Introduction
Colorectal cancer (CRC) is the third most commonly
diagnosed type of cancer worldwide, and represents a leading cause
of mortality in men and women (1,2). Tumor
invasion and distant metastasis are the main causes of
cancer-associated mortality in patients with CRC (3). The liver is the most frequent site of
metastatic spread in CRC, and 15–25% of patients with CRC present
with liver metastases at the time of diagnosis (4). Although improvements in screening
tests, chemotherapy, surgical techniques and multidisciplinary
approaches have substantially decreased the morbidity and mortality
rates of patients with CRC in the last decades (5,6), the
prognosis of patients with advanced-stage CRC remains
unsatisfactory due to frequent metastasis and CRC recurrence
(7). Understanding the underlying
mechanisms of CRC, particularly during colorectal liver metastasis,
is therefore crucial to improve the clinical outcomes and overall
survival of patients.
Long non-coding RNAs (lncRNAs) are defined as
transcripts of >200 nucleotides in length that are not
translated into proteins, and account for much of the transcribed
genome (8). lncRNAs can promote or
inhibit the expression of various protein-coding genes through
interactions with other cellular macromolecules, including DNA, RNA
and proteins (8). Numerous studies
have reported that lncRNAs are crucial for the regulation of major
physiological and pathological processes, including cell growth,
apoptosis, stem-cell pluripotency, cancer invasion, metastasis,
development, differentiation and the immune response, and serve a
role in early diagnosis, targeted therapy and drug resistance of
cancers (9–15). A previous study has demonstrated that
the lncRNA mir-100-let-7a-2-mir-125b-1 cluster host gene (MIR100HG)
is significantly associated with cetuximab resistance (15); however, other functional roles of
MIR100HG in the development of cancer, particularly CRC, remain
unknown and require further investigation.
In the present study, MIR100HG expression in CRC
tissues and paired normal mucosae was assessed by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR). In
addition, the association between MIR100HG expression and the
clinicopathological characteristics of patients with CRC was
assessed. Furthermore, Kaplan-Meier analysis with the log-rank test
was used to evaluate the prognostic value of MIR100HG in patients
with CRC. In addition, in vitro and in vivo cell
function assays were performed to investigate the impact of
MIR100HG on CRC cell migration and invasion and to determine the
potential underlying mechanisms of CRC progression. The findings of
the present study suggested that MIR100HG may be involved in CRC
invasion and liver metastasis.
Materials and methods
Patients and specimens
A total of 116 paired CRC tissues and matched normal
mucosae were collected from patients with CRC who underwent tissue
biopsy at the Department of Gastroenterology, Shanghai General
Hospital of Nanjing Medical University between September 2010 and
March 2016. Patients comprised 60 men and 56 women (mean age, 64
years; age range, 32–89 years). All specimen were
histopathologically confirmed as CRC. No patients had received
local or other related anticancer therapies prior to tumor biopsy.
The tumor grade and clinical stages were classified according to
the guidelines of the American Joint Committee on Cancer (AJCC)
(16). The present study was
approved by the Ethics Committee of Shanghai General Hospital of
Nanjing Medical University, and written informed consent was
obtained from all patients prior to enrollment in the present
study.
RNA extraction and RT-qPCR
Total RNA was isolated from fresh CRC and adjacent
normal mucosae tissues using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). First strand cDNA was
synthesized from 2 µg RNA using a Prime-Script PCR reagent kit
(Takara Bio, Inc.) according to the manufacturer's protocol. By
using the LightCycler®480 system (Roche Applied
Science), the SYBR Premix Ex-Taq II kit (Takara Bio, Inc.) was
applied to perform qPCR. The cycling conditions were as follows:
Initial denaturation (2 min at 95°C) followed by 40 cycles of
denaturation (10 sec at 95°C), annealing (30 sec at 59°C),
elongation (30 sec at 72°C) and a final extension (30 sec at 72°C).
The amplified samples were then maintained at 4°C. The primer
sequences used for MIR100HG detection were as follows: MIR100HG
forward, 5′-CCCAGTGCAAGGACAAAGA-3′ and reverse,
5′-GCAGAGGAGGTGTCTTCAGG-3′; and GAPDH forward,
5′-GGGAAATTCAACGGCACAGT-3′ and reverse, 5′-AGATGGTGATGGGCTTCCC-3′.
The relative expression levels of MIR100HG were normalized to the
endogenous control GAPDH and expressed as 2−ΔΔCq
(17).
Cell culture and transfection
The human CRC LoVo, DLD-1, RKO, SW48, HCT8 and
HCT116 cell lines, and the normal intestinal mucous epithelium FHC
cell line were obtained from the Institute of Biochemistry and Cell
Biology of the Chinese Academy of Sciences. All cells were cultured
in DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% FBS (Invitrogen; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 µg/ml streptomycin and placed at 37°C in a
humidified incubator containing 5% CO2.
