Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the most
common type of pancreatic cancer and has a 5-year survival rate of
6% was reported in year 2011 worldwide (1). Although advances have been made in the
prevention of PDAC as well as in surgical approaches and adjuvant
therapy, the mortality rates in patients with PDAC remain high
(2,3). Several risk factors such as smoking
(4), diabetes (5) and hereditary pancreatitis (6) have been identified. However, tissue
biomarkers or gene signatures for the prognosis prediction of PDAC
have not been well established (7).
The identification of a reliable prognostic genetic determinant in
PDAC is an area of ongoing research and may aid in individualized
risk assessment and treatment decision-making. Furthermore,
biomarkers might present novel therapeutic targets for the
treatment of PDAC.
The inflammatory process is a key mediator of
pancreatic cancer development and progression, and patients with
chronic pancreatitis have an increased risk of developing
pancreatic cancer (8–10). Trypsin-1, the main trypsinogen
secreted by the pancreas and encoded by the serine protease 1 gene
(PRSS1), has attracted attention in the field of PDAC
research (11). Previous studies
revealed that mutations in PRSS1 are associated with
autoimmune (12) and chronic
pancreatitis (13). Progressive
mutation accumulation and clonal expansion, however, are required
for the development of invasive PDAC (11). Mutations in the PRSS1 gene
have been reported to be associated with an increased incidence of
PDAC (10,14–16). The
−409 C/T PRSS1 genotype has been found to protect against
the development of pancreatic cancer in the Han Chinese population
(17); however, the prognostic value
of the aforementioned PRSS1 genotype in patients with PDAC
remains poorly understood.
The aim of the current study was to investigate the
association of the −409 C/T PRSS1 genotype with the
clinicopathological characteristics and prognosis of patients with
PDAC.
Materials and methods
Patient recruitment
Patients with PDAC from The First Affiliated
Hospital of Fujian Medical University (Fuzhou, China) were enrolled
between 2015 and 2017. The inclusion criteria were as follows: i)
Patients with pathologically confirmed PDAC diagnosis; ii) patients
who had received radical resection; and iii) patients who had not
received any preoperative therapy (including radiotherapy,
chemotherapy or targeted therapy). Patients with any comorbid or
previous malignancies were excluded from the analysis. A total of
124 patients were eligible for inclusion in the current study,
including 76 males and 48 females, with a median age of 60 years
(range, 28–86 years).
The present study was performed in accordance with
the ethical principles described in the Declaration of Helsinki and
was approved by the Ethics and Research Committee of The First
Affiliated Hospital of Fujian Medical University. Written informed
consent was obtained from all patients.
Genotype analysis
Peripheral blood samples were collected from the
peripheral vein. Following anticoagulation with EDTA and
centrifugation in 1,509 × g at 4°C for 5 min, genomic DNA was
extracted from each patient using a TIANamp Blood DNA kit (Tiangen
Biotech Co., Ltd.), and was stored at −20°C. Following the
manufacturer's instructions, the full-length PRSS1 gene was
amplified, and following purification, sequencing was performed and
analyzed by Sangon Biotech Co., Ltd. The 50 µl reaction mixture
used to generate the fragments contained 200 ng genomic DNA, 10
mmol/l Tris-hydrochloric acid (pH 9.0), 50 mmol/l potassium
chloride, 0.1% triton, 2 mmol/l magnesium chloride, 0.25 mmol/l
deoxynucleotide mix, 100 ng upstream primer, 100 ng downstream
primer and 3.0 units of Taq DNA-polymerase (Sangon Biotech Co.,
Ltd). The thermocycling conditions included an initial denaturing
step at 95°C for 5 min, followed by 30 cycles at 95°C for 30 sec,
55°C for 30 sec and 72°C for 1 min, followed by a final extension
step at 72°C for 10 min. PCR products were subsequently run on an
1% agarose gel in 1X TAE buffer and purified by a MinElute Gel
Extraction kit (Qiagen GmbH). Following the manufacturer's
instructions, a Perkin-Elmer Big Dye Sequencing kit (PerkinElmer,
Inc.) and an ABI PRISM7700 sequencer (PerkinElmer, Inc.) were used
for sequencing. The PRSS1 primers as follows: Forward,
5′-GGTCCTGGGTCTCATACCTT-3′ and reverse,
5′-GGGTAGGAGGCTTCACACTT-3′.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 20.0; IBM Corp.). Data are expressed as means ±
standard deviation or number and percentage as appropriate. The
association between the PRSS1 genotype and the
clinicopathological parameters of the patients was evaluated using
the c2 or Fisher's exact tests as appropriate. The
overall survival (OS) was defined as the time period between
surgery and mortality or the last follow-up. Survival outcomes were
evaluated by the Kaplan-Meier method and compared using the
log-rank test. A Cox proportional hazard regression model was used
for univariate and multivariate analyses of prognosis predictive
factors. P<0.05 was considered to indicate a statistically
significant difference.
