Introduction
Gastric cancer (GC) has been considered as a common
primary malignant tumor with the fourth-highest cancer-related
mortality rate worldwide in the last decade (1). In China in 2015, ≥60% of patients with
GC had advanced disease (2). In the
last decade, with the development of GC therapy, many cancer
markers such as programmed death-ligand 1 and human epidermal
growth factor receptor 2 have been assessed as candidate prognostic
factors and therapeutic targets for GC (3,4). The
5-year survival rate and quality of life of patients with GC has
improved markedly (5–7). However, patients in the advanced stage
still have a poor prognosis, and advanced GC poses a higher burden
for patients and society (8). Thus,
markers that accurately predict the prognosis of patients with GC
are needed.
RecQ helicases play a critical role in maintaining
genome stability, as well as DNA recombination, replication and
transcription (9–11). There are five RecQ helicases in human
cells: RecQ-like helicase 1 (RECQL1), Werner syndrome RecQ-like
helicase (WRN), Bloom syndrome RecQ-like helicase (BLM), RecQ-like
helicase 4 (RECQL4) and RecQ-like helicase 5 (RECQL5) (9,10,12)
Mutations in WRN, BLM and RECQL4 proteins can lead to genomic
instability and predisposition to cancers, including colorectal,
prostate and breast cancers (13–16).
Important roles of RECQL5 have been identified in DNA replication
and transcription, base excision repair and homologous
recombination (17,18). Lao et al (19) reported the abrogation of RECQL5
expression in colorectal cancer. Another study demonstrated that
RECQL5 acts as a tumor suppressor in osteosarcoma, and increased
expression of RECQL5 can inhibit the progression of osteosarcoma
(20). Conversely, other studies
showed that RECQL5 is overexpressed in breast cancer and bladder
carcinoma, and that depletion of RECQL5 can significantly reduce
the progression of cancer (21,22).
However, the roles of RECQL5 in GC remains unclear.
In the present study, expression of RECQL5 was
investigated by mining the publicly available Oncomine database,
combined with validation in samples from patients with GC and
normal adjacent tissues using immunohistochemistry. The
clinicopathological and prognostic significance of RECQL5 in
patients with GC was also evaluated.
Materials and methods
Bioinformatics prediction
The RECQL5 mRNA data from GC and normal gastric
tissues were extracted from the Oncomine online database
(https://www.oncomine.org). The filtered datasets
were analyzed separately. RECQL5 expression values between normal
gastric tissues and GC tissues were extracted and compared from the
Chen Gastric, DErrico Gastric and Cho Gastric datasets (23–25). The
Kaplan-Meier plotter online (http://kmplot.com/analysis/) was used to predict the
overall survival (OS) outcomes of patients with GC (26). This software contains a public
database of Affymetrix microarray data from 1,065 patients with GC
(ID, 211468_s_at). To analyze the prognostic value of RECQL5 in GC,
the samples in the database were divided into 2 groups: High and
low expression of RECQL5. The relationship between RECQL5
expression and survival data was analyzed using Kaplan-Meier
survival curves. The log rank P-value and hazard ratio (HR) with
95% confidence intervals (CIs) were calculated.
GC tissue specimens and
clinicopathological data
Informed consent was obtained from all individual
participants included in the study, and the specimens were
collected after approval from the Institute Research Medical Ethics
Committee of The Sixth Affiliated Hospital, Sun Yat-sen University
(Guangzhou, China). A total of 78 cancer specimens (age range,
38–76 years) were collected from patients with GC and matched with
adjacent normal gastric tissues. The distance between tumor and
normal tissues was >1 cm. The patients with GC underwent radical
surgery between January 2009 and August 2011 at the Sixth
Affiliated Hospital, Sun Yat-sen University (Guangzhou, China).
Immunohistochemistry
Paraffin embedded sections were used for
immunohistochemistry. The thickness of the slides was 4 µm.
Biotin-Streptavidin HRP Detection Systems (cat. no. SP-9001;
Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.) was used
to detect RECQL5 expression in GC samples. Staining was performed
according to an established protocol using a rabbit polyclonal
antibody against human RECQL5 (Sigma-Aldrich; Merck KGaA; cat no.
