Introduction
Small-cell lung cancer (SCLC) usually presents as a
bulky conglomerated mass with mediastinal invasion, with
approximately two-thirds of patients presenting with metastatic
disease at the time of initial diagnosis (1). Thus, rather than open thoracic surgery,
chemotherapy is used as the first-line treatment, even in patients
with apparently early-stage lesions. Despite the well-documented
chemosensitivity of this tumor type, chemotherapy alone leads to
high intrathoracic failure rates; however, a combination of
thoracic radiotherapy (TRT) with chemotherapy offers benefits in
controlling disease in patients with limited-stage SCLC (LS-SCLC)
(2). Meta-analyses have reported
early concurrent TRT as being beneficial in maximizing tumor
regression compared with sequential chemoradiotherapy, resulting in
favorable survival outcomes (3–5).
With respect to TRT protocols, the optimal intensity
of radiation dose, including fraction size and total dose, is still
not fully determined, despite well-designed clinical trials
(6–10). Twice-daily TRT at a dose of 45 Gy and
once-daily TRT at a high dose of up to 70 Gy in combination with
chemotherapy show promising survival outcomes; however,
radiation-related esophageal toxicity is remarkably high compared
to that associated with a once-daily 45–50 Gy TRT regimen (6–9). Despite
advances in radiation delivery enabling radiation dose escalation
to >60 Gy in 2 Gy fractions (11), twice-daily TRT at 45 Gy is still
considered the standard of care for patients with LS-SCLC (10).
The superiority of twice-daily TRT at 45 Gy may be
explained by the shortened treatment duration and increased
biologically effective dose (BED) of radiation, which are important
predictors of survival (12,13). Currently, panels of National
Comprehensive Cancer Network recommend that TRT should be
administered early, concurrent with the first or second cycle of
chemotherapy. However, TRT could be delayed over the second cycle
of chemotherapy due to huge tumor burden or poor performance. Thus,
we aimed to evaluate the correlation of radiation parameters, such
as dose intensity and treatment time, with survival outcomes in
patients who had undergone concurrent chemoradiation therapy with
curative intent.
Materials and methods
Patient inclusion
The medical records of 101 patients with LS-SCLC
treated with definitive concurrent chemoradiotherapy (CCRT) between
August 2005 and March 2014 were reviewed. Staging was determined
using pretreatment computed tomography (CT), whole-body
18F-fluorodeoxyglucose positron emission tomography
(18FDG-PET), and brain magnetic resonance imaging (MRI).
All patients were restaged according to the 7th American Joint
Committee on Cancer staging system (14). Patients who received a TRT dose
<45 Gy were not included in this analysis. This retrospective
study was approved by the Institutional Review Board of Chonnam
National University Hwasun Hospital (CNUHH-2018-181).
Treatments
All patients received either chemotherapy or TRT
with chemotherapy on the first day of treatment. All patients
received 4–6 cycles of etoposide (100 mg/m2 per day on
days 1–3) and cisplatin (60 mg/m2 on day 1),
administered every 3 weeks. Hematologic toxicity and performance
were monitored after each cycle of chemotherapy.
Patients received three-dimensional conformal
radiotherapy using the Eclipse Treatment Planning System version
13.0 (Varian Medical Systems, Palo Alto, CA, US). Gross tumor
volume (GTV) was defined as the total volume of the primary tumor
apparent at the time of planning the first TRT and of the lymph
nodes that were initially involved. Clinical target volume (CTV)
was defined as GTV plus a minimum 7-mm margin and included the
first-echelon draining lymph node station. No elective irradiation
was performed on lymph node stations that were not involved. Timing
of TRT was determined by the tumor board, and RT dose depended on
the treatment protocol. When administering TRT at a 1.5 Gy dose
twice-daily (BID), the total dose of 45 Gy was administered in 3
weeks. When the simultaneous integrated boost (SIB) technique was
used, a CTV of 36–40 Gy in 1.8–2 Gy fractions and a GTV of 48–65 Gy
in 2.2–2.4 Gy fractions were administered. For patients who were
administered conventional fractions of 2 Gy, a CTV of 36 Gy and a
GTV of 50 Gy were administered using the shrinking field technique.
Upon administration of 50 Gy, a follow-up chest CT was conducted
and a booster dose of 60–65 Gy was administered to the apparently
visible tumor. Prophylactic cranial irradiation (PCI) was performed
for patients who consented to the treatment, completed all
treatment regimens, exhibited no disease progression, and
maintained good performance. The total dose and fraction schedule
of PCI was 25 Gy in 10 fractions. Tumor response was re-evaluated
using the response evaluation criteria in solid tumors after the
completion of chemoradiation (15).
The BED for TRT was calculated according to
following formula (16): BED=(nd)[1
+ d/(α/β)]-(0.639/α)[(T-Tk)/Tpot], where ‘n’
denotes the number of fractions, ‘d’ denotes the fraction size,
‘α/β’ is 10 Gy, ‘α’ is 0.3 Gy, ‘T’ denotes the overall duration of
TRT with the first fraction administered on day 1, ‘Tk’
denotes the delay in tumor proliferation (‘kick-off time’ was
assumed to be 21 days), and ‘Tpot’ the denotes potential
doubling time of tumor clonogenic cells, which was set to 3 days
for SCLC (17).