Results
PRSS1 genotype in PDAC
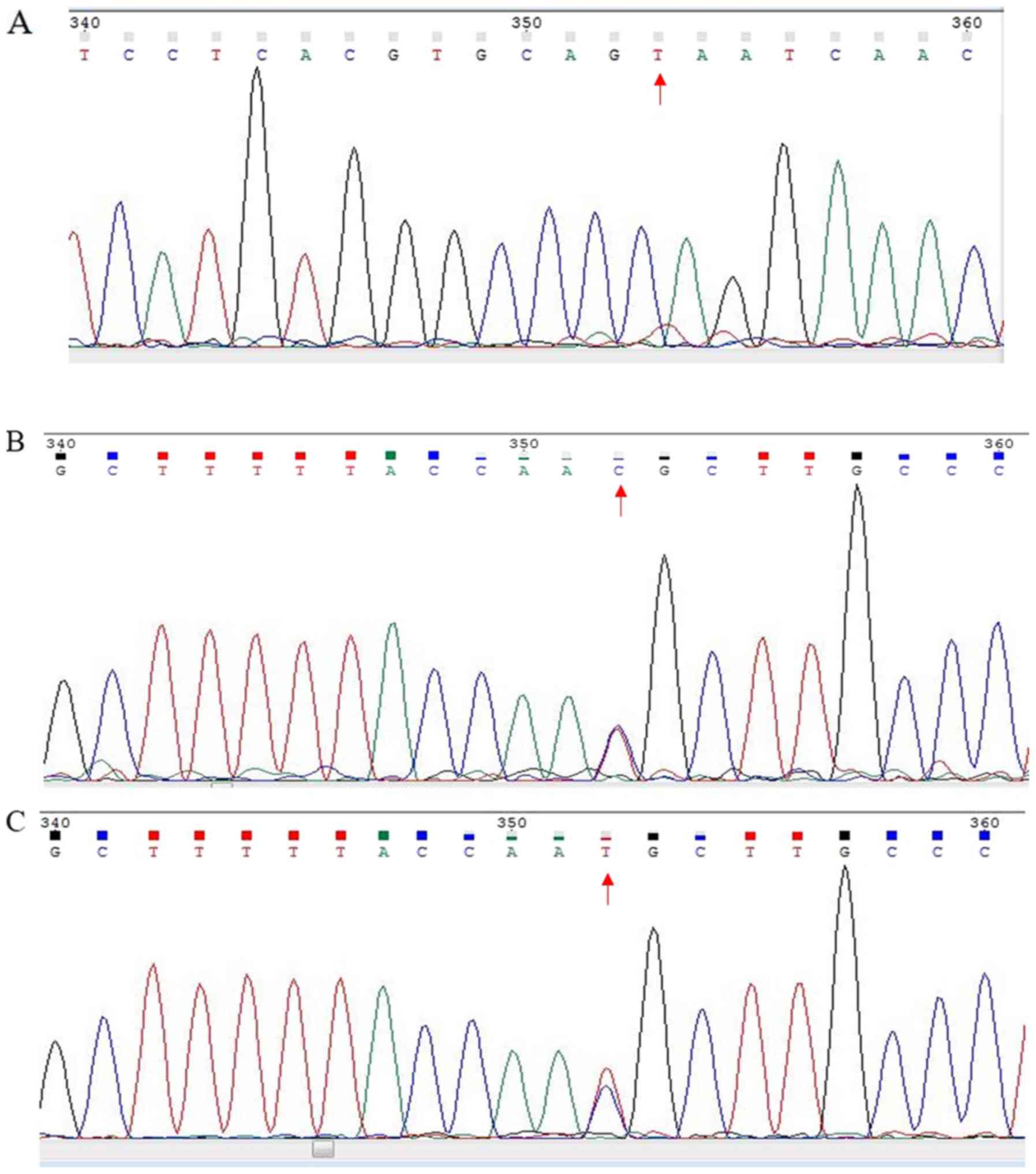
In the current study, PCR was used to determine the
PRSS1 genotype in the enrolled patients. The results
revealed that multiple loci contained one of three genotypes: C/C,
C/T or T/T (Fig. 1), which is
similar to results that have been previously reported (18,19).
