Introduction
Lung cancer is recognized as a leading cause of
cancer-associated mortality and morbidity worldwide. According to
the latest report of the American Cancer Society, ~2.09 million new
cases of lung cancer and ~1.76 million associated deaths were
reported in the world in 2018 (1).
For patients with lung cancer who cannot undergo surgery and
radiotherapy, chemotherapy is still the major treatment option.
However, chemotherapy only offers a modest survival advantage due
to chemoresistance.
One of the molecular mechanisms that drive the
development of chemoresistance is permeability-glycoprotein
(P-gp)-mediated drug resistance (2).
P-gp is often highly expressed in drug-resistant tumor cells.
Several hydrophobic antitumor drugs, such as paclitaxel (PTX) and
vinblastine, are subject to efflux out of the cells by P-gp
(3). Therefore, the inhibition of
P-gp-mediated drug efflux will presumably resensitize resistant
cancer cells to chemotherapy drugs, which may lead to favorable
chemotherapeutic outcomes. Various P-gp inhibitors, such as
verapamil and cyclosporine, have been identified to reverse
chemoresistance and sensitize tumor cells to chemotherapeutic
agents (4). However, the clinical
use of first-generation reversal agents (verapamil and
cyclosporine) and second-generation inhibitors (dexverapamil and
PSC-833) failed, due to undesired side effects and toxicity issues
(5). Thus, no P-gp inhibitor has yet
been applied for clinical use.
Natural compounds have great potential in inhibiting
tumor growth and reversing chemotherapy resistance (6,7).
Chalcones are the most common group of flavonoid/isoflavonoid
compounds found in vegetables and fruits. Earlier studies have
demonstrated that numerous chalcones and their derivatives exhibit
antitumor potential (8–10) with almost no toxic side effects to
normal cells (11). Flavokawain A
(FKA), a novel chalcone from the kava plant, induced apoptosis and
G2/M arrest in different tumor cells (12–14).
However, the potential therapeutic effect and the underlying
molecular mechanism of FKA in paclitaxel (PTX)-resistant A549 cells
are yet to be elucidated.
In the present study, the PTX-resistant cell line
A549/T was established. The efficacy of FKA in the inhibition of
A549/T cell viability in vitro was assessed. Additionally,
the capacity of FKA in reversing P-gp-mediated PTX resistance and
the potential underlying mechanisms were also investigated.
Materials and methods
Reagents
FKA of ≥99% purity was purchased from Sigma-Aldrich
(Merck KGaA). FKA was dissolved in dimethyl sulfoxide (DMSO) to
form a 30 mM stock solution. Cell Counting Kit-8 was purchased from
Dojindo Molecular Technologies, Inc. PTX, LY294002 and DAPI were
all obtained from Sigma-Aldrich (Merck KGaA). Insulin-like factor-1
(IGF-1) was purchased from Abcam (cat. no. 128524). Monoclonal
rabbit anti-human P-gp (cat. no. 13342), monoclonal rabbit
anti-human Akt (cat. no. 4691), polyclonal rabbit anti-human
phosphorylated (p)-Akt (Ser 473; cat. no. 9271), monoclonal rabbit
anti-human PARP (46D11; cat. no. 9532) and polyclonal rabbit
anti-human β-actin (cat. no. 4970) were obtained from Cell
Signaling Technology, Inc. The monoclonal mouse anti-human GAPDH
antibody (cat. no. 60004-1-Ig) was obtained from ProteinTech Group,
Inc. Horseradish peroxidase (HRP)-labelled goat anti-rabbit
immunoglobulin G (cat. no. TA130023) and HRP-labelled goat
anti-mouse immunoglobulin G (cat. no. TA130003) were obtained from
OriGene Technologies, Inc.
Cell culture
Human lung adenocarcinoma cells A549 and
PTX-resistant A549 (A549/T) cells were kindly gifted by the Central
Research Laboratory of the Second Hospital of Shandong University
(Jinan, China). Human hepatic epithelial cells THLE-3 were
purchased from The Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences. All cells were cultured in RPMI-1640
(HyClone; GE Healthcare Life Sciences) containing 10% (v/v) fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.),
penicillin-streptomycin (100 U/ml) and 2 mM glutamine. The cells
were cultured at 37°C in an incubator with 5% CO2. The
A549/T cells were maintained in medium with 3 nM PTX to maintain
PTX resistance in this cell line. Before the experiment, cells were
cultured in drug-free medium for ≥2 weeks.
Cell viability assay
The effect of PTX or FKA on the viability of A549
and A549/T cells was evaluated by Cell Counting Kit-8 assay. The
toxicity effect of FKA was also evaluated in human hepatic
epithelial THLE-3 cells. A549, A549/T and THLE-3 cells were
cultured in 96-well plates (4×103 cells/well) and incubated
overnight. Subsequently, the cells were stimulated for 48 h with
increasing concentrations of PTX or FKA. The controls were treated
with equal volume of DMSO. Cell proliferation inhibition was
assayed by the Cell Counting Kit-8 assay (CCK-8; Dojindo Molecular
Technologies, Inc.) and the methods used were performed according
to manufacturer's protocol. The absorbance was measured at 450 nm
using a microplate reader.
Cell apoptosis assay
Cells were plated at a density of 2×105 cells/2 ml
medium on 6-well plates for 24 h. Following treatment with various
concentrations of FKA (0, 5, 10 and 30 µM) for 24 h, cell apoptosis
was detected using DAPI staining. Cells were fixed with 90%
ethanol/5% acetic acid for 1 h at room temperature. Following 2
washes with PBS, cells were incubated with DAPI solution (1.5 mg/ml
in PBS) for 30 min at room temperature. Images of DAPI fluorescence
were captured using a fluorescence microscope (magnification, ×200;
Nikon Corporation). After treated by different concentrations of
FKA (0, 5, 10 and 30 µM) for 24 h at 37°C, cells were digested with
trypsin and centrifuged at 120 × g for 5 min at 4°C. Following 2
washes with PBS, levels of apoptosis were analyzed using an Annexin
V-fluorescein isothiocyanate/propidium iodide apoptosis detection
kit (BD Biosciences), according to the manufacturer's protocol.
Quantification of fluorescence was determined using flow cytometry
(FACSCalibur™; BD Biosciences), and the data were analyzed using
WinMDI software v2.8 (Purdue University Cytometry
Laboratories).
Reverse transcription-semi
quantitative PCR (RT-PCR)
Cells were treated with 0, 3 and 30 µM FKA for 24 h
at 37°C. Total mRNA was subsequently extracted with
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Complementary (c)DNA was generated using a reverse
transcription kit reagent kit (Promega Corporation). The mixture
was incubated at 42°C for 10 min. The resultant cDNA was then
amplified using primers specific for P-gp (forward primer,
5′-TGACCCGCACTTCAGCTACAT-3′; and reverse primer,
5′-ACTGGGCTTCCCGATGATGTA-3′). As an internal control, GAPDH
expression was detected (forward primer,
5′-TGACTTCAACAGCGACACCCA-3′ and reverse primer,
5′-CACCCTGTTGCTGTAGCCAA-3′). The PCR conditions were as follows:
94°C for 5 min; 20 cycles at 94°C for 60 sec, 60°C for 30 sec and
72°C for 60 sec; and 94°C for 5 min. The PCR products were analyzed
by electrophoresis on a 1.5% agarose gel and stained using ethidium
bromide, then quantified using the ChemiImager 400 software
(ProteinSimple). Experiments were conducted in triplicate and
normalized to the expression of GAPDH.
