Introduction
Radiotherapy is a significant adjuvant therapy for
breast cancer. Post-mastectomy radiation therapy (PMRT) is always
recommended for patients at high risk of recurrence, including
those with ≥4 positive axial lymph nodes (ALNs) or a tumor >5
cm, independent of the nodal status and resection margins (1). Adjuvant PMRT has been shown to be
extremely useful at improving the survival of high-risk patients,
however the benefits and demerits of radiotherapy for breast cancer
patients have not been established (2).
In 2014, the Early Breast Cancer Trialists'
Collaborate Group published a study on the value of PMRT for breast
cancer patients (3). The results of
that systematic review and meta-analysis of 22 trials demonstrated
that PMRT significantly reduced not only the local recurrence rate,
but also the breast cancer mortality rate in patients with 1–3
positive ALNs (3). The 2015 European
Society for Medical Oncology guidelines recommend PMRT for
high-risk patients and also suggest the routine use of PMRT for
patients with 1–3 positive ALNs (4).
However, the primary limitation of relevant studies is that they
were not randomized control studies. Whether patients with
Tumor-Node-Metastasis (TNM) stage of T1-2N1M0 require PMRT remains
controversial (5,6). T1-2N1M0 refers to: T1-2, maximum tumor
diameter ≤50 mm; N1, micrometastasis (maximum diameter >0.2 mm,
or >200 tumor cells in a single lymph node tissue section, but
the maximum diameter ≤2 mm), 1–3 axillary lymph node metastasis, at
least 1 metastatic lesion >2 mm and transfer (including micro
transfer); M0, no distant metastasis (7).
In brief, selection of breast cancer patients for
PMRT is based on established clinical pathology parameters
including the size of the mass and lymph node (LN) status, factors
which contribute to the baseline risk of local recurrence (8). Nevertheless, a growing body of data has
demonstrated the importance of molecular subtypes in treating
patients with breast cancer and predicting their prognoses
(9).
Breast cancer has been demonstrated to be a
heterogeneous group of diseases (10). Perou et al (11) first discovered the intrinsic subtypes
of breast cancer using bioinformatics analysis of gene expression
profiling data. The different molecular subtypes of breast cancer
have distinct outcomes, and therefore, breast cancer subtypes have
been widely used clinically to select adjuvant systemic therapies
and predict patient prognosis (12).
Comprehensive treatment strategies for breast cancer are based on
molecular subtypes, but do not take the individualization of
radiotherapy into account (13).
There is lack of evidence for making firm recommendations about
PMRT in the various breast cancer subgroups. The precise
relationship between the intrinsic sensitivity of radiotherapy and
the molecular subtypes is not yet known and the mechanisms
underlying the different responses of the subtypes have not been
elucidated.
As the concept and techniques of genotyping continue
to develop, molecular typing has become a standardized treatment
for the guidance of chemotherapy and endocrine therapy for patients
with breast cancer (14,15). These advances raise the question of
whether molecular subtypes can be used to predict the response to
PMRT and the prognosis. The present study was conducted to assess
the effects of PMRT administered to patients with T1-2N1M0 breast
cancer and to evaluate the treatment-predictive effect of breast
cancer molecular subtypes among patients in the Surveillance,
Epidemiology, and End Results (SEER) registry who underwent
PMRT.
Patients and methods
Patient selection
The SEER registry of the National Cancer Institute
(USA) is a comprehensive source of information about the occurrence
of all new cancer cases among people residing in areas that take
part in the SEER program (https://seer.cancer.gov/). Of the 181,878 patients
with a pathology-based diagnosis of breast cancer between 2010 and
2013, this study restricted analysis to females with a diagnosis of
a single primary and malignant breast neoplasm. The median
follow-up time was 34 months (range, 0–59 months). Among these,
2,760 patients were treated with radiotherapy (36.97%; PMRT group).
The other 4,706 patients (63.03%) were treated without radiotherapy
and were classified as no-PMRT group. As the SEER registry began
tracking information regarding HER2/neu status in 2010, this date
was used as the earliest period for this study. Inclusion criteria
for this study were as follows: i) Diagnosis confirmed by
histology; ii) female patients with unilateral breast lesions; iii)
mastectomy was performed (surgery of primary site variable values
of 50–74); and iv) patients were diagnosed with breast cancer
defined as T1-2N1M0 stage, according to the 7th American Joint
Committee on Cancer (AJCC) cancer staging manual (7).
The following cases were excluded: i) Patients
diagnosed based on an autopsy or death certificate; ii) patients
whose PMRT was uncertain; iii) patients who did not undergo a
mastectomy; iv) patients with an unknown molecular subtype, unknown
age at diagnosis, unknown year of diagnosis, unknown laterality or
unknown survival months; and v) patients who received preoperative
systemic therapy (radiotherapy and/or chemotherapy). After these
exclusions, a total of 7,466 patients were included in the present
study for analysis.
Table I represents
the demographic variables of the patients selected: Ethnicity
(white, black, others); age at diagnosis (<55, ≥55 years); year
of diagnosis (2010–2013); and marital status (married, unmarried
but domestic partner, unmarried, separated, widowed, divorced,
unknown). The cancer characteristics included the following:
Laterality (left, right); AJCC T-stage (T1, T2); number of positive
LNs (1, 2 or 3); histological type (code 8500/3, infiltrating duct
carcinoma; code 8520/3, lobular carcinoma; code 8522/3,
infiltrating duct and lobular carcinoma; others); histological
grade (well differentiated, moderately differentiated, poorly
differentiated, undifferentiated, unknown); hormone receptor (HR)
status (positive, negative); and human epidermal growth factor
receptor 2 (HER2) status (positive, negative) (Table I).
 | Table I.Clinicopathological characteristics
of the patients in PMRT group and no-PMRT group. |
Table I.
