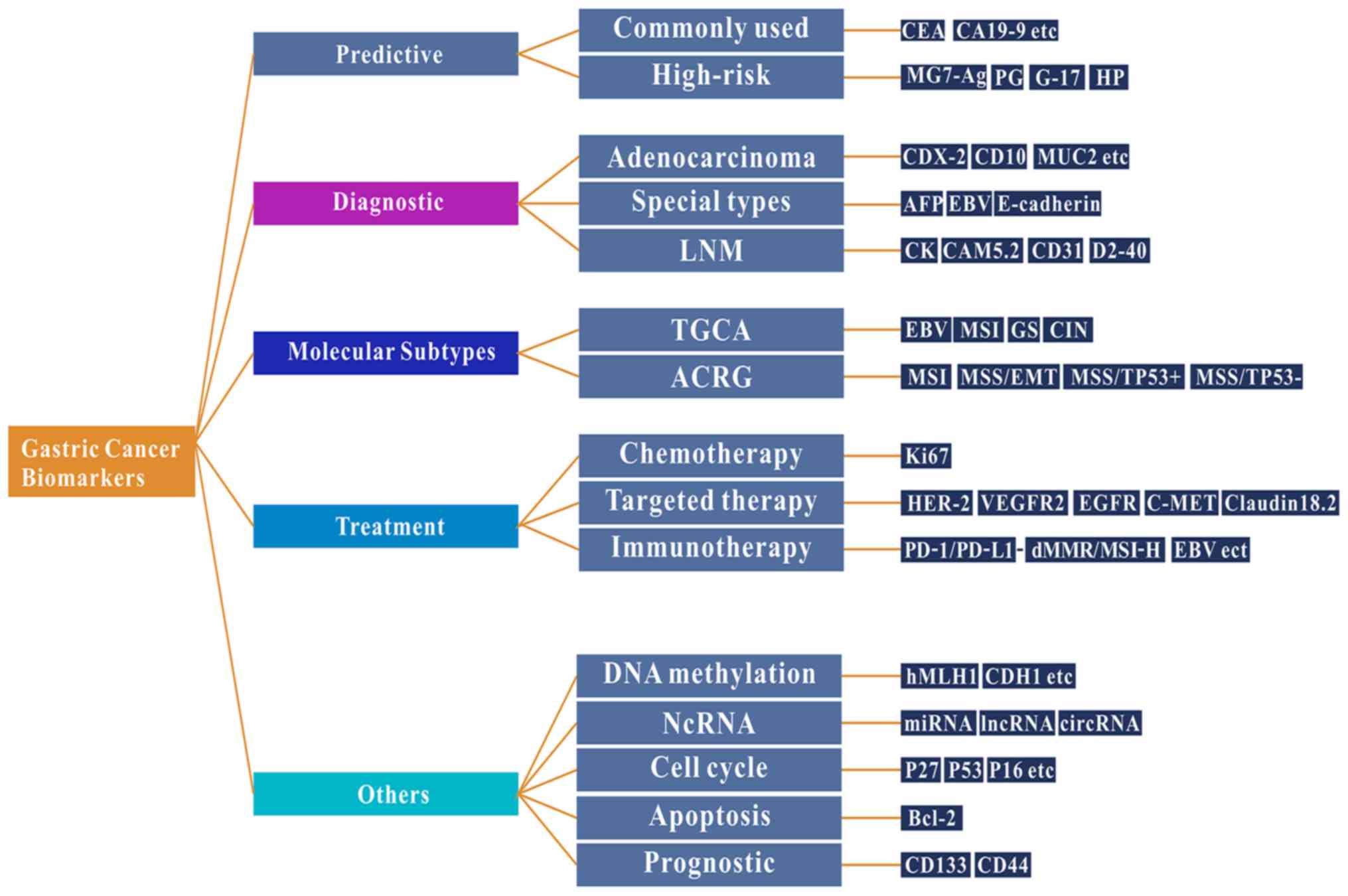
Gastric cancer is the third leading cause of
cancer-associated mortality and the fifth most common malignant
tumor type globally, with ~50% of all cases emerging from Eastern
Asia, where China has the highest incidence rate, and where novel
cases of gastric cancer and mortalities account for 42.6–45.0% of
all cases globally (1,2). The majority of cases of gastric cancer
are usually not diagnosed until an advanced stage, and therefore
the outcome is often poor, with a 5-year survival rate of no more
than 30%, including patients who have undergone surgery (3). However, the 5-year survival likelihood
following early gastric cancer treatment may be >90%, and even
may result in being cured (4). The
rate of diagnosis and treatment of early-stage gastric cancer in
China is <10%, considerably lower than Japan (70%) and South
Korea (50%) (5). Therefore, it is
worthwhile investigating methods for the early diagnosis and
management of gastric cancer, including biomarkers associated with
pathogenesis and pathological type. Understanding these biomarkers
may assist in providing the ideal therapeutic option for an
individuals' specific case of gastric cancer. Detecting the
presence of biomarkers may have promising potential to detect
cancer at earlier stages and thus improve the monitoring of the
progression of a cancer. Furthermore, by obtaining information on
the specific biomarker expression profile of a patient, treatment
options may be better tailored for each patient, thus improving
prognosis. The aim of the review was to focus on providing a
comprehensive overview of the biomarkers in patients who suffer
from stomach cancer, their diagnostic, prognostic and clinical
value and therapeutic application for future prospects.
Gastric cancer is a type of epithelial malignant
neoplasm. A number of various factors contribute to the
pathogenesis of gastric cancer, including environmental factors and
genetic factors (6). Environmental
factors and lifestyle choices include obesity, smoking (7), bile reflux and chronic infections,
particularly with Helicobacter pylori (H. pylori),
which contribute to the development of stomach cancer (8). Globally, ~50% of all patients with
gastric cancer present with evidence of a H. pylori
infection (9), and H. pylori
was considered to be the first carcinogen by the World Health
Organization (WHO) and International Agency for Research on Cancer
(IACR) in 1994 (10). There are
hereditary factors, in addition to environmental factors, including
a germline mutation in the cadherin-1 (CDH1) gene, which results in
hereditary diffuse gastric cancer (11). Patients with inherited conditions,
including Lynch syndrome, familial adenomatous polyps and
Peutz-Jeghers syndrome result in a substantially higher risk of
developing gastric carcinoma (12).
The treatment of gastric cancer is dependent on the
morphology of the cancer tissue at the earliest stage. The
pathological classification of gastric cancer is based on the
histological structure and cell biological characteristics.
Different classifications of gastric cancer types have different
morphological structures, biological behaviors and underlying
molecular mechanisms (8). At
present, gastric cancer is primarily classified using the Borrmann,
Lauren or WHO classification systems, although there are numerous
pathological classification systems for gastric cancer (13,14).
Advanced cancer types may be classified into four macroscopic types
on the basis of the criteria proposed by Borrmann: Polypoid,
fungating, ulcerated and infiltrative (13). The Lauren classification is the most
widely used histological classification, for either early or
advanced cancer types (14), which
classifies gastric cancer as two major subtypes: Intestinal and
diffuse. The diffuse variant may affect the majority of the stomach
and is frequently called linitis plastica or leather bottle
stomach. Intestinal-type gastric cancer occurs more frequently in
elderly male patients and is thought to be associated with better
survival rates (15). In 2010, WHO
published an additional histological classification system for
stomach cancer, which is divided into five categories: Tubular,
papillary, mucinous, poorly cohesive (signet ring cell carcinoma
belongs to this group) and mixed (8). Histological classification has no
substantial impact on the treatment options available for patients
with gastric cancer, therefore, novel biomarkers to aid in the
early diagnosis and treatment of gastric cancer are required. In
the present review, the following topics are discussed: i)
Well-known and emerging biomarkers of gastric cancer; ii) the
impact that high-throughput technologies have had on identifying
biomarkers; and iii) biomarkers associated with the immunotherapy
of gastric cancer and their value as predictors of prognosis
(Fig. 1).
With the advancement of medicine, the definition of
a biomarker has also changed accordingly. In 1998, the National
Institutes of Health Biomarker Definition Working Group defined
biomarker as ‘a feature of objective measurement and assessment of
pharmacological responses to normal biological processes,
pathogenic processes or therapeutic interventions’ (16). Then, Becking and Chen
(17) defined a biomarker as ‘any
material structure or process that can be measured in the body or
its products and effect or predict the incidence of prognosis
disease’. Each of these definitions are similar. Common biomarkers
include carbohydrates, proteins, nucleic acids, small metabolite
lipids and cytogenetics, in addition to all tumor cells identified
in body fluids that are involved in the regulation of physiological
and pharmacological processes (18).
However, Strimbu and Tavel (19) summarized the importance of biomarkers
by stating ‘the foremost issue at present is determined by the link
between any given measurable biomarker and relevant clinical
endpoints’. With the development of molecular biology, an
increasing number of tumor markers have been discovered. Tumor
markers, not only with high sensitivity but also specificity, are
still being discovered. Similarly, numerous molecular biomarkers
have demonstrated their potential efficacy as diagnostic and
prognostic tools in gastric cancer, yet they still require further
confirmation of their use in day-to-day clinical practice (20).
South Korea and Japan have the most complete
prevention and screening gastric cancer program globally, which
result in a high detection rate of early gastric cancer in these
countries (21). At present,
carcinoembryonic antigens including CEA (22), CA19-9 (23) and CA72-4 (24) are the most widely used markers for
detecting gastric cancer in the clinical practice. These markers
lack the sensitivity and specificity required for evaluating the
diagnosis and prognosis of gastric cancer, making their efficacy
questionable. Therefore, the screening value for early gastric
cancer is limited. However, the positive expression of monoclonal
gastric cancer 7 antigen (MG7-Ag) indicates a high risk of gastric
cancer (25). Nevertheless, the
sensitivity and specificity of MG7-Ag as a single marker in the
diagnosis of gastric cancer may not be sufficient, and further
clinical research is required to evaluate its value in diagnosis
for the early screening of gastric cancer.
Pepsinogen (PG) may be divided into two subtypes,
PGI and PGII, according to its biochemical and immunological
activity characteristics (26). PG
is a good indicator of the exocrine function of the gastric antral
mucosa and may be called ‘serological biopsy’ (26). When gastric mucosal atrophy occurs,
serum PGI and/or the PGI/II ratio (PGR) level are decreased
(27). Furthermore, H. pylori
infection is a necessary condition for the occurrence of intestinal
subtype gastric cancer which accounts for the majority of cases of
gastric cancer, but it is not a sufficient condition (28,29).
Thus, previously combining serum PG levels with H. pylori
antibodies (including the ‘ABC method’) were used to assess the
risk of gastric cancer and screened for patients with a high-risk
of developing gastric cancer. Konturek et al (30) reported that elevated levels of serum
gastrin-17 (G-17) may indicate the risk of gastric cancer.