Follow-up and statistics
For the first 2 years after completion of treatment,
patients were followed up every 3–4 months. After 2 years, patients
were followed up at 6-month intervals. Local progression was
defined as tumor recurrence within the radiation port areas. Tumor
recurrence beyond the initial treatment site was defined as distant
metastasis. We defined elective nodal failure as any regional nodal
recurrence, including both supraclavicular lymph nodes outside the
initial radiation port area.
Overall survival (OS) was defined as the duration
between the first day of treatment and the day of patient death or
the date of final follow-up. Progression-free survival (PFS) was
defined as the duration between the first day of treatment and the
day of detection of any discernible tumor on chest CT, whole-body
18FDG-PET, or MRI or the day of patient death. Adverse
events were defined according to the National Cancer Institute
Common Terminology Criteria for Adverse Events, version 4.0
(18). During the follow-up period,
physicians observed the respiratory symptoms by physical
examination and chest CT was also checked regularly.
Statistical analysis
Kaplan-Meier models were used for survival analysis
of all potential factors affecting treatment. To assess the
statistical significance of treatment parameters between groups,
the Chi-square test was used. With respect to each prognostic and
predictive parameter, the log-rank test was used to estimate LC,
PFS, and OS rates. To avoid bias, we performed multivariate
analysis with treatment factors that were significant with respect
to survival outcomes, with a P-value <0.05. The Cox
regression model was used for multivariate analysis, and the Cox
proportional-hazards model was used to calculate hazard ratios.
Statistical analyses were performed using SPSS version 20.0
(IBM).
Results
Patient characteristics
Table I lists patient
characteristics. The median age of the patients was 64 years. Among
the 101 patients, 85 (84.2%) were restaged as having stage IIIA or
IIIB disease. The median dose of TRT administered to the patients
was 50 (range, 45–65) Gy. The median duration of TRT was 38 (range,
23–60) days, and the median duration from the start date of any
therapy to the end of TRT (SER) was 60 (range, 27–154) days.
Overall, 68 patients (67.3%) started TRT before the third cycle of
chemotherapy; 62 patients (61.4%) achieved complete response (CR)
and 39 patients (38.6%) exhibited partial response (PR) or stable
disease after chemoradiation. PCI was performed in 56 patients
(55.4%).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | Value | % of total
patients |
|---|
| Age, years |
|
|
|
Median | 64 |
|
|
Range | 43–80 |
|
| Sex |
|
|
| Male | 88 | 87.1 |
|
Female | 13 | 12.9 |
| ECOG performance
status |
|
|
| 0 | 31 | 30.7 |
| 1 | 68 | 67.3 |
| 2 | 2 | 2.0 |
| Smoking status |
|
|
| None or
past smoking | 63 | 62.4 |
| Current
smoking | 38 | 37.6 |
| T-stage |
|
|
| 1 | 7 | 6.9 |
| 2 | 20 | 19.9 |
| 3 | 37 | 36.6 |
| 4 | 37 | 36.6 |
| N-stage |
|
|
| 0 | 7 | 6.9 |
| 1 | 17 | 16.8 |
| 2 | 29 | 28.7 |
| 3 | 48 | 47.6 |
| Overall stage |
|
|
|
IIA | 8 | 7.9 |
|
IIB | 8 | 7.9 |
|
IIIA | 30 | 29.7 |
|
IIIB | 55 | 54.5 |
| Timing of TRT |
|
|
| <3
cycles of chemotherapy | 68 | 67.3 |
| ≥3
cycles of chemotherapy | 33 | 32.7 |
| Fractionation of
TRT |
|
|
|
Conventional
fractionation | 75 | 74.2 |
|
BID | 2 | 2.0 |
|
SIB | 24 | 23.8 |
| Dose of TRT |
|
|
| Median,
Gy | 50 |
|
|
Range | 45–65 |
|
| Dose of TRT,
BED10 |
|
|
| Median,
Gy | 50 |
|
|
Range | 32.2–65.4 |
|
| Duration of
TRT |
|
|
| Median,
days | 38 |
|
|
Range | 23–60 |
|
| SER |
|
|
| Median
duration, days | 60 |
|
|
Range | 27–154 |
|
| Total cycles of
chemotherapy |
|
|
| 4 | 5 | 5.0 |
| 5 | 12 | 11.9 |
| 6 | 84 | 83.1 |
| Total treatment
duration |
|
|
| Median
duration, days | 124 |
|
|
Range | 66–170 |
|
| PCI |
|
|
|
Yes | 56 | 55.4 |
| No | 45 | 44.6 |
The characteristics of each group of patients
according to tumor response are shown in Table II. A lower dose or a BED lower than
50 Gy (P<0.001, P=0.020, respectively) and short
duration of TRT (≤ 40 days, P=0.020) were correlated with
CR.
 | Table II.Cancer and treatment characteristics
according to the tumor response. |
Table II.