Moreover, 58 patients with PDAC (43.75%) had the T/C genotype
(Table I), which was regarded as a
mutation.
 | Table I.Prognostic impact of PRSS1
genotype on the prognosis of patients with pancreatic ductal
adenocarcinoma. |
Table I.
Prognostic impact of PRSS1
genotype on the prognosis of patients with pancreatic ductal
adenocarcinoma.
|
| PRSS1
genotype |
|
|---|
|
|
|
|
|---|
| Variable | T/C | T/T and C/C | P-value |
|---|
| Age, years (mean ±
SD) | 61.5±12.7 | 59.7±10.3 | 0.253 |
| Sex, n (%) |
|
| 0.534 |
| Male | 36 (64.3) | 40 (58.8) |
|
|
Female | 20 (35.7) | 28 (41.2) |
|
| Tumor size, cm (mean
± SD) | 3.9±2.6 | 3.2±1.5 | 0.027 |
| Tumor location, n
(%) |
|
| 0.688 |
| Head | 41 (73.2) | 53 (77.9) |
|
| Body and
tail | 15 (26.8) | 15 (22.1) |
|
| CEA, ng/ml (mean ±
SD) | 7.7±15.1 | 15.9±48.7 | 0.038 |
| CA19-9, U/ml (mean ±
SD) | 291.6±375.9 | 302.2±361.5 | 0.717 |
| CA125, U/ml (mean ±
SD) | 50.1±89.3 | 45.7±78.7 | 0.460 |
| TNM stage, n
(%) |
|
| 0.041 |
| I | 11 (19.6) | 19 (27.9) |
|
| II | 13 (23.2) | 27 (39.7) |
|
|
III | 27 (48.2) | 20 (29.4) |
|
| IV | 5 (8.9) | 2 (2.9) |
|
| Perineural
invasion, n (%) |
|
| 0.874 |
|
Yes | 19 (33.9) | 24 (35.3) |
|
| No | 37 (66.1) | 44 (64.7) |
|
| Vascular invasion,
n (%) |
|
| 0.442 |
|
Yes | 43 (76.8) | 56 (82.4) |
|
| No | 13 (23.2) | 12 (17.6) |
|
Associations between the PRSS1
genotype and clinicopathological characteristics
As shown in Table I,
the T/C PRSS1 genotype was associated with larger tumor
sizes (P=0.027), higher tumor-node-metastasis (TNM) stages
(P=0.041) and higher levels of serum carcinoembryonic antigen (CEA)
(P=0.038) compared with the C/C or T/T PRSS1 genotypes.
However, no associations between the T/C PRSS1 genotype and
age, sex, tumor location, serum carbohydrate antigen (CA) 19-9 and
12-5 levels, perineural invasion or lymphovascular invasion were
observed (all P>0.05).