Additionally, Shiotani et al (31) reported that serum G-17 combined with
PG may enhance the diagnostic value of gastric cancer. Previously,
when five serological markers markers were combined with PGI, PGII,
PGR, (H. pylori) antibody and G-17 as a screening strategy
for gastric cancer, it was revealed that a decrease in the levels
of PGI and PGR were associated with a high risk of gastric cancer,
whereas low (<0.5 pmol/l) and high (>4.7 pmol/l) G-17 levels
were associated with a higher risk of suffering from gastric cancer
(32). Therefore, it has been
indicated that screening strategies which combine these serological
markers may assist in identifying high-risk individuals, in
addition to guiding targeted screening and precision prevention
(32).
Common types of adenocarcinoma, including tubular,
papillary, mucinous, low adhesion carcinoma or mixed
adenocarcinoma, often present with phenotypic features with
intestinal epithelium [expressing mucin 2, CDX-2 and cluster of
differentiation (CD)10] or gastric epithelium (expressing mucin 1,
cell surface associated, mucin 5AC, olugomeric mucus/gel-forming
and mucin 6, oligomeric mucus/gel forming) (33). In fact, it is frequently unnecessary
to diagnose these common types of adenocarcinoma using
immunohistochemistry (IHC). However, certain unique types of
gastric cancer, including poorly differentiated neuroendocrine
carcinoma, hepatoid adenocarcinoma, adenocarcinoma producing
α-fetoprotein, gastric cancer with lymphoid stroma [typically
associated with Epstein Barr virus (EBV) infection] and
choriocarcinoma require specific biomarkers to confirm the
diagnosis (34). Prior to a case of
hereditary diffuse gastric cancer being diagnosed, IHC detection of
E-cadherin and detection of mutations in the CDH1 gene mutations
are required for screening or confirmation (11).
The specific pathological features of a patients'
unique case of gastric cancer will have a substantial effect on the
therapeutic regimen used and the patients' outcome (15). Lymph node micrometastasis (LNM) is
one of the most important prognostic factors in patients with
gastric cancer, including in patients who do present with evidence
of lymph node metastasis (35).
Comparatively speaking, IHC may be satisfactorily accurate for the
detection of LNM compared with that of haemotoxylin and eosin
staining. Cytokeratin AE1/AE3 and CAM5.2 are reliable biomarkers
for the detection of epithelial tissues or cells in lymph nodes
(36). If it is suspected that the
cancer cells are present in the vasculature, the biomarkers CD31
and D2-40 are available (37).
Additionally, the markers NF or S-100 may be used for the detection
of nerve invasion (38).
Cell proliferation is closely associated with tumor
progression, reflecting the invasiveness and final prognosis of
various malignant tumor types including gastric cancer (39). Ki67 is a nuclear antigen that may be
expressed at all stages of the cell proliferation cycle except in
G0 cells. Cytotoxic chemotherapeutic agents are effective against
tumor cells that have entered the cell division cycle (G1, S, G2
and M phases). A high level of Ki67 indicates that a greater number
of cancer cells are entering the cell division cycle and may be the
most effective indicator for chemotherapeutic drug therapies.
Therefore, the Ki-67 antibody is widely used to evaluate the
proliferative activity of tumor cells and to identify the presence
of circulating cells to measure tissue growth (40). Although there is no consensus on the
prognosis and predictive value of Ki-67 in malignant tumor types,
studies have revealed that the Ki-67 index has notable implications
for the prognosis of cancer (41).
Therefore, the high expression of Ki-67 may be used as a marker for
predicting poor prognosis in patients with gastric cancer. The
levels of Ki-67 expression should thus be considered when selecting
a suitable treatment option and comprehensive treatment. Thus, it
is recommended to perform routine Ki67 detection on gastric cancer
tissues to evaluate the proliferative status of the cancer cells
and provide a reference for determining the efficacy of
chemotherapy.
Human epidermal growth factor receptor-2 (HER-2) is
a proto-oncogene located on chromosome 17, encoding a 185-kDa
tyrosine kinase receptor which belongs to the epidermal growth
factor receptor family. The phosphorylation of HER-2 initiates a
signaling pathway resulting in cell division, proliferation,
differentiation and anti-apoptotic signaling (42,43).
Previous research has predicted that between 7–38% of gastric
cancer types exhibit the amplification and/or overexpression of
HER-2 (44–46). In 2010, the trastuzumab for gastric
cancer study, a phase III, open-label, randomized controlled
clinical trial, revealed that patients with gastric cancer who
exhibited of HER-2 upregulation and treated with trastuzumab had
significantly improved overall survival (OS) and disease-free
survival (DFS) times, and a significantly increased objective
response proportion, compared with chemotherapy alone (cisplatin +
5-fluoropyricil or capecitabine) (44). Therefore, on October 20, 2010, the
U.S. Food and Drug Administration (FDA) granted approval for
trastuzumab (Herceptin) in conjunction with cisplatin and a
fluoropyrimidine (cisplatin or 5-fluoropyricil), for treating
patients with HER-2 amplification or upregulation in metastatic
gastric or gastroesophageal junction adenocarcinoma, who were
untreated for metastatic tumor (44). The National Comprehensive Cancer
Network (NCCN) Clinical Practice Guidelines in Oncology for gastric
cancer suggest the assessment of HER-2 overexpression by IHC and/or
gene amplification through fluorescence in situ
hybridization or another in situ hybridization method in
tumor samples for patients with an unresectable locally advanced,
recurrent or metastatic stomach cancer for whom trastuzumab may be
beneficial (47). A consensus for
HER-2 detection in patients with gastric cancer is thus required to
improve individualized treatment for patients (48). Additionally, it is recommended that
there is routine detection of HER-2 expression in patients with
gastric cancer, so that a greater number of patients do not forego
the opportunity for targeted therapy with the aim of improving the
outcome of these patients. The vascular endothelial growth factor
(VEGF) family is an important regulator of angiogenesis and
lymphangiogenesis, which specifically binds to VEGFR receptor
(VEGFR), promotes vascular and lymphangiogenesis and participates
in the development and progression of a tumor (22). Therefore, VEGF antibody and VEGFR
antagonists are used to block the blood supply to gastric cancer
tissues when treating patients with gastric cancer. The anti-VEGF
drug, bevacizumab, is a recombinant humanized monoclonal antibody
against VEGF-A. Phase III clinical trials in the AVAGAST study
revealed that bevacizumab, combined with capecitabine and
cisplatin, as a first-line treatment for patients with advanced
gastric cancer did not improve overall survival time. However, the
results of the study revealed that there was a significant
difference in survival benefits in the US, but not in Asia
(49). Therefore, it is necessary to
consider individual differences and other factors in future
research to improved individualized treatment.
VEGFR belongs to the tyrosine kinase receptors
family, which includes VEGFR-1, VEGFR-2 and VEGFR-3, of which
VEGFR-2 is the primary receptor mediating increased vascular
permeability (50). Ramucirumab is a
completely humanized immunoglobulin G (IgG1) monoclonal antibody
which targets the extracellular domain of VEGFR2, blocks the
interaction with VEGFR ligands and inhibits receptor activation. It
was demonstrated that in two global, randomized, double-blind phase
III clinical trials, using ramucirumab combined with platinum
and/or fluorouracil (REGARD trial) or ramucirumab combined with
paclitaxel (RAINBOW trial), that the OS and DFS times in patients
with gastric cancer whose cancer had deteriorated following the
initial treatment, were significantly increased in the treatment
group compared with the placebo, who were treated with the
corresponding chemotherapeutics alone (51,52). In
2014, the FDA approved ramucirumab as a second-line therapy for the
treatment of patients with advanced gastric or gastroesophageal
junction adenocarcinoma with evidence of disease progression during
or following treatment with fluoropyrimidine- or
platinum-containing chemotherapeutics (53). The RAINBOW study revealed that Asian
patients did not benefit significantly from anti-angiogenic therapy
compared with patients treated with ramucirumab or ramucirumab
combined with paclitaxel. However, there remain certain issues
which need to be addressed. For example, anti-angiogenic drugs
result in alterations to physiological vasoactive activity.
Additionally, the mechanisms underlying the resistance to drugs
targeting angiogenesis require further study which may partly be
achieved through the identification of more reliable biomarkers
(54). Epidermal growth factor
receptor (EGFR) (also known as HER1) is a member of the human
epidermal growth factor receptor family, which is a multifunctional
glycoprotein on the human cell membrane with a relative molecular
mass of 1.70×104 (55).
The mutation and overexpression of EGFR is closely associated with
malignant tumor types including gastric cancer (56). EGFR-targeting drugs may exhibit
anti-tumor effects by inhibiting the binding of ligands to EGFR or
exerting effects on the intracellular regions of EGFR and
interfering with tyrosine kinase phosphorylation. Cetuximab is a
human-mouse chimeric IgG1 monoclonal antibody against EGFR
(57). In EXPAND, a phase III
clinical trial, it was revealed that the addition of cetuximab to
capecitabine-cisplatin provided no additional benefits to
progression-free survival (PFS) time compared with chemotherapy
alone as a first-line treatment of advanced gastric cancer
(57). Panitumumab is a humanized
IgG2 monoclonal antibody against EGFR. In a large-scale clinical
phase III trial (REAL-3), it was demonstrated that the addition of
panitumumab to epirubicin, oxaliplatin and capecitabine (EOC)
chemotherapy significantly worsened the OS time from 11.3 to 8.8
months (58). Therefore, it was not
recommended for patients with advanced esophageal adenocarcinoma,
who were not selected. However, a large number of studies (59–61) have
revealed inconsistent results, highlighting the need for a
large-scale clinical study to validate whether patients with
gastric cancer with evidence of EGFR expression benefit from
targeted drugs. However, at present, there are no guidelines
recommending the routine use of EGFR for the detection of gastric
cancer.
Mesenchymal-epithelial transition factor gene
(c-MET) is located on chromosome 7q21-31, which encodes for a
protein tyrosine kinase belonging to the hepatocyte growth factor
receptor family, and participates in regulating important cellular
processes, including proliferation, differentiation, motility, cell
cycle and apoptosis (62).
Additionally, numerous studies have demonstrated that c-MET
overexpression is associated with a poorer survival prognosis and
predicted shorter PFS time in various tumor types, including
gastric cancer (63–65). Multi-center retrospective studies in
Japan have revealed that there is a significant difference in the
OS time of patients who had high c-Met expression compared with
those with no/low levels of c-Met expression (66). Catenacci et al (67) observed that when the overexpression
of the MET protein in patients with chemotherapy-insensitive
gastric cancer was present, these patients were able to remain in
remission in a two year follow-up period when treated with a
monovalent MET antibody therapy. Therefore, it is expected that
examining the expression of MET in gastric carcinoma tissues using
IHC may have potential clinical application value for gastric
cancer c-Met targeted therapy. However, a phase III, randomized,
double-blind, multicenter trial (the RILOMET-1 study), 609 patients
with advanced MET-positive gastric or gastroesophageal junction
tumor types were divided into a rilotumumab group (rilotumumab in
combination with epirubicin, cisplatin and capecitabine) or a
placebo group (placebo + epirubicin, cisplatin and capecitabine).