Clinicopathological characteristics
of the patients in PMRT group and no-PMRT group.
|
Characteristics | Cases, n (%) | PMRT, n (%) | No PMRT, n (%) | P-value |
|---|
| Total | 7,466 (100) | 2,760 (37) | 4,706 (63) |
|
| Ethnicity |
|
|
| 0.500 |
|
White | 5,699 (76) | 2,095 (37) | 3,604 (63) |
|
|
Black | 973 (13) | 376 (39) | 597 (61) |
|
|
Others | 794 (11) | 289 (36) | 505 (64) |
|
| Age at diagnosis,
years |
|
|
| <0.001 |
|
<55 | 2,626 (35) | 1,231 (47) | 1,395 (53) |
|
|
≥55 | 4,840 (65) | 1,529 (32) | 3,311 (68) |
|
| Year of
diagnosis |
|
|
| 0.002 |
|
2010 | 2,032 (27) | 685 (34) | 1,347 (66) |
|
|
2011 | 1,931 (26) | 760 (39) | 1,171 (61) |
|
|
2012 | 1,834 (25) | 699 (38) | 1,135 (62) |
|
|
2013 | 1,669 (22) | 616 (37) | 1,053 (63) |
|
| Marital status |
|
|
| <0.001 |
|
Married/unmarried or domestic
partner | 4,152 (56) | 1,631(39) | 2,521 (61) |
|
| Never
married | 1,153 (15) | 442 (38) | 711 (62) |
|
|
Unmarried/separated/widowed | 1,783 (24) | 552 (31) | 1,135 (69) |
|
|
Unknown | 378 (5) | 135 (36) | 1,053 (64) |
|
| Laterality |
|
|
| 0.353 |
|
Left | 3,807 (51) | 1,388 (36) | 2,419 (64) |
|
|
Right | 3,659 (49) | 1,372 (38) | 2,287 (62) |
|
| T stage |
|
|
| <0.001 |
| T1 | 2,791 (37) | 867 (31) | 1,924 (69) |
|
| T2 | 4,675 (63) | 1,893 (40) | 2,782 (60) |
|
| Positive lymph
node, n |
|
|
| <0.001 |
| 1 | 3,922 (53) | 1,189 (30) | 2,733 (70) |
|
| 2 | 2,264 (30) | 922 (41) | 1,342 (59) |
|
| 3 | 1,280 (17) | 649 (51) | 631 (49) |
|
| Histological
type |
|
|
| 0.150 |
|
IDC | 5,956 (80) | 2,239 (38) | 3,717 (62) |
|
|
ILC | 564 (7) | 200 (35) | 364 (65) |
|
|
IDC+ILC | 434 (6) | 146 (34) | 288 (66) |
|
|
Others | 512 (7) | 175 (34) | 337 (66) |
|
| Histological
grade |
|
|
| <0.001 |
| I | 828 (11) | 231 (28) | 597 (72) |
|
| II | 3,198 (43) | 1,111 (35) | 2,087 (65) |
|
|
III | 3,233 (43) | 1,337 (41) | 1,896 (59) |
|
|
Unknown | 207 (3) | 81 (39) | 126 (61) |
|
| HR status |
|
|
| <0.001 |
|
HR+ | 6,138 (82) | 2,204 (36) | 3,934 (64) |
|
|
HR− | 1,328 (18) | 556 (42) | 772 (58) |
|
| HER2 status |
|
|
| 0.035 |
|
HER2+ | 1,477 (20) | 581 (39) | 896 (61) |
|
|
HER2− | 5,989 (80) | 2,179 (36) | 3,810 (64) |
|
| Subtype |
|
|
| <0.001 |
|
HR+/HER2− | 5,102 (68) | 1,781 (35) | 3,321 (65) |
|
|
HR−/HER2+ | 441 (6) | 158 (36) | 283 (64) |
|
|
HR+/HER2+ | 1,036 (14) | 423 (41) | 613 (59) |
|
|
HR−/HER2− | 887 (12) | 398 (45) | 489 (55) |
|
| Chemotherapy |
|
|
| <0.001 |
|
Yes | 5,318 (71) | 2,462 (46) | 2,856 (54) |
|
|
No/unknown | 2,148 (29) | 298 (14) | 1,850 (86) |
|
The treatment characteristics of the patients were
chemotherapy (yes, no/unknown) and radiotherapy (PMRT group,
no-PMRT group). The tumor molecular subtypes were classified as 4
mutually exclusive categories: HR+/HER2−,
HR−/HER2+, HR+/HER2+
and HR−/HER2− [defined as triple-negative
breast cancer (TNBC)]. HR+ was defined as estrogen
receptor (ER)+, progesterone receptor (PR)+
or borderline positive (those that could not be defined as
ER+ or PR+). In contrast, HR− was
defined as ER− and PR−. Individuals who had a
borderline HER2 status were grouped in another category ‘unknown
HER2 status’ (16).
Statistical analysis
The baseline characteristics of the patients were
assessed using a Pearsons χ2−test and the aforementioned
factors were compared between the PMRT group and the no-PMRT group.
The breast cancer-specific survival (BCSS) was extracted from the
SEER database. Kaplan-Meier survival curves were generated and the
log-rank test was used to identify significant differences between
the curves. The prognostic value of PMRT was analyzed by Cox
univariate and multivariate regression analyses. Due to the
statistical non-significance of the diagnosis year in the
univariate regression analysis, this factor was excluded from the
multivariate regression analysis. The HR and HER2 statuses of the
patients were excluded to avoid a repetition in the analysis. Tests
of interaction were used in the Cox multivariate regression
analysis. Hazard ratios and 95% confidence intervals (95% CIs) were
calculated.
To adjust for potential confounding factors in
patients with TNBC, individual propensity score matching (PSM) was
performed, in which randomly selected individuals in the PMRT group
were paired with comparable individuals in the no-PMRT group. The
confounding factors were ethnicity, age, year of diagnosis, marital
status, laterality, T stage, positive LN status, histological type,
histological grade and chemotherapy. All data analyses were
performed using SPSS version 22 (IBM Corp.). All the statistics
tests performed were double-sided, and P<0.05 was considered to
indicate a statistically significant difference.
Outcome measurement
The main endpoint of this study was 5-year BCSS. The
patients were recorded as alive or dead in the SEER database, and
the option of ‘completed months of follow-up’ contained the
patients survival time in months. The BCSS was calculated from the
date of diagnosis to the date of death due to breast cancer or the
last follow-up. Patients who were alive were censored on the date
of their last visit.
