Introduction
Osteosarcoma is a primary bone tumor originating in
mesenchymal tissues, with a high prevalence and high rates of
malignancy. The results of a survey in 2016 indicated that each
year, 20,000-30,000 patients with osteosarcoma succumb to the
disease globally, with the highest incidence rates (70,000–80,000)
among adolescents aged 15–19 years (1). While standard methods of treatment,
including chemotherapy, palliative radiotherapy and limb amputation
or sparing can be effective, there is a high probability (80–90%)
that pulmonary metastasis (PM) will develop (1,2). PM is
common in osteosarcoma and is an important prognostic indicator for
patients. The five-year survival rate of patients with PM was ~20%
in 2014, despite extensive research that has been undertaken to
identify effective treatments for these patients (3,4). The
enhanced chest CT technique is a standard method used to evaluate
PM in patients with osteosarcoma; however, the limited sensitivity
of CT imaging can mean that PM is not detected during early stages
(5). Currently, accurate and
reliable pre-operative PM identification is not possible for
patients with osteosarcoma, and a more detailed understanding of PM
would be conducive to assessing patient prognosis, as well as to
formulate effective treatment plans. Therefore, identification of
PM in patients with osteosarcoma relies on future development of
objective markers.
Encoded by the human PDPN gene, podoplanin is
recognized as a type I transmembrane lymphoid glycoprotein
(6). As a lymphatic endothelium
marker, podoplanin is regulated by the lymphoid-specific homologous
transformant gene, prospero homeobox 1. Podoplanin contains a
platelet aggregation (PLAG)-inducing domain, which can activate the
PLAG enzyme (6,7). Since PDPN induces platelet aggregation
(8), it serves a crucial function in
cell migration, as well as tumor cell dissemination (9,10).
Increased PDPN expression has been observed in a variety of human
cancer cells (11,12), and multiple studies have reported
high PDPN expression in human osteosarcoma tissues (13) and various human osteosarcoma cell
lines, including U2OS, HOS and MG63 (7). However, to the best of our knowledge,
the association between PDPN expression and PM in patients with
osteosarcoma has not yet been investigated, which was the primary
focus of the present study.
Materials and methods
Ethics statement
The current study was performed in strict accordance
with the Declaration of Helsinki. The Institutional Ethics
Committees of Harbin Medical University (Harbin, China) and the
Harbin Medical University Cancer Hospital (Harbin, China) approved
the present study and written informed consent was obtained from
patients directly or their legal guardians. All methods conformed
to the associated institutional regulations and guidelines.
Cell line preparation and
cultivation
The human fetal osteoblastic cell line, hFOB 1.19,
and human osteosarcoma cell lines, MG63, Saos2, HOS and U2OS, were
purchased from the American Type Culture Collection, and were
maintained in a 5% CO2 atmosphere at 37°C. The cells
were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc.) containing 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), 0.6% kanamycin sulfate (Gibco; Thermo Fisher
Scientific, Inc.) and 1% antibiotic-antimycotic (Gibco; Thermo
Fisher Scientific, Inc.). The medium was refreshed two or three
times per week. Harvested cells were used for reverse
transcription-quantitative PCR (RT-qPCR) analysis.
Patients and tissue specimens
A total of 20 pairs of fresh-frozen primary
osteosarcoma tissue (POT) and adjacent non-cancerous bone tissue
(NCBT) samples were obtained from patients undergoing resection
surgery at the Department of Orthopedic Surgery, Among the 20
patients, 11 were male and 9 were female, with an average age of
21.5 years. Samples were obtained from patients at The Affiliated
Cancer Hospital of Harbin Medical University between January 1 2017
and June 31 2017. The samples were used for RT-qPCR analysis.
Immunohistochemical (IHC) analyses were performed
using 168 verified, paraffin-embedded osteosarcoma tissues
collected from patients admitted to the Department of Orthopedics
Surgery, Harbin Medical University Cancer Hospital between January
2003 and December 2012. These consisted of 98 male and 70 female
patients (age range, 7–71 years; mean, 25.1 years). A total of 35
patients received preoperative anticancer treatment, and 23
patients exhibited synchronous distant PM. Clinicopathological
parameters, including age, sex, maximum tumor diameter, Enneking
stage (14), pre-operative serum
alkaline phosphatase (ALP) levels and pre-operative PM status, were
obtained from clinical and pathological records. Osteosarcoma was
pathologically confirmed in all patients according to the World
Health Organization bone tumor diagnosis and staging criteria
(15). All patients were followed up
until 31 December 2017 and monitored over a period of 1–126 months.
Patients with whom contact was lost or had died from causes other
than osteosarcoma were excluded. All patients included in the
present study were still alive or were confirmed to have succumbed
to the cause or complications associated with this disease until
the follow-up deadline. Prognosis of patients was represented by
statistics of patients who survived until the date of follow-up.
For statistics of patient survival time, the unit was months.
RNA extraction and RT-qPCR
analysis
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to extract total RNA from fresh
osteosarcoma tissues and cell lines, according to the
manufacturer's protocols. Total RNA was quantified by
spectrophotometry analysis (Shimadzu Corporation). A universal cDNA
synthesis kit (Toyobo Life Science) was employed to reverse
transcribe RNA into cDNA, which was then analyzed by qPCR analysis
using a SYBR Green PCR kit (Toyobo Life Science) and a Prism 7300
Sequence Detection system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The thermocycling conditions were: Denaturation
at 95°C for 30 sec; followed by 40 cycles of 95°C for 5 sec and
60°C for 30 sec; and a final extension step of 95°C for 15 sec,
60°C for 1 min, 95°C for 15 sec and 60°C for 15 sec. The following
primers were used: PDPN, forward 5′-AGCGAAGACCGCTATAAGTCTG-3′ and
reverse 5′-CTTTCTGAAGTTGGCAGATCCT-3′; GAPDH, forward
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse 5′-TGGTGAAGACGCCAGTGGA-3′.
GAPDH served as the reference gene. The relative expression of PDPN
was calculated using the following formulae: i) ΔCq=Cq(target
gene)-Cq(reference gene); and ii)
ΔΔCq=ΔCq(experiment)-ΔCq(control).
Subsequently, the relative fold-change in gene expression was
calculated as the 2−ΔΔCq value (16). Experiments were performed in
triplicate.
IHC analysis
PDPN protein expression in paraffin-embedded
osteosarcoma tissues (n=168) and normal bone tissue specimens
(n=23) was determined by IHC analysis. Tissues were fixed prior to
embedding in paraffin using 4% paraformaldehyde at room temperature
for 6 h. Briefly, tissue sections (thickness, 4 µm) were dewaxed
using two washed with xylene (5 min each at room temperature) and
dehydrated in a graded series of alcohol (95, 90, 80 and 70%; 5 min
each at room temperature). Slides were prepared by boiling in
citrate buffer for 5 min (95–100°C, pH 6.0) prior to being cooled
for 20 min at room temperature). In order to reduce non-specific
antigen binding and prevent infection, slides were incubated with
0.2% trypsin in a CO2 incubator at 37°C for 50 min. The
slides were then incubated in 0.3% hydrogen peroxide for 3 min at
room temperature to inhibit the activity of endogenous peroxidases.