It was demonstrated that the OS time of the rilotumumab group was
worse compared with the placebo group, and the incidence of
negative effects was increased. The study was aborted early due to
an imbalance in mortalities between the groups (62). In conclusion, the exact efficacy of a
targeted MET monoclonal antibody in patients with gastric cancer
requires additional study to determine the reason for the less
favorable results observed in the RILOMET-1 study. Identifying
suitable molecular markers and investigating the optimum
combinatorial solutions may ultimately improve patient outcomes.
Mammalian target of rapamycin (mTOR) is a member of the
phosphoinositide-3-kinase-associated kinase family, which is highly
expressed in gastric cancer tissues. Hence, blocking the mTOR
signaling pathway may inhibit the proliferation and metastasis of
tumor cells (68). Everolimus is an
oral rapamycin derivative that blocks the phosphorylation of mTOR
and functions as an antitumor agent. A phase III clinical trial of
Granite-1 revealed that everolimus did not improve the prognosis of
patients with advanced gastric cancer compared with matching
placebo (69). Additionally, one
previous study revealed that the efficacy of everolimus was
associated with the level of p-S6 (70). At present, the potential efficacy of
anti-mTOR targeted therapy and biomarkers are still being
determined.
The newly reported research regarding Claudin18.2
(CLDN18.2), a member of the Claudin family, has revealed that
CLDN18.2 is associated with tumor development and progression and
is located on the outer cell membrane. It is expressed in a variety
of tumor types, in particular gastric cancer cells. These
biological characteristics suggest that CLDN18.2 may be a potential
therapeutic target. A monoclonal antibody against CLDN 18.2.
Claudiximab (previously IMAB362), was the first targeted therapy
for CLDN 18.2 expression (71). The
antibody exerted an anti-tumor effect primarily through activating
antibody-dependent cytotoxicity, complement-dependent cytotoxicity
and regulation of the tumor microenvironment, in a recent phase II
clinical study (FAST) that revealed that Claudiximab combined with
EOX (epirubicin 50 mg/m2, oxaliplatin 130
mg/m2 d1 and capecitabine 625 mg/m2 bid,
d1-21, every 21 days) significantly improved the PFS and OS times
compared with EOX in patients with gastric cancer (72). Treatment with IMAB362 plus EOX in
patients with advanced or metastatic gastroesophageal cancer was
considered safe and effective. Therefore, this may be a promising
target therapeutic option for patients with typically difficult
malignancies to treat. However, it is still in phase II trials and
will undergo further research.
As the identification of novel biomarkers has
resulted in an increase in potential therapeutic regimens, it is no
longer sufficient to design treatment options that are not unique
for a patients unique expression profile. For example, Herceptin
may be used for human epidermal growth factor receptor 2
(HER2)-positive breast cancer, but not for the negative type.
However, this may not be completely accurate, as patients who are
positive for this marker may exhibit additional mutations.
Interestingly, high-throughput technologies have brought tremendous
changes, including next generation sequencing and gene array chips,
which have allowed for the detection of a large number of markers
at the same time, thus making it substantially easier to tailor a
specific treatment regimen to an individuals' profile (73). It is well known that there are
general classifications by Borrmann and histological
classifications by Lauren and WHO in gastric cancer. In previous
years, developments in the field of cancer genomics have been
revolutionized by the molecular characterization of the different
varieties of carcinomas including gastric cancer. Initially, there
were only two molecular subtypes identified (74). Subsequently, Lei et al
(75) at the Duke National
University of Singapore identified three subtypes of gastric
adenocarcinoma: Proliferative, metabolic and mesenchymal. In 2014,
The Cancer Genome Atlas (TCGA) integration analysis based on
somatic cell copy number array analysis, full exon sequence
analysis, DNA methylation degree array analysis, mRNA sequence
analysis, microRNA sequence analysis and anti-phase protein array
analysis resulted in the identification of four different subtypes
of gastric cancer: EBV-positive, microsatellite instability (MSI)
type, stable genome (GS) and chromosomal instability (CIN)
(76). Tumor types expressing EBV
frequently underwent recurrent extreme DNA hypermethylation, and
exhibit phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic
subunit-α mutations, Janus kinase 2 amplification, CD274
[programmed death receptor ligand-1 antibody (PD-L1)] and
programmed cell death 1 ligand 2 (PD-L2). The MSI subtype exhibited
mutations in its DNA sequences, including the mutations of genes
which encode targetable oncogenic signaling proteins. The GS tumor
types were more common in the diffuse histological variant and
mutations of ras homolog family member A or fusions involving
RHO-family GTPase-activating proteins. Tumor types with CIN usually
exhibited substantial aneuploidy and focal amplification of
receptor tyrosine kinases. Each subtype could be distinguished by
different biomarkers, as different markers represent different gene
mutations. Meanwhile, they are caused by different factors, which
should corresponding with different treatments (77). The Asian Gastric Cancer Research
Group also divided gastric cancer into four molecular subtypes,
including MSI and microsatellite stable (MSS) tumor types with
either MSS/epithelial-mesenchymal transition, TP53 activity
(MSS/TP53+) or TP53 inactivity (MSS/TP53−)
(77) based on the analysis of major
components, in 2015. These subtypes are similar to the TCGA
subtypes, as the two studies identified MSI immunotherapy. As
gastric cancer is a multifaceted and highly heterogeneous disease,
these molecular classifications allow researchers to further
understand gastric cancer and provide a good basis for innovative
targeted therapies for treating patients with gastric cancer.
Immunotherapy has received growing attention as
potential mechanism for treating patients with gastric cancer, due
to its favorable curative effect and improved survival time.
Discussion of immunotherapy always involves a discussion of
biomarkers. The programmed death receptor-1 (PD-1)/PD-L1 was
considered a breakthrough for the treatment of numerous tumor
types. PD-1 is a negative co-stimulatory receptor, which is
primarily expressed on activated T cells (78), and suppresses an excessive immune
response through binding to its ligands, PD-L1 and PD-L2 (79,80). In
addition to gastric cancer, PD-L1 is expressed in a range of
tissues and is also expressed in certain other malignant tumor
types (78,80–84). In
tumor tissues, effector T-cell function may be inhibited by the
binding of PD-1 to PD-L1, which results in the inhibition of the
antitumor immune response and even accelerates neoplastic growth
(78,79).
Nivolumab is a human IgG4 monoclonal antibody
inhibitor of PD-1. The ATTRACTION-2 (ONO-4538-12) study was a
randomized, double-blind, placebo-controlled, phase III clinical
trial, which compared the efficacy and safety of nivolumab and a
placebo in patients with advanced gastric cancer who underwent
second line systemic treatment (85). Nivolumab treatment had previously
been shown to significantly improve survival (69). In the clinical trial, nivolumab
significantly reduced the risk of mortality by 37% compared with
the placebo group. In addition, the 12-month OS rate was
significantly higher in the nivolumab group compared with the
placebo group. The OS rates were 26.2 and 10.9%, respectively
(69). Nivolumab is now approved for
the treatment of unresectable advanced or recurrent gastric cancer,
based on the results of the ATTRACTION-2 study, and nivolumab has
become an important treatment option for a variety of tumor types
including gastric cancer. Pembrolizumab is an IgG4-κ humanized
monoclonal antibody, which is selective and has a high-affinity,
and binds to PD-1, preventing PD-1 from binding to PD-L1.
Pembrolizumab is relatively safe and has exhibited potential
anti-tumor activity in certain types of advanced solid tumor types
and hematological malignancies (86). A number of countries have approved
pembrolizumab for the treatment of advanced melanoma, and in the
USA it is used for the treatment of metastatic non-small-cell lung
cancer, which expresses PD-L1 and non-small-cell lung cancer with
failed platinum-containing chemotherapeutics (86). Multiple studies have revealed that
tumor types with an upregulated expression of PD-L1 have a higher
rate of response when treated with pembrolizumab (87,88). In
addition, nivolumab and atezolizumab also produced similar results
(89,90). Furthermore, the higher the expression
rate was of PD-L1, the higher the remission rate of the tumor was,
and even resulted in complete cure in certain cases (88). Biomarkers which will help predict the
response to inhibitors of PD-1 or PD-L1 are required. The detection
of PD-L1 protein expression by IHC is a currently available
method.
In a number of studies, PD-L1 overexpression was
observed in >40% of human gastric cancer samples (81,82,91,92). The
KEYNOTE-021 and KEYNOTE-059 clinical trials revealed an improved
outcome in patients with advanced gastric cancer treated with
pembrolizumab+5-FU+cisplatin compared with 5-FU+cisplatin and thus
highlighting the potential use of immunotherapy for the treatment
of patients with gastric cancer (93,94).
PD-L1 Combined Positive Score (CPS) ≥1% in tumor types or
mesenchymal cells was considered to be the cut-off value for
treatment with pembrolizumab. Unfortunately, in the majority of
solid tumor types, the effectiveness of a PD-1 inhibitor alone is
10–30%. One exception is the classic Hodgkin's lymphoma, where the
efficiency is >60%. Although PD-1 inhibitors are not so
efficient, they have a long-lasting effect (94). As the immune system has a functional
‘memory’, once the PD-1 inhibitor has exerted its effects, patients
with certain early tumor types, including patients with malignant
melanoma, kidney cancer and non-small cell lung cancer, are able to
achieve clinical cure, that is, no recurrence, no progression and
long-term survival (95). Whether
this effect may be achieved in gastric cancer requires further
study. In addition, certain patients may also achieve improved
results with a combination of PD-1 inhibitors with other forms of
therapy. At present, PD-1 inhibitors combined with chemotherapy
have been approved for treating gastric cancer (96); however, additional studies are
required to determine its efficacy.
Based on these studies, in September 2017,
pembrolizumab was approved by the FDA as a third-line treatment for
metastatic carcinoma or patients with recurrent locally advanced,
gastric or gastroesophageal junction adenocarcinoma whose tumor
types expressed PD-L1 as determined by an FDA-approved test in
addition to use for any cancer with MSI-high (H) (97). A meta-analysis and systematic review
of the literature have demonstrated that MSI-H and EBV-positive
gastric cancer are often associated with improved prognosis and
longer survival times (98). Derks
et al (99) additionally
reported that interferon-driven genes are abundant in MSI-H and
EBV-positive gastric cancer types, suggesting that these patients
are sensitive for PD-1 or PD-L1 inhibitors. Researchers have
revealed that MSI-H and EBV positive cancer types have upregulated
expression levels of PD-L1 (100).
