
(8,9). Few studies focused on the prognostic
value of EGFR mutation status in patients with advanced
NSCLC with chemotherapy.
In this present analysis of OS, there were no
statistically significant differences between MT patients with PBAC
and those without PBAC. The reason for this outcome may be the
effectiveness of EGFR-TKIs in MT patients in general. Prolonged
survival after recurrence in both groups of MT patients might have
obscured the difference in OS between the groups. Kudo et al
reported that the presence of EGFR mutations is a good
prognostic factor in MT patients with postoperative recurrence
(10). In contrast, in WT patients,
EGFR-TKIs have not been approved for the treatment of NSCLC except
erlotinib and have less effectiveness than in MT patients.
EGFR positive lung cancer is effective by
EGFR-TKI, and the benefits of PBAC might been diminished due to the
longer treatment period after relapse compared to the time to
relapse.
Instead of PBAC in MT patients whose effect may be
less effective, EGFR-TKI treatment as postoperative adjuvant
chemotherapy for MT patients has been investigated. Several studies
suggested a possible benefit of EGFR-TKI as postoperative adjuvant
chemotherapy for MT patients. In an analysis of 167 patients with
resected stages I to IIIA NSCLC with EGFR mutations,
patients who received EGFR-TKI treatment showed a more favorable 2
year DFS rate than an untreated group (HR, 0.53; 95% CI, 0.28–1.03;
P=0.06) (16). Another study, a
single-arm, multi-institutional, prospective phase II study called
the SELECT trial, showed that 100 patients with resected stages IA
to IIIA NSCLC and EGFR mutations receiving adjuvant
erlotinib for 2 years after standard-of-care treatment had a 2 year
DFS rate of 89% with a median follow-up of 3.4 years (17).
The above studies suggest the possible benefit of
EGFR-TKI adjuvant chemotherapy in MT patients. On the other hand, a
randomized, double-blind, placebo-controlled phase III trial called
the RADIANT study using erlotinib as adjuvant EGFR-TKI analyzed a
total of 278 patients and failed to demonstrate a DFS benefit of
erlotinib in EGFR mutation-positive patients (18).
In the analysis of subtypes, 19del patients with
PBAC showed significantly longer DFS than 19del patients without
PBAC, but 21L858R patient groups showed no statistically
significant difference in DFS. There are likely differences in
clinical and biological features between patients with 19del and
those with L858R. It was reported that the patterns of both
EGFR amplification and EGFR autophosphorylation were
shown to differ between cell lines harboring the two most common
EGFR mutation types of 19del and L858R mutation (19). Several studies have reported data
regarding these two common EGFR mutations. In an analysis of
MT patients receiving no EGFR-TKI therapy, those with the 19 del
mutation had worse survival than those with the L858R mutation
(11). Yamashita et al
reported that among NSCLC patients receiving platinum-based
chemotherapy, those with exon 19 deletion have a longer PFS and OS.
This result supported the significant effect of PBAC in DFS rates
in 19 del patient (20).
A multicenter, international, open-label,
exploratory, randomized controlled trial called the LUX-Lung7
showed a difference in OS between patients with the common
EGFR mutations (21) and
suggested that 19del patients might be more sensitive to EGFR-TKIs
than those with 21L858R.
On the basis of previous reports, we speculated that
the number of patients with the 19del mutation who remain at the
micro-metastasis stage after complete cancer resection would be
larger than those with the 21L858R mutation. In terms of
postoperative recurrence, EGFR-TKIs showed good effectiveness among
19del patients (16,21). We found a statistically significant
difference in DFS but not OS between 19del patients with PBAC and
those without PBAC.
We must acknowledge three limitations in the present
study. First, the number of patients was small and data analysis
may not have been maximally effective. In future studies, we plan
to collect more data regarding surgically resected adenocarcinoma
with pathologically confirmed lymph node metastasis. Second, this
study was retrospective and single institutional. Third, we
excluded patients with unknown EGFR mutation status; even
though the number of such excluded patients was small, it may have
contributed to selection bias.