HPA029971) diluted in PBS (1:150). Slides were incubated at 4°C in
a moist chamber overnight with the primary antibody. Slides stained
with PBS instead of primary antibody were used as negative
controls. The visual immunoreactivity score (IRS) was calculated by
using the following formula: Staining intensity (SI) × percentage
of stained cells with that intensity. IRS values were used to
determine the expression level of RECQL5. The SI scores were as
follows: 0, negative; 1, weak; 2, moderate; and 3, strong. The
percentage of stained cells was calculated as the percentage of
positively-stained tumor cells in the field, and was expressed as
follows: 0, negative; 1, 0–25%; 2, 26–50; 3, 51–75%; and 4,
>76%. Based on the SI scores, the RECQL5 expression level was
classified as high (grades 4–12) or low (grades 0–3). Patients were
classified into 2 groups, RECQL5 high and low. The tissues were
independently scored by 2 pathologists who were blinded to the
origin of each tissue. For any discrepancy, the 2 pathologists
reassessed the slides together to reach an agreement.
Statistical analysis
SPSS version 22.0 (IBM Corp.) was used for
statistical analyses. Ordinary one-way ANOVA was used to analyze
the expression difference of RECQL5 from the Oncomine database. The
association between clinicopathological features and RECQL5 protein
expression was assessed using a χ2 test. The survival
rate was assessed using Kaplan-Meier curves and the log-rank test.
Cox proportional hazards regression model was applied for
multivariate analysis to determine independent prognostic factors
of GC. P<0.05 was considered to indicate a statistically
significant difference.
Results
RECQL5 mRNA and protein expression is
low in patients with GC
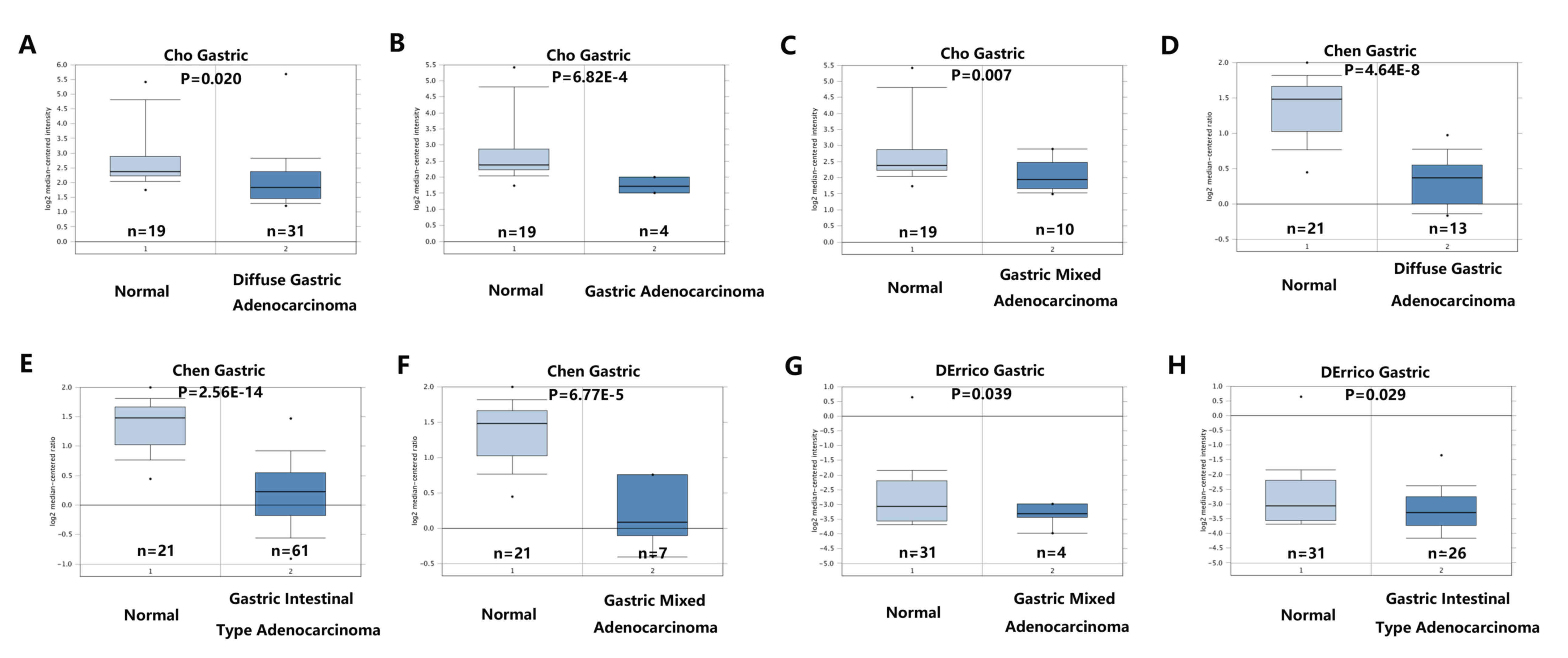
Oncomine database analysis demonstrated that RECQL5
mRNA was downregulated in GC tissues compared with normal gastric
tissues. The Cho Gastric dataset indicated that expression of
RECQL5 was downregulated in diffuse gastric adenocarcinoma (n=31;
P=0.020), gastric adenocarcinoma (n=4; P=6.82×10−4), and
gastric mixed adenocarcinoma (n=10; P=0.007) compared with that in
normal gastric tissues (n=19; Fig.