To reduce nonspecific binding, the slides were incubated in PBS
supplemented with 10% goat serum (Dako; Agilent Technologies, Inc.)
at room temperature for 30 min. The slides were subsequently
incubated with monoclonal mouse antibodies against human PDPN (cat.
no. D2-40; dilution, 1:100; Dako; Agilent Technologies, Inc.)
overnight at 4°C in the refrigerator. The following day, slides
were incubated with a goat anti-mouse antibody (cat. no. ZB-2305;
1:500; Histofine Simple Stain MAX PO-M; Nichirei Biosciences, Inc.)
at room temperature for 30 min. The slides were developed using
3,3′-diaminobenzidine solution. Slides were counterstained with
hematoxylin and sealed with neutral gum. Negative controls were
prepared by incubating with PBS instead of the primary antibody and
were utilized to verify the immunostaining specificity. Light
microscopy was used to observe the stained tissues ×100 or ×400 as
indicated.
IHC assessment
The scoring system for PDPN expression was based on
the percentage of positively stained tumor cells and the staining
intensity. Initial scores for the percentage of positively stained
cells were as follows: 0, 0; 1, 1–25; 2, 26–50; and 3, 51–100%.
Staining intensity was scored as follows: 0, negative; 1, weakly
positive; 2, moderately positive; and 3, strongly positive. The
immunostaining score, or immunoreactive score, was calculated as
the product of the aforementioned two scores and was determined for
all samples. IHC scoring was conducted in duplicate by two
individual pathologists with extensive experience, that were
blinded to the clinicopathological details of the patients and the
identity of the slides. The percentage of positively stained cells
in each sample was determined using >5 randomly selected fields
of view (magnification, ×400). If different scoring results were
reported by each pathologist, a third pathologist was consulted to
reach a consensus regarding the final result. Scores ranged between
0 and 9, where 0–3 was considered to indicate low protein
expression levels of PDPN, while scores of 4–9 represented high
expression levels.
Statistical analysis
The SPSS statistical software package (version,
19.0; IBM Corp.) was used for statistical analysis of the data. The
data were compared by one-way ANOVA followed by Dunnett's multiple
comparisons post hoc test. All data are expressed as the means ±
standard deviation. A paired t-test was used to compare differences
in expression levels in tumor and adjacent tissues. A χ2
test was utilized to investigate the association between
clinicopathological features and PDPN expression. The Kaplan-Meier
method was used to plot survival curves. Further analysis of the
survival plots was achieved using the log-rank test. Univariate
analysis was used to analyze differences between prognostic groups,
and factors deemed significant by the univariate analysis were
further analyzed by multivariate analysis. P<0.05 was considered
to indicate a statistically significant difference.
Results
PDPN mRNA expression levels are
increased in human osteosarcoma tissues and cell lines
RT-qPCR analysis was employed to assess PDPN
expression levels in human osteosarcoma tissue samples and cell
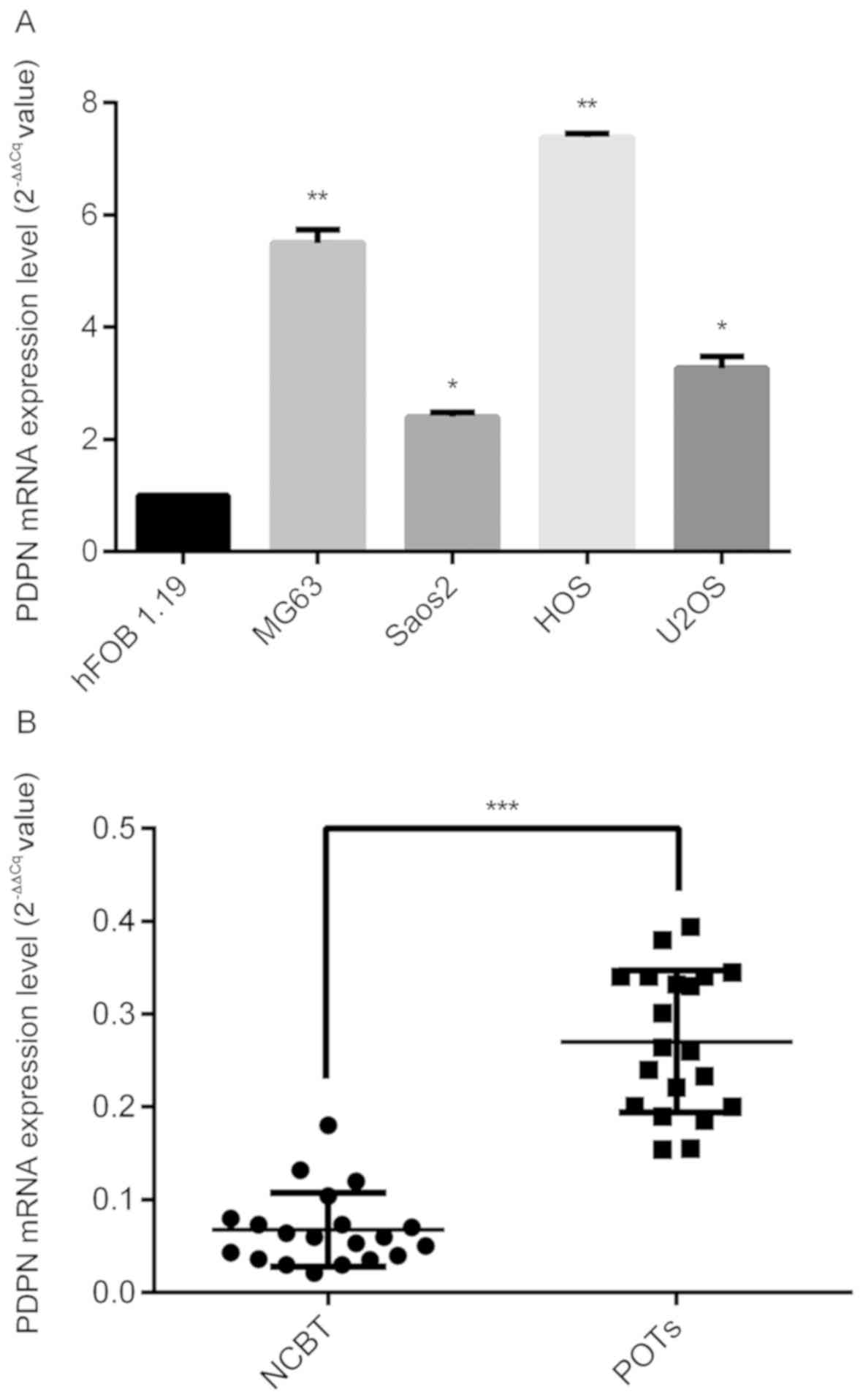
lines. As shown in Fig. 1A, PDPN
mRNA levels were observed to be higher in osteosarcoma cell lines
compared with the normal hFOB 1.19 cell line. In addition,
comparison of PDPN expression in 20 pairs of fresh POT and matched
NCBT samples revealed significantly higher mRNA expression levels
of PDPN in POT samples compared with in NCBTs (P<0.001; Fig. 1B).