Prognostic impact of the PRSS1
genotype
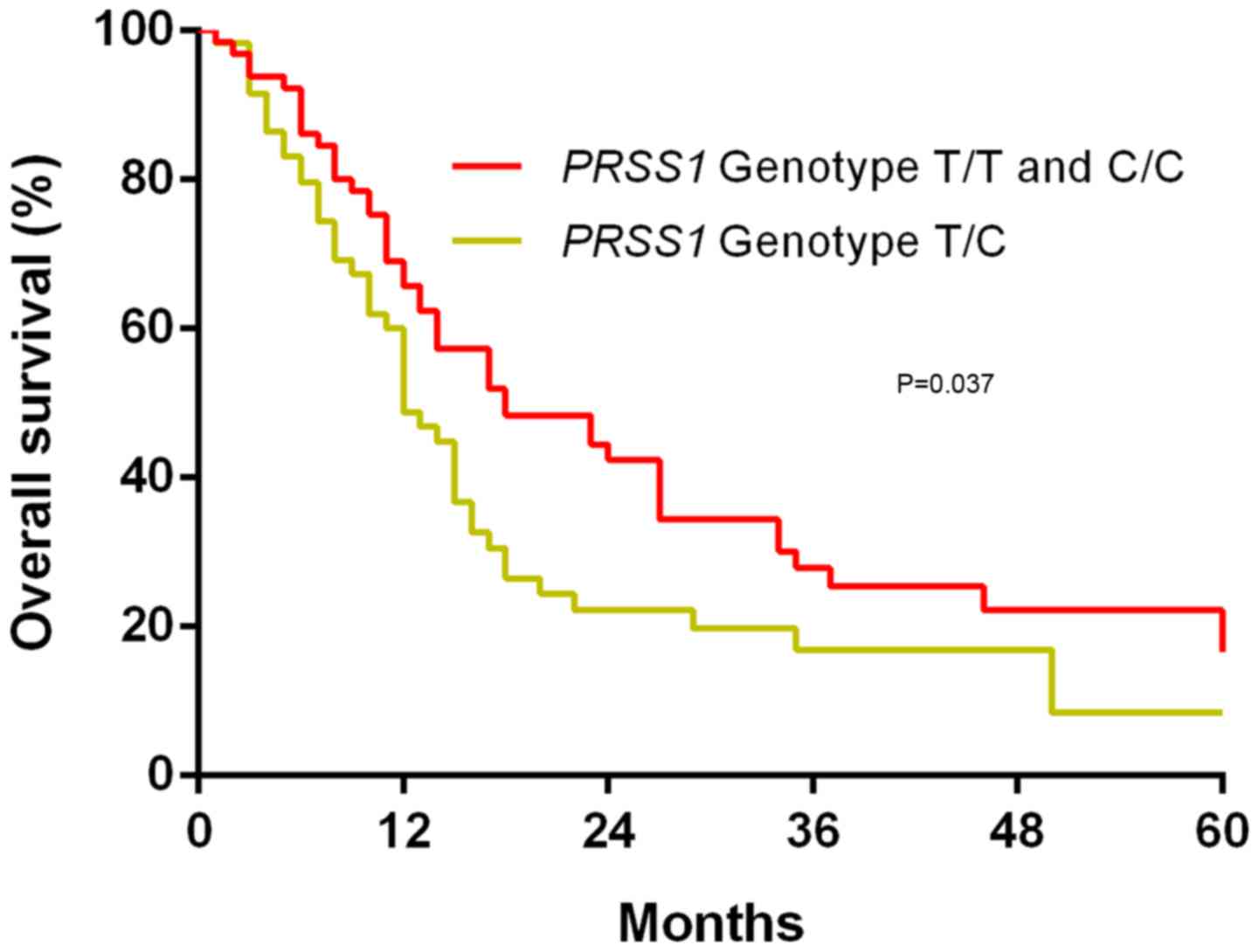
The possible association of the PRSS1
genotype with OS in patients with PDAC was investigated using
Kaplan-Meier survival curves and log-rank tests. It was revealed
that following a median follow-up period of 19 months, the T/C
PRSS1 genotype was associated with a worse OS time compared
with the T/T and C/C PRSS1 genotypes (P=0.037; Fig. 2).
Univariate and multivariate analyses
of predictive factors of OS
Univariate analysis revealed that surgical type
(P=0.032), serum CA19-9 level (P=0.001), serum CEA level (P=0.034),
TNM stage III (P=0.005), TNM stage IV (P<0.001), and
PRSS1 genotype (P=0.002) were significantly associated with
the OS time of patients with PDAC (Table II).
 | Table II.Univariate analysis of factors
affecting the overall survival time in patients with pancreatic
ductal adenocarcinoma. |
Table II.