Results
Clinicopathological characteristics of
breast cancer patients
A total of 7,466 T1-2N1M0 breast cancer patients
treated with a mastectomy were identified from the SEER database.
The clinical characteristics of the patients, and the comparison
between the PMRT and no-PMRT group are summarized in Table I. As presented in Table I, 65% (n=4,840) of patients were
diagnosed after the age of 55 years. Of these 4,840 patients, 32%
received PMRT and 68% did not. Analysis of the data also revealed
that 68% (n=5,102) of the patients were
HR+/HER2−, 6% (n=441) were
HR−/HER2+, 14% (n=1,036) were
HR+/HER2+ and 12% (n=887) were
HR−/HER2− (TNBC). Using the Pearson
χ2−test, significant differences were observed between
the PMRT and the no-PMRT groups with regard to age at diagnosis
(P<0.001), year of diagnosis (P=0.002), marital status
(P<0.001), T stage (P<0.001), positive LN (P<0.001),
histological grade (P<0.001), HR status (P<0.001), HER2
status (P=0.035), subtype (P<0.001) and chemotherapy
(P<0.001) (Table I). Ethnicity
(P=0.500), laterality (P=0.353) and histological type (P=0.150)
were not significantly different between the PMRT and no-PMRT group
(Table I).
Prognostic factors
Univariate and multivariate analyses identified the
following independent prognostic factors: Ethnicity (P=0.002;
P=0.031); age at diagnosis (P=0.006; P=0.028); T stage (P<0.001;
P<0.001); histological grade (P<0.001; P<0.001); molecular
subtype (P<0.001; P<0.001); and PMRT (P=0.025; P=0.005)
(Table II). The multivariate
analysis examining subtypes demonstrated that PMRT was an
independent prognostic factor for TNBC (Hazard ratio, 1.519; 95%
CI, 1.044–2.208; P=0.029) (Table
III).
 | Table II.Univariate and multivariate analysis
to evaluate breast cancer-specific survival according to
clinicopathological variables from the SEER database. |
Table II.
Univariate and multivariate analysis
to evaluate breast cancer-specific survival according to
clinicopathological variables from the SEER database.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Ethnicity |
| 0.002 |
| 0.031 |
|
White | Reference |
| Reference |
|
|
Black | 1.380
(1.823–1.823) | 0.023 | 1.036
(0.778–1.379) | 0.811 |
|
Others | 0.556
(0.349–0.886) | 0.014 | 0.54
(0.339–0.862) | 0.010 |
| Age at diagnosis,
years |
|
|
|
|
|
<55 | Reference |
| Reference |
|
|
≥55 | 1.385
(1.098–1.747) | 0.006 | 1.314
(1.029–1.677) | 0.028 |
| Year of
diagnosis |
| 0.690 |
|
|
|
2010 | Reference |
|
|
|
|
2011 | 0.89
(0.685–1.156) | 0.382 |
|
|
|
2012 | 1.065
(0.786–1.444) | 0.683 |
|
|
|
2013 | 0.945
(0.588–1.518) | 0.815 |
|
|
| Marital status |
| 0.009 |
| 0.204 |
|
Married/unmarried but domestic
partner | Reference |
| Reference |
|
|
Unmarried | 1.344
(0.997–1.812) | 0.053 | 1.217
(0.895–1.654) | 0.210 |
|
Separated/widowed | 1.479
(1.156–1.891) | 0.002 | 1.254
(0.972–1.617) | 0.082 |
|
Unknown | 1.467
(0.932–2.310) | 0.098 | 1.394
(0.884–2.198) | 0.153 |
| Laterality |
|
|
|
|
|
Left | Reference |
|
|
|
|
Right | 0.815
(0.66–1.007) | 0.058 |
|
|
| T stage |
|
|
|
|
| T1 | Reference |
| Reference |
|
| T2 | 2.625
(2.011–3.427) | <0.001 | 2.356
(1.796–3.09) | <0.001 |
| Positive lymph
nodes, n |
| 0.267 |
| 0.202 |
| 1 | Reference |
| Reference |
|
| 2 | 0.935
(0.73–1.196) | 0.590 | 0.916
(0.715–1.174) | 0.489 |
| 3 | 1.199
(0.91–1.58) | 0.197 | 1.212
(0.916–1.603) | 0.179 |
| Histological
type |
| 0.006 |
| 0.337 |
|
IDC | Reference |
| Reference |
|
|
ILC | 0.42
(0.241–0.733) | 0.002 | 0.604
(0.34–1.073) | 0.085 |
|
IDC+ILC | 0.603
(0.353–1.03) | 0.064 | 0.933
(0.54–1.610) | 0.802 |
|
Others | 0.899
(0.593–1.362) | 0.615 | 0.848
(0.557–1.292) | 0.444 |
| Histological
grade |
| <0.001 |
| <0.001 |
| I | Reference |
| Reference |
|
| II | 1.420
(0.845–2.384) | 0.185 | 1.264
(0.749–2.133) | 0.380 |
|
III | 3.809
(2.328–6.232) | <0.001 | 2.213
(1.307–3.747) | 0.003 |
|
Unknown | 1.762
(0.731–4.249) | 0.207 | 1.275
(0.521–3.119) | 0.595 |
| HR status |
|
|
|
|
|
HR+ | Reference |
|
|
|
|
HR− | 4.176
(3.382–5.156) | <0.001 |
|
|
| HER2 status |
|
|
|
|
|
HER2+ | Reference |
|
|
|
|
HER2− | 1.361
(1.016–1.823) | 0.039 |
|
|
| Subtype |
| <0.001 |
| <0.001 |
|
HR+/HER2− | Reference |
| Reference |
|
|
HR−/HER2+ | 1.911
(1.263–2.892) | 0.002 | 1.464
(0.951–2.254) | 0.083 |
|
HR+/HER2+ | 0.842
(0.56–1.266) | 0.410 | 0.711
(0.469–1.08) | 0.110 |
|
HR−/HER2− | 5.208
(4.141–6.550) | <0.001 | 3.828
(2.94–4.983) | <0.001 |
| Chemotherapy |
|
|
|
|
|
Yes | Reference |
| Reference |
|
|
No/unknown | 1.23
(0.982–1.542) | 0.071 | 1.518
(1.182–1.949) | 0.001 |
| PMRT |
|
|
|
|
|
Yes | Reference |
| Reference |
|
| No | 1.294
(1.033–1.622) | 0.025 | 1.413
(1.112–1.796) | 0.005 |
 | Table III.Multivariate analysis to evaluate
breast cancer-specific survival by molecular subtype. |
Table III.