In the present study, compared with patients with
WT, EGFR mutation-positive patients showed less influence of
PBAC on improvement of survival rate. PBAC might not be necessary
for Mt patients with pathological stage II/III lung
adenocarcinoma
On the basis of the EGFR mutation subtype,
patients with the 19 del mutation undergoing PBAC showed
significantly more favorable DFS than those without PBAC. It may
therefore be necessary to consider postoperative strategy based on
the presence of EGFR mutations and their subtype in patients
with completely resected adenocarcinoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by AMED [grant no.
JP17ck0106295].
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YK, TeO and NI designed the study; YK performed the
research, with the assistance of TeO, KI, SM, JM, KY, MH, MK, NK
TaO and JM. YK and TeO analyzed the data. YK and TOK generated the
figures and wrote the paper. TeO and NI revised the figures and
critically revised the manuscript for important intellectual
content. All authors have read and approved the manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of the Tokyo Medical University (approval number:
2016-167).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CT
|
computed tomography
|
|
DFS
|
disease-free survival
|
|
EGFR
|
epidermal growth factor receptor
|
|
NSCLC
|
non-small-cell lung cancer
|
|
OS
|
overall survival
|
|
PBAC
|
platinum-based adjuvant
chemotherapy
|
|
TKI
|
tyrosine kinase inhibitors
|
|
WT
|
wild-type
|
References
|
1
|
Arriagada R, Bergman B, Dunant A, Le
Chevalier T, Pignon JP and Vansteenkiste J; International Adjuvant
Lung Cancer Trial Collaborative Group, : Cisplatin-based adjuvant
chemotherapy in patients with completely resected non-small-cell
lung cancer. N Engl J Med. 350:351–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
NSCLC Meta-analyses Collaborative Group, ;
Arriagada R, Auperin A, Burdett S, Higgins JP, Johnson DH, Le
Chevalier T, Le Pechoux C, Parmar MK, Pignon JP, et al: Adjuvant
chemotherapy, with or without postoperative radiotherapy, in
operable non-small-cell lung cancer: Two meta-analyses of
individual patient data. Lancet. 375:1267–1277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Strauss GM, Herndon JE II, Maddaus MA,
Johnstone DW, Johnson EA, Harpole DH, Gillenwater HH, Watson DM,
Sugarbaker DJ, Schilsky RL, et al: Adjuvant paclitaxel plus
carboplatin compared with observation in stage IB non-small-cell
lung cancer: CALGB 9633 with the cancer and leukemia group B,
radiation therapy oncology group, and north central cancer
treatment group study groups. J Clin Oncol. 26:5043–5051. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Winton T, Livingston R, Johnson D, Rigas
J, Johnston M, Butts C, Cormier Y, Goss G, Inculet R, Vallieres E,
et al: Vinorelbine plus cisplatin vs. observation in resected
non-small-cell lung cancer. N Engl J Med. 352:2589–2597. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Douillard JY, Rosell R, De Lena M,
Carpagnano F, Ramlau R, Gonzáles-Larriba JL, Grodzki T, Pereira JR,
Le Groumellec A, Lorusso V, et al: Adjuvant vinorelbine plus
cisplatin versus observation in patients with completely resected
stage IB-IIIA non-small-cell lung cancer [Adjuvant Navelbine
International Trialist Association (ANITA)]: A randomised
controlled trial. Lancet Oncol. 7:719–727. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pignon JP, Tribodet H, Scagliotti GV,
Douillard JY, Shepherd FA, Stephens RJ, Dunant A, Torri V, Rosell
R, Seymour L, et al: LACE Collaborative group, lung adjuvant
cisplatin evaluation: A pooled analysis by the LACE collaborative
group. J Clin Oncol. 26:3552–3559. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Waller D, Peake MD, Stephens RJ, Gower NH,
Milroy R, Parmar MK, Rudd RM and Spiro SG: Chemotherapy for
patients with non-small cell lung cancer: The surgical setting of
the big lung trial. Eur J Cardiothorac Surg. 26:173–182. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): A multicentre, open-label, randomised phase 3 trial.