Increased mRNA expression levels of
PDPN in patients with osteosarcoma and PM
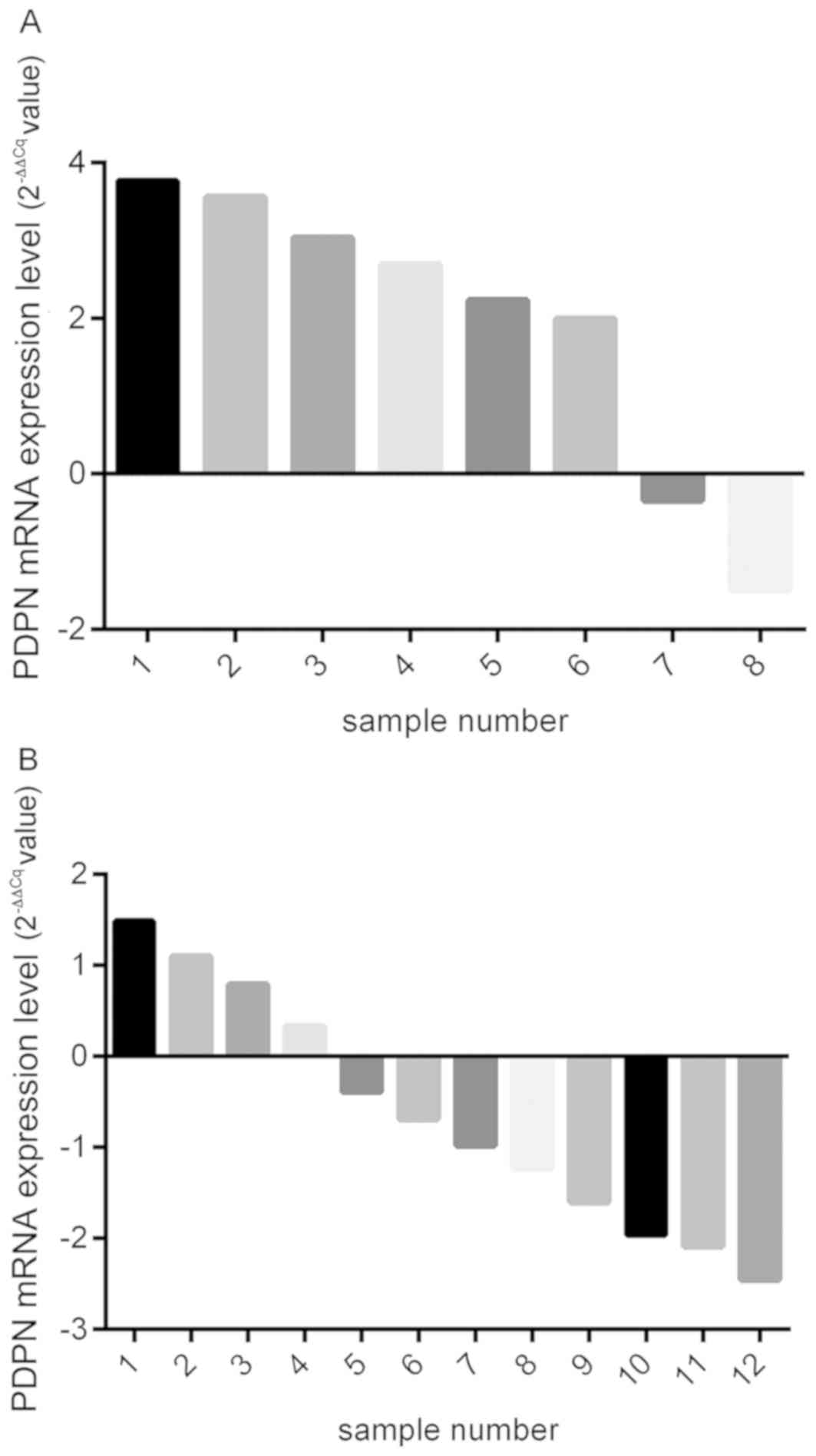
In order to verify the results, PDPN mRNA levels in
the 20 pairs of osteosarcoma and matched adjacent normal tissue
samples were analyzed, noting that 8 of these patients presented
PM. RT-qPCR analysis demonstrated higher PDPN expression in the
osteosarcoma tissues in 6 out of the 8 cases with PM (Fig. 2A), and statistical significance was
reached when the high and low expressors in the PM+
group were compared (t=2.546, P=0.014). Out of the remaining 12
patients with osteosarcoma and without PM, high levels of PDPN
expression were observed in four patients (Fig. 2B); however, no significant difference
between the high and low expressors in the PM− group was
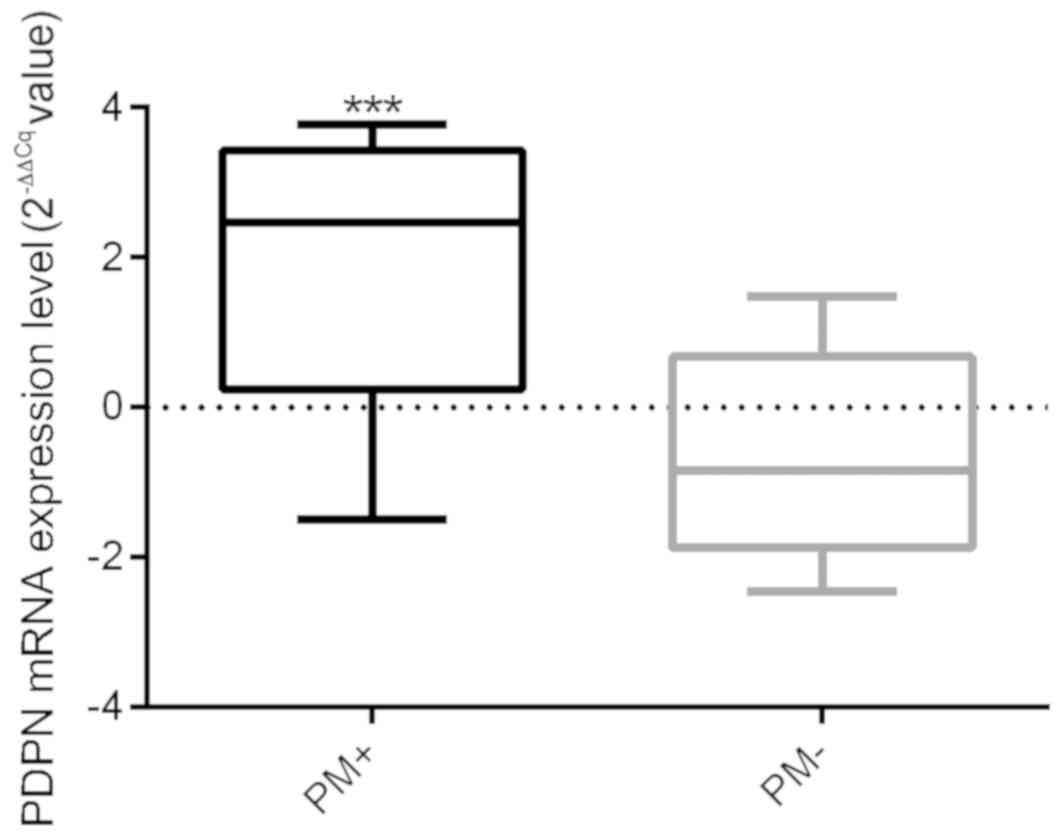
observed (t=0.495, P=0.749). The difference between the
PM+ and PM− groups regarding PDPN mRNA
expression of tissue samples was calculated. The results revealed
that the PDPN mRNA expression in the PM+ group was
significantly higher compared with the PM− group. The
mRNA expression levels of PDPN in the eight patients with
PM+ osteosarcoma and in the 12 patients with
PM− osteosarcoma were significantly different
(P<0.001; Fig. 3).