Multivariate analysis to evaluate
breast cancer-specific survival by molecular subtype.
| Subtypes | Hazard ratio (95%
CI) | P-value |
|---|
|
HR+/HER2−, PMRT vs.
no PMRT | 1.189
(0.836–1.692) | 0.335 |
|
HR−/HER2+−, PMRT vs.
no PMRT | 1.108
(0.429–2.857) | 0.833 |
|
HR+/HER2+−, PMRT vs.
no PMRT | 2.391
(0.845–6.763) | 0.100 |
|
HR−/HER2−, PMRT vs.
no PMRT | 1.519
(1.044–2.208) | 0.029 |
Survival analysis
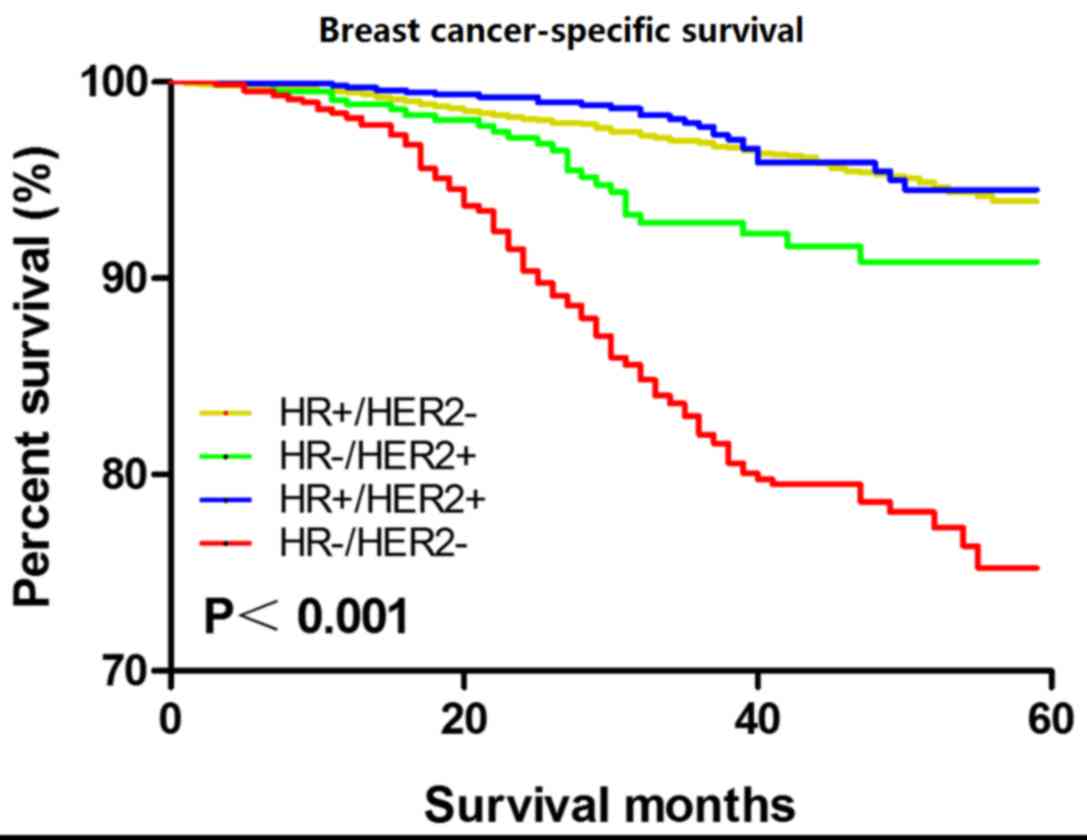
Kaplan-Meier analysis revealed that, among the 4
subtypes of patients with breast cancer, TNBC was associated with
the worst BCSS (P<0.001; Fig. 1).