Therefore, the NCCN clinical practice guidelines for gastric cancer
2017 version 5 and 2018 version 1 have recommended that mismatch
repair (MMR)/MSI-H, PD-L1 and tumor EBV status should be considered
in patients with locally advanced, recurrent or distant metastases
who are candidates for treatment with PD-1 inhibitors (101). The expression of the MMR proteins
[mutL homolog 1 (MLH1), PMS1 homolog 2, mismatch repair system
component, mutS homolog 2 and mutS homolog 6] and PD-L1 expression
are assessed using IHC in clinical practice.
In addition, tumor mutation burden (TMB) is also an
important biomarker for predicting the effect of PD-1 inhibitors
(102). The definition of TMB is
the number of somatic mutations in the whole genome subsequent to
counting germline DNA variants. For efficacy analysis, patients may
be divided into three groups: TMB high burden (≥248), medium burden
(143–247) and low burden (<143) groups (103). Studies have revealed that the
efficacy of PD-1/PD-L1 inhibitors in a tumor are significantly
associated with TMB (102,104). Hence, patients with a high TMB may
be more likely to benefit from immunotherapy. However, there are
some difficulties in assessing TMB in clinical practice, including
a long detection period, poor platform accessibility, high costs
and inconsistent standards. TMB testing requires strict
experimental conditions and has a low success rate at present.
Furthermore, there is insufficient data at present to support the
notion that TMB benefits OS (104).
Therefore, additional research is required to fully understand the
implications and clinical applications of TMB.
DNA methylation is a frequent epigenetic event that
serves an important function in cancer development (105). Hypermethylation of gastric cancer
in CPG islands are interrelated with the gene silencing of numerous
tumor suppressor genes, including human MLH1, p16, CDH1
(E-cadherin) and RUNX family transcription factor 3 (RUNX3) genes.
Cancer-derived DNA methylation may be detected easily in the serum
of patients with gastric cancer (106). A number of studies have reported
that methylation of the p16 gene and the CDH1 gene were detectable
in the serum of 20–50% of patients with gastric cancer, but not in
the cancer-free and control patients (107,108).
Thirty percent of patients with gastric cancer had RUNX3
methylation, detected in the peripheral circulation, and it was
associated with tumor stage, vascular invasion and lymph node
metastasis (109). However, serum
RUNX3 methylation levels were significantly reduced in patients
with gastric cancer following surgery (110). Therefore, the detection of abnormal
DNA methylation in serum is an effective tool for cancer screening,
disease monitoring and prognosis determination.
Among the epigenetic changes involved, DNA
methylation is associated with anticancer drug resistance (111). A number of methylation alterations
to apoptotic genes have been identified and used as epigenetic
biomarkers for determining either chemoresistance or
chemosensitivity to anticancer drugs (111). According to Choi et al
(112), the hypermethylation of
cyclin dependent kinase inhibitor 2A (p16INK4a) may be a
useful biomarker for 5-florouracil-sensitive and resistant gastric
cancer, respectively. Furthermore, the hypomethylations of adhesion
G protein-couples receptor L2 and G2 and S-phase expressed 1 are
potential biomarkers for cisplatin-sensitive gastric cancer.
Additionally, the hypomethylation of ATP binding cassette subfamily
B member 1 may be a useful biomarker, regardless of the drug type,
for chemotherapy-resistant gastric cancer.
In previous years, ncRNA, including microRNA
(miR/miRNA), long ncRNA (lncRNA) and circular RNA (circRNA), have
been identified as important regulators of protein-coding genes,
and are involved in the regulation of cell development,
differentiation and the occurrence of a tumor. They have also been
demonstrated to serve key functions in the evolution and
development of gastric cancer (113–115).
miRNAs are small ncRNA molecules that regulate gene
expression at the post-transcriptional level (116). miRNAs have a large range of gene
regulatory functions and are involved in a series of biological
processes including regulating apoptosis, cell proliferation, cell
differentiation and development, epigenetic genetic regulation and
serve a notable function in the development and progression of
cancer (117,118). Numerous miRNAs have been revealed
to be expressed at varying degrees in gastric cancer, regulating
the signaling pathways, and certain specific miRNAs are associated
with the occurrence, progression and prognosis of gastric cancer
(119,120). Using the microarray analysis of
miRNAs, 22 types of miRNAs were demonstrated to be upregulated and
13 species were downregulated, compared with non-tumor gastric
tissue (119,120). miR125b, miR199a and miR-433 are
miRNAs that serve an important function in the progression of
cancer. Furthermore, the low expression of miR-433 and high
expression of miR214 are independent predictors of poor prognosis
(121). In gastric cancer, the
downregulation of miR-148a results in reduced tumor metastasis and
causes lymph node metastasis and disease progression when miR-148a
is downregulated (122). miR-335
inhibits metastasis by regulating BCL2 like 2 and specificity
protein-1 in gastric cancer (123).
Furthermore, miRNA-421 is a promising tumor biomarker with
diagnostic potential to monitor a number of different types of
cancer. Gastric juice levels of miR421 in patients with gastric
cancer were significantly different compared with patients with a
benign gastric disease (124).
Therefore, miRNAs may function as potential biomarker targets for
the molecular diagnosis of gastric cancer.
For the past few years, the dysregulated expression
of numerous lncRNAs (ncRNAs that are >200 nucleotides long) have
been demonstrated to be associated with tumorigenesis using
next-generation sequencing; and these lncRNAs perform basic
regulatory functions by modulating tumor cell proliferation,
apoptosis, invasion and metastasis (125). Differential expression of lncRNAs
serve critical functions in the carcinogenesis of gastric cancer. A
number of studies have revealed that lncRNA H19 is upregulated and
highly expressed in gastric cancer and is involved in the complex
molecular regulation of gastric cancer, and thus may serve as a
potential diagnostic marker and molecular therapeutic target,
particularly for early tumor screening (126,127).
Pang et al (128)
illuminated that the expression levels of LINC00152 in gastric
cancer tissues was significantly higher compared with normal
tissues and normal healthy controls, and was associated with
invasion. Additionally, this previous study demonstrated that the
lncRNA LINC00152 may be a novel biomarker for predicting gastric
cancer (99). Furthermore, high
urothelial cancer associated 1 (UCA1) expression is associated with
tumor size, reduced differentiation, adavanced
Tumor-Node-Metastasis stages and increased invasion depth in
gastric cancer. Therefore UCA1 may also serve as a potential marker
for the early detection and prognostic prediction of gastric cancer
(129). It was demonstrated that a
three-lncRNA signature, including UCA1, long stress-induced
non-coding transcript 5 and phosphatase and tensin homolog
pseudogene 1, was confirmed to be a potential diagnostic biomarker
for detecting gastric cancer (130). Hence, it is clear that the study of
the pathophysiology of lncRNA expression in gastric cancer is a
novel research trend which may improve the diagnosis and treatment
of patients with gastric cancer.
CircRNA, a novel type of ncRNA which is dissimilar
to the well-known linear RNA, forms a covalently closed continuous
loop. In circular RNA, the 3′ end and 5′ end are usually joined
together (131). At present,
research on the function of circRNA in cancer remains in its
infancy. Although a large number of circRNAs have been demonstrated
to be downregulated by using next-generation RNA sequencing and
bioinformatics analysis, there is emerging evidence that numerous
circRNAs are abnormally expressed in various diseases, including
certain types of cancer, which may function as oncogenes or tumor
suppressor genes (132,133). Certain circRNAs serve a function in
various aspects of biology and disease, particularly in cancer
(134,135). Notable, studies have revealed that
select circRNAs are upregulated or downregulated in patients with
gastric cancer. Chen et al (136) revealed that circRNA Pvt1 oncogene
was upregulated in gastric cancer and functioned as an miRNA
sponge, regulating the expression of the target genes of the miRNA
in gastric cancer, and ultimately demonstrated a proliferative
effect and potential as a prognostic marker. In addition, three
independent studies demonstrated that hsa_circ_0000190 (137), hsa_circ_0000096 (138) and hsa_circ_002059 (139) were downregulated in gastric cancer
tissues compared with the adjacent noncancerous tissues. Due to the
tissue, timing and disease specificity of circRNA expression,
circRNAs have attracted substantial interest in cancer research. At
present, circRNAs present high specificity in gastric cancer, which
indicates that they may have promise as prospective novel
biomarkers. However, their function and mechanism remain yet to be
elucidated.
The normal progression of the cell cycle is
regulated by positive regulators of cyclins, cyclin-dependent
kinase (CDK) complexes, and negative regulators of cell
cycle-dependent kinase inhibitors (CKI). The overexpression of cell
cycle positive regulators, abnormal activity and lack of expression
of CKI result in disorders of cell proliferation (140). Hence, any defects in cell cycle
regulators affect the outcome of patients with gastric cancer, as
cell-cycle regulators are involved in a number of processes,
including the proliferation of cancer. An important regulator is
cyclin E, which is also a useful prognostic factor in gastric
cancer. The activity of CDK is inhibited by CKIs, including P16,
P21 and P27 (141). The
downregulation of P27 serves as a negative predictor of cyclin
E-positive tumor types (142–144).
In addition, the function of P53, a tumor suppressor gene, is
pivotal to cell cycle regulation, DNA repair and apoptosis.
Multivariate analysis has revealed that the upregulation of P53 was
an independent prognostic factor in patients with advanced gastric
cancer and it was associated with poor prognosis (145). However, P53 has no substantial
prognostic value in the initial phase of gastric cancer in patients
(146).
The growth of a tumor depends on the ratio of cell
proliferation to apoptosis. In addition to the function of
oncogenes and tumor suppressor genes, factors that regulate cell
apoptosis also serve a crucial function in the development of a
number of tumor types, including gastric cancer. Certain programmed
cell death factors are good prognostic indicators for gastric
cancer. Apoptosis is achieved through the interaction between
pro-apoptotic and anti-apoptotic molecules (147). The BCL2 apoptosis regulator (Bcl-2)
gene, which is a crucial antiapoptotic gene, influences the
intrinsic apoptosis pathway (147).
At the same time, Bcl-2 may facilitate tumor invasion and
metastasis (148). It has been
suggested that the inhibition of Bcl-2 expression is an important
strategy for the treatment of various cancer types, including
gastric cancer (149,150). The sensitivity of tumor cells to
apoptosis may be detected and evaluated by assessing the levels of
the proto-oncogene Bcl-2, which is a useful prognostic factor of
survival in patients with advanced gastric cancer (151). The newest inhibitory apoptotic
family, survivin, may suppress apoptosis via pathways other than
those associated with the Bcl-2 family (152). One study hypothesized that survivin
may be expressed in gastric cancer, although the nuclear
localization of survivin is thought to delay physiological
development (153). Thus, survivin
may be used as a prognostic factor for poor outcome in patients
with gastric cancer (154).
In previous years, the association between certain
potentially enriched markers of gastric cancer stem cells including
CD133, CD44 and leucine rich repeat containing G protein-coupled
receptor 5 and the biological behavior and prognosis of gastric
cancer in patients has attracted increasing attention. If CD133 is
highly expressed in gastric cancer cells, the cells display
increased malignant biological behaviors, as its upregulation is
associated with tumor progression, chemotherapy resistance, relapse
and poor prognosis (155).