1A-C). The Chen Gastric dataset revealed low expression of
RECQL5 in diffuse gastric adenocarcinoma (n=12;
P=4.64×10−8), gastric intestinal type adenocarcinoma
(n=63; P=2.56×10−4), gastric mixed adenocarcinoma (n=8;
P=6.77×10−5) and compared with normal gastric tissues
(n=26; Fig. 1D-F). The DErrico
Gastric dataset revealed that RECQL5 was downregulated in gastric
mixed adenocarcinoma (n=4; P=0.039), gastric intestinal type
adenocarcinoma (n=26; P=0.029) compared with normal gastric tissues
(n=31; Fig. 1G and H).
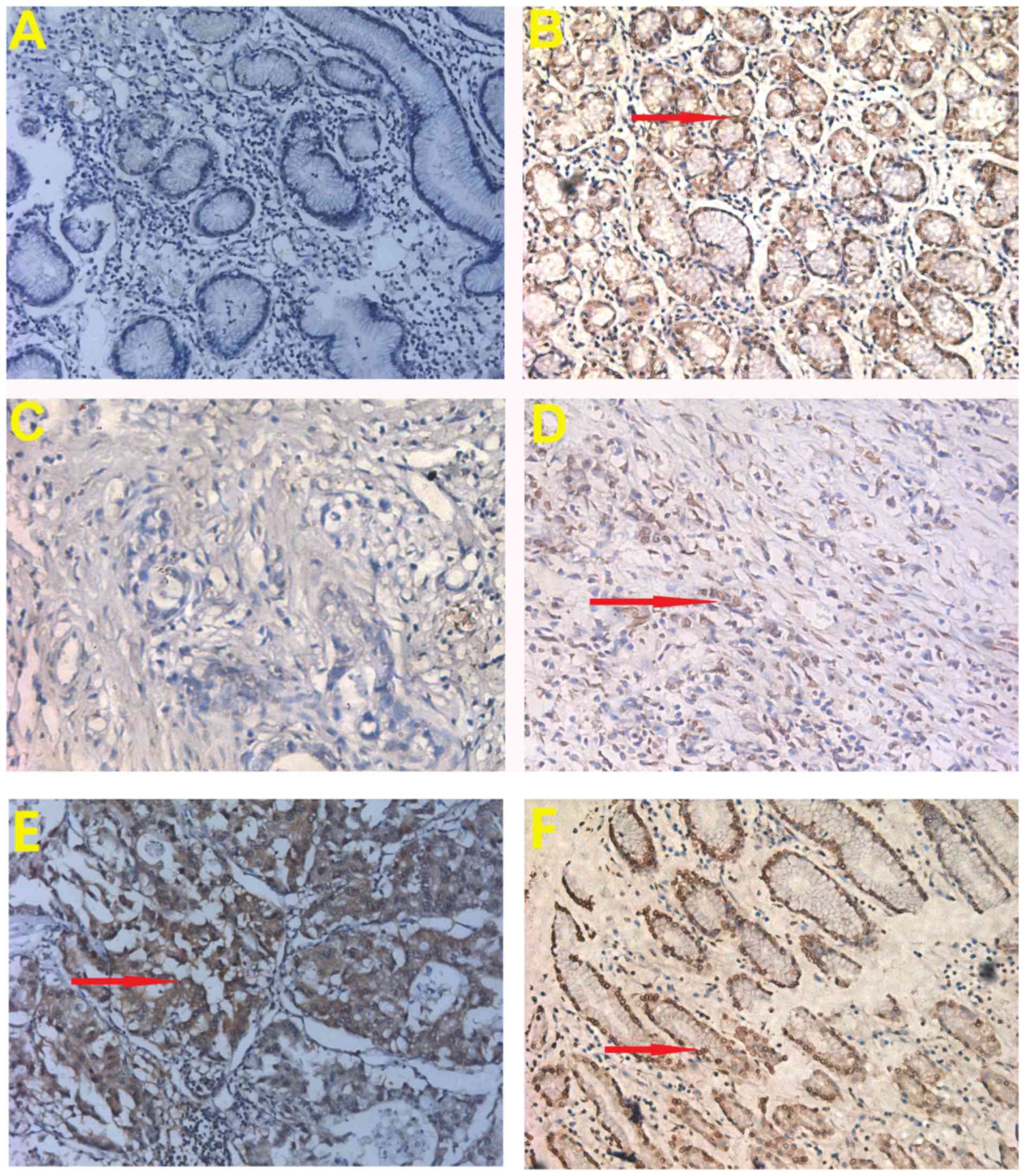
Immunohistochemistry was used to verify RECQL5
protein expression in GC and normal tissues. Expression of RECQL5
was found in GC tissues (Fig. 2D and
E). Overall, 71.8% (56/78 samples) of patients with GC
displayed low RECQL5 expression in GC samples, while 28.2% (22/78)
displayed high RECQL5 expression. In the matched normal gastric
tissues, 32.1% (25/78) of the patients displayed low RECQL5
expression and 67.9% (53/78) of the patients had high RECQL5
expression. The results indicated that RECQL5 expression was
downregulated in GC tissues compared with normal gastric tissues
(P<0.05; Table I), consistent
with the results of RECQL5 mRNA expression from the Oncomine
database.
 | Table I.Expression of RECQL5 in normal gastric
mucosa and primary gastric cancer tissues. |
Table I.
Expression of RECQL5 in normal gastric
mucosa and primary gastric cancer tissues.
|
|
| Expression of
RECQL5 |
|
|---|
|
|
|
|
|
|---|
| Samples | Patients, n | Low, n (%) | High, n (%) | P-value |
|---|
| Gastric cancer | 78 | 56 (71.8) | 22 (28.2) | <0.001 |
| Normal gastric
tissue | 78 | 25 (32.1) | 53 (67.9) |
|
Association of RECQL5 differential
expression and clinicopathological parameters of patients with
GC
Low expression of RECQL5 was associated with depth
of tumor invasion, histological differentiation and TNM stage
(P<0.05), but not with patient age or sex, tumor size, lymph