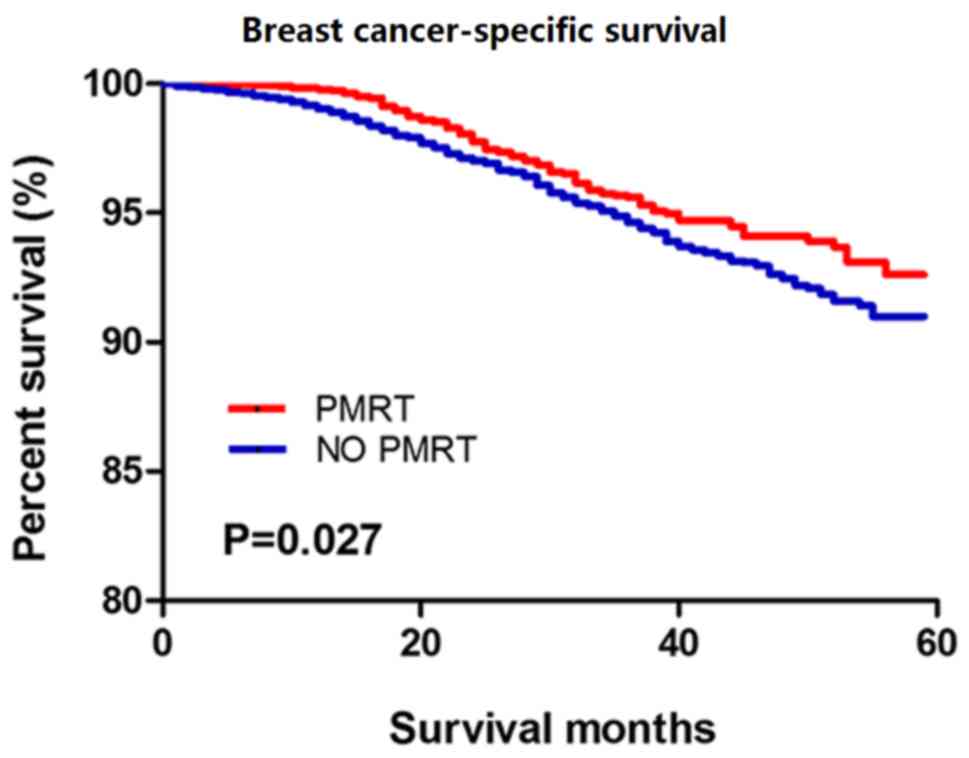
Patients with T1-2N1M0 breast cancer treated with PMRT showed
improved BCSS compared with those not treated with PMRT (P=0.027;
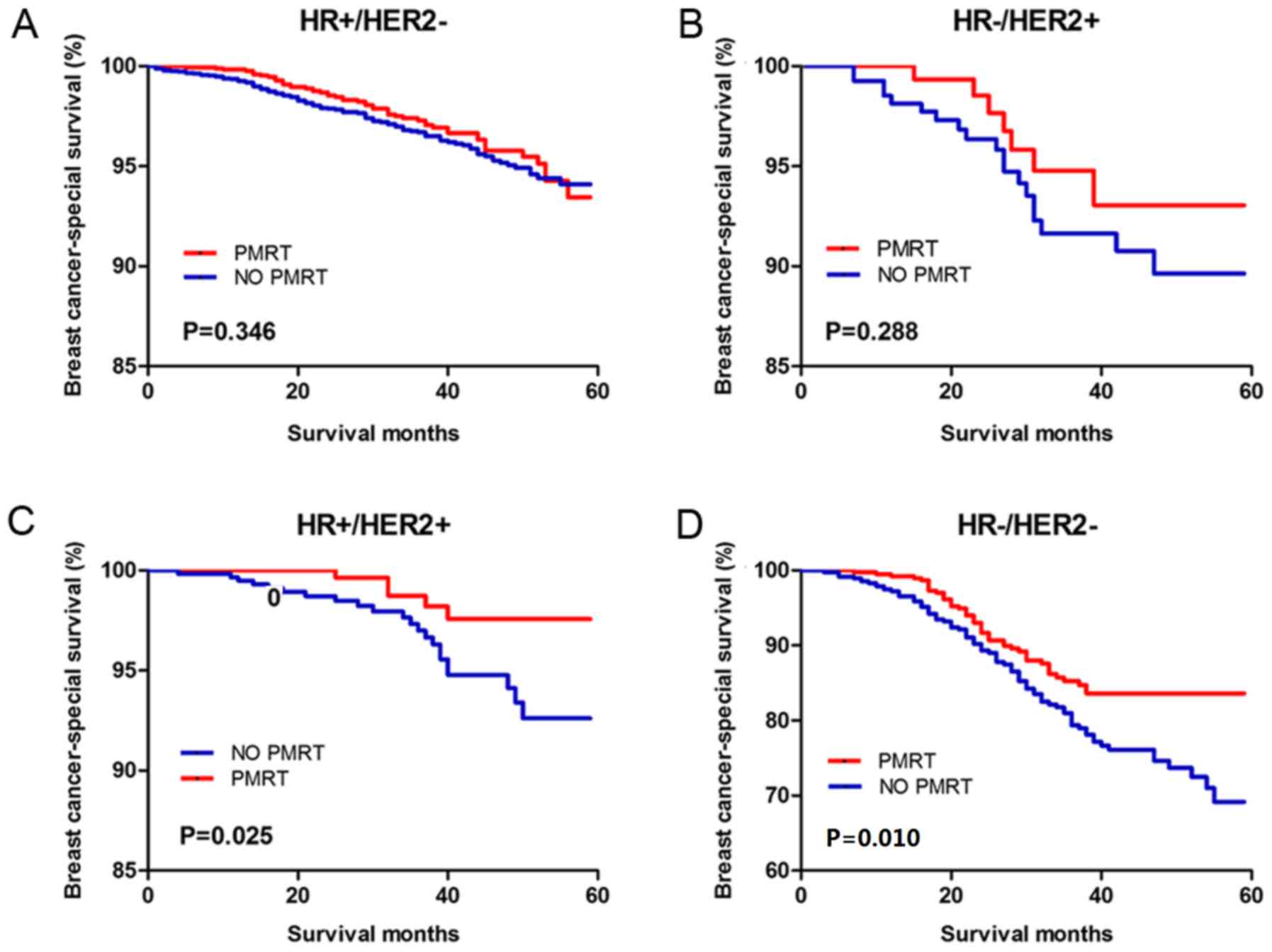
Fig. 2). The Kaplan-Meier analysis
of the 4 molecular subtypes revealed that both the
HR+/HER2+ (Hazard ratio, 5.208; 95% CI,
4.141–6.550; P=0.025) and HR−/HER2− (Hazard
ratio, 3.828; 95% CI, 2.940–4.983; P=0.010) patients benefited from
PMRT (Fig. 3). However, no
significant statistical difference was observed in the
HR+/HER2− (hazard ratio, 0.857; 95% CI,
0.621–1.182; P=0.346) and HR−/HER2+ (hazard
ratio, 0.649; 95% CI, 0.292–1.442; P=0.288).