Univariate analysis of factors
affecting the overall survival time in patients with pancreatic
ductal adenocarcinoma.
|
| Univariate
analysis |
|
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value |
|---|
| Sex (male vs.
female) | 0.785 | 0.510–1.208 | 0.271 |
| Age | 1.016 | 0.996–1.036 | 0.113 |
| Surgery type
(Whipple surgery vs. pancreatectomy) | 1.626 | 1.042–2.537 | 0.032 |
| Resection margin
(yes vs. no) | 1.049 | 0.476–2.315 | 0.905 |
| Tumor size | 1.155 | 1.038–1.285 | 0.008 |
| Serum CA19-9
level | 1.001 | 1.000–1.002 | 0.001 |
| Serum CEA
level | 1.007 | 1.001–1.014 | 0.034 |
| Serum CA125
level | 1.001 | 0.999–1.004 | 0.194 |
| TNM stage |
|
|
|
| I | Reference
value |
| 0.001 |
| II vs.
I | 1.691 | 0.931- 3.072 | 0.085 |
| III vs.
I | 2.257 | 1.272–4.005 | 0.005 |
| IV vs.
I | 5.320 | 2.186–12.947 | <0.001 |
| Perineural invasion
(yes vs. no) | 1.303 | 0.841–2.020 | 0.237 |
| Vascular invasion
(yes vs. no) | 1.487 | 0.921–2.398 | 0.104 |
| PRSS1
genotype (T/C vs. C/C or T/T) | 1.961 | 1.293–2.973 | 0.002 |
A multivariate analysis using the Cox regression
model was subsequently performed, and the results revealed that the
serum CA19-9 level [hazard ratio (HR)=1.001; P=0.001], TNM stage
III (HR=2.078; P=0.031), TNM stage IV (HR=3.090; P=0.041), and
PRSS1 genotype (HR=2.196; P=0.002) were independent
predictors of the OS time in patients with PDAC (Table III).
 | Table III.Multivariate analysis of factors
affecting the overall survival time in patients with pancreatic
ductal adenocarcinoma. |
Table III.
Multivariate analysis of factors
affecting the overall survival time in patients with pancreatic
ductal adenocarcinoma.
| Variable | Regression
coefficient | SE | HR | 95% CI | P-value |
|---|
| Surgery type
(Whipple surgery vs. pancreatectomy) | −0.009 | 0.274 | 0.991 | 0.579–1.695 | 0.973 |
| Tumor size | 0.081 | 0.067 | 1.085 | 0.952–1.236 | 0.223 |
| Serum CA19-9
level | 0.001 | <0.001 | 1.001 | 1.000–1.002 | 0.001 |
| Serum CEA
level | 0.006 | 0.005 | 1.006 | 0.996–1.016 | 0.230 |
| TNM stage |
|
|
|
|
|
| I | Reference
value |
|
|
| 0.110 |
| II vs.
I | 0.613 | 0.345 | 1.847 | 0.938–3.634 | 0.076 |
| III vs.
I | 0.731 | 0.339 | 2.078 | 1.068–4.041 | 0.031 |
| IV vs.
I | 1.128 | 0.551 | 3.090 | 1.049–9.104 | 0.041 |
| PRSS1
genotype (T/C vs. C/C or T/T) | 0.786 | 0.249 | 2.196 | 1.347–3.580 | 0.002 |
Discussion
The current study revealed that the T/C PRSS1
genotype was statistically associated with larger tumor size and
higher TNM stage compared with other genotypes. To the best of the
authors' knowledge, the present study was the first to demonstrate
that the T/C PRSS1 genotype was associated with poor OS, and
that the aforementioned genotype may serve as an independent
prognostic factor of OS in patients with PDAC, together with
factors including serum CA19-9 level and TNM stage.
Previous studies revealed that the processes of
genotype accumulation and clonal expansion are required for the
development of invasive PDAC (11,16,20).
Many efforts have been made to identify gene mutations associated
with pancreatic cancer, including mutL homolog 1 and PMS1 homolog
1, mismatch repair system component (21), and BRCA1/2 DNA repair (22). Increasing numbers of studies have
demonstrated that mutations in the PRSS1 gene are associated
with an increased incidence of PDAC (10,14–16).
Furthermore, the −409 C/T PRSS1 genotype was found to exert
a protective effective against pancreatic cancer in the Han Chinese
population (17). PRSS1
encodes trypsin-1, the main trypsinogen enzyme secreted by the
pancreas. PRSS1 mutations have been associated with
increased trypsin-1 serum levels as well as the trypsin and
α1-antitrypsin imbalance observed in patients with pancreatic
cancer, which may alter the pancreatic microenvironment and
increase immunological escape and clonal proliferation of
pancreatic cancer cells (19).