node metastasis, venous or lymphatic invasion or distant metastasis
(P>0.05; Table II).
 | Table II.Association between RECQL5 expression
and clinicopathological features of patients with gastric
carcinoma. |
Table II.
Association between RECQL5 expression
and clinicopathological features of patients with gastric
carcinoma.
|
|
| RECQL5 protein
expression |
|
|---|
|
|
|
|
|
|---|
| Characteristics | Patients, n | Low, n (%) | High, n (%) | P-value |
|---|
| Sex |
|
|
| 0.203 |
| Male | 48 | 32 (66.7) | 16 (32.3) |
|
|
Female | 30 | 24 (80.0) | 6 (20.0) |
|
| Age, years |
|
|
| 0.240 |
|
≥60 | 45 | 30 (66.7) | 15 (33.3) |
|
|
<60 | 33 | 26 (78.8) | 7 (21.2) |
|
| Tumor size, cm |
|
|
| 0.094 |
| ≥5 | 31 | 19 (61.3) | 12 (38.7) |
|
|
<5 | 47 | 37 (78.7) | 10 (21.3) |
|
| Histological
differentiation |
|
|
| 0.002 |
| Well,
moderate | 29 | 15 (51.7) | 14 (48.3) |
|
| Poorly,
others | 49 | 41 (83.7) | 8 (16.3) |
|
| Depth of tumor
invasion |
|
|
| 0.019 |
|
T1-T2 | 30 | 17 (56.7) | 13 (43.3) |
|
|
T3-T4 | 48 | 39 (81.3) | 9 (18.8) |
|
| Lymphatic
invasion |
|
|
| 0.957 |
|
Yes | 28 | 20 (60.7) | 8 (39.3) |
|
| No | 50 | 36 (78.0) | 14 (22.0) |
|
| Vascular
invasion |
|
|
| 0.881 |
|
Yes | 30 | 22 (73.3) | 8 (26.7) |
|
| No | 48 | 34 (70.8) | 14 (29.2) |
|
| Lymph node
metastases |
|
|
| 0.060 |
| N0 | 33 | 20 (60.6) | 13 (39.4) |
|
| N1,
N2 | 45 | 36 (80.0) | 9 (20.0) |
|
| Distant
metastasis |
|
|
| 0.571 |
| M0 | 53 | 37 (69.8) | 16 (30.2) |
|
| M1 | 25 | 19 (76.0) | 6 (24.0) |
|
| TNM stage |
|
|
| 0.002 |
|
I–II | 32 | 17 (53.1) | 15 (46.9) |
|
|
III–IV | 46 | 39 (84.8) | 7 (15.2) |
|
Low expression of RECQL5 predicts poor
prognosis in patients with GC
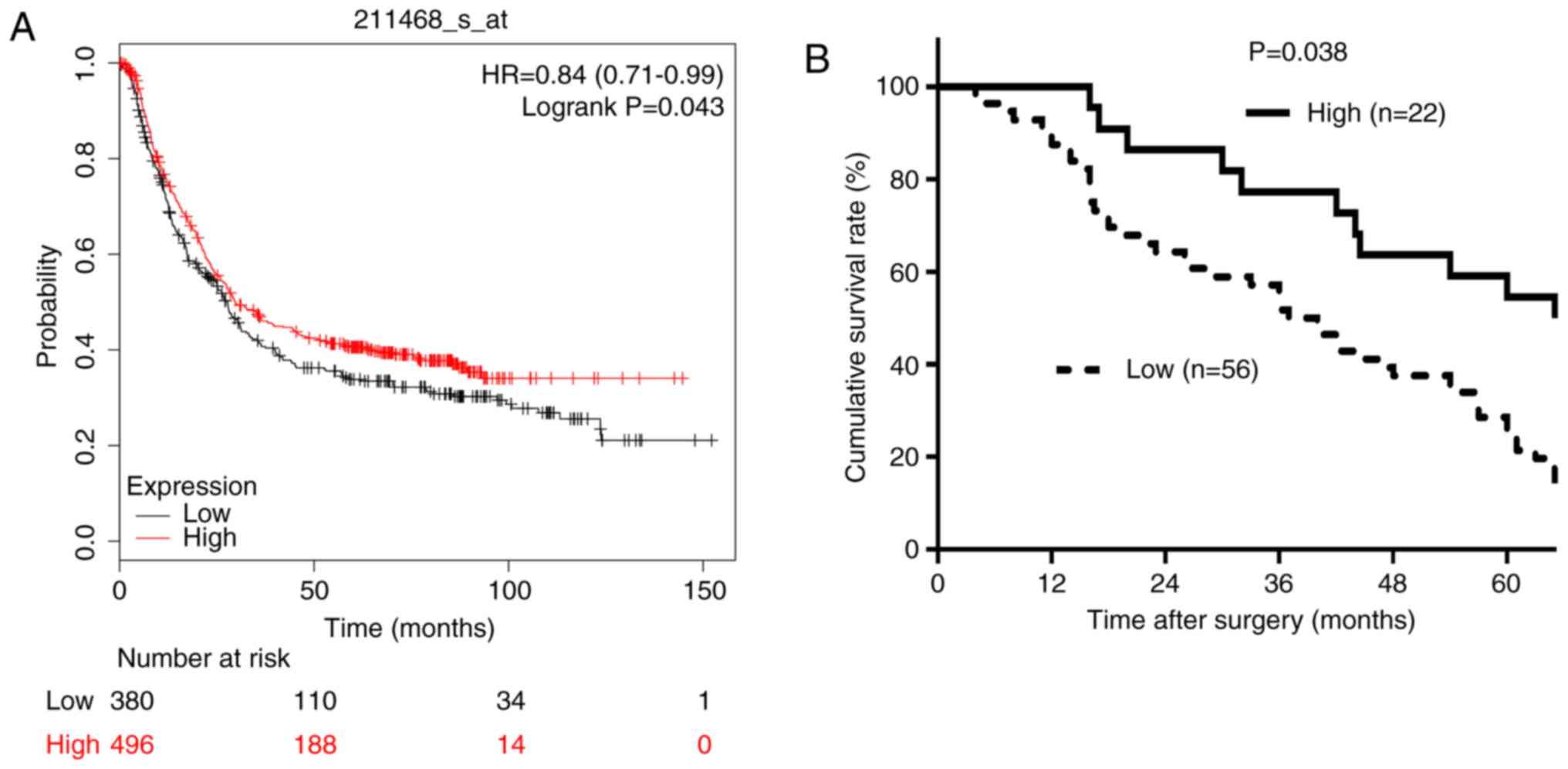
The association between RECQL5 mRNA expression
levels and OS time in patients with GC was investigated using the
Kaplan-Meier plotter software. Patients with a low expression of