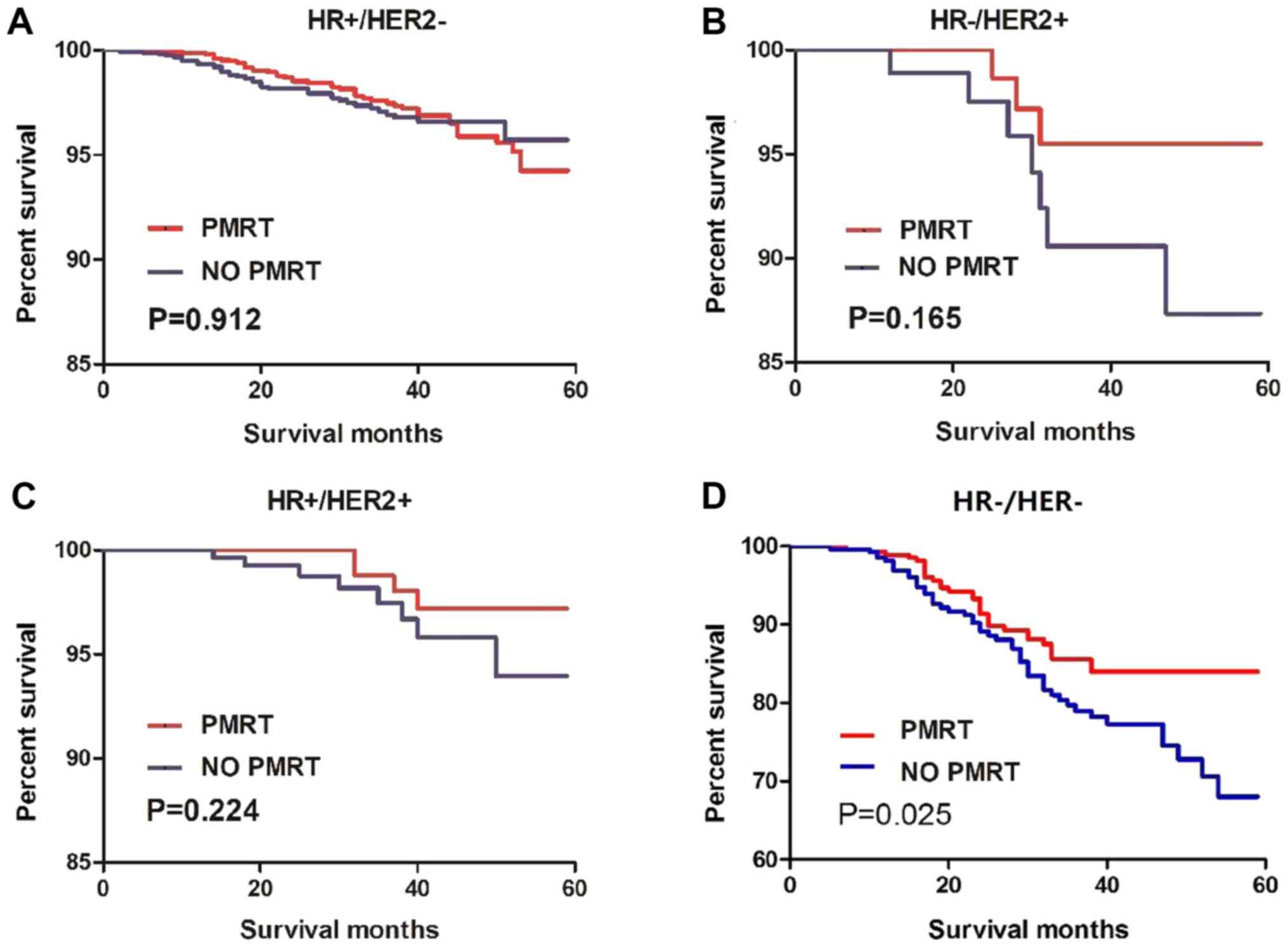
PSM analysis
To decrease the influence of potential confounding
factors, a PSM analysis was conducted between the PMRT and no-PMRT
group of the 4 molecular subtypes of T1-2N1M0 patients. The
Kaplan-Meier analysis after PSM demonstrated that only patients
with TNBC benefited from PMRT (hazard ratio, 0.6208; 95% CI,
0.4009–0.9615; P=0.025) while patients with the other 3 molecular
subtypes did not (Fig. 4). The PSM
analysis assigned 271 patients with T1-2N1M0 TNBC to the PMRT
group, matched with 271 patients in the no-PMRT group (Fig. S1). Of the 542 patients with T1-2N1M0
TNBC, no factors differed significantly between the 2 groups
(Table IV).
 | Table IV.Clinicopathological characteristic
before and after PSM in patients with triple-negative breast cancer
with and without PMRT. |
Table IV.
Clinicopathological characteristic
before and after PSM in patients with triple-negative breast cancer
with and without PMRT.
|
| Before PSM | After PSM |
|---|
|
|
|
|
|---|
|
Characteristics | PMRT, n | No PMRT, n | χ2 | P-value | PMRT, n | No PMRT, n | χ2 | P-value |
|---|
| Ethnicity |
|
| 0.537 | 0.764 |
|
| 0.162 | 0.922 |
|
White | 286 | 350 |
|
| 192 | 191 |
|
|
|
Black | 75 | 99 |
|
| 51 | 54 |
|
|
|
Others | 37 | 40 |
|
| 28 | 26 |
|
|
| Age at diagnosis,
years |
|
| 8.551 | 0.003 |
|
| 3.411 | 0.065 |
|
<55 | 175 | 168 |
|
| 117 | 96 |
|
|
|
≥55 | 223 | 321 |
|
| 154 | 175 |
|
|
| Year of
diagnosis |
|
| 3.37 | 0.338 |
|
| 0.785 | 0.853 |
|
2010 | 97 | 142 |
|
| 66 | 73 |
|
|
|
2011 | 110 | 129 |
|
| 81 | 75 |
|
|
|
2012 | 108 | 113 |
|
| 65 | 61 |
|
|
|
2013 | 83 | 105 |
|
| 59 | 62 |
|
|
| Marital status |
|
| 2.93 | 0.403 |
|
| 2.583 | 0.461 |
|
Married/unmarried or domestic
partner | 228 | 252 |
|
| 164 | 146 |
|
|
| Never
married | 59 | 83 |
|
| 37 | 46 |
|
|
|
Unmarried/separated/widowed | 91 | 126 |
|
| 57 | 65 |
|
|
|
Unknown | 20 | 28 |
|
| 13 | 14 |
|
|
| Laterality |
|
| 1.563 | 0.211 |
|
| 0.119 | 0.731 |
|
Left | 217 | 246 |
|
| 146 | 142 |
|
|
|
Right | 181 | 243 |
|
| 125 | 129 |
|
|
| T stage |
|
| 6.643 | 0.010 |
|
| 0 | 1.000 |
| T1 | 95 | 155 |
|
| 63 | 63 |
|
|
| T2 | 303 | 334 |
|
| 208 | 208 |
|
|
| Positive lymph
nodes, n |
|
| 7.984 | 0.018 |
|
| 1.598 | 0.450 |
| 1 | 201 | 285 |
|
| 166 | 152 |
|
|
| 2 | 114 | 134 |
|
| 72 | 84 |
|
|
| 3 | 83 | 70 |
|
| 33 | 35 |
|
|
| Histologic
type |
|
| 0.581 | 0.446 |
|
| 0 | 1.000 |
|
IDC | 358 | 432 |
|
| 246 | 246 |
|
|
|
ILC/IDC+ILC/others | 40 | 57 |
|
| 25 | 25 |
|
|
| Histological
grade |
|
| 11.514 | 0.003 |
|
| 1.811 | 0.404 |
|
I/II | 34 | 79 |
|
| 24 | 33 |
|
|
|
III | 352 | 395 |
|
| 239 | 232 |
|
|
|
Unknown | 12 | 15 |
|
| 8 | 6 |
|
|
| Chemotherapy |
|
| 72.69 | <0.001 |
|
| 0.31 | 0.861 |
|
Yes | 379 | 361 |
|
| 253 | 254 |
|
|
|
No/Unknown | 19 | 128 |
|
| 18 | 17 |
|
|
Discussion
According to the National Comprehensive Cancer
Network (USA), after a patient with breast cancer has undergone a
total mastectomy with N2/3 ALNs or a T3/4 primary tumor, PMRT is a
standard adjuvant therapy (17). The
application of PMRT for T1-2N1M0 breast cancer remains
controversial (18–21). The St. Gallen Breast Cancer
Conference pointed out that ~64% of experts did not recommend PMRT
as a routine treatment for T1-2N1M0 breast cancer (22). Among these experts, 62% agreed that
PMRT can be beneficial for patients with adverse prognostic factors
(23). In the present study, the
molecular subtype of breast cancer was a significant predictor for
radiosensitivity.
Breast tumors are heterogeneous, and the
heterogeneity determines the strategy for cancer follow-up
treatment (24). Previous studies
have investigated the relationships between histopathological
patterns, including tumor size, histological type and histological
grade, and therapy and prognosis (25). Molecular subtypes of cancer are based
on gene expression profiling, which reflects the intrinsic nature
of the tumor cells (26). Recent
studies have demonstrated that these molecular subtypes are
associated with different clinical characteristics and outcomes