Therefore, the positive expression of CD133 in gastric cancer cells
may contribute to the prognosis of patients (156).
The CDH1 gene is a tumor suppressor gene which
encodes E-cadherin, a transmembrane glycoprotein involved in cell
adhesion and epithelial differentiation (157). Mutation of this gene are associated
with the occurrence and progression of hereditary diffuse gastric
cancer and sporadic gastric cancer. A lack of expression of
E-cadherin is an independent prognostic factor for gastric cancer,
and predicts a worse prognosis in patients (158). E-cadherin-negative and nuclear
β-catenin-positive gastric cancer are often associated with the
poor differentiation of gastric cancer, loss of adhesion, increased
infiltration ability, increased tumor progression and a poorer
prognosis (159). Consequently, the
expression of CD133 or E-cadherin may be used to predict the
prognosis of patients with gastric cancer.
Gastric cancer is a malignant tumor type with high
rates of incidence and mortality globally (1). Gastric cancer is a group of tumor types
which are diverse in their biology and genetics; and furthermore,
there are various factors at work in the etiology of this cancer,
including environmental and genetic factors (6). Although endoscopy and imaging
technology have improved the detection of early-stage gastric
carcinoma-associated lesions, gastric cancer has a wide range of
morphological heterogeneity. It is easy to overlook certain cases
of this disease if only the morphology is relied upon, and advances
in the field of understanding biomarkers have helped to improve the
early diagnosis and accuracy of diagnosis of gastric cancer early
as well as the prognosis and treatment of various diseases
including cancer.
The majority of cases of gastric cancer are either
moderately or severely advanced at first diagnosis; however, the
application of biomarkers may improve the early detection of
gastric cancer screening and diagnostic accuracy. As the correct
treatment may improve the prognosis of patients, it is important to
tailor treatments according the unique biomarker expression profile
of each patient. In particular, certain markers may guide the
individual and precise therapy of gastric cancer in order to
maximize a patient's survival time, instead of or in addition to
relying on histological classification and chemotherapy completely.
So far, targeted therapies for a small number of patients with
gastric cancer have been performed instead of or in addition to
using trastuzumab and ramucirumab, which are the second-line
treatments for advanced gastric cancer when either HER2 or VEGFR2
expression are upregulated, respectively. The PD-1 inhibitor
pembrolizumab has been approved by the FDA as a third-line (or
higher) treatment for patients with recurrent locally advanced or
metastatic gastric adenocarcinoma. The NCCN guidelines recommend
the detection of PD-L1 or MSI/MMR, as PD-1 inhibitors are effective
in patients with MSI-H/deficient MMR and a high expression of PD-L1
(76). Targeted therapy and
immunotherapy have presented clinicians with a novel method for
treating patients with advanced gastric cancer.
Numerous markers have now been identified for
targeted therapy and immunotherapy, and each marker exhibits
advantages and disadvantages (44,50,56).
With the development of high-throughput technologies, various
markers will be identified in the future, not only at the organ
level but also at the genetic level. All tumor types that express
the same markers may be treated using similar therapeutic regimens,
despite the location of the tumor. Conversely, tumor types which
are located in the same organ but exhibit different biomarker
profiles may be treated with using different therapeutic regimens.
In summary, the identification of novel and effective biomarkers is
required to improve the diagnosis of gastric cancer, in order to
strengthen the accuracy of gastric cancer diagnosis, determine the
prognosis and predict the pathogenesis, and establish a novel and
effective treatment option for patients with gastric cancer.
Not applicable.
The present review was supported by the Hunan
Provincial Groundbreaking Platform Open Fund of University of South
China (grant no. 18K076), the Doctoral Research Fund of the
University of South China (grant no. 2016XQD21), the Student
Research Learning and Innovative Experimental Project of University
of South China (grant nos. 2016NH055XJXZ and 2017XJXZ030), the
Hunan Provincial Key Subject Fund of Basic Medical Sciences and the
University of South China and Horizontal Cooperation Project of
Yueyang Maternal and Child Health Hospital (grant no.
2018KHX43).
Not applicable.
ZZ and DMY drafted the manuscript. DMY, GX and YX
acquired the data. WM, YXL, WL and YL interpreted the data. DY, ZZ
and YL edited the manuscript and revised it. All authors read and
approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Katai H, Ishikawa T, Akazawa K, Isobe Y,
Miyashiro I, Oda I, Tsujitani S, Ono H, Tanabe S, Fukagawa T, et
al: Five-year survival analysis of surgically resected gastric
cancer cases in Japan: A retrospective analysis of more than
100,000 patients from the nationwide registry of the Japanese
Gastric Cancer Association (2001–2007). Gastric Cancer. 21:144–154.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sumiyama K: Erratum to: Past and current
trends in endoscopic diagnosis for early stage gastric cancer in
Japan. Gastric Cancer. 20:5622017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ren W, Yu J, Zhang ZM, Song YK, Li YH and
Wang L: Missed diagnosis of early gastric cancer or high-grade
intraepithelial neoplasia. World J Gastroenterol. 19:2092–2096.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nie Y, Wu K, Yu J, Liang Q, Cai X, Shang
Y, Zhou J, Pan K, Sun L, Fang J, et al: A global burden of gastric
cancer: The major impact of China. Expert Rev Gastroenterol
Hepatol. 11:651–661. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fock KM: Review article: The epidemiology
and prevention of gastric cancer. Aliment Pharmacol Ther.
40:250–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li ZS and Li Q: The latest 2010 WHO
classification of tumors of digestive system. Zhonghua Bing Li Xue
Za Zhi. 40:351–354. 2011.(In Chinese). PubMed/NCBI
|
|
9
|
Plummer M, Franceschi S, Vignat J, Forman
D and de Martel C: Global burden of gastric cancer attributable to
Helicobacter pylori. Int J Cancer. 136:487–490. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schistosomes, liver flukes and
Helicobacter pylori. IARC working group on the evaluation of
carcinogenic risks to humans. Lyon, 7–14 June 1994. IARC Monogr
Eval Carcinog Risks Hum. 61:1–241. 1994.PubMed/NCBI
|
|
11
|
van der Post RS, Vogelaar IP, Carneiro F,
Guilford P, Huntsman D, Hoogerbrugge N, Caldas C, Schreiber KE,
Hardwick RH, Ausems MG, et al: Hereditary diffuse gastric cancer:
Updated clinical guidelines with an emphasis on germline CDH1
mutation carriers. J Med Genet. 52:361–374. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saigusa S, Tanaka K, Mohri Y, Ohi M,
Shimura T, Kitajima T, Kondo S, Okugawa Y, Toiyama Y, Inoue Y and
Kusunoki M: Clinical significance of RacGAP1 expression at the
invasive front of gastric cancer. Gastric Cancer. 18:84–92. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Catalano V, Labianca R, Beretta GD, Gatta
G, de Braud F and Van Cutsem E: Gastric cancer. Crit Rev Oncol
Hematol. 71:127–164. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lauren P: The two histological main types
of gastric carcinoma: Diffuse and so-called intestinal-type
carcinoma. An attempt at a histo-clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luu C, Thapa R, Woo K, Coppola D, Almhanna
K, Pimiento JM, Chen DT, Marquez DD and Hodul PJ: Does histology
really influence gastric cancer prognosis? J Gastrointest Oncol.
8:1026–1036. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
NIH-FDA conference: Biomarkers and
surrogate endpoints: Advancing clinical research and applications.
Abstracts. Dis Markers. 14:187–334. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Becking GC and Chen BH: International
programme on chemical safety (IPCS) environmental health criteria
on boron human health risk assessment. Biol Trace Elem Res.
66:439–452. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abbas M, Habib M, Naveed M, Karthik K,
Dhama K, Shi M and Dingding C: The relevance of gastric cancer
biomarkers in prognosis and pre- and post-chemotherapy in clinical
practice. Biomed Pharmacother. 95:1082–1090. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Strimbu K and Tavel JA: What are
biomarkers? Curr Opin HIV AIDS. 5:463–466. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meng FP, Ding J, Yu ZC, Han QL, Guo CC,
Liu N and Fan DM: Oral attenuated Salmonella typhimurium vaccine
against MG7-Ag mimotope of gastric cancer. World J Gastroenterol.
11:1833–1836. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ohtsu A, Yoshida S and Saijo N:
Disparities in gastric cancer chemotherapy between the East and
West. J Clin Oncol. 24:2188–2196. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tatsuta M, Itoh T, Okuda S, Yamamura H,
Baba M and Tamura H: Carcinoembryonic antigen in gastric juice as
an aid in diagnosis of early gastric cancer. Cancer. 46:2686–2692.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ishigami S, Natsugoe S, Hokita S, Che X,
Tokuda K, Nakajo A, Iwashige H, Tokushige M, Watanabe T, Takao S
and Aikou T: Clinical importance of preoperative carcinoembryonic
antigen and carbohydrate antigen 19-9 levels in gastric cancer. J
Clin Gastroenterol. 32:41–44. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yin LK, Sun XQ and Mou DZ: Value of
combined detection of serum CEA, CA72-4, CA19-9 and TSGF in the
diagnosis of gastric cancer. Asian Pac J Cancer Prev. 16:3867–3870.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ren J, Chen Z, Juan SJ, Yong XY, Pan BR
and Fan DM: Detection of circulating gastric carcinoma-associated
antigen MG7-Ag in human sera using an established single
determinant immuno-polymerase chain reaction technique. Cancer.
88:280–285. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miki K, Ichinose M, Shimizu A, Huang SC,
Oka H, Furihata C, Matsushima T and Takahashi K: Serum pepsinogens
as a screening test of extensive chronic gastritis. Gastroenterol
Jpn. 22:133–141. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miki K: Gastric cancer screening using the
serum pepsinogen test method. Gastric Cancer. 9:245–253. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rugge M, Genta RM, Di Mario F, El-Omar EM,
El-Serag HB, Fassan M, Hunt RH, Kuipers EJ, Malfertheiner P, Sugano
K and Graham DY: Gastric cancer as preventable disease. Clin
Gastroenterol Hepatol. 15:1833–1843. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Graham DY: Helicobacter pylori
update: Gastric cancer, reliable therapy, and possible benefits.
Gastroenterology. 148:719–731.e3. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Konturek SJ, Starzynska T, Konturek PC,
Karczewska E, Marlicz K, Lawniczak M, Jaroszewicz-Heigelman H,
Bielanski W, Hartwich A, Ziemniak A and Hahn EG: Helicobacter
pylori and CagA status, serum gastrin, interleukin-8 and
gastric acid secretion in gastric cancer. Scand J Gastroenterol.
37:891–898. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shiotani A, Iishi H, Uedo N, Kumamoto M,
Nakae Y, Ishiguro S, Tatsuta M and Graham DY: Histologic and serum
risk markers for noncardia early gastric cancer. Int J Cancer.