Cancer and treatment characteristics
according to the tumor response.
|
| Tumor response |
|
|---|
|
|
|
|
|---|
|
Characteristics | CR (n=62) | Non-CR (n=39) | P-value |
|---|
| T-stage |
|
| 0.250 |
|
1–3 | 42 | 22 |
|
| 4 | 20 | 17 |
|
| N-stage |
|
| 0.849 |
|
0–2 | 33 | 20 |
|
| 3 | 29 | 19 |
|
| Overall stage |
|
| 0.257 |
|
IIA-IIIA | 31 | 15 |
|
|
IIIB | 31 | 24 |
|
| Timing of TRT,
cycles |
|
| 0.911 |
|
<3 | 42 | 26 |
|
| ≥3 | 20 | 13 |
|
| Dose of TRT,
Gy |
|
| <0.001 |
|
≤50 | 45 | 14 |
|
|
>50 | 17 | 25 |
|
| Dose of TRT
(BED10), Gy |
|
| 0.020 |
|
≤50 | 37 | 14 |
|
|
>50 | 25 | 25 |
|
| Duration of TRT,
days |
|
| 0.020 |
|
≤40 | 43 | 18 |
|
|
>40 | 19 | 21 |
|
| SER, days |
|
| 0.576 |
|
≤70 | 40 | 23 |
|
|
>70 | 22 | 16 |
|
Treatment outcomes
Median follow-up period for all patients was 26.9
(range, 2.5–162.7) months and median survival was 26.9 months.
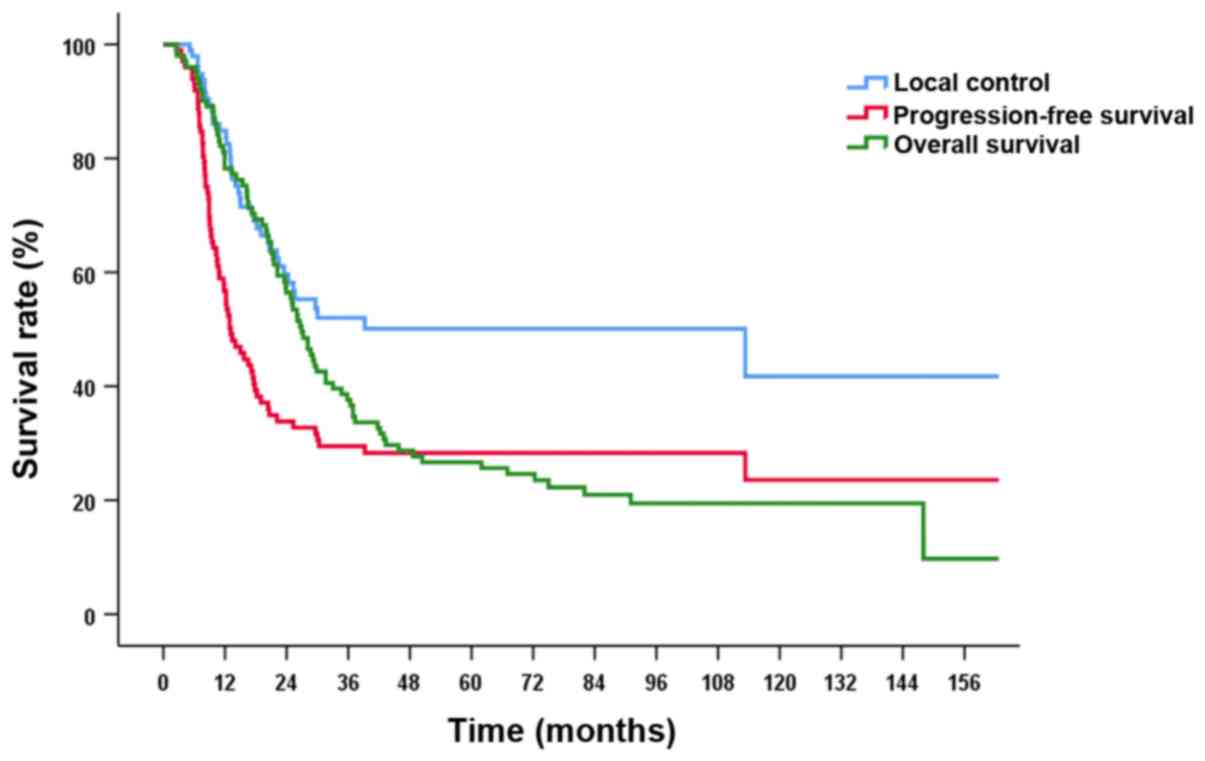
3-year LC, PFS, and OS rates were 52.0, 29.5 and 37.6%,
respectively. 5-year LC, PFS, and OS rates were 50.1, 28.3 and
26.7%, respectively (Fig. 1). Local
failure occurred in 41 patients (40.5%), and distant metastasis was
detected in 54 patients (53.4%). Elective nodal failure was
observed in 12 patients (11.8%). Sites of distant metastasis were
located in the brain in 28 (27.7%), bone in 11 (10.8%), ipsilateral
or contralateral lung in 5 (4.9%), supraclavicular and cervical
lymph nodes in 5, liver in 5, adrenal gland in 1, and diaphragm in
1 patients. Grade 3 radiation pneumonitis and esophagitis occurred
in 7 (6.9%) and 7 (6.9%) patients, respectively. There were no
cases of grade 4 or 5 radiation esophagitis. One patient died due
to radiation pneumonitis after 6 months of TRT with a radiation
dose of 60 Gy (Table III).
 | Table III.Treatment-related toxicity. |
Table III.