Western blot analysis
Briefly, A549/T cells (3×105) were
incubated with different concentrations of FKA (0, 2.5, 5, 10 and
30 µM) for 24 h. Cell lysates were prepared using
radioimmunoprecipitation assay lysis buffer according to the
manufacturer's protocol (Beyotime Institute of Biotechnology,
Inc.). Total protein was quantified using the BCA protein assay
(Beyotime Institute of Biotechnology, Inc.). Samples containing
equal amounts of protein (50 µg) from the lysates were separated
using 8 and 12% SDS-PAGE and transferred to polyvinylidene
difluoride membranes (EMD Millipore; Merck KGaA). The blot was
incubated with 5% skimmed milk for 1 h at room temperature. Next,
the membrane was probed with monoclonal rabbit anti-human
antibodies against P-gp, Akt and a polyclonal rabbit anti-human
antibody against p-Akt (Ser 473) (all 1:1,000) overnight at 4°C.
Following washes with TBS/Tween-20 buffer, the membranes were
incubated with either the HRP-labelled goat anti-rabbit secondary
antibody (1:10,000) or the HRP-labelled goat anti-mouse secondary
antibody (1:10,000) for 2 h at room temperature. GAPDH served as a
protein loading control. For poly (ADP-ribose) polymerase (PARP)
expression detection, the western blot method used is
aforementioned however monoclonal rabbit anti-human PARP (cat. no.
46D11; 1:1,000) was used. β-actin served as a protein loading
control. The ECL detection system (GE Healthcare Life Sciences) was
used to detect the signal. Protein levels were quantified using
densitometry using ImageJ v1.6 softwate (National Institutes of
Health).
Inhibition or activation of PI3K/Akt
signaling
The PI3K inhibitor LY294002 and the PI3K-specific
agonist IGF-1 were used to suppress or activate the PI3K/Akt
signaling pathway, respectively, in A549/T cells. Cells (1×104
cells/well) were incubated with the following: DMSO; 30 µM FKA for
24 h; LY294002 (10 µM) for 1 h; IGF-1 (12.5 nM) (15) for 1 h; 30 µM FKA for 24 h, followed
by LY294002 for 1 h; or 30 µM FKA for 24 h, followed by IGF-1 for 1
h. The protein expression levels of p-Akt (Ser 473) and P-gp were
measured by western blot analysis. Each experiment was conducted in
triplicates to determine the means and standard deviations
(SDs).
Statistical analyses
The data are presented as the mean ± SD of ≥3
independent experiments and analyzed by GraphPad Prism 8 (GraphPad
Software, Inc.). Comparisons of cell viability of human
PTX-resistant A549/T cells and parental A549 cells following PTX or
FKA treatment were evaluated by one-way analysis of variance
(ANOVA) with Tukey's post-hoc test. Other multiple group
comparisons were performed with one-way ANOVA, followed by
Dunnett's multiple comparison test. P<0.05 was considered to
indicate a statistically significant difference.
Results
FKA significantly inhibits A549/T cell
viability
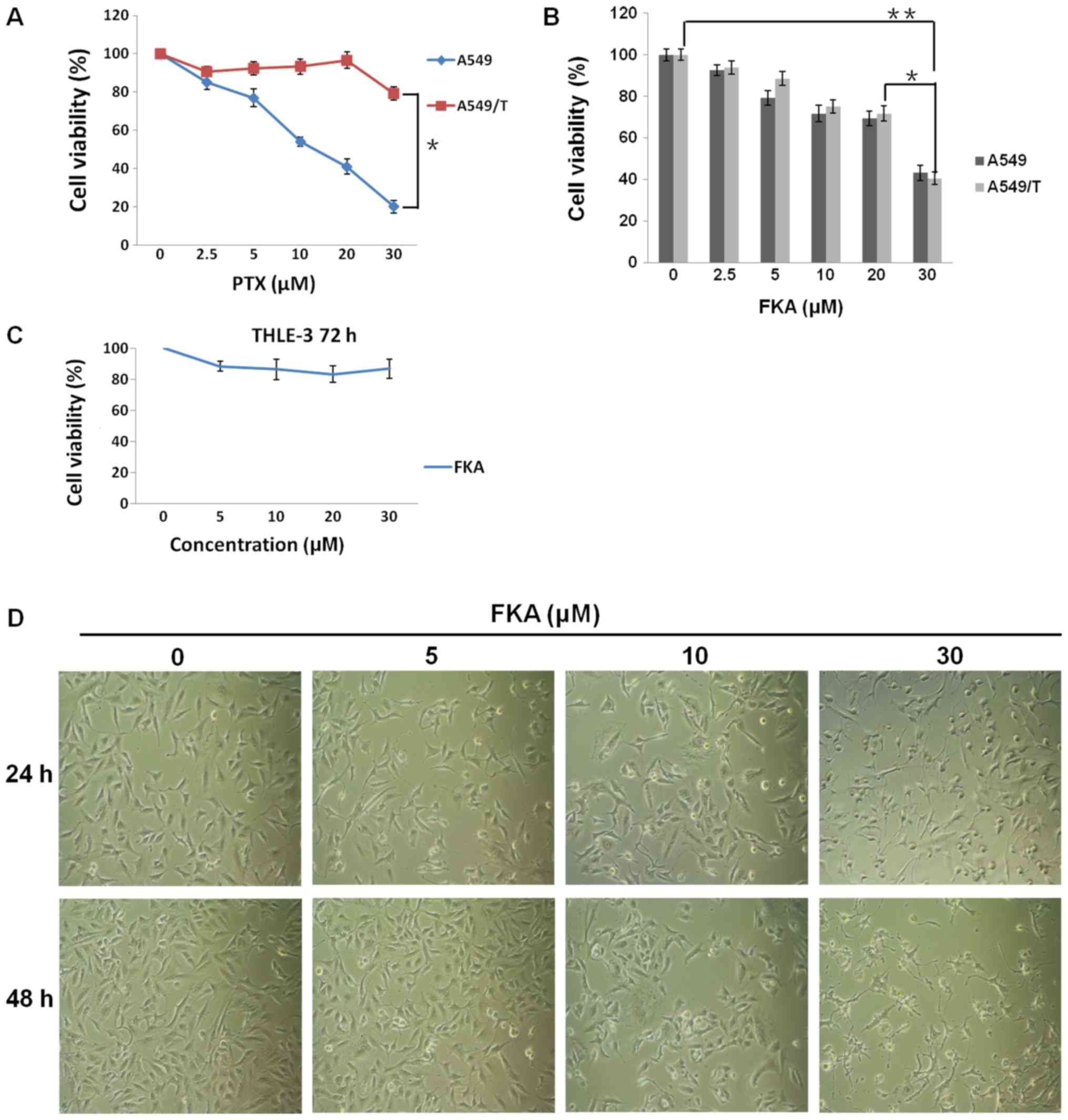
As shown in Fig. 1A,
PTX dose-dependently inhibited cell proliferation in A549 cells,
with an IC50 value of 0.3 µM. However, the cell
viability of PTX-resistant A549/T cells was 79% following treatment
with 30 µM PTX, and the IC50 was 34.64 µM. As shown in
Fig. 1B, A549/T cells were sensitive
to FKA, with a marginally decreased IC50 value of ~21 µM
compared with ~26 µM in A549 cells. FKA at increasing
concentrations of 2.5, 5, 10, 20 and 30 µM resulted in decreased
cell viability in a dose-dependent manner, inhibiting A549/T cell
growth by ~6.2, 11.4, 25.0, 28.3 and 59.4%, while inhibiting A549
cell growth by ~7.3, 20.7, 28.3, 30.5 and 56.8%, respectively
(Fig. 1B). In addition, FKA at 30 µM
exhibited almost no toxic effects on normal human hepatic
epithelial THLE-3 cells (Fig. 1C).