RECQL5 had a shorter OS time (HR, 0.84; 95% CI,0.71–0.99; P=0.043;
Fig. 3A). The prognostic value of
RECQL5 expression in GC was confirmed using the prognosis data of
patients with GC from the Sixth Affiliated Hospital, Sun Yat-sen
University (Guangzhou, China). The follow-up time ranged between 4
months and 9.5 years. The 5-year OS rate was 48.7% (38/78
patients). The 5-year OS rate of the RECQL5-low and RECQL5-high
groups was 63.6% (14/22) and 42.9% (24/56), respectively. Patients
in the low RECQL5 expression group had a significantly shorter OS
time (P=0.038; Fig. 3B). The
prognosis data was consistent with results from the Kaplan-Meier
analysis. Multivariate analysis indicated that the independent
prognostic factors were low expression of RECQL5 and depth of
invasion (P<0.05; Table
III).
 | Table III.Multivariate Cox regression analysis
in patients with gastric cancer. |
Table III.
Multivariate Cox regression analysis
in patients with gastric cancer.
| Parameter | P-value | Hazard ratio | 95% CI |
|---|
| RECQL5, low vs.
high | 0.002 | 2.922 | 1.504–5.679 |
| Histological
differentiation, well, moderate vs. poorly, others | 0.326 | 1.533 | 0.653–3.598 |
| Depth of tumor
invasion, T1-T2 vs. T3-T4 | 0.019 | 0.463 | 0.243–0.880 |
| TNM stage, I–II vs.
III–IV | 0.544 | 0.772 | 0.336–1.778 |
Discussion
Defects of WRN, BLM and RECQL4 may increase cancer
predisposition in humans (10).
However, whether RECQL5 is associated with cancer predisposition
syndrome is unclear. Previous studies have shown that RECQL5 is an
essential factor for maintenance of genomic stability, and that
RECQL5 may act as an oncogene in various types of cancer (20–22,27). Hu
et al (28,29) reported that RECQL5 regulates
homologous recombination in mouse embryonic stem cells and
downregulates the expression of RECQL5 in mice, which can increase
susceptibility to colon carcinoma. Lao et al (19) demonstrated that loss of RECQL5
expression contributes to the pathogenesis of colorectal cancer.