The CRC cell line LOVo overexpressing MIR100HG was
established by transfection with lentiviral vectors encoding human
MIR100HG (PCDH-MIR100HG). In addition, MIR100HG-knockdown CRC cell
line HCT116 were constructed by transfection with MIR100HG short
hairpin RNA (shRNA). The sequences used for overexpression and
knockdown were previously described (18).
In vitro Transwell assay
By using the two transfected CRC cell lines LoVo and
HCT116 and their control groups, transwell assays were used to
assess tumor cell migration and invasion. A Transwell 24-well
Boyden chamber with a polycarbonate membrane (pore size, 8.0 µm;
Corning Inc.) was used for cell migration (without
Matrigel® coating; BD Biosciences) and invasion (with
Matrigel® coating, prepared on ice) assays, according to
the manufacturer's protocol. Briefly, 5×105 cells/ml
were seeded in 200 µl serum-free DMEM medium in the upper chamber,
whereas DMEM medium supplemented with 500 µl 10% FBS was added to
the lower chamber. Following incubation for 24 h, cells were fixed
with 4% polyoxymethylene and stained with 0.1% crystal violet at
room temperature. Cells that had moved to the lower side of the
membrane were counted in 10 visual fields and the average value was
calculated. Cells images were captured using a light microscope
(Olympus Corporation; magnification, ×200). Each experiment was
performed independently three times.
In vivo metastatic assay
Nude mice were purchased from Shanghai Research
Center for Model Organisms, Inc. Mice had an average weight of 20
g. Mice were placed in plastic cages with airtight air filter at
the temperature of 18–22°C, humidity of 40–60%, under a light/dark
cycle of 10/14 h each day, and had free access to food and water.
For the in vivo cell metastatic assay, following anesthesia
with ether inhalation, MIR100HG-overexpressing CRC cells LoVo,
sh-MIR100HG knockdown CRC cells HCT116 and their control groups
were injected (200 µl at the density of 1×106/ml in PBS)
into the tail vein of nude mice (4 weeks old male BALB/C nude mice;
n=3 for each group). After 4 weeks, mice were euthanized by
cervical dislocation, and livers were collected and immediately
fixed with 4% formaldehyde at room temperature for 24–48 h. After
paraffin embedding, liver tissue was cut into 4–7 µm-thick
sections. After hematoxylin-eosin staining at room temperature for
3–5 min, the tumor colonies formed in the livers were detected by
light microscopy and quantified using GraphPad Prim 7 (GraphPad
Software, Inc.). All experimental procedures and animal studies
involving mice were in accordance with the Shanghai General
Hospital of Nanjing Medical University Animal Care and Use
guidelines. Ethical approval for the animal study was obtained from
the Ethics Committee of Shanghai General Hospital of Nanjing
Medical University.
Statistical analysis
Each experiment was performed independently three
times. Data were expressed as the means ± standard deviation and
were analyzed using SPSS v22.0 software (IBM Corp.). Student's
t-test or one-way analysis of variance followed by
Student-Newman-Keuls post hoc test were used to analyze the
differences in MIR100HG expression among the continuous variables.
A χ2 test or Fisher's exact test was appropriately used
to determine the statistical significance between MIR100HG
expression and the patients' clinicopathological characteristics.