However, the clinicopathological and prognostic significance of
PRSS1 mutations in patients with PDAC remains unclear.
PRSS1 mutations have been previously shown to
be associated with autoimmune (12)
and chronic pancreatitis (13).
Additionally, the mean serum trypsin level was increased in
patients with pancreatic cancer compared with healthy controls
(19). While the PRSS1 gene
is not currently considered to be an oncogene, tumor-suppressor
gene or genome-maintenance gene, PRSS1 genotypes were
strongly correlated with the trypsin and α1-antitrypsin imbalance
in patients with pancreatic cancer (13).
Traditional prognostic factors, including serum
CA19-9 level and TNM stage, were identified as independent
prognostic factors of OS in patients with PDAC in the current
study, similarly to previously published studies (7,23). In
the current study, the T/C PRSS1 genotype indicated a poor
prognosis and a shorter OS time in patients with PDAC, as
demonstrated by the Kaplan-Meier method. Furthermore, associations
between the PRSS1 genotype and clinicopathological
parameters such as large tumor size and higher TNM stage were
identified in the current study, suggesting that the PRSS1
genotype may be involved in the progression and aggressiveness of
pancreatic cancer. Moreover, by using univariate and Cox regression
analysis, the results obtained in the current study demonstrated
that the PRSS1 genotype was an independent prognostic factor
of OS in patients with PDAC. The PRSS1 genotype may aid the
selection of treatment plans and the identification of patients who
may benefit from additional adjuvant therapy.
The current study had a number of limitations.
Firstly, the present study was a single-centre retrospective study.
Secondly, a limited sample size was included in the current study.
Thirdly, patients with unresectable PDAC were not included in the
current study due to insufficient data. Finally, all the patients
enrolled in the current study were of Chinese descent, therefore
the association between the mutation status of PRSS1 and
prognosis of patients of different ethnicities was not
investigated. Nevertheless, the current study contributed to the
understanding of the association between the PRSS1 genotype
and prognosis of patients with PDAC.
In conclusion, the current study demonstrated that
the T/C PRSS1 genotype was associated with large tumor size,
higher TNM stage and poor OS compared with other genotypes. The
PRSS1 genotype, together with other factors including serum
CA19-9 level and TNM stage, may therefore be used as an independent
prognostic factor of OS in patients with PADC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HW, ZZ and YC conceived and designed the study. FD
and XC acquired data. HW, SC, ZS and QL analysed the data. HW, ZZ
and YC interpretated, drafted and revised the manuscript. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics and
Research Committee of The First Affiliated Hospital of Fujian
Medical University (approval no. 2018-053). Written informed
consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ryan D, Hong T and Bardeesy N: Pancreatic
adenocarcinoma. N Engl J Med. 371:2140–2141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Uesaka K, Boku N, Fukutomi A, Okamura Y,
Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto
H, et al: Adjuvant chemotherapy of S-1 versus gemcitabine for
resected pancreatic cancer: A phase 3, open-label, randomised,
non-inferiority trial (JASPAC 01). Lancet. 388:248–257. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Picozzi VJ, Oh SY, Edwards A, Mandelson
MT, Dorer R, Rocha FG, Alseidi A, Biehl T, Traverso LW, Helton WS,
et al: Five-year actual overall survival in resected pancreatic
cancer: A contemporary single-institution experience from a
multidisciplinary perspective. Ann Surg Oncol. 24:1722–1730. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rulyak S, Lowenfels A, Maisonneuve P and
Brentnall T: Risk factors for the development of pancreatic cancer
in familial pancreatic cancer kindreds. Gastroenterology.
124:1292–1299. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huxley R, Ansary-Moghaddam A, Berrington
De González A, Barzi F and Woodward M: Type-II diabetes and
pancreatic cancer: A meta-analysis of 36 studies. Br J Cancer.
92:2076–2083. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu QC, Zhuang ZH, Zeng K, Cheng ZJ, Gao F
and Wang ZQ: Prevalence of pancreatic diabetes in patients carrying
mutations or polymorphisms of the PRSS1 gene in the Han population.