(12,24–29). Due
to the high cost of gene expression tests, immunohistochemistry,
which is a cheaper alternative, was proposed along with criteria
set by the expert panel of the 13th St. Gallen International Breast
Cancer Conference (23). However,
very few studies of patients with T1-2N1M0 cancer have evaluated
the role of molecular subtyping in guiding decisions regarding
radiotherapy after mastectomy.
In the Swedish Breast Cancer Group 91 Radiotherapy
trial, radiotherapy showed a trend to improve the BCSS for patients
with TNBC; however, this trend did not reach significance (30). The results of the present study
demonstrated that PMRT can improve the BCSS of patients with
T1-2N1M0 TNBC. Of patients with BRCA-1 mutant breast cancer, 60–80%
are TNBC, which implies a high association between these types of
breast cancer (31). When the BRCA-1
gene is mutated, damaged DNA cannot be repaired by homologous
recombination, which is the main method for the repair of
double-stranded DNA breaks (31).
The dysfunction or deficiency of BRCA-1 may increase the
susceptibility to radiotherapy (31).
The main strength of a SEER analysis is that the
SEER database has access to a much larger cohort of patients
compared with that of a single institution. In the present study,
PSM was also conducted to reduce the effects of confounding
factors. However, this study had several limitations. Firstly, the
SEER registry does not provide any information on the details of
treatments such as chemotherapy regimens, HER2-targeted therapy,
endocrine therapy or methods of PMRT. In addition, the SEER
registry lacks information on the specific positive rates of ER/PR
and Ki-67, and therefore, the 4 molecular types examined in this
study are only a molecular subtype estimation. Due to HER2 status
only being available after 2010 in the SEER database, this resulted
in a lack of samples and insufficient follow-up in the present
study.
As research on TNBC progresses, patients with the
T1-2N1M0 subtype, which has no therapeutic targets to date, may
benefit from radiotherapy, although guidelines and current
international consensuses do not recommend the routine use of PMRT
for patients with this subtype (19,32).
Clinical trials should be conducted to validate the effectiveness
of radiotherapy after mastectomy in patients with T1-2N1M0
TNBC.
In conclusion, patients with T1-2N1M0 TNBC can
benefit from PMRT. Despite limitations, the findings of the current
study will help clinicians identify patients with T1-2N1M0 breast
cancer who may benefit from PMRT.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Jiangsu Natural
Science Foundation (grant no. BK20180274).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the SSER repository (https://seer.cancer.gov/).
Authors' contributions
XW, YX and LZ made substantial contributions to the
conception, design and acquisition of data. SG was involved in
collecting data, drafting the manuscript and revising it critically
for important intellectual content. YX, SG and MA made substantial
contributions to the acquisition, analysis and interpretation of
data. LZ, PC, SW, HT and JZ contributed to the data analysis. LZ,
SW, PC, MA, HT and JZ revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shi W, Luo Y, Zhao D, Huang H and Pang W:
Evaluation of the benefit of post-mastectomy radiotherapy in
patients with early-stage breast cancer: A propensity score
matching study. Oncol Lett. 17:4851–4858. 2019.PubMed/NCBI
|
|
2
|
Voduc KD, Cheang MC, Tyldesley S, Gelmon
K, Nielsen TO and Kennecke H: Breast cancer subtypes and the risk
of local and regional relapse. J Clin Oncol. 28:1684–1691. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
EBCTCG (Early Breast Cancer Trialists'
Collaborative Group), ; McGale P, Taylor C, Correa C, Cutter D,
Duane F, Ewertz M, Gray R, Mannu G, Peto R, et al: Effect of
radiotherapy after mastectomy and axillary surgery on 10-year
recurrence and 20-year breast cancer mortality: Meta-analysis of
individual patient data for 8135 women in 22 randomised trials.