Association between PDPN expression
and clinical parameters in patients with primary osteosarcoma
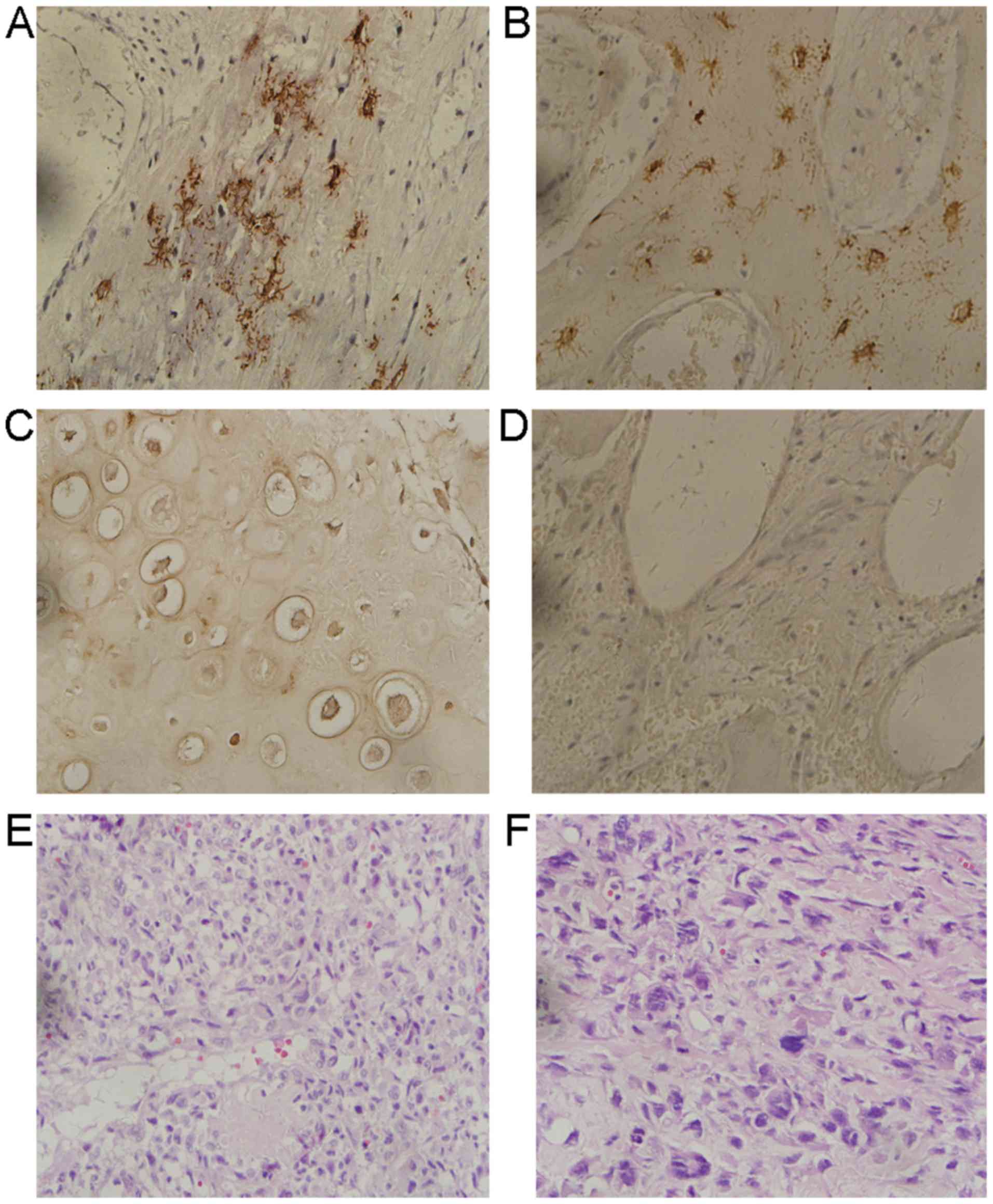
IHC analysis was used to determine PDPN expression
in samples collected from 168 patients with osteosarcoma (Fig. 4). Significantly higher levels of PDPN
expression were recorded in POTs (n=74/168; 44.0%) compared with
the NCBTs (n=2/23; 8.7%; P=0.009). Specific analysis of the
association between PDPN expression in POTs and the clinical
features of patients (Table I)
revealed a significant association between high PDPN expression and
Enneking stage (P<0.001) and PM (P<0.001); however, no
significant association was observed with patient age (P=0.196),
sex (P=0.173), maximum tumor diameter (P=0.713), preoperative
chemotherapy (P=0.635) and ALP levels in preoperative serum samples
(P=0.119).
 | Table I.Association between PDPN protein
expression and the clinicopathological characteristics of patients
with osteosarcoma. |
Table I.