RECQL5 expression is also downregulated in osteosarcoma, and can
inhibit proliferation and promote apoptosis of osteosarcoma cells
(20). Conversely, a tumor-promoting
function of RECQL5 was reported by several studies. Arora et
al (21) reported the
upregulation of RECQL5 in breast cancer due to gene amplification
and described a critical role for RECQL5 in cancer progression and
demonstrated that small interfering RNA-mediated knockdown of
RECQL5 can significantly inhibit in vivo tumorigenicity and
in vitro clonogenic survival of breast cancer cells
(30). Patterson et al
(22) identified a positive
association between upregulated expression of RECQL5 with invasion
of human urothelial bladder carcinoma. However, RECQL5 in patients
with GC has not been fully investigated in previous studies.
In the present study, RECQL5 expression at the mRNA
and proteins levels was significantly lower in GC tissues compared
with normal gastric tissues. RECQL5 was expressed in 28.2% of GC
samples and 67.9% of matched normal gastric tissues. RECQL5 was
localized mainly in the nucleus, similar to other studies (19,20). In
addition, the low expression of RECQL5 protein was associated with
poor histological differentiation, deep tumor invasion and high
tumor stage, indicating a prognostic role for RECQL5 in preventing
GC progression. Furthermore, patients with high expression of
RECQL5 had a higher 5-year OS rate compared with patients with low
expression. Thus, low expression of RECQL5 might be a potential
prognostic factor in GC. This was verified by multivariate
analysis, which indicated that low expression of RECQL5 is an
independent marker of poor prognosis, strengthening the hypothesis
that RECQL5 may play an important role in preventing the
progression of GC. The collective results of this study indicate
that low expression of RECQL5 may be a predictor of poor prognosis
in patients with GC.