115:463–469. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tu H, Sun L, Dong X, Gong Y, Xu Q, Jing J,
Bostick RM, Wu X and Yuan Y: A serological biopsy using five
stomach-specific circulating biomarkers for gastric cancer risk
assessment: A multi-phase study. Am J Gastroenterol. 112:704–715.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shiroshita H, Watanabe H, Ajioka Y,
Watanabe G, Nishikura K and Kitano S: Re-evaluation of mucin
phenotypes of gastric minute well-differentiated-type
adenocarcinomas using a series of HGM, MUC5AC, MUC6, M-GGMC, MUC2
and CD10 stains. Pathol Int. 54:311–321. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Camargo MC, Murphy G, Koriyama C, Pfeiffer
RM, Kim WH, Herrera-Goepfert R, Corvalan AH, Carrascal E, Abdirad
A, Anwar M, et al: Determinants of Epstein-Barr virus-positive
gastric cancer: An international pooled analysis. Br J Cancer.
105:38–43. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou Y, Zhang GJ, Wang J, Zheng KY and Fu
W: Current status of lymph node micrometastasis in gastric cancer.
Oncotarget. 8:51963–51969. 2017.PubMed/NCBI
|
|
36
|
Hata M, Machi J, Mamou J, Yanagihara ET,
Saegusa-Beecroft E, Kobayashi GK, Wong CC, Fung C, Feleppa EJ and
Sakamoto K: Entire-volume serial histological examination for
detection of micrometastases in lymph nodes of colorectal cancers.
Pathol Oncol Res. 17:835–841. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Giorgadze TA, Baloch ZW, Pasha T, Zhang PJ
and Livolsi VA: Lymphatic and blood vessel density in the
follicular patterned lesions of thyroid. Mod Pathol. 18:1424–1431.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Moore BW: A soluble protein characteristic
of the nervous system. Biochem Biophys Res Commun. 19:739–744.
1965. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Abdel-Aziz A, Ahmed RA and Ibrahiem AT:
Expression of pRb, Ki67 and HER 2/neu in gastric carcinomas:
Relation to different histopathological grades and stages. Ann
Diagn Pathol. 30:1–7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yerushalmi R, Woods R, Ravdin PM, Hayes MM
and Gelmon KA: Ki67 in breast cancer: Prognostic and predictive
potential. Lancet Oncol. 11:174–183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Genc CG, Falconi M, Partelli S, Muffatti
F, van Eeden S, Doglioni C, Klumpen HJ, van Eijck C and Nieveen
VDE: Recurrence of pancreatic neuroendocrine tumors and survival
predicted by Ki67. Ann Surg Oncol. 25:2467–2474. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Akiyama T, Sudo C, Ogawara H, Toyoshima K
and Yamamoto T: The product of the human c-erbB-2 gene: A
185-kilodalton glycoprotein with tyrosine kinase activity. Science.
232:1644–1646. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu L, Wu N and Li J: Novel targeted
agents for gastric cancer. J Hematol Oncol. 5:312012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tanner M, Hollmén M, Junttila TT, Kapanen
AI, Tommola S, Soini Y, Helin H, Salo J, Joensuu H, Sihvo E, et al:
Amplification of HER-2 in gastric carcinoma: Association with
topoisomerase IIalpha gene amplification, intestinal type, poor
prognosis and sensitivity to trastuzumab. Ann Oncol. 16:273–278.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gravalos C and Jimeno A: HER2 in gastric
cancer: A new prognostic factor and a novel therapeutic target. Ann
Oncol. 19:1523–1529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wood DE, Kazerooni E, Baum SL, Dransfield
MT, Eapen GA, Ettinger DS, Hou L, Jackman DM, Klippenstein D, Kumar
R, et al: Lung cancer screening, version 1.2015: Featured updates
to the NCCN guidelines. J Natl Compr Canc Netw. 13:23–34. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ruschoff J, Hanna W, Bilous M, Hofmann M,
Osamura RY, Penault-Llorca F, van de Vijver M and Viale G: HER2
testing in gastric cancer: A practical approach. Mod Pathol.
25:637–650. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ohtsu A, Shah MA, Van Cutsem E, Rha SY,
Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B, et al:
Bevacizumab in combination with chemotherapy as first-line therapy
in advanced gastric cancer: A randomized, double-blind,
placebo-controlled phase III study. J Clin Oncol. 29:3968–3976.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hu X, Cao J, Hu W, Wu C, Pan Y, Cai L,
Tong Z, Wang S, Li J, Wang Z, et al: Multicenter phase II study of
apatinib in non-triple-negative metastatic breast cancer. Bmc
Cancer. 14:8202014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Derde LPG, Cooper BS, Goossens H,
Malhotra-Kumar S, Willems RJL, Gniadkowski M, Hryniewicz W, Empel
J, Dautzenberg M, Annane D, et al: Interventions to reduce
colonisation and transmission of antimicrobial-resistant bacteria
in intensive care units: An interrupted time series study and
cluster randomised trial. Lancet Infect Dis. 14:31–39. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lee J and Ou SH: Towards the goal of
personalized medicine in gastric cancer-time to move beyond HER2
inhibition. Part II: Targeting gene mutations and gene
amplifications and the angiogenesis pathway. Discov Med. 16:7–14.
2013.PubMed/NCBI
|
|
53
|
Javle M, Li Y, Tan D, Dong X, Chang P, Kar
S and Li D: Biomarkers of TGF-β signaling pathway and prognosis of
pancreatic cancer. PLoS One. 9:e859422014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Elice F, Rodeghiero F, Falanga A and
Rickles FR: Thrombosis associated with angiogenesis inhibitors.
Best Pract Res Clin Haematol. 22:115–128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Westover D, Zugazagoitia J, Cho BC, Lovly
CM and Paz-Ares L: Mechanisms of acquired resistance to first- and
second-generation EGFR tyrosine kinase inhibitors. Ann Oncol. 29
(Suppl 1):i10–i19. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wainberg ZA, Anghel A, Desai AJ, Ayala R,
Luo T, Safran B, Fejzo MS, Hecht JR, Slamon DJ and Finn RS:
Lapatinib, a dual EGFR and HER2 kinase inhibitor, selectively
inhibits HER2-amplified human gastric cancer cells and is
synergistic with trastuzumab in vitro and in vivo. Clin Cancer Res.
16:1509–1519. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lordick F, Kang YK, Chung HC, Salman P, Oh
SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V, et
al: Capecitabine and cisplatin with or without cetuximab for
patients with previously untreated advanced gastric cancer
(EXPAND): A randomised, open-label phase 3 trial. Lancet Oncol.
14:490–499. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Waddell T, Chau I, Cunningham D, Gonzalez
D, Okines AF, Okines C, Wotherspoon A, Saffery C, Middleton G,
Wadsley J, et al: Epirubicin, oxaliplatin, and capecitabine with or
without panitumumab for patients with previously untreated advanced
oesophagogastric cancer (REAL3): A randomised, open-label phase 3
trial. Lancet Oncol. 14:481–489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liu X, Guo W, Zhang W, Yin J, Zhang J, Zhu
X, Liu T, Chen Z, Wang B, Chang J, et al: A multi-center phase II
study and biomarker analysis of combined cetuximab and modified
FOLFIRI as second-line treatment in patients with metastatic
gastric cancer. BMC Cancer. 17:1882017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tebbutt NC, Price TJ, Ferraro DA, Wong N,
Veillard AS, Hall M, Sjoquist KM, Pavlakis N, Strickland A, Varma
SC, et al: Panitumumab added to docetaxel, cisplatin and
fluoropyrimidine in oesophagogastric cancer: ATTAX3 phase II trial.
Br J Cancer. 114:505–509. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kim MA, Lee HS, Lee HE, Jeon YK, Yang HK
and Kim WH: EGFR in gastric carcinomas: Prognostic significance of
protein overexpression and high gene copy number. Histopathology.
52:738–746. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Catenacci D, Tebbutt NC, Davidenko I,
Murad AM, Al-Batran SE, Ilson DH, Tjulandin S, Gotovkin E,
Karaszewska B and Bondarenko I: Rilotumumab plus epirubicin,
cisplatin, and capecitabine as first-line therapy in advanced
MET-positive gastric or gastro-oesophageal junction cancer
(RILOMET-1): A randomised, double-blind, placebo-controlled, phase
3 trial. Lancet Oncol. 18:1467–1482. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ha SY, Lee J, Kang SY, Do IG, Ahn S, Park
JO, Kang WK, Choi MG, Sohn TS and Bae JM: MET overexpression
assessed by new interpretation method predicts gene amplification
and poor survival in advanced gastric carcinomas. Mod Pathol.
26:1632–1641. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lee SJ, Lee J, Park SH, Park JO, Lim HY,
Kang WK, Park YS and Kim ST: c-MET overexpression in colorectal
cancer: A poor prognostic factor for survival. Clin Colorectal
Cancer. 17:165–169. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Pasquini G and Giaccone G: C-MET
inhibitors for advanced non-small cell lung cancer. Expert Opin
Investig Drugs. 27:363–375. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kuboki Y, Matsusaka S, Minowa S, Shibata
H, Suenaga M, Shinozaki E, Mizunuma N, Ueno M, Yamaguchi T and
Hatake K: Circulating tumor cell (CTC) count and epithelial growth
factor receptor expression on CTCs as biomarkers for cetuximab
efficacy in advanced colorectal cancer. Anticancer Res.
33:3905–3910. 2013.PubMed/NCBI
|
|
67
|
Catenacci DV, Henderson L, Xiao SY, Patel
P, Yauch RL, Hegde P, Zha J, Pandita A, Peterson A and Salgia R:
Durable complete response of metastatic gastric cancer with
anti-Met therapy followed by resistance at recurrence. Cancer
Discov. 1:573–579. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Al-Batran SE, Ducreux M and Ohtsu A: mTOR
as a therapeutic target in patients with gastric cancer. Int J
Cancer. 130:491–496. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ohtsu A, Ajani JA, Bai YX, Bang YJ, Chung
HC, Pan HM, Sahmoud T, Shen L, Yeh KH, Chin K, et al: Everolimus
for previously treated advanced gastric cancer: Results of the
randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol.
31:3935–3943. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wainberg ZA, Soares HP, Patel R, DiCarlo
B, Park DJ, Liem A, Wang HJ, Yonemoto L, Martinez D, Laux I, et al:
Phase II trial of everolimus in patients with refractory metastatic
adenocarcinoma of the esophagus, gastroesophageal junction and
stomach: Possible role for predictive biomarkers. Cancer Chemother
Pharmacol. 76:61–67. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Al-Batran SE, Hofheinz RD, Pauligk C, Kopp
HG, Haag GM, Luley KB, Meiler J, Homann N, Lorenzen S, Schmalenberg
H, et al: Histopathological regression after neoadjuvant docetaxel,
oxaliplatin, fluorouracil, and leucovorin versus epirubicin,
cisplatin, and fluorouracil or capecitabine in patients with
resectable gastric or gastro-oesophageal junction adenocarcinoma
(FLOT4-AIO): Results from the phase 2 part of a multicentre,
open-label, randomised phase 2/3 trial. Lancet Oncol. 17:1697–1708.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Singh P, Toom S and Huang Y: Anti-claudin
18.2 antibody as new targeted therapy for advanced gastric cancer.