Association between PDPN protein
expression and the clinicopathological characteristics of patients
with osteosarcoma.
|
| PDPN, n (%) |
|
|
|---|
|
|
|
|
|
|---|
| Clinical
parameters | High
expression | Low expression | χ2 | P-value |
|---|
| Age, years |
|
| 1.904 | 0.196 |
|
≤25 | 43 (39.8) | 65 (60.2) |
|
|
|
>25 | 31 (51.7) | 29 (48.3) |
|
|
| Sex |
|
| 1.857 | 0.173 |
|
Male | 45 (45.9) | 53 (54.1) |
|
|
|
Female | 29 (41.4) | 41 (58.6) |
|
|
| Tumor diameter,
cm |
|
| 0.015 | 0.713 |
| ≤5 | 32 (42.1) | 44 (57.9) |
|
|
|
>5 | 42 (45.7) | 50 (54.3) |
|
|
| Enneking stage |
|
| 9.805 | <0.001 |
| I and
II | 56 (38.6) | 89 (61.4) |
|
|
|
III | 18 (78.3) | 5 (21.7) |
|
|
| Preoperative
chemotherapy |
|
| 0.159 | 0.635 |
|
Yes | 23 (65.7) | 12 (34.3) |
|
|
| No | 51 (38.3) | 82 (61.7) |
|
|
| Preoperative serum
ALP |
|
| 2.217 | 0.119 |
|
High | 43 (45.3) | 52 (54.7) |
|
|
|
Normal | 31 (42.5) | 42 (57.5) |
|
|
| PM |
|
| 9.805 | <0.001 |
|
Yes | 18 (78.3) | 5 (21.7) |
|
|
| No | 56 (38.6) | 89 (61.4) |
|
|
Univariate and multivariate analyses
of clinical outcome prediction for patients with osteosarcoma
In univariate analysis, a significant association
between overall survival and tumor size [hazard ratio (HR)=2.185;
95% CI=1.506–3.130; P<0.001], Enneking stage (HR=3.476; 95%
CI=2.438–4.859; P<0.001), PM (HR=4.369; 95% CI=2.891–6.338;
P<0.001) and PDPN expression (HR=1.933; 95% CI=1.302–2.540;
P<0.001) was observed. In addition, Enneking stage (HR=1.718;
95% CI=1.116–2.650; P=0.013) and PM (HR=3.164; 95% CI=1.817–5.413;
P<0.001) were identified as significant factors by multivariate
analysis (Table II). In the
prognostic analysis, there was no statistical difference identified
between the high PDPN expression group and the low PDPN expression
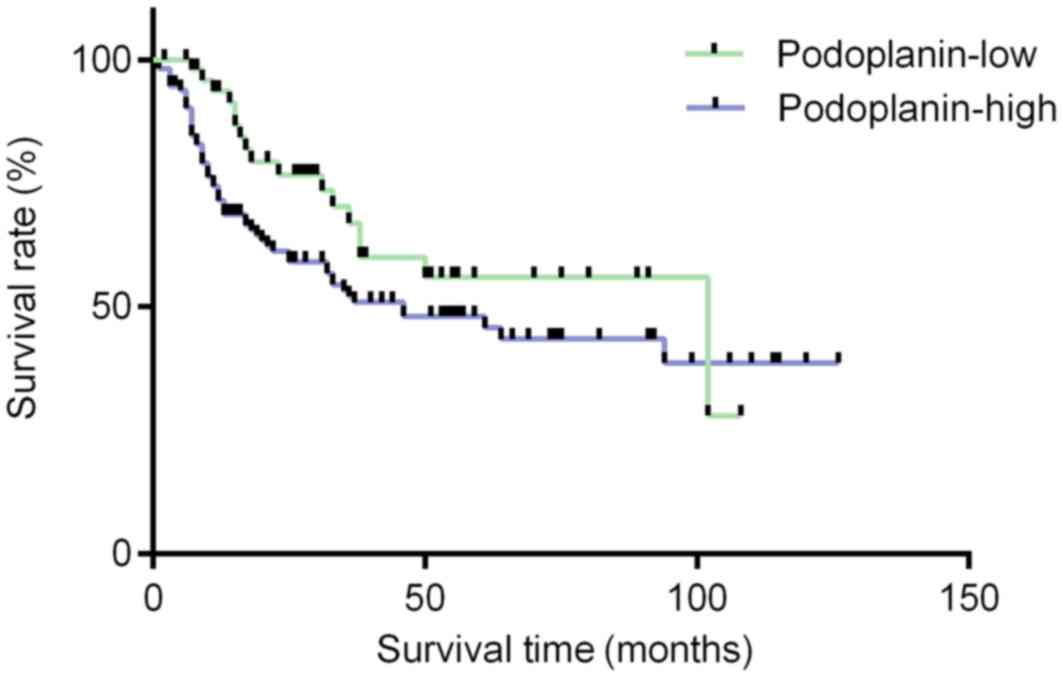
group (P=0.683; Fig. 5). Notably,
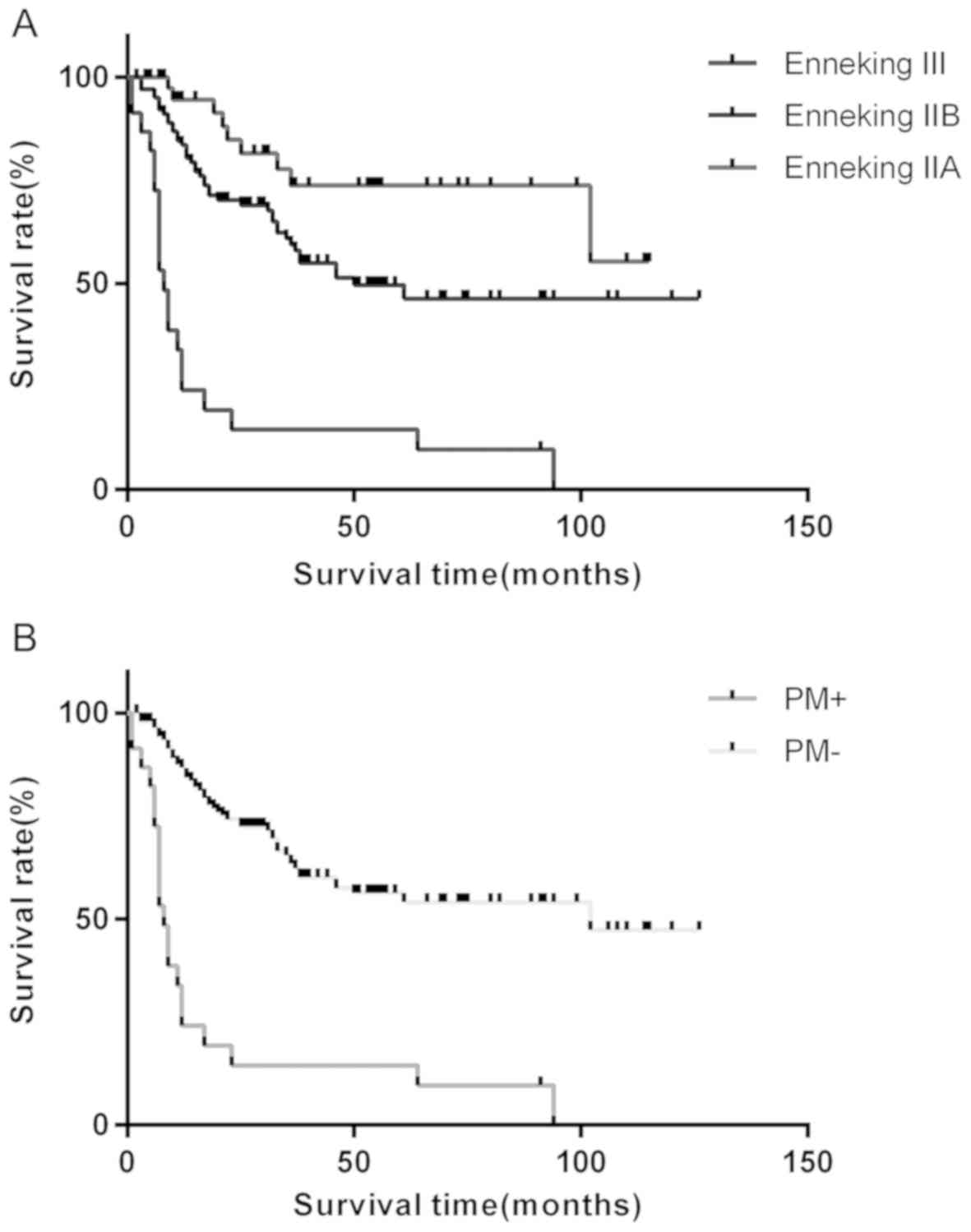
significantly shorter overall survival rates for patients with
Enneking stage III were observed compared with those with Enneking
stage II (P=0.013; Fig. 6A), as well
as for patients with PM compared with those without (P<0.001;
Fig. 6B), as determined using
Kaplan-Meier analysis and the log-rank test.