RECQL5 is essential for maintaining genome stability
and reducing cancer risk (28).
RECQL5 has a tumor-suppressive role in the mouse gastrointestinal
tract (29). The results of the
present study indicate that the RECQL5 gene may be a candidate
tumor suppressor gene in the stomach, and that high expression of
RECQL5 may limit tumor growth. The present study also demonstrated
that RECQL5 expression was high in normal gastric tissues, which
may indicate that RECQL5 plays a role in maintaining genome
stability and reducing cancer risk in the stomach. Moreover, low
expression of RECQL5 may be a predictor of poor prognosis in
patients with GC, which is consistent with previous reports
(29,30).
The present study has several limitations. This is a
preliminary small-scale bioinformatics and clinical study. As the
patient and normal samples were collected non-sequentially from a
single center, a selection bias may exist in the study, which may
have influenced the findings. Thus, further large-scale studies are
required to validate the findings of the present study. In
addition, in this study, only the expression of RECQL5 was
investigated, and therefore, detailed studies to understand the
molecular mechanisms of RECQL5 in GC are required. In conclusion,
downregulation of RECQL5 was observed in GC samples. Low expression
of RECQL5 was indicative of a more aggressive disease and might be
an independent factor of poor prognosis in patients with GC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Key
Clinical Discipline, Guangdong Province Science and Technology Plan
Project (grant no. 2017A010105004) and the Guangzhou Science and
Technology Project (grant no. 201803010040).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL, HC and JP designed the study. YL and HW analyzed
and interpreted the patients' data and were the major contributors
in writing the manuscript. HW analyzed and interpreted the patient
data. XW and ML performed the histological experiments and were
major contributors in writing the manuscript. All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The work was approved by the Institute Research
Medical Ethics Committee of The Sixth Affiliated Hospital, Sun
Yat-sen University (Guangzhou, China). This study has been
performed in accordance with the Declaration of Helsinki. Informed
consent was obtained from all individual participants included in
the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Balakrishnan M, George R, Sharma A and
Graham DY: Changing trends in stomach cancer throughout the world.
Curr Gastroenterol Rep. 19:362017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kankeu Fonkoua L and Yee NS: Molecular
characterization of gastric carcinoma: Therapeutic implications for
biomarkers and targets. Biomedicines. 6(pii): E322018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roberto M, Romiti A, Onesti CE, Zullo A,
Falcone R and Marchetti P: Evolving treatments for advanced gastric
cancer: Appraisal of the survival trend. Expert Rev Anticancer
Ther. 16:717–729. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Imano M and Okuno K: Treatment strategies
for gastric cancer patients with peritoneal metastasis. Surg Today.
44:399–404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yazici O, Sendur MA, Ozdemir N and Aksoy
S: Targeted therapies in gastric cancer and future perspectives.
World J Gastroenterol. 22:471–489. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Charalampakis N, Economopoulou P,
Kotsantis I, Tolia M, Schizas D, Liakakos T, Elimova E, Ajani JA
and Psyrri A: Medical management of gastric cancer: A 2017 update.
Cancer Med. 7:123–133. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arnold M, Moore SP, Hassler S,
Ellison-Loschmann L, Forman D and Bray F: The burden of stomach
cancer in indigenous populations: A systematic review and global
assessment. Gut. 63:64–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bernstein KA, Gangloff S and Rothstein R:
The RecQ DNA helicases in DNA repair. Annu Rev Genet. 44:393–417.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Monnat RJ Jr: Human RECQ helicases: Roles
in DNA metabolism, mutagenesis and cancer biology. Semin Cancer
Biol. 20:329–339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rezazadeh S: RecQ helicases; at the
crossroad of genome replication, repair, and recombination. Mol
Biol Rep. 39:4527–4543. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ouyang KJ, Woo LL and Ellis NA: Homologous
recombination and maintenance of genome integrity: Cancer and aging
through the prism of human RecQ helicases. Mech Ageing Dev.