Moreover, morphological analysis revealed that cell shrinkage was
observed following FKA treatment in A549/T cells, and cell
shrinkage became more noticeable as the time and dose of FKA
increased, suggesting that apoptosis was induced by FKA (Fig. 1D). The above data demonstrated that
FKA was capable of inhibiting A549/T cell proliferation.
FKA induces apoptosis in A549/T
cells
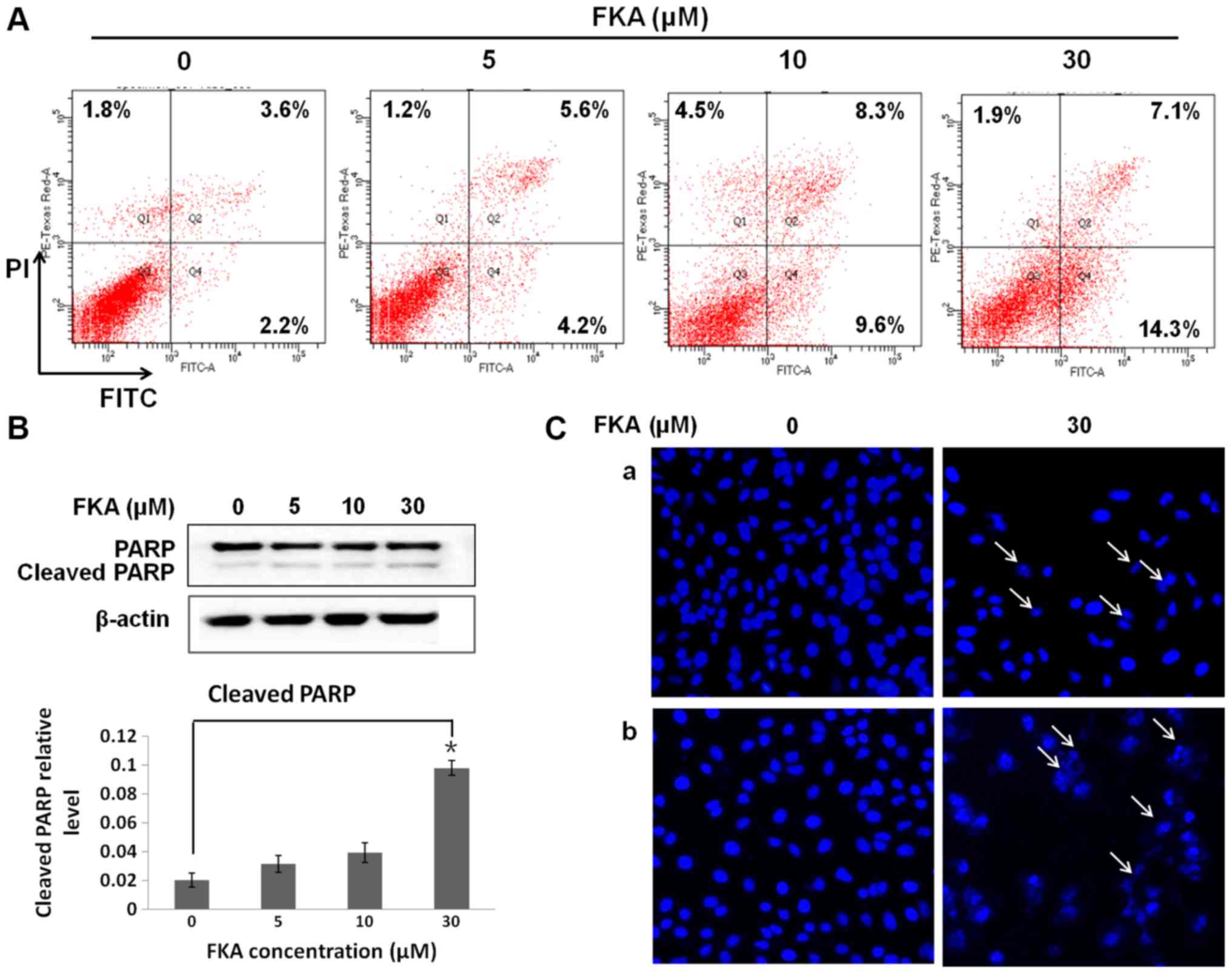
Flow cytometry was used to analyze apoptosis in
A549/T cells treated with FKA. The results revealed that FKA caused
a markedly dose-dependent increase in the proportion of apoptotic
cells at 24 h, with ~9.2, 17.9 and 21.4% early and late apoptotic
cells following treatment with 5, 10 and 30 µM FKA, respectively
(Fig. 2A). In addition, increased
cleavage of PARP, a hallmark of apoptosis, was observed in A549/T
cells exposed to 30 µM FKA, (Fig.
2B). The nucleic morphological changes associated with
apoptosis were assessed by staining nuclear DNA with DAPI. FKA
treatment (30 µM) at 48 h resulted in a marked increase in the
number of apoptotic cells (indicated using arrows) with condensed
and fragmented DNA (Fig. 2Ca)
compared with A549/T cells treated with 30 µM FKA for 24 h
(Fig. 2Cb). Together, these results
suggest that FKA induced apoptosis in A549/T cells.