Kaplan-Meier analysis and the log-rank test were used for the
comparison of patients' survival curves. The hazard ratio with 95%
confidence interval in the Cox proportional hazards model was
calculated to measure the hazard risk of individual factors for
disease-free survival (DFS) and overall survival (OS). All graphs
were plotted using GraphPad Prism v5.0 software (GraphPad Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
High MIR100HG expression is associated
with an aggressive phenotype in patients with CRC
A total of 40 paired CRC and normal specimen were
randomly selected from the 116 patients to detect MIR100HG
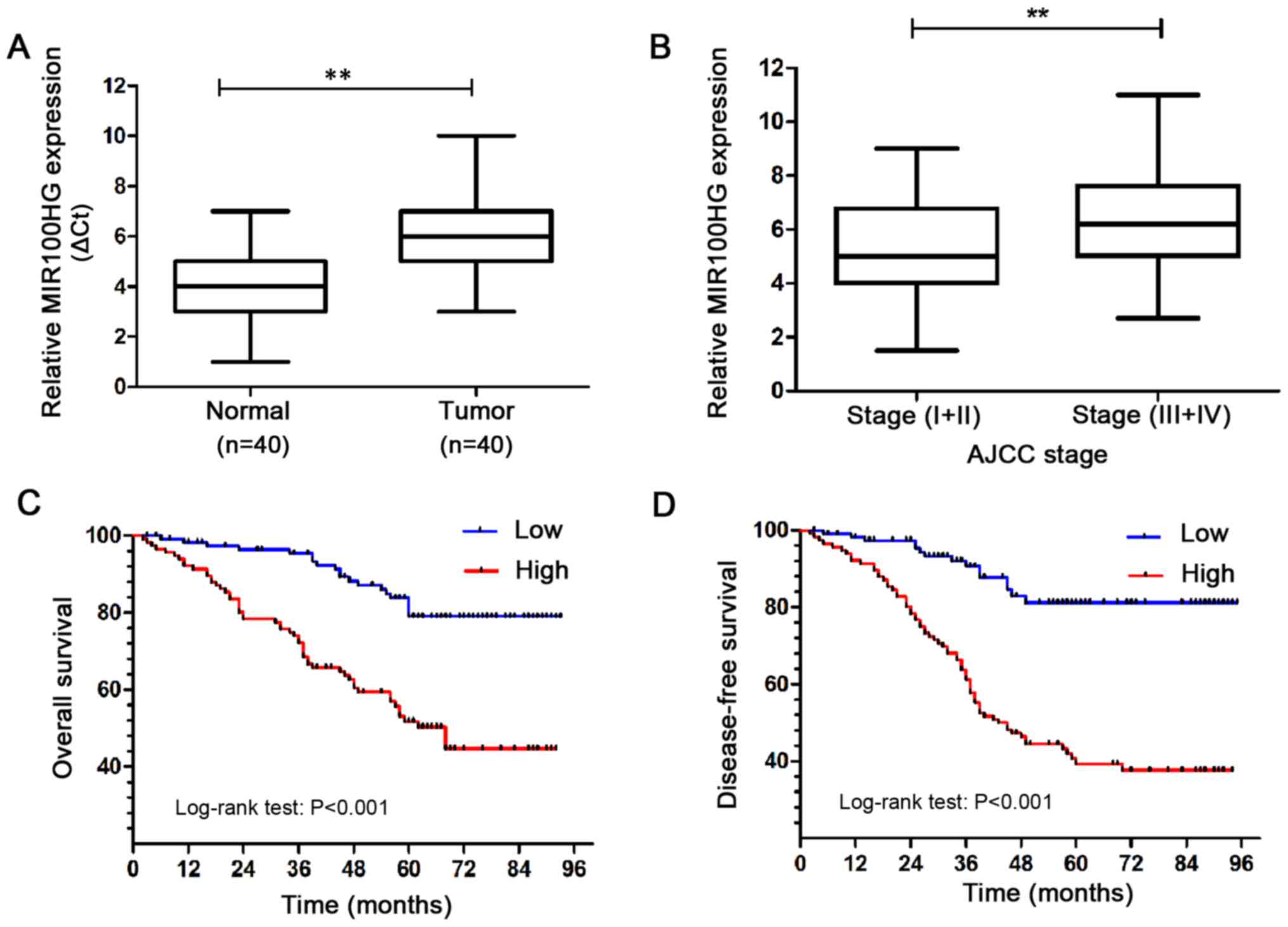
expression by RT-qPCR. The results demonstrated that MIR100HG
expression was significantly increased in CRC tissues compared with
adjacent normal colon mucosae (Fig.
1A). Furthermore, MIR100HG expression was examined in CRC
tissues at different stages. The results revealed that MIR100HG
expression was significantly higher in advanced-stage (III+IV) CRC
tissues compared with low stage (I+II) CRC tissues (Fig. 1B). These results demonstrated that
MIR100HG was significantly elevated in CRC and may be associated
with an aggressive phenotype in patients with CRC.
Subsequently, the association between MIR100HG
expression and TNM stage in the 116 patients with CRC was
investigated (19). MIR100HG
expression was first evaluated in the 116 paired samples by
RT-qPCR. A mean value of 5.55 was calculated. CRC samples with an
expression level of MIR100HG ≥5.55 and <5.55 were classified in
the high and low expression groups, respectively. Briefly, 75.0%
(87/116) of specimens exhibited low MIR100HG expression in normal
colorectal mucosa tissues. Furthermore, 25.0% (29/116) of normal
colorectal mucosa tissues presented with high MIR100HG expression,
whereas 68.1% (79/116) of CRC samples presented with high MIR100HG
expression (Table I). In addition,
high MIR100HG expression was positively associated with T stage,
lymph node metastasis, distant metastasis, AJCC stage and
histological differentiation in CRC samples (Table II). These results further indicated
that MIR100HG overexpression may serve a role in CRC metastasis and
in the prognosis of patients with CRC.
 | Table I.MIR100HG expression in paired normal
colorectal mucosa and tumor tissues. |
Table I.