Diabetes Technol Ther. 11:799–804. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Winter JM, Yeo CJ and Brody JR:
Diagnostic, prognostic, and predictive biomarkers in pancreatic
cancer. J Surg Oncol. 107:15–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hausmann S, Kong B, Michalski C, Erkan M
and Friess H: The role of inflammation in pancreatic cancer. Adv
Exp Med Biol. 816:129–151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pinho A, Chantrill L and Rooman I: Chronic
pancreatitis: A path to pancreatic cancer. Cancer Lett.
345:203–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weiss F: Pancreatic cancer risk in
hereditary pancreatitis. Front Physiol. 5:702014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Whitcomb DC: Genetic risk factors for
pancreatic disorders. Gastroenterology. 144:1292–1302. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao F, Li YM, Hong GL, Xu ZF, Liu QC, He
QL, Lin LQ and Weng SH: PRSS1_p.Leu81Met mutation results in
autoimmune pancreatitis. World J Gastroenterol. 19:3332–3338. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao F, Liu QC, Zhang S, Zhuang ZH, Lin CZ
and Lin XH: PRSS1 intron mutations in patients with pancreatic
cancer and chronic pancreatitis. Mol Med Rep. 5:449–451.
2012.PubMed/NCBI
|
|
14
|
Hengstler JG, Bauer A, Wolf HK, Bulitta
CJ, Tanner B, Oesch F, Gebhard S and Boettger T: Mutation analysis
of the cationic trypsinogen gene in patients with pancreatic
cancer. Anticancer Res. 20:2967–2974. 2000.PubMed/NCBI
|
|
15
|
Rebours V, Boutron-Ruault M, Schnee M,
Férec C, Maire F, Hammel P, Ruszniewski P and Lévy P: Risk of
pancreatic adenocarcinoma in patients with hereditary pancreatitis:
A national exhaustive series. Am J Gastroenterol. 103:111–119.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen J and Férec C: Chronic pancreatitis:
Genetics and pathogenesis. Annu Rev Genomics Hum Genet. 10:63–87.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Q, Lin X, Liu J, Liu A and Gao F: The
−409 C/T genotype of PRSS1 protects against pancreatic cancer in
the Han Chinese population. Dig Dis Sci. 57:573–579. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeng K, Liu QC, Lin JH, Lin XH, Zhuang ZH,
Gao F and Ou QS: Novel mutations of PRSS1 gene in patients with
pancreatic cancer among Han population. Chin Med J (Engl).
124:2065–2067. 2011.PubMed/NCBI
|
|
19
|
Yi Q, Dong F, Lin L, Liu Q, Chen S, Gao F
and He Q: PRSS1 mutations and the proteinase/antiproteinase
imbalance in the pathogenesis of pancreatic cancer. Tumour Biol.
37:5805–5810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Campa D, Pastore M, Capurso G, Hackert T,
Di Leo M, Izbicki JR, Khaw KT, Gioffreda D, Kupcinskas J, Pasquali
C, et al: Do pancreatic cancer and chronic pancreatitis share the
same genetic risk factors? A PANcreatic Disease ReseArch (PANDoRA)
consortium investigation. Int J Cancer. 142:290–296. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gargiulo S, Torrini M, Ollila S, Nasti S,
Pastorino L, Cusano R, Bonelli L, Battistuzzi L, Mastracci L, Bruno
W, et al: Germline MLH1 and MSH2 mutations in Italian pancreatic
cancer patients with suspected Lynch syndrome. Fam Cancer.
8:547–553. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fogelman D, Wolff R, Kopetz S, Javle M,
Bradley C, Mok I, Cabanillas F and Abbruzzese J: Evidence for the
efficacy of Iniparib, a PARP-1 inhibitor, in BRCA2-associated
pancreatic cancer. Anticancer Res. 31:1417–1420. 2011.PubMed/NCBI
|
|
23
|
Berger AC, Garcia M Jr, Hoffman JP, Regine
WF, Abrams RA, Safran H, Konski A, Benson AB III, MacDonald J and
Willett CG: Postresection CA 19-9 predicts overall survival in
patients with pancreatic cancer treated with adjuvant
chemoradiation: A prospective validation by RTOG 9704. J Clin
Oncol. 26:5918–5922. 2008. View Article : Google Scholar : PubMed/NCBI
|