FKA inhibits the protein expression of
P-gp
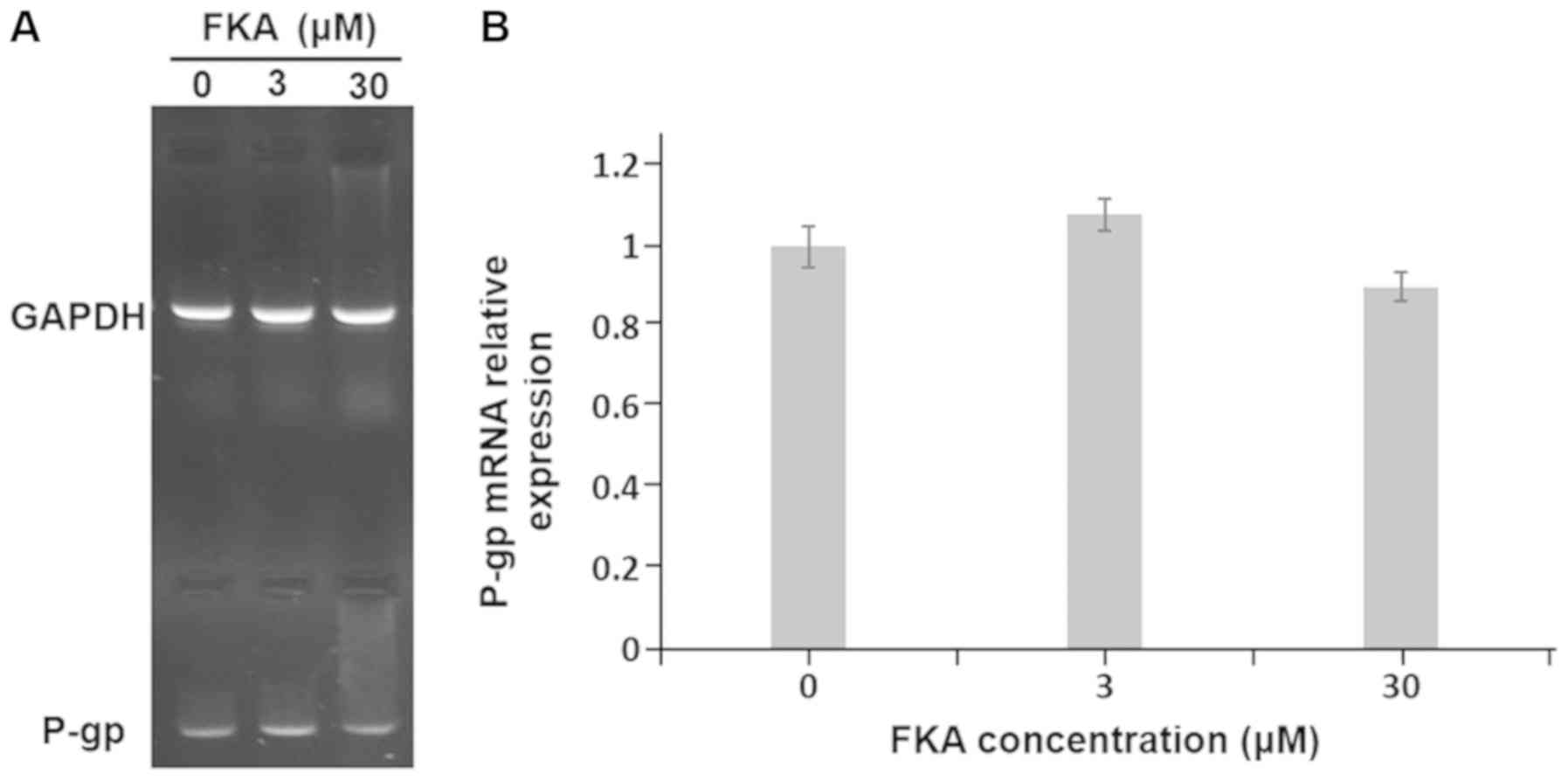
RT-semi quantitative PCR and western blot analysis
were performed to evaluate the effects of FKA on P-gp expression at
the mRNA and protein level. The results demonstrated that the mRNA
expression of P-gp slightly decreased to 90 and 80% compared with
the control cells, following treatment with 3 and 30 µM FKA,
respectively (Fig. 3). However, the
differences were not statistically significant. The results from
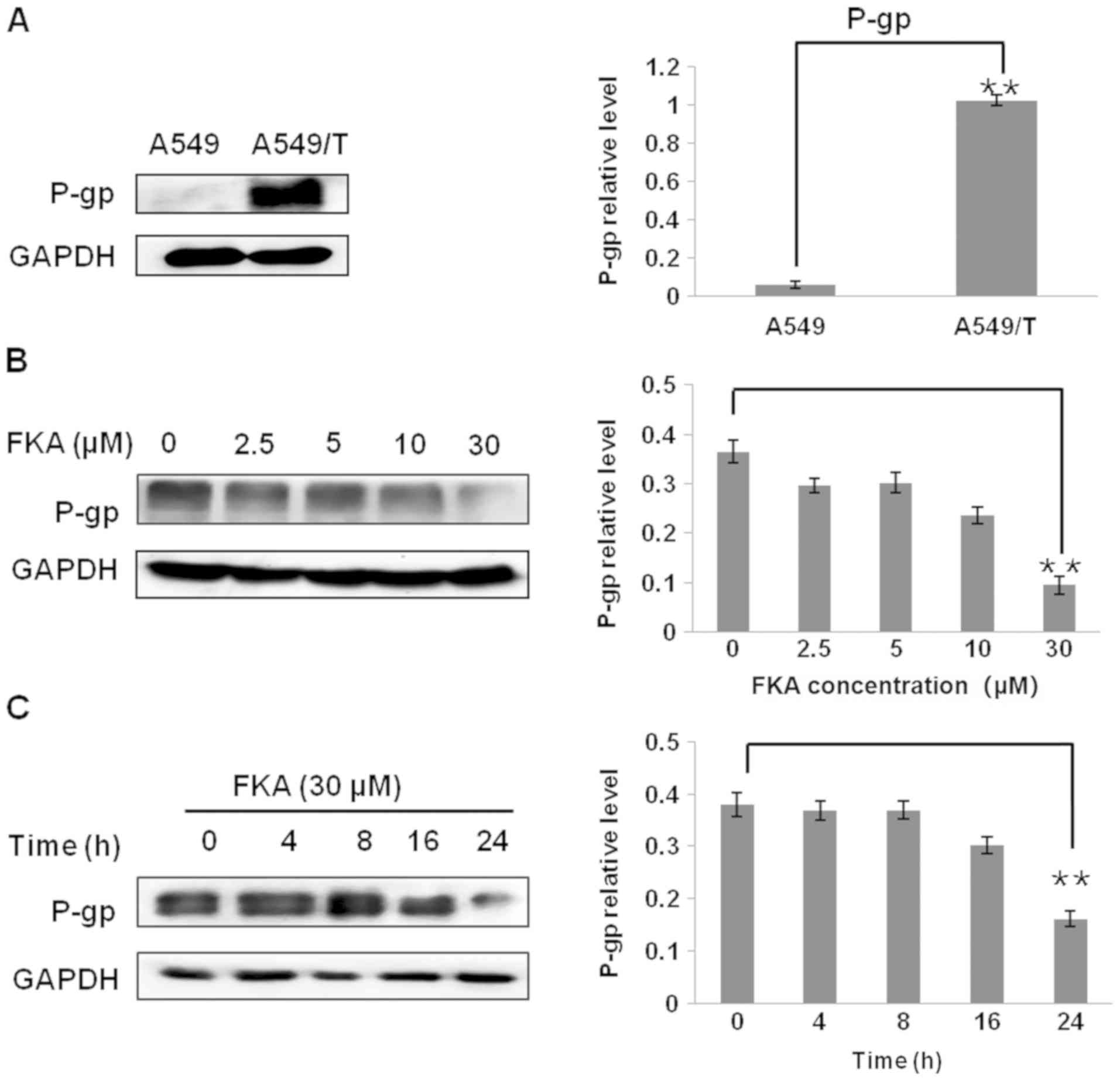
the western blot analysis revealed that P-gp was not expressed in
A549 cells but it was highly expressed in A549/T cells (P<0.01;
Fig. 4A). Subsequently, the protein
levels of P-gp in A549/T cells were measured in response to various
concentration of FKA. The expression of P-gp was significantly
decreased, ~3.0-fold, following treatment with 30 µM FKA for 24 h
(Fig. 4B). Subsequently, the changes
in P-gp expression with respect to treatment duration were
investigated. As indicated in Fig.