MIR100HG expression in paired normal
colorectal mucosa and tumor tissues.
|
|
| Relative MIR100HG
expression |
|
|---|
|
|
|
|
|
|---|
| Tissue samples | Number | Low, n (%) | High, n (%) | P-value |
|---|
| Normal tissues | 116 | 87 (75.0) | 29 (25.0) | <0.001 |
| Tumor tissue | 116 | 37 (31.9) | 79 (68.1) |
|
 | Table II.Association between MIR100HG
expression and clinicopathological characteristics of patients with
colorectal cancer. |
Table II.
Association between MIR100HG
expression and clinicopathological characteristics of patients with
colorectal cancer.
|
|
| Relative MIR100HG
expression |
|
|---|
|
|
|
|
|
|---|
| Variables | Number (%) | Low, n (n=37, %) | High, n (n=79,
%) | P-value |
|---|
| Age, years |
|
|
| 0.596 |
|
<65 | 54 (46.2) | 20 (54.1) | 34 (43.0) |
|
| ≥65 | 62 (53.8) | 17 (45.9) | 45 (57.0) |
|
| Sex |
|
|
| 0.629 |
| Male | 60 (51.7) | 16 (43.2) | 44 (55.7) |
|
|
Female | 56 (48.3) | 21 (56.8) | 35 (44.3) |
|
| Tumor size, cm |
|
|
| 0.067 |
|
<3 | 62 (53.4) | 25 (67.6) | 37 (46.8) |
|
| ≥3 | 54 (46.6) | 12 (32.4) | 42 (53.2) |
|
| Location |
|
|
| 0.416 |
|
Right | 29 (25.0) | 6 (16.2) | 23 (29.1) |
|
|
Transverse | 27 (23.3) | 17 (45.9) | 10 (12.7) |
|
|
Left | 60 (51.7) | 14 (37.9) | 46 (58.2) |
|
| AJCC stage |
|
|
| 0.024a |
|
I–II | 45 (38.8) | 24 (64.9) | 21 (26.6) |
|
|
III–IV | 71 (61.2) | 13 (35.1) | 58 (73.4) |
|
| pT stage |
|
|
| 0.048a |
| T1 | 17 (14.6) | 10 (27.0) | 7 (8.9) |
|
| T2 | 46 (39.7) | 12 (32.4) | 34 (43.0) |
|
| T3 | 37 (31.9) | 11 (29.8) | 26 (32.9) |
|
| T4 | 16 (13.8) | 4 (10.8) | 12 (15.2) |
|
| pN stage |
|
|
| 0.020a |
| N0 | 45 (38.8) | 24 (64.9) | 21 (26.6) |
|
|
N1-2 | 71 (61.2) | 13 (35.1) | 58 (73.4) |
|
| pM stage |
|
|
| 0.023a |
| M0 | 101 (87.1) | 37 (100) | 64 (81.0) |
|
| M1 | 15 (12.9) | 0 (0.0) | 15 (19.0) |
|
|
Differentiation |
|
|
| 0.035a |
|
Well | 31 (26.7) | 19 (51.4) | 12 (15.2) |
|
|
Moderate | 40 (34.5) | 10 (27.0) | 30 (38.0) |
|
|
Poor | 45 (38.8) | 8 (21.6) | 37 (46.8) |
|
| Vascular
invasion |
|
|
| 0.862 |
|
Yes | 3 (2.6) | 0 (0.0) | 3 (3.8) |
|
| No | 113 (97.4) | 37 (100) | 76 (96.2) |
|
MIR100HG upregulation predicts an
unfavorable prognosis and poor survival for patients with CRC
The prognostic value of MIR100HG expression in CRC
was evaluated by assessing the DFS and OS of the 116 patients with
CRC using Kaplan-Meier analysis and log-rank test. The results
demonstrated that the DFS and OS of patients with CRC and high
MIR100HG expression were shorter compared with those of patients
with CRC and low MIR100HG expression (Fig. 1C and D). These results suggested that
high MIR100HG expression may predict a poor prognosis and survival.
Furthermore, the results from univariate and multivariate Cox
regression analyses demonstrated that MIR100HG expression, AJCC
stage, T classification, N classification, M classification and
tumor differentiation may be considered as independent prognostic
factors for DFS and OS (Tables III
and IV). These findings suggested
that high MIR100HG expression in CRC tissues may be considered as a
prognostic factor for patients with CRC.
 | Table III.Univariate and multivariate analyses
of disease-free survival in patients with colorectal cancer. |
Table III.