J Hematol Oncol. 10:1052017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chen X, Wang M, Yang X, Wang Y, Yu L, Sun
J and Ding J: Injectable hydrogels for the sustained delivery of a
HER2-targeted antibody for preventing local relapse of
HER2+ breast cancer after breast-conserving surgery.
Theranostics. 9:6080–6098. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Tan IB, Ivanova T, Lim KH, Ong CW, Deng N,
Lee J, Tan SH, Wu J, Lee MH, Ooi CH, et al: Intrinsic subtypes of
gastric cancer, based on gene expression pattern, predict survival
and respond differently to chemotherapy. Gastroenterology.
141:476–485, 485.e1-e11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lei Z, Tan IB, Das K, Deng N, Zouridis H,
Pattison S, Chua C, Feng Z, Guan YK, Ooi CH, et al: Identification
of molecular subtypes of gastric cancer with different responses to
PI3-kinase inhibitors and 5-fluorouracil. Gastroenterology.
145:554–565. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Cancer Genome Atlas Research Network, .
Comprehensive molecular characterization of gastric adenocarcinoma.
Nature. 513:202–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Cristescu R, Lee J, Nebozhyn M, Kim KM,
Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, et al: Molecular
analysis of gastric cancer identifies subtypes associated with
distinct clinical outcomes. Nat Med. 21:449–456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Keir ME, Butte MJ, Freeman GJ and Sharpe
AH: PD-1 and its ligands in tolerance and immunity. Annu Rev
Immunol. 26:677–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Topalian SL, Drake CG and Pardoll DM:
Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor
immunity. Curr Opin Immunol. 24:207–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kim JW, Nam KH, Ahn SH, Park DJ, Kim HH,
Kim SH, Chang H, Lee JO, Kim YJ, Lee HS, et al: Prognostic
implications of immunosuppressive protein expression in tumors as
well as immune cell infiltration within the tumor microenvironment
in gastric cancer. Gastric Cancer. 19:42–52. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Qing Y, Li Q, Ren T, Xia W, Peng Y, Liu
GL, Luo H, Yang YX, Dai XY, Zhou SF and Wang D: Upregulation of
PD-L1 and APE1 is associated with tumorigenesis and poor prognosis
of gastric cancer. Drug Des Devel Ther. 9:901–909. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Taube JM, Anders RA, Young GD, Xu H,
Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL
and Chen L: Colocalization of inflammatory response with B7-h1
expression in human melanocytic lesions supports an adaptive
resistance mechanism of immune escape. Sci Transl Med.
4:127ra372012. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Sun J, Xu K, Wu C, Wang Y, Hu Y, Zhu Y,
Chen Y, Shi Q, Yu G and Zhang X: PD-L1 expression analysis in
gastric carcinoma tissue and blocking of tumor-associated PD-L1
signaling by two functional monoclonal antibodies. Tissue Antigens.
69:19–27. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y,
Kato K, Chung HC, Chen JS, Muro K, Kang WK, et al: Nivolumab in
patients with advanced gastric or gastro-oesophageal junction
cancer refractory to, or intolerant of, at least two previous
chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet.
390:2461–2471. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Patnaik A, Kang SP, Rasco D, Papadopoulos
KP, Elassaiss-Schaap J, Beeram M, Drengler R, Chen C, Smith L,
Espino G, et al: Phase I study of pembrolizumab (MK-3475; Anti-PD-1
Monoclonal Antibody) in patients with advanced solid tumors. Clin
Cancer Res. 21:4286–4293. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Giuliani J and Bonetti A:
Immune-checkpoint inhibitors in head and neck squamous cell
carcinoma: Cost-efficacy in second-line treatment based on
programmed death-ligand 1 (PD-L1) level. Oral Oncol. 97:143–145.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Kerr KM and Hirsch FR: Programmed death
ligand-1 immunohistochemistry: Friend or foe? Arch Pathol Lab Med.
140:326–331. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Boyiadzis M, Bishop MR, Abonour R,
Anderson KC, Ansell SM, Avigan D, Barbarotta L, Barrett AJ, Van
Besien K, Bergsagel PL, et al: The society for immunotherapy of
cancer consensus statement on immunotherapy for the treatment of
hematologic malignancies: Multiple myeloma, lymphoma, and acute
leukemia. J Immunother Cancer. 4:902016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Gettinger S, Horn L, Jackman D, Spigel D,
Antonia S, Hellmann M, Powderly J, Heist R, Sequist LV, Smith DC,
et al: Five-year follow-up of nivolumab in previously treated
advanced non-small-cell lung cancer: Results from the CA209-003
study. J Clin Oncol. 36:1675–1684. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Hou J, Yu Z, Xiang R, Li C and Wang L,
Chen S, Li Q, Chen M and Wang L: Correlation between infiltration
of FOXP3+ regulatory T cells and expression of B7-H1 in
the tumor tissues of gastric cancer. Exp Mol Pathol. 96:284–291.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Geng Y, Wang H, Lu C, Li Q, Xu B, Jiang J
and Wu C: Expression of costimulatory molecules B7-H1, B7-H4 and
Foxp3+ Tregs in gastric cancer and its clinical
significance. Int J Clin Oncol. 20:273–281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Muro K, Chung HC, Shankaran V, Geva R,
Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, et al:
Pembrolizumab for patients with PD-L1-positive advanced gastric
cancer (KEYNOTE-012): A multicentre, open-label, phase 1b trial.
Lancet Oncol. 17:717–726. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Fuchs CS, Doi T, Jang RW, Muro K, Satoh T,
Machado M, Sun W, Jalal SI, Shah MA, Metges JP, et al: Safety and
efficacy of pembrolizumab monotherapy in patients with previously
treated advanced gastric and gastroesophageal junction cancer:
Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 4:e1800132018.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Hamid O, Puzanov I, Dummer R, Schachter J,
Daud A, Schadendorf D, Blank C, Cranmer LD, Robert C, Pavlick AC,
et al: Final analysis of a randomised trial comparing pembrolizumab
versus investigator-choice chemotherapy for ipilimumab-refractory
advanced melanoma. Eur J Cancer. 86:37–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Fashoyin-Aje L, Donoghue M, Chen H, He K,
Veeraraghavan J, Goldberg KB, Keegan P, McKee AE and Pazdur R: FDA
approval summary: Pembrolizumab for recurrent locally advanced or
metastatic gastric or gastroesophageal junction adenocarcinoma
expressing PD-L1. Oncologist. 24:103–109. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Joshi SS, Maron SB and Catenacci DV:
Pembrolizumab for treatment of advanced gastric and
gastroesophageal junction adenocarcinoma. Future Oncol. 14:417–430.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Sohn BH, Hwang JE, Jang HJ, Lee HS, Oh SC,
Shim JJ, Lee KW, Kim EH, Yim SY, Lee SH, et al: Clinical
significance of four molecular subtypes of gastric cancer
identified by the cancer genome atlas project. Clin Cancer Res. Jul
26–2017.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Derks S, Liao X, Chiaravalli AM, Xu X,
Camargo MC, Solcia E, Sessa F, Fleitas T, Freeman GJ, Rodig SJ, et
al: Abundant PD-L1 expression in epstein-barr virus-infected
gastric cancers. Oncotarget. 7:32925–32932. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ma C, Patel K, Singhi AD, Ren B, Zhu B,
Shaikh F and Sun W: Programmed death-ligand 1 expression is common
in gastric cancer associated with epstein-barr virus or
microsatellite instability. Am J Surg Pathol. 40:1496–1506. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Qiu H and Zhou Z: Updates and
interpretation on NCCN clinical practice guidelines for gastric
cancer 2017 version 5. Zhonghua Wei Chang Wai Ke Za Zhi.
21:160–164. 2018.(In Chinese). PubMed/NCBI
|
|
102
|
Yarchoan M, Hopkins A and Jaffee EM: Tumor
mutational burden and response rate to PD-1 inhibition. N Engl J
Med. 377:2500–2501. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Hellmann MD, Callahan MK, Awad MM, Calvo
E, Ascierto PA, Atmaca A, Rizvi NA, Hirsch FR, Selvaggi G,
Szustakowski JD, et al: Tumor mutational burden and efficacy of
nivolumab monotherapy and in combination with ipilimumab in
small-cell lung cancer. Cancer Cell. 33:853–861.e4. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Hellmann MD, Ciuleanu TE, Pluzanski A, Lee
JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers
S, Salman P, et al: Nivolumab plus ipilimumab in lung cancer with a
high tumor mutational burden. N Engl J Med. 378:2093–2104. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Das PM and Singal R: DNA methylation and
cancer. J Clin Oncol. 22:4632–4642. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Mitsuno M, Kitajima Y, Ide T, Ohtaka K,
Tanaka M, Satoh S and Miyazaki K: Aberrant methylation of p16
predicts candidates for 5-fluorouracil-based adjuvant therapy in
gastric cancer patients. J Gastroenterol. 42:866–873. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Leung WK, To KF, Chu ES, Chan MW, Bai AH,
Ng EK, Chan FK and Sung JJ: Potential diagnostic and prognostic
values of detecting promoter hypermethylation in the serum of
patients with gastric cancer. Br J Cancer. 92:2190–2194. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Ikoma H, Ichikawa D, Daito I, Nobuyuki T,
Koike H, Okamoto K, Ochiai T, Ueda Y, Yamagishi H and Otsuji E:
Clinical application of methylation specific-polymerase chain
reaction in serum of patients with gastric cancer.
Hepatogastroenterology. 54:946–950. 2007.PubMed/NCBI
|
|
109
|
Sakakura C, Hamada T, Miyagawa K, Nishio
M, Miyashita A, Nagata H, Ida H, Yazumi S, Otsuji E and Chiba T:
Quantitative analysis of tumor-derived methylated RUNX3 sequences
in the serum of gastric cancer patients. Anticancer Res.
29:2619–2625. 2009.PubMed/NCBI
|
|
110
|
Homma N, Tamura G, Honda T, Matsumoto Y,
Nishizuka S, Kawata S and Motoyama T: Spreading of methylation
within RUNX3 CpG island in gastric cancer. Cancer Sci. 97:51–56.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Chen CC, Lee KD, Pai MY, Chu PY, Hsu CC,
Chiu CC, Chen LT, Chang JY, Hsiao SH and Leu YW: Changes in DNA
methylation are associated with the development of drug resistance
in cervical cancer cells. Cancer Cell Int. 15:982015. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Choi SJ, Jung SW, Huh S, Chung YS, Cho H
and Kang H: Alteration of DNA methylation in gastric cancer with
chemotherapy. J Microbiol Biotechnol. 27:1367–1378. 2017.PubMed/NCBI
|
|
113
|
Djebali S, Davis CA, Merkel A, Dobin A,
Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F,
et al: Landscape of transcription in human cells. Nature.