4C, the level of P-gp expression decreased from 16 to 24 h
(~2.5-fold) following treatment with 30 µM FKA. Thus, treatment
with FKA decreased the protein expression of P-gp in A549/T
cells.
FKA downregulates P-gp expression via
inhibition of the PI3K/Akt signaling pathway
The involvement of PI3K/Akt signaling pathway in
P-gp-mediated multidrug resistance has been reported (16). High protein expression of P-gp, Akt
and p-Akt (Ser 473) was detected in A549/T cells. FKA downregulated
the protein expression of P-gp, Akt and p-Akt (Ser 473). Notably,
the levels of P-gp, Akt and p-Akt (Ser 473) were significantly
decreased following treatment with 30 µM FKA, as compared with the
control group (P<0.05; Fig. 5).
To confirm whether the suppression or activation of the PI3K/Akt
pathway influenced the levels of P-gp, the effect of the PI3K
inhibitor LY294002 and the PI3K agonist IGF-1 on P-gp and PI3K/Akt
signaling was investigated. The expression of p-Akt (Ser 473) was
decreased following treatment with LY294002 for 1 h compared with
the untreated control (P<0.01; Fig.
6A and B). A549/T cells treated with 30 µM FKA for 24 h
exhibited decreased expression of P-gp and p-Akt (Ser 473). In
addition, the combined treatment of FKA and LY294002 (pretreatment
with 30 µM FKA for 24 h, followed by treatment with 10 µM LY294002
for 1 h) exerted additive inhibitory effects on P-gp and p-Akt (Ser
473) expression. As illustrated in Fig.
6B, the expression levels of P-gp and p-Akt (Ser 473) were not
influenced by PI3K agonist IGF-1 but inhibited by 30 µM FKA. The
combined treatment of FKA and IGF-1 (pretreatment with 30 µM FKA
for 24 h, followed by treatment with IGF-1 for 1 h) also inhibited
the expression of p-Akt (Ser 473). Thus, 30 µM FKA could reverse
IGF-1-induced activation of the PI3K/Akt signaling pathway.
Overall, FKA was demonstrated to inhibit P-gp expression via the
PI3K/Akt signaling pathway.
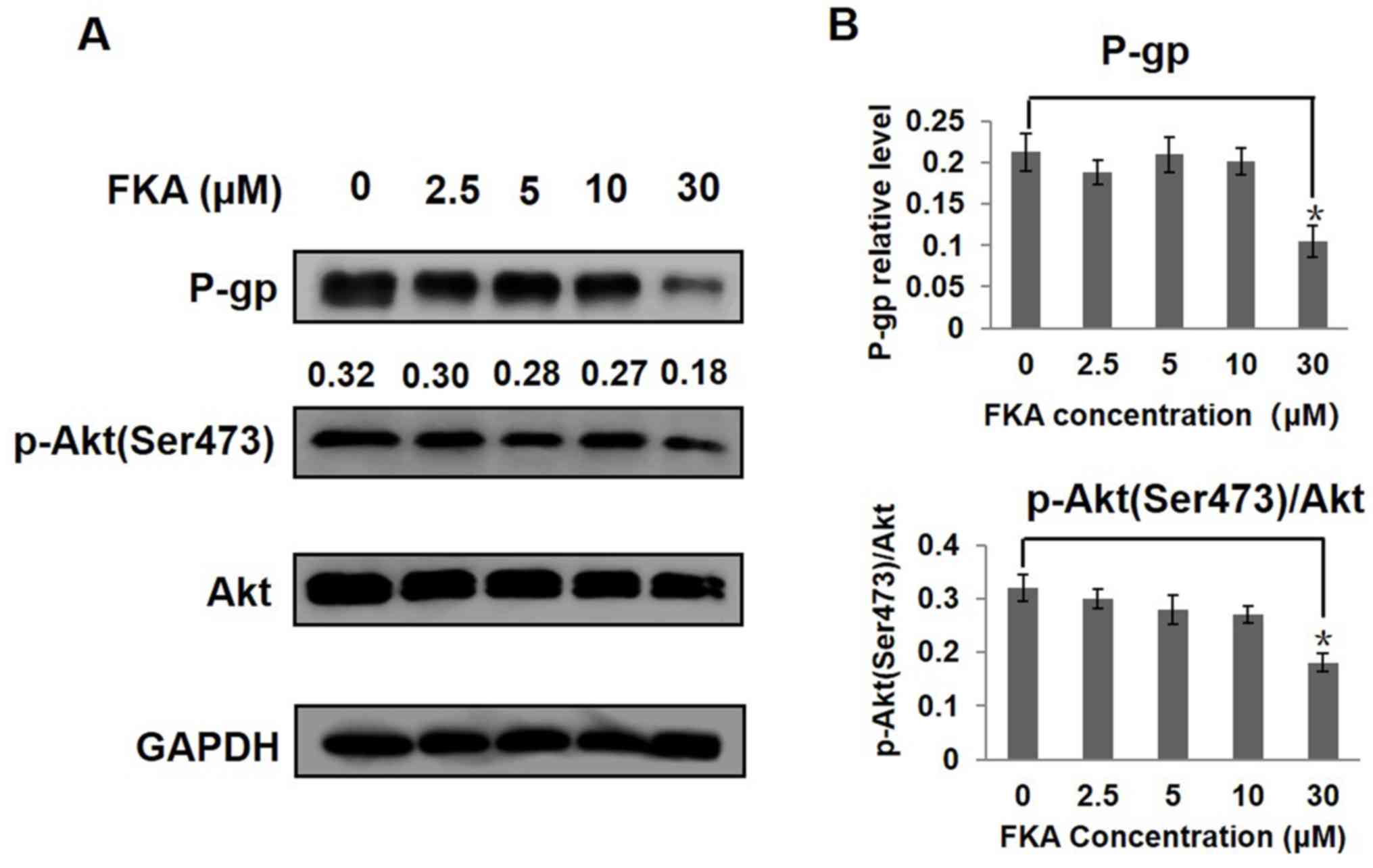 | Figure 5.FKA inhibits the PI3K/Akt signaling
pathway in A549/T cells. Western blot analysis of the (A) protein
expression levels of P-gp, Akt and p-Akt (Ser 473) in A549/T cells
treated with various concentrations of FKA for 24 h. GAPDH served
as an internal control for P-gp and total Akt was used for p-Akt,
The ratios of p-Akt (Ser 473) to Akt after treated with 0, 2.5, 5,
10 and 30 µM FKA were 0.32, 0.3, 0.28, 0.27, 0.18 respectively. (B)
The relative protein level of P-gp, Akt and p-Akt (Ser 473) was
quantified using densitometry. *P<0.05 vs. 0 µM FKA. FKA,
flavokawain A; P-gp, permeability-glycoprotein, p-,
phosphorylated. |
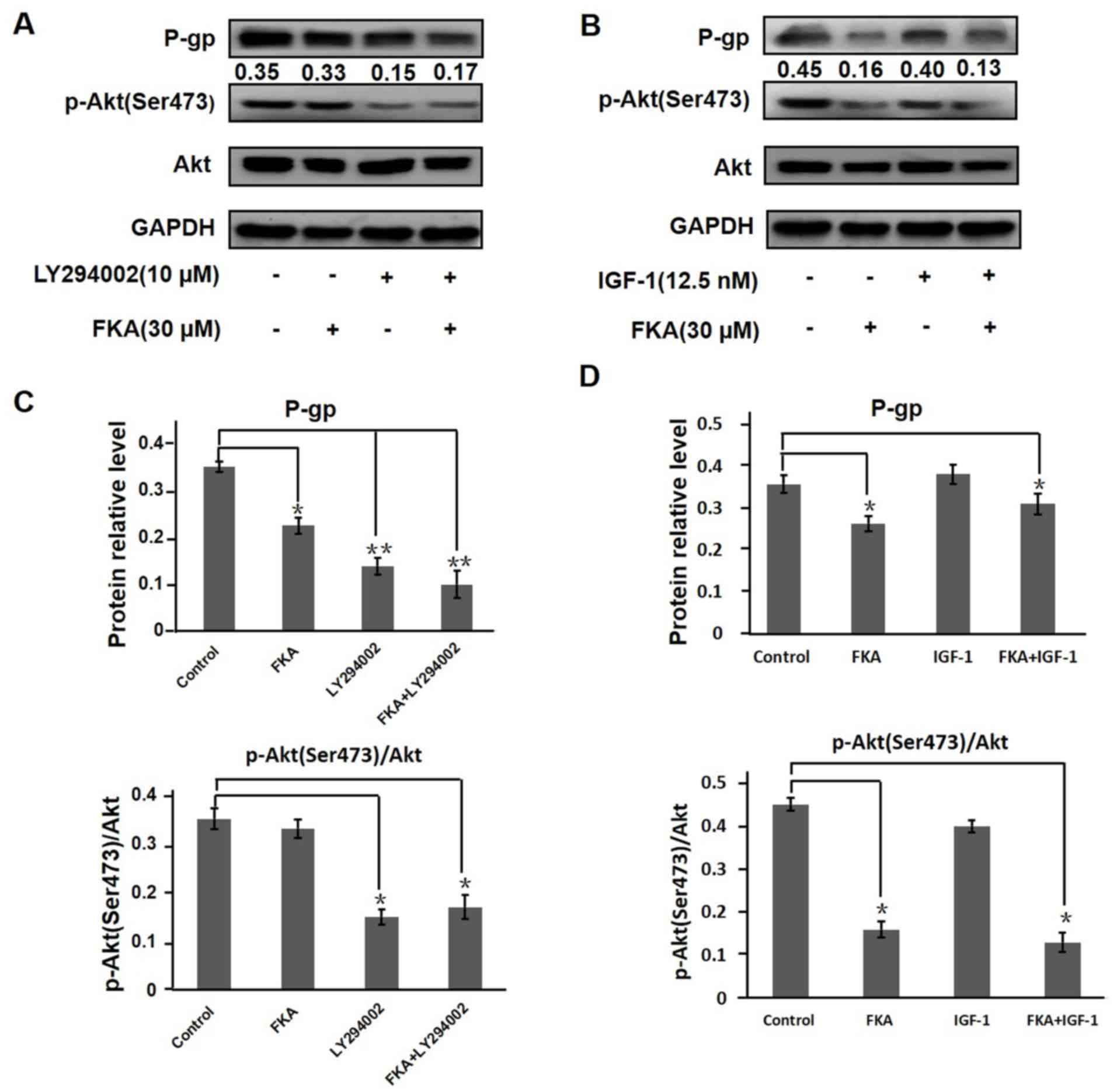 | Figure 6.FKA downregulates P-gp expression via
inhibition of the PI3K/Akt signaling pathway. A549/T cells were
either untreated or treated with (A) FKA, LY294002 or co-treated
with FKA and LY294002 or (B) FKA, IGF-1 or co-treated with FKA and
IGF-1 using western blot analysis. GAPDH served as the internal