Univariate and multivariate analyses
of disease-free survival in patients with colorectal cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| MIR100HG | 3.658 | 1.956–6.893 | 0.001a | 4.105 | 1.809–6.842 | 0.001a |
| Age | 1.165 | 0.758–2.345 | 0.456 | – | – | – |
| Sex | 1.208 | 0.765–1.998 | 0.547 | – | – | – |
| Tumor size | 1.077 | 0.657–1.954 | 0.658 | – | – | – |
| Location | 0.895 | 0.384–1.848 | 0.506 | – | – | – |
| AJCC Stage | 4.924 | 1.264–5.831 | 0.002a | 5.264 | 1.249–6.048 | 0.001a |
| T
classification | 1.216 | 1.851–2.536 | 0.035a | 1.768 | 1.959–2.833 | 0.027a |
| N
classification | 1.659 | 1.181–2.611 | 0.032a | 1.937 | 1.349–3.117 | 0.022a |
| M
classification | 3.786 | 1.854–4.594 | 0.003a | 3.853 | 2.432–4.897 | 0.001a |
|
Differentiation | 1.821 | 1.125–2.543 | 0.021a | 2.018 | 1.275–3.958 | 0.023a |
| Vascular
invasion | 1.354 | 0.872–1.685 | 0.183 | – | – | – |
 | Table IV.Univariate and multivariate analyses
of overall survival in patients with colorectal cancer. |
Table IV.
Univariate and multivariate analyses
of overall survival in patients with colorectal cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| MIR100HG | 3.241 | 2.843–7.231 | <0.001 | 3.533 | 1.877–5.958 | <0.001 |
| Age | 1.072 | 0.694–2.017 | 0.442 | – | – | – |
| Sex | 1.128 | 0.911–1.333 | 0.526 | – | – | – |
| Tumor size | 0.987 | 0.733–2.142 | 0.083 | – | – | – |
| Location | 1.035 | 0.767–1.842 | 0.723 | – | – | – |
| AJCC Stage | 3.951 | 2.523–5.015 | <0.001 | 4.017 | 2.349–3.483 | <0.001 |
| T
classification | 1.212 | 1.017–2.335 | 0.047a | 2.012 | 1.954–2.874 | 0.031a |
| N
classification | 1.537 | 1.061–4.938 | 0.043a | 1.977 | 1.575–3.213 | 0.044a |
| M
classification | 3.596 | 2.427–4.938 | <0.001 | 3.672 | 2.731–5.376 | <0.001 |
|
Differentiation | 2.017 | 1.029–3.240 | 0.033a | 1.996 | 1.233–2.841 | 0.042a |
| Vascular
invasion | 1.282 | 1.134–2.047 | 0.097 | – | – | – |
Association between MIR100HG
expression and clinicopathological characteristics
To further confirm the clinical significance of
MIR100HG expression in CRC, the association between MIR100HG
expression and the clinicopathological characteristics of patients
with CRC was assessed (Table II).
The results demonstrated that high MIR100HG expression was
significantly associated with AJCC stage (P=0.024), pathological T
stage (P=0.048), N stage (P=0.020), M stage (P=0.023) and tumor
differentiation (P=0.035). However, no association was identified
between high MIR100HG expression and other clinical parameters,
including age, sex, tumor size, tumor location and vascular
invasion status (P>0.05; Table
II). These findings suggested that high MIR100HG expression may
be associated with CRC migration and invasion.
Effect of MIR100HG on CRC cell
migration and invasion in vitro and in vivo