129:425–440. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qian X, Feng S, Xie D, Feng D, Jiang Y and
Zhang X: RecQ helicase BLM regulates prostate cancer cell
proliferation and apoptosis. Oncol Lett. 14:4206–4212. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chun SG, Shaeffer DS and Bryant-Greenwood
PK: The Werner's Syndrome RecQ helicase/exonuclease at the nexus of
cancer and aging. Hawaii Med J. 70:52–55. 2011.PubMed/NCBI
|
|
15
|
Frank B, Hoffmeister M, Klopp N, Illig T,
Chang-Claude J and Brenner H: Colorectal cancer and polymorphisms
in DNA repair genes WRN, RMI1 and BLM. Carcinogenesis. 31:442–445.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu X, Chen H, Yang Y, Xu C, Zhou J, Zhou
J and Chen Y: Distinct prognosis of mRNA expression of the five
RecQ DNA-helicase family members-RECQL, BLM, WRN, RECQL4, and
RECQL5-in patients with breast cancer. Cancer Manag Res.
10:6649–6668. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schwendener S, Raynard S, Paliwal S, Cheng
A, Kanagaraj R, Shevelev I, Stark JM, Sung P and Janscak P:
Physical interaction of RECQ5 helicase with RAD51 facilitates its
anti-recombinase activity. J Biol Chem. 285:15739–15745. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aygün O and Svejstrup JQ: RECQL5 helicase:
Connections to DNA recombination and RNA polymerase II
transcription. DNA Repair (Amst). 9:345–353. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lao VV, Welcsh P, Luo Y, Carter KT,
Dzieciatkowski S, Dintzis S, Meza J, Sarvetnick NE, Monnat RJ Jr,
Loeb LA and Grady WM: Altered RECQ helicase expression in sporadic
primary colorectal cancers. Transl Oncol. 6:458–469. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu J, Zhi L, Dai X, Cai Q and Ma W:
Decreased RECQL5 correlated with disease progression of
osteosarcoma. Biochem Biophys Res Commun. 467:617–622. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arora A, Abdel-Fatah TM, Agarwal D,
Doherty R, Croteau DL, Moseley PM, Hameed K, Green A, Aleskandarany
MA, Rakha EA, et al: Clinicopathological and prognostic
significance of RECQL5 helicase expression in breast cancers.
Carcinogenesis. 37:63–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Patterson K, Arya L, Bottomley S, Morgan
S, Cox A, Catto J and Bryant HE: Altered RECQL5 expression in
urothelial bladder carcinoma increases cellular proliferation and
makes RECQL5 helicase activity a novel target for chemotherapy.
Oncotarget. 7:76140–76150. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen X, Leung SY, Yuen ST, Chu KM, Ji J,
Li R, Chan AS, Law S, Troyanskaya OG, Wong J, et al: Variation in
gene expression patterns in human gastric cancers. Mol Biol Cell.
14:3208–3215. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
D'Errico M, de Rinaldis E, Blasi MF, Viti
V, Falchetti M, Calcagnile A, Sera F, Saieva C, Ottini L, Palli D,
et al: Genome-wide expression profile of sporadic gastric cancers
with microsatellite instability. Eur J Cancer. 45:461–469. 2009.
View Article : Google Scholar
|
|
25
|
Cho JY, Lim JY, Cheong JH, Park YY, Yoon
SL, Kim SM, Kim SB, Kim H, Hong SW, Park YN, et al: Gene expression
signature-based prognostic risk score in gastric cancer. Clin
Cancer Res. 17:1850–1857. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Szász AM, Lánczky A, Nagy Á, Förster S,
Hark K, Green JE, Boussioutas A, Busuttil R, Szabó A and Győrffy B:
Cross-validation of survival associated biomarkers in gastric
cancer using transcriptomic data of 1,065 patients. Oncotarget.
7:49322–49333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dong YZ, Huang YX and Lu T: Single
nucleotide polymorphism in the RECQL5 gene increased osteosarcoma
susceptibility in a Chinese Han population. Genet Mol Res.
14:1899–1902. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu Y, Raynard S, Sehorn MG, Lu X, Bussen
W, Zheng L, Stark JM, Barnes EL, Chi P, Janscak P, et al:
RECQL5/Recql5 helicase regulates homologous recombination and
suppresses tumor formation via disruption of Rad51 presynaptic
filaments. Genes Dev. 21:3073–3084. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu Y, Lu X and Luo G: Effect of Recql5
deficiency on the intestinal tumor susceptibility of Apc(min) mice.
World J Gastroenterol. 16:1482–1486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He YJ, Qiao ZY, Gao B, Zhang XH and Wen
YY: Association between RECQL5 genetic polymorphisms and
susceptibility to breast cancer. Tumour Biol. 35:12201–12204. 2014.
View Article : Google Scholar : PubMed/NCBI
|