control for P-GP and total AKT served as an internal control for
p-AKT. The ratios of p-Akt (Ser 473) to Akt treated with control,
FKA, LY294002 or FKA combined with LY294002 were 0.35, 0.33, 0.15,
0.17 respectively. The ratios of p-Akt (Ser 473) to Akt treated
with control, FKA, IGF-1 or FKA combined with IGF-1 were 0.45,
0.16, 0.4, 0.13 respectively. The relative level of P-gp, Akt and
p-Akt (Ser 473) after treatment with (C) FKA, LY294002 or FKA
combined with LY294002 and (D) FKA, IGF-1 or FKA combined with
IGF-1 treatment. *P<0.05. **P<0.01. FKA, flavokawain A; P-gp,
permeability-glycoprotein, p-, phosphorylated; IGF-1, insulin-like
factor-1. |
Discussion
Drug resistance is the main obstacle in cancer
chemotherapy and results in poor therapeutic outcome (17). P-gp overexpression is one of the main
mechanisms underlying drug resistance. Inhibiting the expression or
function of P-gp is a key step towards improved treatment of
patients with cancer. Inhibitors of the drug-efflux pump have been
reported as effective in increasing the sensitivity to anticancer
drugs (18). However, no potential
modulators are currently licensed for clinical application, due to
the associated toxicities or unacceptable side effects.
Chalcones and their derivatives have great
advantages in overcoming chemotherapeutic drug resistance by
inhibiting the protein expression or functions of ATP-binding
cassette (ABC) transporters, including P-gp and multidrug
resistance (MDR)-associated protein 1 (MRP1) (19). 2′-Hydroxy-2,4,6′-trimethoxychalcone
was shown to significantly inhibit the protein expression of P-gp
in MES-SA/DX5 cells and overcome P-gp-mediated MDR in
drug-resistant uterine sarcoma cells (20). Komoto et al (21) found that the chalcone licochalcone A
decreased the protein expression of P-gp in BT20 breast cancer
cells. The present study demonstrated that FKA did not block the
efflux of Rh-123 out of cells (data not shown), which demonstrated
that FKA had no influence on the efflux function of ABC
transporters. Furthermore, the mRNA expression of P-gp was
unaffected following treatment with 30 µM FKA. Conversely, the
protein level of P-gp was significantly inhibited by 30 µM FKA. In
summary, to the best of our knowledge, the present study is the
first to report that FKA decreased the viability of A549/T cells by
inhibiting the protein expression of P-gp.
The PI3K/Akt pathway is a commonly activated
signaling pathway in cancer, which plays an important role in
inhibiting cancer cell apoptosis and promoting cancer cell
proliferation, invasion and angiogenesis (22). As the central node of the PI3K/Akt
signaling cascade, Akt activates multiple oncogenic signaling
pathways to promote cancer, and Akt hyperactivation has been
associated with poor differentiation and worse prognosis (23). Akt also regulates the expression of
the MDR1 gene, while P-gp is one of the downstream factors
of the PI3K/Akt pathway (24).