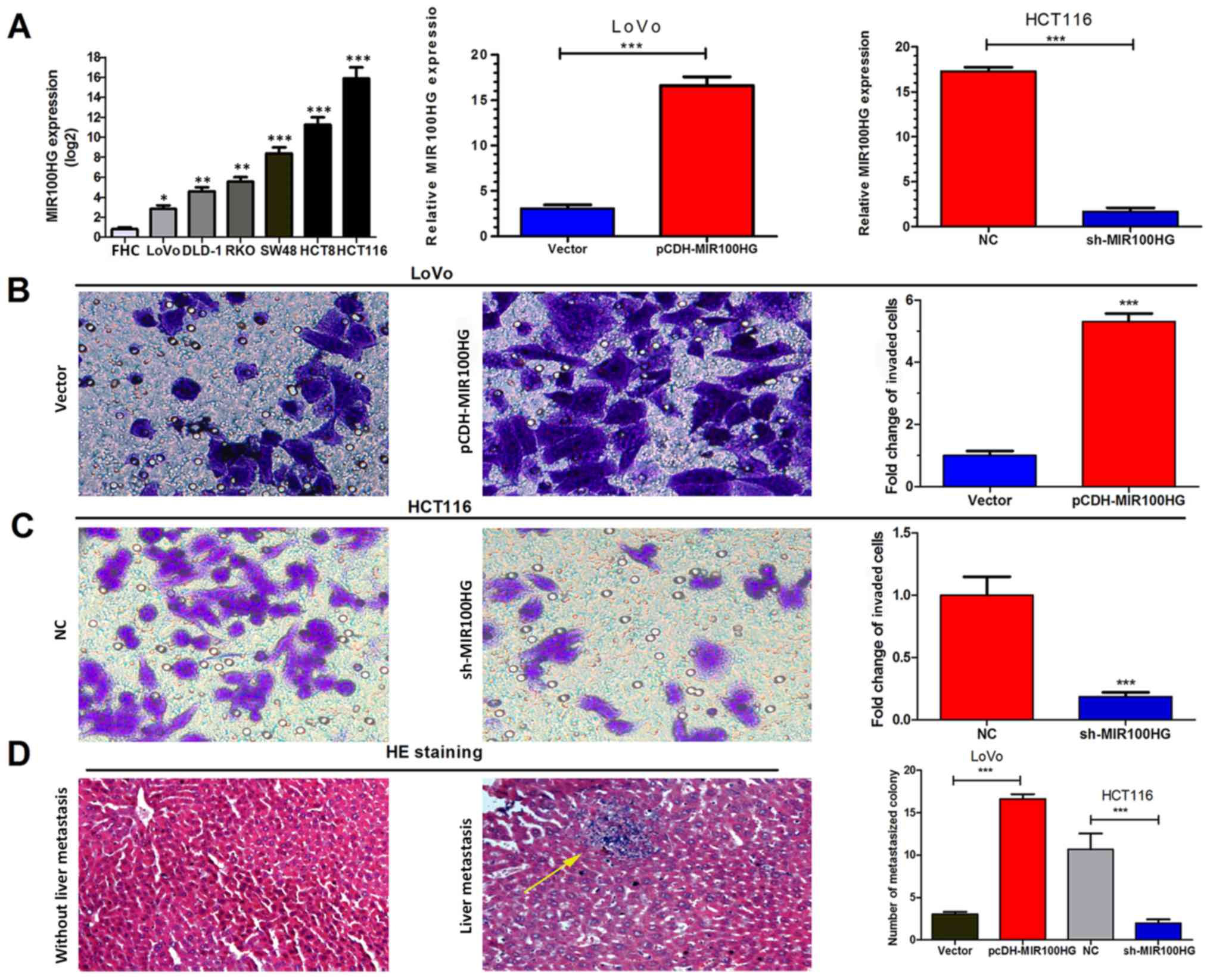
To further investigate the role of MIR100HG in CRC
cells, MIR100HG expression in various CRC cell lines (LoVo, DLD-1,
RKO, SW48, HCT8 and HCT116) and the normal intestinal mucous
epithelium FHC cell line was assessed by RT-qPCR. The results
demonstrated that CRC cell lines exhibited higher MIR100HG
expression compared with the FHC cell line (Fig. 2A). Subsequently, the HCT116 cell
line, which exhibited the highest expression levels of MIR100HG,
was selected to establish a cell line with knockdown of MIR100HG.
Conversely, the LoVo cell line, which displayed the lowest MIR100HG
expression level, was selected to establish an overexpression model
for MIR100HG. The results of RT-qPCR analysis demonstrated that the
transfections were successful (Fig.
2A).
In vitro Transwell assays were used to assess
the function of MIR100HG in CRC progression. MIR100HG
overexpression significantly enhanced the migration and invasion of
LoVo cells (P<0.001; Fig. 2B),
whereas MIR100HG-knockdown significantly inhibited the migration
and invasion of HCT116 cells (P<0.001; Fig. 2C). These results suggested that
MIR100HG overexpression may promote the aggressive phenotype of CRC
cells in vitro.
Subsequently, by injecting CRC cells LoVo and HCT116
into tail veins of nude mice, the impact of MIR100HG on the
migratory and invasive capacities of CRC cells was investigated.
After 4 weeks, the results demonstrated that higher and lower
numbers of liver metastatic colonies were formed in the
MIR100HG-overexpression and knockdown groups, respectively,
compared with their corresponding controls (P<0.001; Fig. 2D). MIR100HG overexpression may
therefore enhance the migratory and invasive capacities of CRC
cells in vitro and in vivo.
Discussion
Recent studies have reported that distant metastasis
remains the main cause of mortality in patients with CRC, and that
the liver is the most common metastatic site (4,6). In
total, 15–25% of patients with CRC have liver metastases at the
time of diagnosis (20). In
addition, 30–50% of patients with CRC who undergo radical resection
will have local and systemic recurrences, and their risk of
recurrence following metastatic resection is ~75% (4–7). It is
therefore crucial to understand the mechanism of CRC and metastasis
development. The present study demonstrated that MIR100HG
expression was higher in CRC tissues compared with adjacent normal
mucosa, and that MIR100HG overexpression was associated with poor
prognosis and survival in patients with CRC. In addition, MIR100HG
overexpression enhanced the migratory and invasive capacities of
CRC cells in vitro and liver metastasis in vivo,
which indicated that MIR100HG expression may be crucial in CRC
progression, particularly in colorectal liver metastasis.