Treatment-related toxicity.
| Toxicity | Grade 3, n (%) | Grade 4, n (%) | Grade 5, n (%) |
|---|
|
Anaphylaxisa | 1 (0.9) | 0 | 0 |
| Dysphagia | 7 (6.9) | 0 | 0 |
| Pneumonitis | 7 (6.9) | 0 | 1 (0.9) |
| Leukopenia | 34 (33.6) | 13 (12.8) | 0 |
| Febrile
neutropenia | 9 (8.9) | 1 (0.9) | 0 |
| Anemia | 14 (13.8) | 0 | 0 |
|
Thrombocytopenia | 6 (13.8) | 4 (3.9) | 0 |
Predictive factors affecting treatment
outcomes
In the univariate analysis, age (P=0.002),
stage (P<0.001), timing of TRT (P=0.017), dose of
TRT (P=0.028), SER (P=0.025), PCI (P=0.003),
and tumor response (P=0.015) were significant predictors of
OS. Stage (P<0.001) and PCI (P=0.017)
significantly affected PFS. Stage (P=0.009) was the only
prognostic factor related to LC (Table
IV).
 | Table IV.Univariate analysis of clinical and
treatment factors based on treatment outcomes. |
Table IV.
Univariate analysis of clinical and
treatment factors based on treatment outcomes.
| Variable | Patients, n | 3-year LC, % | P-value | 3-year PFS, % | P-value | 3-year OS, % | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
|
<65 | 54 | 62.1 | 0.212 | 35.9 | 0.298 | 46.3 | 0.002 |
|
≥65 | 47 | 37.2 |
| 20.7 |
| 27.7 |
|
| Sex |
|
|
|
|
|
|
|
|
Male | 88 | 51.3 | 0.615 | 27.6 | 0.508 | 34.1 | 0.068 |
|
Female | 13 | 58.9 |
| 42.0 |
| 61.5 |
|
| Smoking |
|
|
|
|
|
|
|
|
None-past | 63 | 50.9 | 0.980 | 29.8 | 0.695 | 34.9 | 0.996 |
|
Current | 38 | 54.3 |
| 28.9 |
| 42.1 |
|
| ECOG performance
status |
|
|
|
|
|
|
|
| 0 | 31 | 51.4 | 0.859 | 27.6 | 0.436 | 29.0 | 0.095 |
|
1–2 | 70 | 52.3 |
| 30.5 |
| 41.4 |
|
| Overall stage |
|
|
|
|
|
|
|
|
IIA-IIIA | 46 | 63.0 | 0.009 | 48.9 | <0.001 | 58.7 | <0.001 |
|
IIIB | 55 | 38.0 |
| 11.0 |
| 20.0 |
|
| Timing of TRT,
cycles |
|
|
|
|
|
|
|
|
<3 | 68 | 53.5 | 0.471 | 32.7 | 0.133 | 45.6 | 0.017 |
| ≥3 | 33 | 49.3 |
| 22.8 |
| 21.2 |
|
| Dose of TRT,
Gy |
|
|
|
|
|
|
|
|
≤50 | 59 | 51.9 | 0.411 | 31.1 | 0.157 | 44.1 | 0.028 |
|
>50 | 42 | 53.7 |
| 27.5 |
| 28.6 |
|
| Dose of TRT
(BED10), Gy |
|
|
|
|
|
|
|
|
≤50 | 51 | 56.7 | 0.318 | 36.0 | 0.108 | 45.1 | 0.082 |
|
>50 | 50 | 46.9 |
| 21.9 |
| 30.0 |
|
| Duration of TRT,
days |
|
|
|
|
|
|
|
|
≤40 | 61 | 47.2 | 0.723 | 28.9 | 0.367 | 41.0 | 0.249 |
|
>40 | 40 | 62.7 |
| 31.1 |
| 32.5 |
|
| SER, days |
|
|
|
|
|
|
|
|
≤70 | 63 | 55.7 | 0.081 | 32.0 | 0.101 | 46.0 | 0.025 |
|
>70 | 38 | 45.8 |
| 25.3 |
| 23.7 |
|
| PCI |
|
|
|
|
|
|
|
| No | 56 | 37.9 | 0.095 | 19.1 | 0.017 | 23.2 | 0.003 |
|
Yes | 45 | 63.7 |
| 41.0 |
| 55.6 |
|
| Tumor response |
|
|
|
|
|
|
|
| CR | 62 | 50.9 | 0.898 | 30.1 | 0.363 | 41.9 | 0.015 |
|
Non-CR | 39 | 54.0 |
| 28.6 |
| 30.8 |
|
Multivariate analysis revealed that stage was the
only significant factor affecting all treatment outcomes, including
LC (P=0.011), PFS (P<0.001), and OS
(P<0.001). Other factors, such as SER (P=0.005),
PCI (P=0.007), and tumor response (P=0.014) were
significantly associated with OS (Table
V).
 | Table V.Multivariate analysis of clinical and
treatment factors based on treatment outcomes. |
Table V.