Increased Akt phosphorylation has been reported to be associated
with chemotherapeutic resistance (25). However, the association between the
inhibition of P-gp expression and the PI3K/Akt pathway by FKA is
still unclear. In the present study, the effect of FKA on P-gp
expression via the PI3K/Akt pathway was investigated. PTX-resistant
A549/T cells displayed high expression levels of p-Akt and P-gp,
which represented high PI3K/Akt activity, as well as an association
between PI3K signaling and the resistant phenotype. Following
treatment with 30 µM FKA, the expression levels of P-gp, Akt and
p-Akt (Ser 473) decreased significantly. In addition, it was found
that LY294002 and IGF-1 promoted and inhibited Akt phosphorylation,
respectively. These results indicated that FKA inhibited P-gp
expression by inhibiting the PI3K/Akt pathway.
Numerous small molecule Akt inhibitors have been
developed and tested in preclinical or clinical models.
Ipatasertib, a novel selective ATP competitive small-molecule
inhibitor of Akt, preferentially targets active p-Akt and exerts
anti-tumor activity (26). LY294002,
a flavonoid-derived PI3K/Akt inhibitor, inhibits PI3K by competing
with ATP for binding to the PI3K active site (27). LY294002 also inhibits Akt
phosphorylation (28). Furthermore,
it was reported that LY294002 had inhibitory effects on ABC
transporters, including breast cancer resistance protein (BCRP),
MRP1 and P-gp. LY294002 competitively inhibited the transport
activity of BCRP, while exerting inhibitory effects on MRP1
function via competitive inhibition of substrate transport and
modulation of expression (29).
LY294002 also antagonized the transport activity of P-gp without
influencing its expression (29).
Since FKA belongs to flavonoid/isoflavonoid compounds, its chemical
structure is similar to that of LY294002. The present study
demonstrated the inhibition of P-gp protein expression, rather than
its pumping function, by FKA. The inhibitory effect of FKA on BCRP
and MRP1 requires further investigation.
The kinase function of Akt is activated following
the phosphorylation of two residues, threonine 308 (Thr 308) and
serine 473 (Ser 473). It was reported that FKA downregulates Akt
activation by suppressing Akt phosphorylation in
HER2-overexpressing breast cancer cells (14). In the present study, FKA suppressed
Akt activation by decreasing p-Akt (Ser 473) expression level. The
Akt (pan) (C67E7) monoclonal anitbody (cat. no. 4691; Cell
Signaling Technology, Inc.) detects endogenous levels of total
(both phosphorylated and non-phosphorylated) Akt1, Akt2 and Akt3
protein. The target undergoes a wide range of post-translational
modifications (PTMs). These PTMs can lead to a slight shift in
signal. Each Akt isoform can have a unique expression pattern
depending on the specific treatment and specific cells. The
existing two bands of Akt (in Fig.
5A and Fig. 6) are close in size
to 60 kDa is common in A549 cells. The same double band pattern was
also observed in A549 cells in other publications detecting total
Akt (30,31).
A previous report claimed that kava extracts may
cause hepatotoxicity (32).
Interestingly, it was reported that liver toxicity cases occurred
in the South Pacific region only, and this may be due to the
tropical humidity and temperature as the kava plants may have been
contaminated by mould hepatotoxins instead (33). A previous study also confirmed that
kava alone did not affect mouse development and induced no signs of
hepatotoxicity (34). In order to
detect whether FKA has hepatotoxicity, the effect of FKA on human
normal liver cells was investigated. The result demonstrated that
FKA exerted no obvious hepatotoxicity to normal liver epithelial
cells. Overall, there is a need to determine the acute and
subchronic toxicity of FKA in vivo.
The effect of FKA on the expression of other ABC
transporters such as MRP1 and BCRP has not been studied. Thus,
further investigation is required in order to fully understand the
mechanism by which FKA inhibits the function of ABC transporters
and to determine the signaling pathway that inhibits P-gp
expression. In addition, the inhibitory activity of FKA alone or
combined with chemotherapy drugs in resistant tumor cells requires
investigation in future in vivo studies.
In summary, the present study demonstrated the
capacity of FKA to decrease the viability in PTX-resistance cells
and inhibit P-gp expression. Furthermore, FKA inhibits the protein
expression of P-gp via inhibition of the PI3K/Akt pathway. The
findings of the present study may provide a foundation for the
development of better strategies for reversing chemotherapy
resistance.
Acknowledgements
The authors would like to thank Dr Wen Jiang for
flow cytometry data analysis and Dr Lu Zhang for providing the
cells (both Central Research Laboratory at the Second Hospital of
Shandong University, Shandong, China).
Funding
The present study was funded by the Shandong Natural
Science Foundation (grant no. ZR2017BH014), the Shandong Key
Research and Development Program (grant nos. 2018GSF118010 and
2018GSF118075) and the Shandong Province Medical and Health
Technology Development Plan (grant no. 2016WS0334).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL and RW designed the study. JL, MY, YL, JW and XT
performed the experiments. JL and RW contributed new reagents and
analytic tools. LeZ WJ and LuZ analyzed the data. JL wrote the
paper. JL and RW revised the paper.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chakravarty G, Mathur A, Mallade P,
Gerlach S, Willis J, Datta A, Srivastav S, Abdel-Mageed AB and
Mondal D: Nelfinavir targets multiple drug resistance mechanisms to
increase the efficacy of doxorubicin in MCF-7/Dox breast cancer
cells. Biochimie. 124:53–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Podolski-Renić A, Banković J, Dinić J,
Ríos-Luci C, Fernandes MX, Ortega N, Kovačević-Grujičić N, Martín
VS, Padrón JM and Pešić M: DTA0100, dual topoisomerase II and
microtubule inhibitor, evades paclitaxel resistance in
P-glycoprotein overexpressing cancer cells. Eur J Pharm Sci.
105:159–168. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abraham J, Salama NN and Azab AK: The role
of P-glycoprotein in drug resistance in multiple myeloma. Leuk
Lymphoma. 56:26–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Joshi P, Vishwakarma RA and Bharate SB:
Natural alkaloids as P-gp inhibitors for multidrug resistance
reversal in cancer. Eur J Med Chem. 138:273–292. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo Q, Cao H, Qi X, Li H, Ye P, Wang Z,
Wang D and Sun M: Research progress in reversal of tumor multi-drug
resistance via natural products. Anticancer Agents Med Chem.
17:1466–1476. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Y, Yue C, Li J, Wu J, Wang S, Sun D,
Guo Y, Lin Z, Zhang D and Wang R: Enhancement of cisplatin
cytotoxicity by retigeric acid B involves blocking DNA repair and
activating DR5 in prostate cancer cells. Oncol Lett. 15:2871–2880.
2018.PubMed/NCBI
|
|
8
|
Cabral BLS, da Silva ACG, de Ávila RI,
Cortez AP, Luzin RM, Lião LM, de Souza Gil E, Sanz G, Vaz BG,
Sabino JR, et al: A novel chalcone derivative, LQFM064, induces
breast cancer cells death via p53, p21, KIT and PDGFRA. Eur J Pharm
Sci. 107:1–15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Santos MB, Pinhanelli VC, Garcia MAR,
Silva G, Baek SJ, França SC, Fachin AL, Marins M and Regasini LO:
Antiproliferative and pro-apoptotic activities of 2-and
4-aminochalcones against tumor canine cells. Eur J Med Chem.