489:101–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Guttman M and Rinn JL: Modular regulatory
principles of large non-coding RNAs. Nature. 482:339–346. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Kwok ZH and Tay Y: Long noncoding RNAs:
Lincs between human health and disease. Biochem Soc Trans.
45:805–812. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Bartel DP and Chen CZ: Micromanagers of
gene expression: The potentially widespread influence of metazoan
microRNAs. Nat Rev Genet. 5:396–400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Liu N, Chen NY, Cui RX, Li WF, Li Y, Wei
RR, Zhang MY, Sun Y, Huang BJ, Chen M, et al: Prognostic value of a
microRNA signature in nasopharyngeal carcinoma: A microRNA
expression analysis. Lancet Oncol. 13:633–641. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Yasui W, Sentani K, Sakamoto N, Anami K,
Naito Y and Oue N: Molecular pathology of gastric cancer: Research
and practice. Pathol Res Pract. 207:608–612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Wu WK, Lee CW, Cho CH, Fan D, Wu K, Yu J
and Sung JJ: MicroRNA dysregulation in gastric cancer: A new player
enters the game. Oncogene. 29:5761–5771. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Ueda T, Volinia S, Okumura H, Shimizu M,
Taccioli C, Rossi S, Alder H, Liu CG, Oue N, Yasui W, et al:
Relation between microRNA expression and progression and prognosis
of gastric cancer: A microRNA expression analysis. Lancet Oncol.
11:136–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Yang TS, Yang XH, Wang XD, Wang YL, Zhou B
and Song ZS: MiR-214 regulate gastric cancer cell proliferation,
migration and invasion by targeting PTEN. Cancer Cell Int.
13:682013. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zheng B, Liang L, Wang C, Huang S, Cao X,
Zha R, Liu L, Jia D, Tian Q, Wu J, et al: MicroRNA-148a suppresses
tumor cell invasion and metastasis by downregulating ROCK1 in
gastric cancer. Clin Cancer Res. 17:7574–7583. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Xu Y, Zhao F, Wang Z, Song Y, Luo Y, Zhang
X, Jiang L, Sun Z, Miao Z and Xu H: MicroRNA-335 acts as a
metastasis suppressor in gastric cancer by targeting Bcl-w and
specificity protein 1. Oncogene. 31:1398–1407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Zhang X, Cui L, Ye G, Zheng T, Song H, Xia
T, Yu X, Xiao B, Le Y and Guo J: Gastric juice microRNA-421 is a
new biomarker for screening gastric cancer. Tumour Biol.
33:2349–2355. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Bhan A and Mandal SS: Long noncoding RNAs:
Emerging stars in gene regulation, epigenetics and human disease.
ChemMedChem. 9:1932–1956. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Zhou X, Yin C, Dang Y, Ye F and Zhang G:
Identification of the long non-coding RNA H19 in plasma as a novel
biomarker for diagnosis of gastric cancer. Sci Rep. 5:115162015.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Yang F, Bi J, Xue X, Zheng L, Zhi K, Hua J
and Fang G: Up-regulated long non-coding RNA H19 contributes to
proliferation of gastric cancer cells. FEBS J. 279:3159–3165. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Pang Q, Ge J, Shao Y, Sun W, Song H, Xia
T, Xiao B and Guo J: Increased expression of long intergenic
non-coding RNA LINC00152 in gastric cancer and its clinical
significance. Tumour Biol. 35:5441–5447. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Zheng Q, Wu F, Dai WY, Zheng DC, Zheng C,
Ye H, Zhou B, Chen JJ and Chen P: Aberrant expression of UCA1 in
gastric cancer and its clinical significance. Clin Transl Oncol.
17:640–646. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Dong L, Qi P, Xu MD, Ni SJ, Huang D, Xu
QH, Weng WW, Tan C, Sheng WQ, Zhou XY, et al: Circulating CUDR,
LSINCT-5 and PTENP1 long noncoding RNAs in sera distinguish
patients with gastric cancer from healthy controls. Int J Cancer.
137:1128–1135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Hentze MW and Preiss T: Circular RNAs:
Splicing's enigma variations. EMBO J. 32:923–925. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Liu H, Liu Y, Bian Z, Zhang J, Zhang R,
Chen X, Huang Y, Wang Y and Zhu J: Circular RNA YAP1 inhibits the
proliferation and invasion of gastric cancer cells by regulating
the miR-367-5p/p27 Kip1 axis. Mol Cancer. 17:1512018.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Lyu D and Huang S: The emerging role and
clinical implication of human exonic circular RNA. Rna Biol.
14:1000–1006. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Qu S, Zhong Y, Shang R, Zhang X, Song W,
Kjems J and Li H: The emerging landscape of circular RNA in life
processes. Rna Biol. 14:992–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Chen J, Li Y, Zheng Q, Bao C, He J, Chen
B, Lyu D, Zheng B, Xu Y, Long Z, et al: Circular RNA profile
identifies circPVT1 as a proliferative factor and prognostic marker
in gastric cancer. Cancer Lett. 388:208–219. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Chen S, Li T, Zhao Q, Xiao B and Guo J:
Using circular RNA hsa_circ_0000190 as a new biomarker in the
diagnosis of gastric cancer. Clin Chim Acta. 466:167–171. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Li P, Chen H, Chen S, Mo X, Li T, Xiao B,
Yu R and Guo J: Circular RNA 0000096 affects cell growth and
migration in gastric cancer. Br J Cancer. 116:626–633. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Li P, Chen S, Chen H, Mo X, Li T, Shao Y,
Xiao B and Guo J: Using circular RNA as a novel type of biomarker
in the screening of gastric cancer. Clin Chim Acta. 444:132–136.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Kawakami M, Mustachio LM,
Rodriguez-Canales J, Mino B, Roszik J, Tong P, Wang J, Lee JJ,
Myung JH, Heymach JV, et al: Next-generation CDK2/9 inhibitors and
anaphase catastrophe in lung cancer. J Natl Cancer Inst. 109:2017.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Zohny SF, Baothman OA, El-Shinawi M,
Al-Malki AL, Zamzami MA and Choudhry H: The KIP/CIP family members
p21^{Waf1/Cip1} and p57^{Kip2} as diagnostic markers for breast
cancer. Cancer Biomark. 18:413–423. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Tenderenda M: A study on the prognostic
value of cyclins D1 and E expression levels in resectable gastric
cancer and on some correlations between cyclins expression,
histoclinical parameters and selected protein products of
cell-cycle regulatory genes. J Exp Clin Cancer Res. 24:405–414.
2005.PubMed/NCBI
|
|
143
|
Nitti D, Belluco C, Mammano E, Marchet A,
Ambrosi A, Mencarelli R, Segato P and Lise M: Low level of
p27(Kip1) protein expression in gastric adenocarcinoma is
associated with disease progression and poor outcome. J Surg Oncol.
81:167–176. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Xiangming C, Natsugoe S, Takao S, Hokita
S, Tanabe G, Baba M, Kuroshima K and Aikou T: The cooperative role
of p27 with cyclin E in the prognosis of advanced gastric
carcinoma. Cancer. 89:1214–1219. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Sgambato A, Migaldi M, Leocata P, Ventura
L, Criscuolo M, Di Giacomo C, Capelli G, Cittadini A and De Gaetani
C: Loss of p27Kip1 expression is a strong independent prognostic
factor of reduced survival in N0 gastric carcinomas. Cancer.
89:2247–2257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Ichiyoshi Y, Oiwa H, Tomisaki S, Sakaguchi
Y, Ohno S, Maehara Y and Sugimachi K: Overexpression of p53 is
associated with growth pattern and prognosis in advanced gastric
cancer. Hepatogastroenterology. 44:546–553. 1997.PubMed/NCBI
|
|
147
|
Lowe SW, Cepero E and Evan G: Intrinsic
tumour suppression. Nature. 432:307–315. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Youle RJ and Strasser A: The BCL-2 protein
family: Opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Long J, Menggen Q, Wuren Q, Shi Q and Pi
X: MiR-219-5p inhibits the growth and metastasis of malignant
melanoma by targeting BCL-2. Biomed Res Int. 2017:90325022017.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Kang MH and Reynolds CP: Bcl-2 inhibitors:
Targeting mitochondrial apoptotic pathways in cancer therapy. Clin
Cancer Res. 15:1126–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Compare D, Rocco A, Liguori E, D'Armiento
FP, Persico G, Masone S, Coppola-Bottazzi E, Suriani R, Romano M
and Nardone G: Global DNA hypomethylation is an early event in
Helicobacter pylori-related gastric carcinogenesis. J Clin
Pathol. 64:677–682. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Kim MA, Lee HE, Lee HS, Yang HK and Kim
WH: Expression of apoptosis-related proteins and its clinical
implication in surgically resected gastric carcinoma. Virchows
Arch. 459:503–510. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Komatsu S, Ichikawa D, Tsujiura M, Konishi
H, Takeshita H, Nagata H, Kawaguchi T, Hirajima S, Arita T,
Shiozaki A, et al: Prognostic impact of circulating miR-21 in the
plasma of patients with gastric carcinoma. Anticancer Res.
33:271–276. 2013.PubMed/NCBI
|
|
154
|
Okada E, Murai Y, Matsui K, Isizawa S,
Cheng C, Masuda M and Takano Y: Survivin expression in tumor cell
nuclei is predictive of a favorable prognosis in gastric cancer
patients. Cancer Lett. 163:109–116. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Ishigami S, Ueno S, Arigami T, Uchikado Y,
Setoyama T, Arima H, Kita Y, Kurahara H, Okumura H, Matsumoto M, et
al: Prognostic impact of CD133 expression in gastric carcinoma.
Anticancer Res. 30:2453–2457. 2010.PubMed/NCBI
|
|
156
|
Hashimoto K, Aoyagi K, Isobe T, Kouhuji K
and Shirouzu K: Expression of CD133 in the cytoplasm is associated
with cancer progression and poor prognosis in gastric cancer.
Gastric Cancer. 17:97–106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Uemura T: The cadherin superfamily at the
synapse: More members, more missions. Cell. 93:1095–1098. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Vleminckx K, Vakaet LJ, Mareel M, Fiers W
and van Roy F: Genetic manipulation of E-cadherin expression by
epithelial tumor cells reveals an invasion suppressor role. Cell.
66:107–119. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Zhou YN, Xu CP, Han B, Li M, Qiao L, Fang
DC and Yang JM: Expression of E-cadherin and beta-catenin in
gastric carcinoma and its correlation with the clinicopathological
features and patient survival. World J Gastroenterol. 8:987–993.
2002. View Article : Google Scholar : PubMed/NCBI
|