Multivariate analysis of clinical and
treatment factors based on treatment outcomes.
|
| LC | PFS | OS |
|---|
|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Stage (IIA-IIIA vs.
IIIB) | 2.29
(1.21–4.36) | 0.011 | 3.61
(2.15–6.07) | <0.001 | 2.49
(1.56–3.98) | <0.001 |
| Timing of TRT
(<3 cycles vs. ≥3 cycles) | 0.74
(0.28–1.95) | 0.550 | 1.11
(0.49–2.50) | 0.798 | 1.68
(0.80–3.54) | 0.168 |
| Dose of TRT (≤50 Gy
vs. >50 Gy) | 1.30
(0.62–2.72) | 0.482 | 1.56
(0.90–2.68) | 0.108 | 1.57
(0.95–2.59) | 0.076 |
| SER (≤70 days vs.
>70 days) | 2.18
(0.89–5.30) | 0.084 | 1.46
(0.67–3.15) | 0.331 | 1.93
(1.22–3.07) | 0.005 |
| PCI (Yes vs.
No) | 1.55
(0.79–3.05) | 0.200 | 1.59
(0.95–2.65) | 0.072 | 1.87
(1.19–3.02) | 0.007 |
| Tumor response (CR
vs. non-CR) | 0.87
(0.42–1.80) | 0.720 | 1.11
(0.65–1.89) | 0.693 | 1.76
(1.12–2.77) | 0.014 |
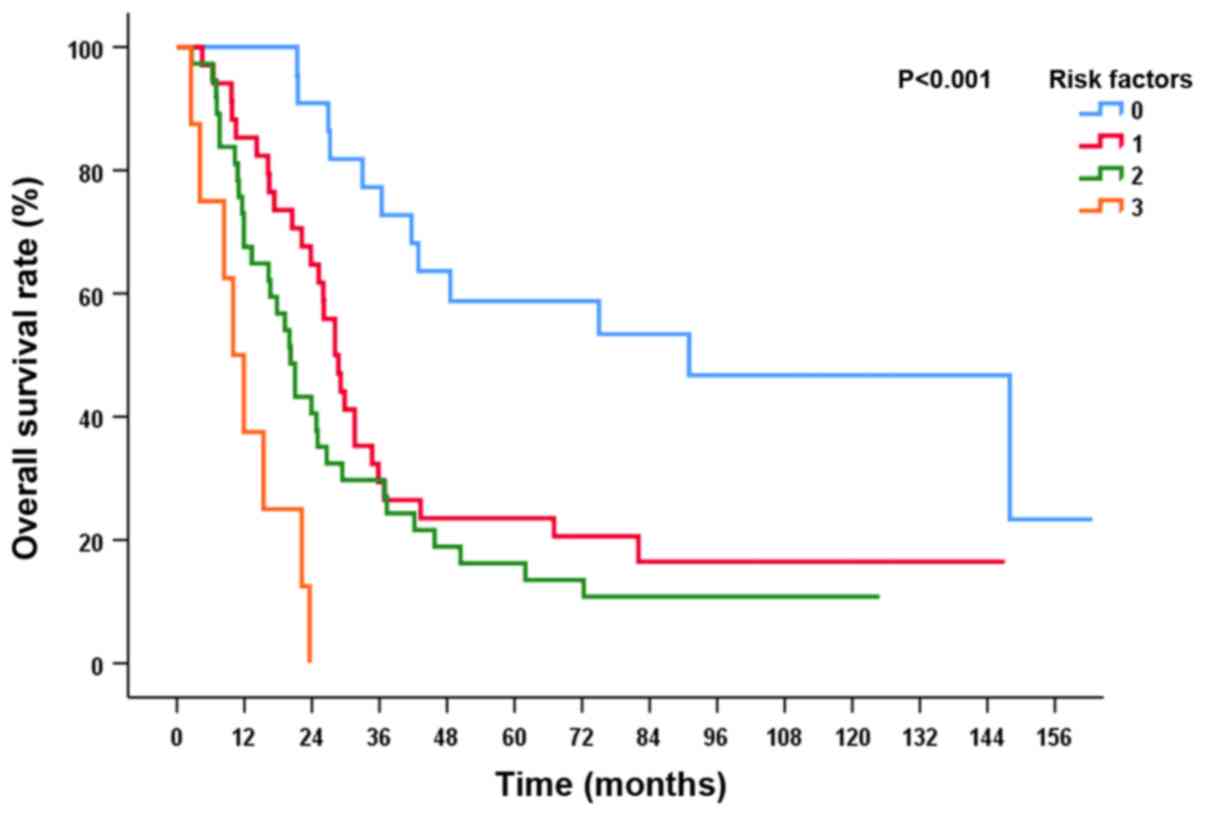
On the basis of multivariate analysis, patients were
stratified into subgroups by risk factor, including stage IIIB,
non-CR, and SER >70 days. There were significant differences in
OS in accordance with the number of risk factors
(P<0.001). Patients with 0, 1, 2, and 3 risk factors
presented with a 5-year OS rate of 58.7, 23.5, 16.2 and 0%,
respectively (Fig. 2). The 5-year OS
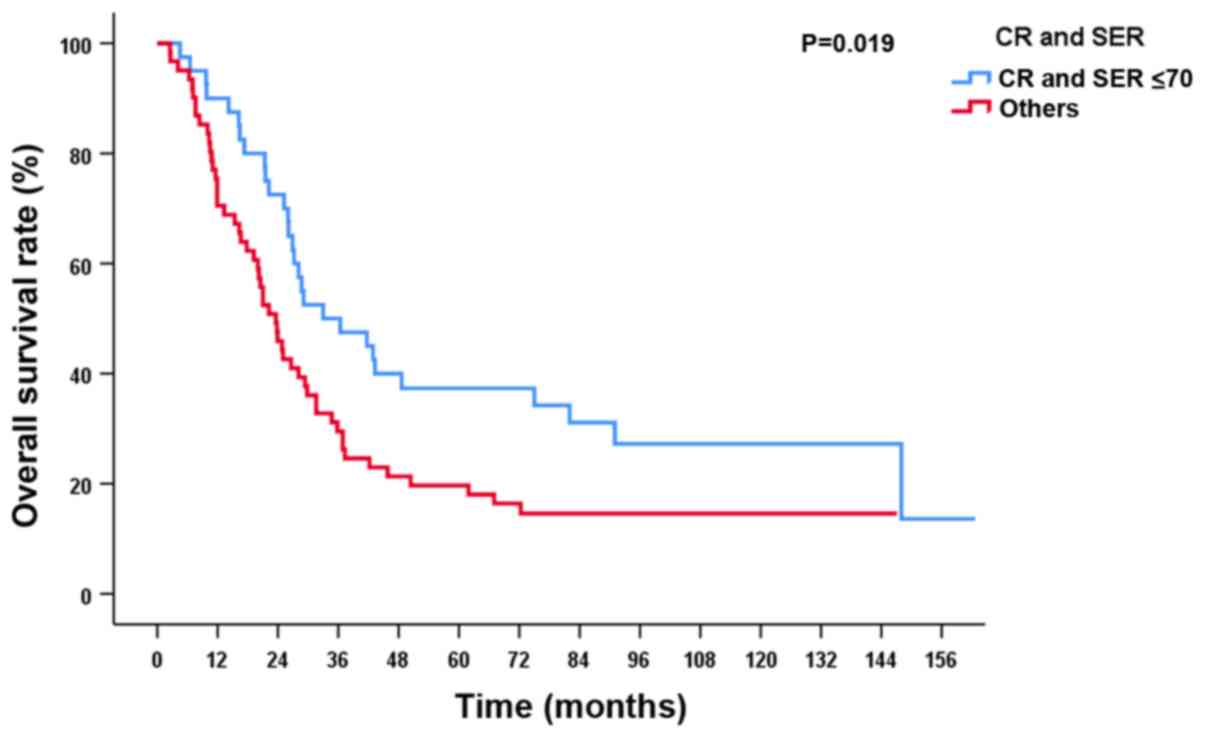
rate was 37.3% in patients who achieved CR with SER ≤70 days and
19.7% in the other patients (P=0.019; Fig. 3).
Discussion
Prolonged duration of treatment in cancer patients
can affect survival outcomes, possibly because of accelerated tumor
repopulation. SER significantly affects survival in patients with
LS-SCLC (19). Our data also
supported the clinical significance of treatment duration to the
end of TRT on survival outcomes. De Ruysscher et al