Lancet Oncol. 13:239–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kudo Y, Shimada Y, Saji H, Kato Y, Yoshida
K, Matsubayashi J, Nagase S, Kakihana M, Kajiwara N, Ohira T, et
al: Prognostic factors for survival after recurrence in patients
with completely resected lung adenocarcinoma: Important roles of
epidermal growth factor receptor mutation status and the current
staging system. Clin Lung Cancer. 16:e213–221. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shigematsu H, Lin L, Takahashi T, Nomura
M, Suzuki M, Wistuba II, Fong KM, Lee H, Toyooka S, Shimizu N, et
al: Clinical and biological features associated with epidermal
growth factor receptor gene mutations in lung cancers. J Natl
Cancer Inst. 97:339–346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Isaka T, Yokose T, Ito H, Nagata M,
Furumoto H, Nishii T, Katayama K, Yamada K, Nakayama H and Masuda
M: Correlations between the EGFR mutation status and
clinicopathological features of clinical Stage I lung
adenocarcinoma. Medicine (Baltimore). 94:e17842015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sobin LH, Gospodarowicz MK and Wittekind
C: International Union against Cancer, TNM Classification of
Malignant Tumours7th. Wiley-Blackwell; Chichester, UK, Hoboken, NJ:
2010
|
|
14
|
Yatabe Y, Hida T, Horio Y, Kosaka T,
Takahashi T and Mitsudomi T: A rapid, sensitive assay to detect
EGFR mutation in small biopsy specimens from lung cancer. J Mol
Diagn. 8:335–341. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoshida K, Yatabe Y, Park JY, Shimizu J,
Horio Y, Matsuo K, Kosaka T, Mitsudomi T and Hida T: Prospective
validation for prediction of gefitinib sensitivity by epidermal
growth factor receptor gene mutation in patients with non-small
cell lung cancer. J Thoracic Oncol. 2:22–28. 2007. View Article : Google Scholar
|
|
16
|
Janjigian YY, Park BJ, Zakowski MF,
Ladanyi M, Pao W, D'Angelo SP, Kris MG, Shen R, Zheng J and Azzoli
CG: Impact on disease-free survival of adjuvant erlotinib or
gefitinib in patients with resected lung adenocarcinomas that
harbor EGFR mutations. J Thorac Oncol. 6:569–575. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pennell NA, Neal JW, Chaft JE, Azzoli CG,
Janne PA, Govindan R, Evans TL, Costa DB, Rosovsky RP, Wakelee HA,
et al: SELECT: A multicenter phase II trial of adjuvant erlotinib
in resected early stage EGFR mutation-positive NSCLC. J Clin Oncol.
32:7514. 2014. View Article : Google Scholar
|
|
18
|
Kelly K, Altorki NK, Eberhardt WE, O'Brien
ME, Spigel DR, Crinò L, Tsai CM, Kim JH, Cho EK, Hoffman PC, et al:
Adjuvant erlotinib versus placebo in patients with stage IB-IIIA
non-small-cell lung cancer (RADIANT): A randomized, double-blind,
Phase III trial. J Clin Oncol. 33:4007–4014. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Okabe T, Okamoto I, Tamura K, Terashima M,
Yoshida T, Satoh T, Takada M, Fukuoka M and Nakagawa K:
Differential constitutive activation of the epidermal growth factor
receptor in non-small cell lung cancer cells bearing EGFR gene
mutation and amplification. Cancer Res. 67:2046–2053. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamashita F, Azuma K, Yoshida T, Yamada K,
Kawahara A, Hattori S, Takeoka H, Zaizen Y, Kawayama T, Kage M and
Hoshino T: Prognostic value of EGFR mutation and ERCC1 in patients
with non-small cell lung cancer undergoing platinum-based
chemotherapy. PLoS One. 8:e713562013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park K, Tan EH, O'Byrne K, Zhang L, Boyer
M, Mok T, Hirsh V, Yang JC, Lee KH, Lu S, et al: Afatinib versus
gefitinib as first-line treatment of patients with EGFR
mutation-positive non-small-cell lung cancer (LUX-Lung 7): A phase
2B, open-label, randomised controlled trial. Lancet Oncol.
17:577–589. 2016. View Article : Google Scholar : PubMed/NCBI
|