MIR100HG, which is located on chromosome 11q24.1, is
a polycistronic micro RNA host gene that is associated with the
progression of several types of tumor (21–23). It
has been reported that MIR100HG overexpression can predict a poor
prognosis and is associated with metastasis in cervical cancer
(21). In addition, MIR100HG
overexpression is associated with OS in patients with oral cavity
cancer (22). MIR100HG is also
overexpressed in acute megakaryoblastic leukemia (23), and aberrant MIR100HG expression has
been reported in patients with CRC and resistant to cetuximab
(24). However, the clinical
significance and prognostic value of MIR100HG expression in CRC
remains unclear. The present study elucidated MIR100HG expression
in CRC samples, examined its association with clinicopathological
characteristics and distant metastasis in patients with CRC, and
determined whether it could be considered as a prognosis
factor.
The results of RT-qPCR demonstrated that MIR100HG
expression was increased in CRC tissues compared with normal
tissues. Furthermore, MIR100HG expression in advanced stage
(III–IV) CRC tissues was significantly increased compared with low
stage (I–II) CRC tissues, which was consistent with a previous
study from The Cancer Genome Atlas data repository (24). Subsequently, the association between
high MIR100HG expression and the clinicopathological
characteristics of patients with CRC was investigated. Previous
studies on acute megakaryoblastic leukemia, early-stage cervical
cancer and head and neck squamous cell carcinoma have reported that
MIR100HG overexpression accelerates tumor malignancy and is
associated with poor prognosis and survival (21–23).
Similarly, the present study reported that MIR100HG overexpression
was associated with poor prognosis and survival in patients with
CRC. Furthermore, univariate and multivariate Cox regression
analyses demonstrated that MIR100HG expression, AJCC stage, N
classification, M classification and tumor differentiation were
significantly associated with DFS and OS. Subsequently, in
vitro cell function assays investigated the impact of MIR100HG
on the migratory and invasive capacities of CRC cells. The results
demonstrated that MIR100HG upregulation enhanced CRC cell migration
and invasion in vitro, and liver metastasis in vivo.
These findings suggested that MIR100HG may have crucial roles in
CRC invasion and liver metastasis.
In conclusion, the present study reported the
crucial roles of MIR100HG in CRC progression, and indicated that
MIR100HG may serve as a novel prognostic biomarker for CRC.
Furthermore, future manipulation of MIR100HG levels may represent a
potential treatment strategy for patients with CRC, particularly
those with colorectal liver metastasis. It has been reported that
MIR100HG is the host gene of the microRNA
(miR)-100/let-7a-2/miR-125b-1 cluster on chromosome 11, and that
MIR100HG overexpression can upregulate miR-100 and miR-125b
(24). The present study did not
examine the impact of MIR100HG on miR-100 and miR-125b-1 expression
levels. Future studies will therefore examine the effect of
modulating MIR100HG expression on miR-100 and miR-125b expression
levels, and will investigate how MIR100HG could promote CRC
development and distant metastasis.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Zhejiang Medical
Technology Plan Project (grant no. 2017ZD003), the Natural Science
Foundation of Jiangsu Province, China (grant no. BK20161168) and
the Social Development Project of Xuzhou, China (grant no.
KC17109).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LL and XT conceived the study and wrote the
manuscript. WL and FY performed the experiments. XZ and WC analyzed
and interpreted the data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Shanghai General Hospital of Nanjing Medical
University. All patients provided written informed consent prior to
the present study. Animal studies involving mice were in accordance
with the Shanghai General Hospital of Nanjing Medical University
Animal Care and Use guidelines. Ethical approval was obtained from
the Ethics Committee of Shanghai General Hospital of Nanjing
Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bhullar DS, Barriuso J, Mullamitha S,
Saunders MP, O'Dwyer ST and Aziz O: Biomarker concordance between
primary colorectal cancer and its metastases. EBioMedicine.
40:363–374. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hur K, Toiyama Y, Okugawa Y, Ide S, Imaoka
H, Boland CR and Goel A: Circulating microRNA-203 predicts
prognosis and metastasis in human colorectal cancer. Gut.