(19) reported that patients with
SER <30 days exhibited improved survival. In the present study,
we report a statistically significant difference at a cutoff of 70
days. The difference may be due to the heterogeneity of
fractionation and timing of TRT in our patient population. This
result reflects the importance of early combination of TRT with one
or two rounds of chemotherapy (20).
The probability of chemoresistance should be proportional to
elapsed treatment time; drug-resistant SCLC is not completely
cross-resistant to radiation (21).
Thus, it is a reasonable strategy to start TRT as early as possible
to prevent negative outcomes due to chemoresistance (22). However, in practice, because of the
presence of bulky tumors, radiation oncologists hesitate to proceed
with the combination of TRT and chemotherapy from the beginning,
and usually, TRT is deferred until after chemotherapy, when the
tumor regresses. Sun et al (23) reported that delaying TRT to the third
cycle of chemotherapy does not result in inferior treatment
outcomes compared with administering TRT with the first cycle of
chemotherapy (median PFS of 11.2 months for late TRT vs. 12.4
months for early TRT, and median OS of 26.8 months vs. 24.1 months,
respectively). In our study, median PFS and OS in patients with SER
≤70 days were 16.5 and 29.4 months, respectively. If TRT was
administered within the third cycle of chemotherapy, and if the
patients exhibited no side effects necessitating interruption,
treatment could be completed within 70 days. Therefore, it may be
reasonable to assume that the important predictive factor in
patients with LS-SCLC with respect to treatment duration is the
completion time of TRT (SER), in addition to initiation of TRT at
an early time point.