138:884–889. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee DH, Jung Jung Y, Koh D, Lim Y, Lee YH
and Shin SY: A synthetic chalcone,
2-hydroxy-2,3,5-trimethoxychalcone triggers unfolded protein
response-mediated apoptosis in breast cancer cells. Cancer Lett.
372:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jandial DD, Blair CA, Zhang S, Krill LS,
Zhang YB and Zi X: Molecular targeted approaches to cancer therapy
and prevention using chalcones. Curr Cancer Drug Targets.
14:181–200. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abu N, Akhtar MN, Yeap SK, Lim KL, Ho WY,
Zulfadli AJ, Omar AR, Sulaiman MR, Abdullah MP and Alitheen NB:
Flavokawain A induces apoptosis in MCF-7 and MDA-MB231 and inhibits
the metastatic process in vitro. PLoS One. 9:e1052442014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abu N, Mohameda NE, Tangarajoo N, Yeap SK,
Akhtar MN, Abdullah MP, Omar AR and Alitheen NB: In vitro toxicity
and in vivo immunomodulatory effects of flavokawain A and
flavokawain B in Balb/C mice. Nat Prod Commun. 10:1199–1202.
2015.PubMed/NCBI
|
|
14
|
Jandial DD, Krill LS, Chen L, Wu C, Ke Y,
Xie J, Hoang BH and Zi X: Induction of G2M arrest by flavokawain A,
a kava chalcone, increases the responsiveness of
HER2-overexpressing breast cancer cells to herceptin. Molecules.
22:E4622017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang S, Kuang G, Zhao G, Wu X, Zhang C,
Lei R, Xia T, Chen J, Wang Z, Ma R, et al: Involvement of the
mitochondrial p53 pathway in PBDE-47-induced SH-SY5Y cells
apoptosis andits underlying activation mechanism. Food Chem
Toxicol. 62:699–706. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Muthusamy G, Gunaseelan S and Prasad NR:
Ferulic acid reverses P-glycoprotein-mediated multidrug resistance
via inhibition of PI3K/Akt/NF-κB signaling pathway. J Nutr Biochem.
63:62–71. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma H, Cheng L, Hao K, Li Y, Song X, Zhou H
and Jia L: Reversal effect of ST6GAL 1 on multidrug resistancein
human leukemia by regulating the PI3K/Akt pathway and the
expression of P-gp and MRP1. PLoS One. 9:e851132014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Calatozzolo C, Gelati M, Ciusani E,
Sciacca FL, Pollo B, Cajola L, Marras C, Silvani A,
Vitellaro-Zuccarello L, Croci D, et al: Expression of drug
resistance proteins Pgp, MRP1, MRP3, MRP5 and GST-pi in human
glioma. J Neurooncol. 74:113–121. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lindamulage IK, Vu HY, Karthikeyan C,
Knockleby J, Lee YF, Trivedi P and Lee H: Novel quinolone chalcones
targeting colchicine-binding pocket kill multidrug-resistant cancer
cells by inhibiting tubulin activity and MRP1 function. Sci Rep.
7:102982017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shin SY, Lee MS, Lee DH, Lee DH, Koh D and
Lee YH: The synthetic compound 2-hydroxy-2,4,6-trimethoxychalcone
overcomes P-glycoprotein-mediated multi-drug resistance in
drug-resistant uterine sarcoma MES-SA/DX5 cells. J Korean Soc Appl
Bi. 58:105–109. 2015. View Article : Google Scholar
|
|
21
|
Komoto TT, Bernardes TM, Mesquita TB,
Bortolotto LFB, Silva G, Bitencourt TA, Baek SJ, Marins M and
Fachin AL: Chalcones repressed the AURKA and MDR proteinsinvolved
in metastasis and multiple drug resistance in breast cancer cell
lines. Molecules. 23:20182018. View Article : Google Scholar
|
|
22
|
Wang H, Jia XH, Chen JR, Wang JY and Li
YJ: Osthole shows the potential to overcome P-glycoprotein-mediated
multidrug resistance in human myelogenous leukemia K562/ADM cells
by inhibiting the PI3K/Akt signaling pathway. Oncol Rep.
35:3659–3668. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao Z, Liao Q, Su M, Huang K, Jin J and
Cao D: AKT and ERK dual inhibitors: The way forward? Cancer Lett.
459:30–40. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han Z, Hong L, Han Y, Wu K, Han S, Shen H,
Li C, Yao L, Qiao T and Fan D: Phospho Akt mediates multidrug
resistance of gastric cancer cells through regulation of P-gp,
Bcl-2 and Bax. J Exp Clin Cancer Res. 26:261–268. 2007.PubMed/NCBI
|
|
25
|
Morishita N, Tsukahara H, Chayama K,
Ishida T, Washio K, Miyamura T, Yamashita N, Oda M and Morishima T:
Activation of Akt is associated with poor prognosis and
chemotherapeutic resistance in pediatric B-precursor acute
lymphoblastic leukemia. Pediatr Blood Cancer. 59:83–89. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saura C, Roda D, Roselló S, Oliveira M,
Macarulla T, Pérez-Fidalgo JA, Morales-Barrera R, Sanchis-García
JM, Musib L, Budha N, et al: A first-in-human phase I study of the
ATP-competitive AKT inhibitor ipatasertib demonstrates robust and
safe targeting of AKT in patients with solid tumors. Cancer Discov.
7:102–113. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Toledo LM, Lydon NB and Elbaum D: The
structure-based design of ATP-site directed protein kinase
inhibitors. Curr Med Chem. 6:775–805. 1999.PubMed/NCBI
|
|
28
|
Ohnishi K, Yasumoto J, Takahashi A and
Ohnishi T: LY294002, an inhibitor of PI-3K, enhances heat
sensitivity independently of p53 status in human lung cancer cells.
Int J Oncol. 29:249–253. 2006.PubMed/NCBI
|
|
29
|
Imai Y, Yamagishi H, Ono Y and Ueda Y:
Versatile inhibitory effects of the flavonoid-derived PI3K/Akt
inhibitor, LY294002, on ATP-binding cassette transporters that
characterize stem cells. Clin Transl Med. 1:242012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cao P, Liu B, Du F, Li D, Wang Y, Yan X,
Li X and Li Y: Scutellarin suppresses proliferation and promotes
apoptosis in A549 lung adenocarcinoma cells via AKT/mTOR/4EBP1 and
STAT3 pathways. Thorac Cancer. 10:492–500. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang W, Chen Y, Song X, Shao Y, Ning Z
and Gu W: Pim-1 inhibitor SMI-4a suppresses tumor growth in
non-small cell lung cancer via PI3K/AKT/mTOR pathway. Onco Targets
Ther. 12:3043–3050. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Teschke R: Kava hepatotoxicity-a clinical
review. Ann Hepatol. 9:251–265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Teschke R, Qiu SX and Lebot V: Herbal
hepatotoxicity by kava: Update on pipermethystine, flavokavain B,
and mould hepatotoxins as primarily assumed culprits. Dig Liver
Dis. 43:676–681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Narayanapillai SC, Leitzman P, O'Sullivan
MG and Xing C: Flavokawains a and B in kava, not
dihydromethysticin, potentiate acetaminophen-induced hepatotoxicity
in C57BL/6 mice. Chem Res Toxicol. 27:1871–1876. 2014. View Article : Google Scholar : PubMed/NCBI
|