 | Table II.Univariate and multivariate analysis
of prognostic factors for patients with osteosarcoma. |
Table II.
Univariate and multivariate analysis
of prognostic factors for patients with osteosarcoma.
| Variables | HR | Univariate 95%
CI | P-value | HR | Multivariate 95%
CI | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
| ≤25 vs.
>25 | 1.297 | 0.926–1.383 | 0.343 |
|
|
|
| Sex |
|
|
|
|
|
|
| Female
vs. male | 1.165 | 0.816–1.407 | 0.175 |
|
|
|
| Tumor diameter,
cm |
|
|
|
|
|
|
| >5
vs. ≤5 | 2.185 | 1.506–3.130 | <0.001 | 1.229 | 0.927–1.853 | 0.143 |
| Enneking stage |
|
|
|
|
|
|
| III vs.
I/II | 3.476 | 2.438–4.859 | <0.001 | 1.718 | 1.116–2.650 | 0.013 |
| Preoperative
Chemotherapy |
|
|
|
|
|
|
| No vs.
yes | 1.152 | 0.667–1.572 | 0.498 |
|
|
|
| Preoperative serum
ALP |
|
|
|
|
|
|
| High
vs. normal | 1.263 | 0.889–1.784 | 0.627 |
|
|
|
| PM |
|
|
|
|
|
|
| Yes vs.
no | 4.369 | 2.891–6.338 | <0.001 | 3.164 | 1.817–5.413 | <0.001 |
| Podoplanin |
|
|
|
|
|
|
| High
vs. low | 1.933 | 1.302–2.540 | <0.001 | 1.122 | 0.830–1.458 | 0.683 |
Discussion
PM is the most reliable prognostic indicator for
patients with resectable osteosarcoma, followed by Enneking stage,
surgical complications, jumping lesions and local recurrence
(17–19). Therefore, accurate assessment of PM
is important for predicting patient prognosis and developing
effective surgical treatment plans. A previous study demonstrated
that positron emission tomography-computed tomography (CT) is a
valuable tool for the detection of PM in patients with osteosarcoma
when PM is suspected (20). However,
tumors <0.2 cm in size in the lung are beyond the limit of
detection, therefore CT imaging has limited sensitivity for the
early detection of PM (5). High
accuracy, preoperative assessment of PM is a vital part of
osteosarcoma treatment. To the best of our knowledge, There are no
studies that have investigated the association between PDPN
expression and PM in patients with osteosarcoma.
In the present study, the mRNA expression levels of
PDPN in human osteosarcoma tissues and four cell lines and its
association with osteosarcoma prognosis were investigated.
Consistent with a previous report (7), increased PDPN expression was observed
in human osteosarcoma tissues and the same four cell lines.
Comparison of PDPN expression in 20 pairs of fresh POT and matched
NCBT samples demonstrated significantly higher levels of PDPN mRNA
in POT samples compared with NCBTs. In addition, the present study
demonstrated that PDPN expression was significantly higher in
patients with osteosarcoma with PM compared with in patients
without PM. Furthermore, high PDPN expression was significantly
associated with Enneking stage and PM. The major difference between
Enneking stages III and II is metastasis to a distant organ. In
osteosarcoma, the most common metastatic site is the lung. The
results of the present study demonstrated that high PDPN expression
is associated with PM, which suggested that increased PDPN
expression may function as a marker of disease progression, whereby
the tumor cells pass through the interventricular barrier, enter
the blood stream and metastasize to the lungs.
High PDPN expression has been previously reported in
several types of cancer and is used as an effective biomarker of
tumor malignancy and prognosis (21–23). A
previous study confirmed that anti-PDPN monoclonal antibodies serve
an inhibitory role in PDPN-expressing tumors in terms of their
growth and hematogenous metastasis (24). A definite association between high
PDPN expression and PDPN-mediated lung metastasis in osteosarcoma
has not been established in previous studies. Thus far, several
hypotheses have been proposed to explain the effect of PDPN on
promoting tumor metastasis, including accelerating
epithelial-mesenchymal transition (EMT) (25), inducing collective cell migration
(26), inducing platelet activation
and aggregation (27–29), and enhancing lymphangiogenesis
(30). Regarding the mechanism of
PDPN in mediating PM in patients with osteosarcoma, the following
hypothesis is speculated based on previous studies (25,27–29): i)
The assumption that PDPN and EMT processes are associated; ii) PDPN
may serve as an endogenous ligand for C-type lectin-like receptor-2
during tumor metastasis; and iii) PDPN may promote
platelet-specific acceleration of PM to some extent. Overall,
further research to understand the mechanisms underlying
PDPN-mediated PM is required in order to develop more effective
treatment strategies for patients with osteosarcoma.
Kunita et al (7) reported that the expression of PDPN in
MG63, HOS and U2OS osteosarcoma cell lines was able to induce
platelet aggregation. Treatment with PDPN small interfering RNA or
specific neutralizing antibodies inhibited PDPN expression
(7). Enhanced migration of Dunn
osteosarcoma cells was observed following overexpression of PDPN,
while cell proliferation remained unaffected. The present study
observed increased PDPN expression in patients with osteosarcoma
and PM. Furthermore, the difference in PDPN mRNA expression levels
of tissue samples between the PM+ group and
PM− group was calculated. The results demonstrated that
the PDPN mRNA expression in the PM+ group was
significantly higher compared with the PM− group. The
differences in PDPN mRNA expression levels between the eight
patients with osteosarcoma with PM and the 12 patients without PM
were statistically significant (P<0.001). Taking the results of
all studies into consideration, it is possible that PDPN may serve
an important role in mediating tumor metastasis in patients with
osteosarcoma, and that high PDPN expression may be involved in the
development of PM in these patients. This is supported by the
observation that PDPN expression was identified as a predictor of
PM in patients with osteosarcoma in the present study. Increased
PDPN expression may therefore serve as an effective and novel
predictor of PM in patients with osteosarcoma in the clinic.