With respect to optimal dosing and fractionation
schedules of TRT, several controversies remain. Turrisi et
al (9) reported superior
survival in LS-SCLC patients with a twice-daily accelerated regimen
than in those administered a once-daily 45 Gy dose of TRT. Despite
superior survival outcomes, twice-daily TRT has not gained
widespread acceptance in routine clinical practice, predominantly
because of the high prevalence of clinically significant
esophagitis and the logistical problems involved. Thus, dose
escalation studies with once-daily TRT in 2 Gy fractions are
designed as experimental group. A pooled analysis of Cancer and
Leukemia Group B studies using a single 2 Gy fraction per day
revealed a median survival of 19.9 months, 2-year PFS rate of 26%,
and 5-year OS rate of 20% (8). It
appears that TRT at 70 Gy once daily results in similar outcomes as
TRT at 45 Gy BID. We obtained similar results, with 2-year OS and
PFS rates of 56.4 and 33.8%, respectively. A meta-analysis on the
dose-response relationship in LS-SCLC patients showed that
increased BEDs of TRT were correlated with prolonged survival and
highlighted the potential clinical benefits of radiation dose
escalation within the limited duration of TRT (13).
Patients with LS-SCLC who achieved CR after combined
chemoradiotherapy demonstrated a higher median OS than patients who
achieved PR (OS of 21.8 months vs. 14.9 months, respectively)
(24). We also observed a median
survival of 29.1 months in patients who achieved CR with concurrent
TRT, compared with 20.5 months reported in patients with non-CR
(data not shown). In a comparison of the underlying characteristics
between patients with CR and non-CR in our study, we observed a
significant difference in treatment duration and BED of TRT,
because we did not escalate the dose of TRT in certain patients
with a good response at administration of 50 Gy in 2 Gy fractions.
Currently, we routinely prescribe a dose of TRT of at least 60 Gy
in 2 Gy QD.
There were several limitations in our study. First,
the timing of TRT was not consistent. Approximately 62% of the
patients received early TRT; the administration of delayed TRT
could affect patient survival and the evaluation of prognostic
factors. Second, in our study, a higher dose of radiation was
administered to patients with slow tumor regression. This resulted
in poorer survival outcomes than in the lower-dose group and
potentially led to a selection bias in the survival analysis.
In conclusion, patients with stage IIIB LS-SCLC who
underwent CCRT demonstrated poorer survival outcomes than did those
with stage IIA or IIIA LS-SCLC. SER was an important factor
associated with the survival of patients who received 45 Gy TRT BID
or 48 Gy TRT SIB, and more than 50 Gy CCRT QD. The best survival
outcomes were expected in patients who exhibited CR with SER ≤70
days.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JUJ and WJ contributed to acquisition of data,
interpretation of data and drafting of the manuscript. SJA designed
and conceived this study, and participated in the analysis and
interpretation of data and drafting of the manuscript. JUJ, MSY and
SJA mainly contributed to administration of radiotherapy. JYS
mainly contributed to radiotherapy planning. YCK, IJO and CKP
mainly contributed to administration of chemotherapy. TKN, JYS,
WKC, MSY, YCK, IJO and CKP contributed to the interpretation of
data and critical revision of the manuscript for important
intellectual content. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This retrospective study was approved by the
Institutional Review Board of Chonnam National University Hwasun
Hospital (approval no. CNUHH-2018-181).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rich AL, Tata LJ, Stanley RA, Free CM,
Peake MD, Baldwin DR and Hubbard RB: Lung cancer in England:
Information from the National Lung Cancer Audit (LUCADA). Lung
Cancer. 72:16–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pignon JP, Arriagada R, Ihde DC, Johnson
DH, Perry MC, Souhami RL, Brodin O, Joss RA, Kies MS, Lebeau B, et
al: A meta-analysis of thoracic radiotherapy for small-cell lung
cancer. N Engl J Med. 327:1618–1624. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takada M, Fukuoka M, Kawahara M, Sugiura
T, Yokoyama A, Yokota S, Nishiwaki Y, Watanabe K, Noda K, Tamura T,
et al: Phase III study of concurrent versus sequential thoracic
radiotherapy in combination with cisplatin and etoposide for
limited-stage small-cell lung cancer: Results of the Japan Clinical
Oncology Group Study 9104. J Clin Oncol. 20:3054–3060. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huncharek M and McGarry R: A meta-analysis
of the timing of chest irradiation in the combined modality
treatment of limited-stage small cell lung cancer. Oncologist.
9:665–672. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fried DB, Morris DE, Poole C, Rosenman JG,
Halle JS, Detterbeck FC, Hensing TA and Socinski MA: Systematic
review evaluating the timing of thoracic radiation therapy in
combined modality therapy for limited-stage small-cell lung cancer.
J Clin Oncol. 22:4837–4845. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schild SE, Bonner JA, Shanahan TG, Brooks
BJ, Marks RS, Geyer SM, Hillman SL, Farr GH Jr, Tazelaar HD, Krook
JE, et al: Long-term results of a phase III trial comparing
once-daily radiotherapy with twice-daily radiotherapy in
limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys.