66:654–65. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cronin KA, Lake AJ, Scott S, Sherman RL,
Noone AM, Howlader N, Henley SJ, Anderson RN, Firth AU, Ma J, et
al: Annual report to the nation on the status of cancer, part I:
National cancer statistics. Cancer. 124:2785–800. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van der Valk MJM, Hilling DE, Bastiaannet
E, Meershoek-Klein Kranenbarg E, Beets GL, Figueiredo NL, Habr-Gama
A, Perez RO, Renehan AG, van de Velde CJH and IWWD Consortium:
Long-term outcomes of clinical complete responders after
neoadjuvant treatment for rectal cancer in the International Watch
& Wait Database (IWWD): An international multicentre registry
study. Lancet. 391:2537–2545. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kopp F and Mendell JT: Functional
classification and experimental dissection of long noncoding RNAs.
Cell. 172:393–407. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leisegang MS, Fork C, Josipovic I, Richter
FM, Preussner J, Hu J, Miller MJ, Epah J, Hofmann P, Gunther S, et
al: Long noncoding RNA MANTIS facilitates endothelial angiogenic
function. Circulation. 136:65–79. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sahakyan A, Kim R, Chronis C, Sabri S,
Bonora G, Theunissen TW, Kuoy E, Langerman J, Clark AT, Jaenisch R
and Plath K: Human naive pluripotent stem cells model X chromosome
dampening and X inactivation. Cell Stem Cell. 20:87–101. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marin-Bejar O, Mas AM, Gonzalez J,
Martinez D, Athie A, Morales X, Galduroz M, Raimondi I, Grossi E,
Guo S, et al: The human lncRNA LINC-PINT inhibits tumor cell
invasion through a highly conserved sequence element. Genome Biol.
18:2022017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grelet S, Link LA, Howley B, Obellianne C,
Palanisamy V, Gangaraju VK, Diehl JA and Howe PH: A regulated PNUTS
mRNA to lncRNA splice switch mediates EMT and tumour progression.
Nature Cell Biol. 19:1105–15. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang M, Zhang S, Yang Z, Lin H, Zhu J,
Liu L, Wang W, Liu S, Liu W, Ma Y, et al: Self-Recognition of an
inducible host LncRNA by RIG-I feedback restricts innate immune
response. Cell. 173:906–19.e13. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Atianand MK, Caffrey DR and Fitzgerald KA:
Immunobiology of long noncoding RNAs. Annu Rev Immunol. 35:177–98.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li L, Lv G, Wang B and Kuang L: The role
of lncRNA XIST/miR-211 axis in modulating the proliferation and
apoptosis of osteoarthritis chondrocytes through CXCR4 and MAPK
signaling. Biochem Biophys Res Commun. 503:2555–2562. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
O'Connell JB, Maggard MA and Ko CY: Colon
cancer survival rates with the new American Joint Committee on
cancer sixth edition staging. J Natl Cancer Inst. 96:1420–1425.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang S, Ke H, Zhang H, Ma Y, Ao L, Zou L,
Yang Q, Zhu H, Nie J, Wu C and Jiao B: LncRNA MIR100HG promotes
cell proliferation in triple-negative breast cancer through triplex
formation with p27 loci. Cell Death Dis. 9:8052018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pages F, Mlecnik B, Marliot F, Bindea G,
Ou FS, Bifulco C, Lugli A, Zlobec I, Rau TT, Berger MD, et al:
International validation of the consensus Immunoscore for the
classification of colon cancer: A prognostic and accuracy study.
Lancet. 391:2128–2139. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van der Geest LG, Lam-Boer J, Koopman M,
Verhoef C, Elferink MA and de Wilt JH: Nationwide trends in
incidence, treatment and survival of colorectal cancer patients
with synchronous metastases. Clin Exp Metastasis. 32:457–465. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shang C, Zhu W, Liu T, Wang W, Huang G,
Huang J, Zhao P, Zhao Y and Yao S: Characterization of long
non-coding RNA expression profiles in lymph node metastasis of
early-stage cervical cancer. Oncol Rep. 35:3185–3197. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wilkins OM, Titus AJ, Salas LA, Gui J,
Eliot M, Butler RA, Sturgis EM, Li G, Kelsey KT and Christensen BC:
MicroRNA-related genetic variants associated with survival of head
and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev.
28:127–136. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Emmrich S, Streltsov A, Schmidt F,
Thangapandi VR, Reinhardt D and Klusmann JH: LincRNAs MONC and
MIR100HG act as oncogenes in acute megakaryoblastic leukemia. Mol
Cancer. 13:1712014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu Y, Zhao X, Liu Q, Li C, Graves-Deal R,
Cao Z, Singh B, Franklin JL, Wang J, Hu H, et al: lncRNA
MIR100HG-derived miR-100 and miR-125b mediate cetuximab resistance
via Wnt/beta-catenin signaling. Nat Med. 23:1331–1341. 2017.
View Article : Google Scholar : PubMed/NCBI
|