However, this requires confirmation in a larger cohort of
patients.
The association between ALP levels and the prognosis
of patients with osteosarcoma has been investigated in numerous
previous studies (31–33); however, no formal consensus has been
reached. According to a recent meta-analysis (34), increased ALP levels are associated
with reduced overall survival rates in patients with osteosarcoma,
and ALP serves as a biomarker. These results are inconsistent with
those of the present study, where no significant association
between these factors was observed. In addition, no association
between preoperative chemotherapy and patient prognosis was
observed in a previous study (35).
It is possible that preoperative chemotherapy reduces the extent of
tumor edema, which enables clear observation of the tumor boundary
and the complete removal of the tumor. However, it may not
significantly impact the survival of patients with
osteosarcoma.
In the present study, a trend was observed for high
PDPN expression to be involved in worse outcome, but this was not
deemed to be significant using Kaplan Meier analysis. PDPN was
significantly associated with PM, and PM was an independent
prognostic factor. Therefore, future studies with larger cohorts
are required to confirm whether PDPN alone can be an independent
prognostic factor for osteosarcoma. A previous study demonstrated
that PDPN immunoreactivity in tumor cells may be an effective
indicator of poor prognosis for patients with non-small cell lung
cancer (21), which is consistent
with the results of the present study. In addition, Enneking stage
and PM were identified as independent prognostic markers in
patients with osteosarcoma in the present study, which is
consistent with previous studies (36,37).
Furthermore, an association between high PDPN expression levels and
PM was observed, and PDPN expression was also identified as a
significant prognostic marker, according to univariate analyses.
Nevertheless, it was not an independent prognostic factor in
osteosarcoma according to multivariate analysis. Therefore, it is
possible that PDPN may mediate PM in patients with osteosarcoma,
which subsequently affects their prognosis. PDPN is not an
independent prognostic factor; however, the present study
demonstrated that PDPN overexpression is associated with lung
metastasis, and one may hypothesize that PDPN-induced lung
metastasis will indirectly affect the prognosis of patients. Future
studies with larger experimental samples will be required to
explore the potential of PDPN expression as a prognostic marker. In
addition, the results of the present study indicated that PDPN
expression was increased in patients with primary osteosarcoma with
PM, and a significant association with the risk of PM development.
Therefore, enhanced PDPN expression was proposed as a molecular
biomarker for PM development and the subsequent prognosis of
patients with osteosarcoma.
The present study had several limitations. First,
surgical specimens rather than biopsy specimens were analyzed;
therefore, it is possible that demineralization or preoperative
chemotherapy may have affected the results of PDPN immunostaining.
Only the sample tissue at a certain time (surgical removal) was
selected in the present study. This represented a single time
point, not a continuous observation. In the present study, only
differences between the PM+ and PM− groups
were observed. If possible, the effect of time factors on PDPN
expression should be examined. Second, tumor size was recorded in
the preoperative medical records or recorded in the surgical
records. This resulted in subjectivity. In further studies,
parameters should be recorded more objectively. Third,
heterogeneity at the protein and mRNA levels is an important
feature of malignant tumors, and the sample size in the present
study was likely too small to accurately determine the differential
expression of PDPN among the various pathological types. Therefore,
additional experiments in a larger sample cohort are required.
In conclusion, high PDPN expression levels were
observed to be significantly associated with PM in patients with
osteosarcoma in the present study. PDPN expression may be a useful
immunological marker for PM in patients with osteosarcoma. However,
further experiments are required to confirm these results.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from The
Science and Technology Talents Program of Harbin (Harbin, China;
grant nos. 2014RFXGJ041 and 2014RFQGJ094), a post-doctoral fund
(grant. no. 160780) and the Heilongjiang Natural Science Foundation
(grant nos. QC2016102 and H2016002).
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW and QM designed the study and wrote the
manuscript. JW and LN collected the specimen and patient data. XW,
WL, JB, and QS performed the experiments and analyzed the data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the Declaration of Helsinki and approved by the institutional
Ethics Committee of Harbin Medical University and Harbin Medical
University Cancer Hospital. Written informed consent was obtained
from patients or their legal guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Biazzo A and De Paolis M:
Multidisciplinary approach to osteosarcoma. Acta Orthop Belg.
82:690–698. 2016.PubMed/NCBI
|
|
2
|
Biermann JS, Chow W, Reed DR, Lucas D,
Adkins DR, Agulnik M, Benjamin RS, Brigman B, Budd GT, Curry WT, et
al: NCCN guidelines insights: Bone cancer, version 2.2017. J Natl
Compr Canc Netw. 15:155–167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nataraj V, Batra A, Rastogi S, Khan SA,
Sharma MC, Vishnubhatla S and Bakhshi S: Developing a prognostic
model for patients with localized osteosarcoma treated with uniform
chemotherapy protocol without high dose methotrexate: A
single-center experience of 237 patients. J Surg Oncol.
112:662–668. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boye K, Del Prever AB, Eriksson M, Saeter
G, Tienghi A, Lindholm P, Fagioli F, Skjeldal S, Ferrari S and Hall
KS: High-dose chemotherapy with stem cell rescue in the primary
treatment of metastatic and pelvic osteosarcoma: Final results of
the ISG/SSG II study. Pediatr Blood Cancer. 61:840–845. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heaton TE, Hammond WJ, Farber BA, Pallos
V, Meyers PA, Chou AJ, Price AP and LaQuaglia MP: A 20-year
retrospective analysis of CT-based pre-operative identification of
pulmonary metastases in patients with osteosarcoma: A single-center
review. J Pediatr Surg. 52:115–119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oki H, Kaneko MK, Ogasawara S, Tsujimoto
Y, Liu X, Sugawara M, Takakubo Y, Takagi M and Kato Y:
Characterization of monoclonal antibody LpMab-7 recognizing
Non-PLAG domain of podoplanin. Monoclon Antib Immunodiagn
Immunother. 34:174–180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kunita A, Kashima TG, Ohazama A,
Grigoriadis AE and Fukayama M: Podoplanin is regulated by AP-1 and
promotes platelet aggregation and cell migration in osteosarcoma.