59:943–951. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bogart JA, Herndon JE II, Lyss AP, Watson
D, Miller AA, Lee ME, Turrisi AT and Green MR; Cancer and Leukemia
Group B study 39808, : 70 Gy thoracic radiotherapy is feasible
concurrent with chemotherapy for limited-stage small-cell lung
cancer: Analysis of Cancer and Leukemia Group B study 39808. Int J
Radiat Oncol Biol Phys. 59:460–468. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salama JK, Hodgson L, Pang H, Urbanic JJ,
Blackstock AW, Schild SE, Crawford J, Bogart JA and Vokes EE: A
pooled analysis of limited-stage small-cell lung cancer patients
treated with induction chemotherapy followed by concurrent
platinum-based chemotherapy and 70 Gy daily radiotherapy: CALGB
30904. J Thorac Oncol. 8:1043–1049. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Turrisi AT III, Kim K, Blum R, Sause WT,
Livingston RB, Komaki R, Wagner H, Aisner S and Johnson DH:
Twice-daily compared with once-daily thoracic radiotherapy in
limited small-cell lung cancer treated concurrently with cisplatin
and etoposide. N Engl J Med. 340:265–271. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Faivre-Finn C, Snee M, Ashcroft L, Appel
W, Barlesi F, Bhatnagar A, Bezjak A, Cardenal F, Fournel P, Harden
S, et al: Concurrent once-daily versus twice-daily
chemoradiotherapy in patients with limited-stage small-cell lung
cancer (CONVERT): An open-label, phase 3, randomised, superiority
trial. Lancet Oncol. 18:1116–1125. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Diwanji TP, Mohindra P, Vyfhuis M, Snider
JW III, Kalavagunta C, Mossahebi S, Yu J, Feigenberg S and Badiyan
SN: Advances in radiotherapy techniques and delivery for non-small
cell lung cancer: Benefits of intensity-modulated radiation
therapy, proton therapy, and stereotactic body radiation therapy.
Transl Lung Cancer Res. 6:131–147. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Ruysscher D, Lueza B, Le Péchoux C,
Johnson DH, O'Brien M, Murray N, Spiro S, Wang X, Takada M, Lebeau
B, et al: Impact of thoracic radiotherapy timing in limited-stage
small-cell lung cancer: Usefulness of the individual patient data
meta-analysis. Ann Oncol. 27:1818–1828. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu L, Zhang S, Xu X, Wang B, Wu K, Deng
Q, Xia B and Ma S: Increased biological effective dose of radiation
correlates with prolonged survival of patients with limited-stage
small cell lung cancer: A systematic review. PLoS One.
11:e01564942016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–2247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xia B, Chen GY, Cai XW, Zhao JD, Yang HJ,
Fan M, Zhao KL and Fu XL: The effect of bioequivalent radiation
dose on survival of patients with limited-stage small-cell lung
cancer. Radiat Oncol. 6:502011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bepler G, Jaques G, Neumann K, Aumuller G,
Gropp C and Havemann K: Establishment, growth properties, and
morphological characteristics of permanent human small cell lung
cancer cell lines. J Cancer Res Clin Oncol. 113:31–40. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Common Terminology Criteria for Adverse
Events (CTCAE) Version 4.0. [Cited 2017 Oct 22]. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5×11.pdf
|
|
19
|
De Ruysscher D, Pijls-Johannesma M,
Bentzen SM, Minken A, Wanders R, Lutgens L, Hochstenbag M, Boersma
L, Wouters B, Lammering G, et al: Time between the first day of
chemotherapy and the last day of chest radiation is the most
important predictor of survival in limited-disease small-cell lung
cancer. J Clin Oncol. 24:1057–1063. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Videtic GM: The role of radiation therapy
in small cell lung cancer. Curr Oncol Rep. 15:405–410. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Willers H, Azzoli CG, Santivasi WL and Xia
F: Basic mechanisms of therapeutic resistance to radiation and
chemotherapy in lung cancer. Cancer J. 19:200–207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Murray N, Coy P, Pater JL, Hodson I,
Arnold A, Zee BC, Payne D, Kostashuk EC, Evans WK, Dixon P, et al:
Importance of timing for thoracic irradiation in the combined
modality treatment of limited-stage small-cell lung cancer. The
National Cancer Institute of Canada Clinical Trials Group. J Clin
Oncol. 11:336–344. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun JM, Ahn YC, Choi EK, Ahn MJ, Ahn JS,
Lee SH, Lee DH, Pyo H, Song SY, Jung SH, et al: Phase III trial of
concurrent thoracic radiotherapy with either first- or third-cycle
chemotherapy for limited-disease small-cell lung cancer. Ann Oncol.
24:2088–2092. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Manapov F, Niyazi M, Gerum S,
Roengvoraphoj O, Eze C and Li M: Evaluation of the role of
remission status in a heterogeneous limited disease small-cell lung
cancer patient cohort treated with definitive chemoradiotherapy.
BMC Cancer. 16:2162016. View Article : Google Scholar : PubMed/NCBI
|