Am J Pathol. 179:1041–1049. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kato Y, Kaneko MK, Kuno A, Uchiyama N,
Amano K, Chiba Y, Hasegawa Y, Hirabayashi J, Narimatsu H, Mishima K
and Osawa M: Inhibition of tumor cell-induced platelet aggregation
using a novel anti-podoplanin antibody reacting with its
platelet-aggregation-stimulating domain. Biochem Biophys Res
Commun. 349:1301–1307. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miyata K, Takemoto A, Okumura S, Nishio M
and Fujita N: Podoplanin enhances lung cancer cell growth in vivo
by inducing platelet aggregation. Sci Rep. 7:40592017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takagi S, Sato S, Oh-hara T, Takami M,
Koike S, Mishima Y, Hatake K and Fujita N: Platelets promote tumor
growth and metastasis via direct interaction between
Aggrus/podoplanin and CLEC-2. PLoS One. 8:e736092013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Swain N, Kumar SV, Routray S, Pathak J and
Patel S: Podoplanin-a novel marker in oral carcinogenesis. Tumour
Biol. 35:8407–8413. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamaki E, Yajima T, Kosaka T, Mogi A,
Tanaka S and Kuwano H: Podoplanin overexpression in human
mesothelioma cell lines enhances the tumorigenic phenotype. Oncol
Rep. 29:932–940. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kaneko MK, Oki H, Ogasawara S, Takagi M
and Kato Y: Anti-podoplanin monoclonal antibody LpMab-7 detects
metastatic lesions of osteosarcoma. Monoclon Antib Immunodiagn
Immunother. 34:154–161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
DePaolo CJ, Foster WS, Dabezies EJ and
D'Ambrosia RD: A case report of malignant mesenchymoma with
discussion of musculoskeletal tumor staging: The Enneking system.
Orthopedics. 11:1263–1276. 1988.PubMed/NCBI
|
|
15
|
Jo VY and Doyle LA: Refinements in sarcoma
classification in the current 2013 world health organization
classification of tumours of soft tissue and bone. Surg Oncol Clin
N Am. 25:621–643. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grimer RJ, Aydin BK, Wafa H, Carter SR,
Jeys L, Abudu A and Parry M: Very long-term outcomes after
endoprosthetic replacement for malignant tumours of bone. Bone
Joint J. 98-B:857–864. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koirala P, Roth ME, Gill J, Chinai JM,
Ewart MR, Piperdi S, Geller DS, Hoang BH, Fatakhova YV, Ghorpade M,
et al: HHLA2, a member of the B7 family, is expressed in human
osteosarcoma and is associated with metastases and worse survival.
Sci Rep. 6:311542016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Puri A, Byregowda S, Gulia A, Crasto S and
Chinaswamy G: A study of 853 high grade osteosarcomas from a single
institution-Are outcomes in Indian patients different? J Surg
Oncol. 117:299–306. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Angelini A, Ceci F, Castellucci P,
Graziani T, Polverari G, Trovarelli G, Palmerini E, Ferrari S,
Fanti S and Ruggieri P: The role of 18F-FDG PET/CT in
the detection of osteosarcoma recurrence. Eur J Nucl Med Mol
Imaging. 44:1712–1720. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kadota K, Huang CL, Liu D, Nakashima N,
Yokomise H, Ueno M and Haba R: The clinical significance of the
tumor cell D2-40 immunoreactivity in non-small cell lung cancer.
Lung Cancer. 70:88–93. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yuan P, Temam S, El-Naggar A, Zhou X, Liu
DD, Lee JJ and Mao L: Overexpression of podoplanin in oral cancer
and its association with poor clinical outcome. Cancer.
107:563–569. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Minardi D, d'Anzeo G, Lucarini G, Filosa
A, Zizzi A, Simonetti O, Polito M Jr, Offidani AM, Di Primio R,
Montironi R and Muzzonigro G: D2-40 immunoreactivity in penile
squamous cell carcinoma: A marker of aggressiveness. Hum Pathol.
42:1596–1602. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakazawa Y, Takagi S, Sato S, Oh-hara T,
Koike S, Takami M, Arai H and Fujita N: Prevention of hematogenous
metastasis by neutralizing mice and its chimeric
anti-Aggrus/podoplanin antibodies. Cancer Sci. 102:2051–2057. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martin-Villar E, Megias D, Castel S,
Yurrita MM, Vilaró S and Quintanilla M: Podoplanin binds ERM
proteins to activate RhoA and promote epithelial-mesenchymal
transition. J Cell Sci. 119:4541–4553. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wicki A, Lehembre F, Wick N, Hantusch B,
Kerjaschki D and Christofori G: Tumor invasion in the absence of
epithelial-mesenchymal transition: Podoplanin-mediated remodeling
of the actin cytoskeleton. Cancer Cell. 9:261–272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kunita A, Kashima TG, Morishita Y,
Fukayama M, Kato Y, Tsuruo T and Fujita N: The platelet
aggregation-inducing factor aggrus/podoplanin promotes pulmonary
metastasis. Am J Pathol. 170:1337–1347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Suzuki-Inoue K, Kato Y, Inoue O, Kaneko
MK, Mishima K, Yatomi Y, Yamazaki Y, Narimatsu H and Ozaki Y:
Involvement of the snake toxin receptor CLEC-2, in
podoplanin-mediated platelet activation, by cancer cells. J Biol
Chem. 282:25993–26001. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakazawa Y, Sato S, Naito M, Kato Y,
Mishima K, Arai H, Tsuruo T and Fujita N: Tetraspanin family member
CD9 inhibits Aggrus/podoplanin-induced platelet aggregation and
suppresses pulmonary metastasis. Blood. 112:1730–1739. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cueni LN, Hegyi I, Shin JW, Albinger-Hegyi
A, Gruber S, Kunstfeld R, Moch H and Detmar M: Tumor
lymphangiogenesis and metastasis to lymph nodes induced by cancer
cell expression of podoplanin. Am J Pathol. 177:1004–1016. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Han J, Yong B, Luo C, Tan P, Peng T and
Shen J: High serum alkaline phosphatase cooperating with MMP-9
predicts metastasis and poor prognosis in patients with primary
osteosarcoma in Southern China. World J Surg Oncol. 10:372012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim SH, Shin KH, Moon SH, Jang J, Kim HS,
Suh JS and Yang WI: Reassessment of alkaline phosphatase as serum
tumor marker with high specificity in osteosarcoma. Cancer Med.
6:1311–1322. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Marais LC, Bertie J, Rodseth R, Sartorius
B and Ferreira N: Pre-treatment serum lactate dehydrogenase and
alkaline phosphatase as predictors of metastases in extremity
osteosarcoma. J Bone Oncol. 4:80–84. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hao H, Chen L, Huang D, Ge J, Qiu Y and
Hao L: Meta-analysis of alkaline phosphatase and prognosis for
osteosarcoma. Eur J Cancer Care (Engl). 26:2017. View Article : Google Scholar
|
|
35
|
Nagano A, Ishimaru D, Nishimoto Y, Akiyama
H and Kawai A: Primary bone sarcomas in patients over 40 years of
age: A retrospective study using data from the bone tumor registry
of Japan. J Orthop Sci. 22:749–754. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lisenda L, Linda ZA, Snyman FPJ, Kyte RD
and Lukhele M: Osteosarcoma patient outcomes at a South African
tertiary hospital. S Afr Med J. 107:754–757. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang B, Pang QJ, Zhang HJ and Yuan Y:
Multivariate analysis for prognostic factors among 43 patients with
osteosarcoma. Zhongguo Gu Shang. 24:982–986. 2011.(In Chinese).
PubMed/NCBI
|