Lancet. 383:2127–2135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Senkus E, Kyriakides S, Ohno S,
Penault-Llorca F, Poortmans P, Rutgers E, Zackrisson S and Cardoso
F; ESMO Guidelines Committee, : Primary breast cancer: ESMO
clinical practice guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 26 (Suppl 5):v8–v30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Whelan TJ, Olivotto IA and Levine MN:
Regional nodal irradiation in early-stage breast cancer. N Engl J
Med. 373:1878–1879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Poortmans PM, Collette S, Kirkove C, Van
Limbergen E, Budach V, Struikmans H, Collette L, Fourquet A,
Maingon P, Valli M, et al: Internal mammary and medial
supraclavicular irradiation in breast cancer. N Engl J Med.
373:317–327. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mukesh MB, Duke S, Parashar D, Wishart G,
Coles CE and Wilson C: The Cambridge post-mastectomy radiotherapy
(C-PMRT) index: A practical tool for patient selection. Radiother
Oncol. 110:461–466. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Valla M, Vatten LJ, Engstrøm MJ, Haugen
OA, Akslen LA, Bjørngaard JH, Hagen AI, Ytterhus B, Bofin AM and
Opdahl S: Molecular subtypes of breast cancer: Long-term incidence
trends and prognostic differences. Cancer Epidemiol Biomarkers
Prev. 25:1625–1634. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zardavas D, Irrthum A, Swanton C and
Piccart M: Clinical management of breast cancer heterogeneity. Nat
Rev Clin Oncol. 12:381–394. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cancer Genome Atlas Network, .
Comprehensive molecular portraits of human breast tumours. Nature.
490:61–70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu CY, Du SL, Zhang J, Liang AL and Liu
YJ: Exosomes and breast cancer: A comprehensive review of novel
therapeutic strategies from diagnosis to treatment. Cancer Gene
Ther. 24:6–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pusztai L, Broglio K, Andre F, Symmans WF,
Hess KR and Hortobagyi GN: Effect of molecular disease subsets on
disease-free survival in randomized adjuvant chemotherapy trials
for estrogen receptor-positive breast cancer. J Clin Oncol.
26:4679–4683. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Prat A, Lluch A, Albanell J, Barry WT, Fan
C, Chacón JI, Parker JS, Calvo L, Plazaola A, Arcusa A, et al:
Predicting response and survival in chemotherapy-treated
triple-negative breast cancer. Br J Cancer. 111:1532–1541. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Howlader N, Altekruse SF, Li CI, Chen VW,
Clarke CA, Ries LA and Cronin KA: US incidence of breast cancer
subtypes defined by joint hormone receptor and HER2 status. J Natl
Cancer Inst. 106:dju0552014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gradishar WJ, Anderson BO, Balassanian R,
Blair SL, Burstein HJ, Cyr A, Elias AD, Farrar WB, Forero A,
Giordano SH, et al: Breast Cancer, Version 4.2017, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
16:310–320. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yin H, Qu Y, Wang X, Ma T, Zhang H, Zhang
Y, Li Y, Zhang S, Ma H, Xing E, et al: Impact of postmastectomy
radiation therapy in T1-2 breast cancer patients with 1–3 positive
axillary lymph nodes. Oncotarget. 8:49564–49573. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nordenskjold AE, Fohlin H, Albertsson P,
Arnesson LG, Chamalidou C, Einbeigi Z, Holmberg E, Nordenskjöld B
and Karlsson P; Swedish Western and Southeastern Breast Cancer
Groups, : No clear effect of postoperative radiotherapy on survival
of breast cancer patients with one to three positive nodes: A
population-based study. Ann Oncol. 26:1149–1154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chitapanarux I, Tharavichitkul E,
Jakrabhandu S, Klunklin P, Onchan W, Srikawin J, Pukanhaphan N,
Traisathit P and Vongtama R: Real-world outcomes of postmastectomy
radiotherapy in breast cancer patients with 1–3 positive lymph
nodes: A retrospective study. J Radiat Res. 55:121–128. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He ZY, Wu SG, Zhou J, Li FY, Lin Q, Lin HX
and Sun JY: Postmastectomy radiotherapy improves disease-free
survival of high risk of locoregional recurrence breast cancer
patients with T1-2 and 1 to 3 positive nodes. PLoS One.
10:e01191052015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gnant M, Harbeck N and Thomssen C: St.
Gallen/Vienna 2017: A brief summary of the consensus discussion
about escalation and de-escalation of primary breast cancer
treatment. Breast Care (Basel). 12:102–107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart- Gebhart M, Thürlimann B and Senn HJ; Panel members, :
Personalizing the treatment of women with early breast cancer:
Highlights of the St gallen international expert consensus on the
primary therapy of early breast cancer 2013. Ann Oncol.
24:2206–2223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen HL, Ding A and Wang FW: Prognostic
effect analysis of molecular subtype on young breast cancer
patients. Chin J Cancer Res. 27:428–436. 2015.PubMed/NCBI
|
|
25
|
Shen H, Zhao L, Wang L, Liu X, Liu X, Liu
J, Niu F, Lv S and Niu Y: Postmastectomy radiotherapy benefit in
Chinese breast cancer patients with T1-T2 tumor and 1–3 positive
axillary lymph nodes by molecular subtypes: An analysis of 1369
cases. Tumour Biol. 37:6465–6475. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang J, Shi M, Ling R, Xia Y, Luo S, Fu X,
Xiao F, Li J, Long X, Wang J, et al: Adjuvant chemotherapy and
radiotherapy in triple-negative breast carcinoma: A prospective
randomized controlled multi-center trial. Radiother Oncol.
100:200–204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moo TA, McMillan R, Lee M, Stempel M, Ho
A, Patil S and El-Tamer M: Impact of molecular subtype on
locoregional recurrence in mastectomy patients with T1-T2 breast
cancer and 1–3 positive lymph nodes. Ann Surg Oncol. 21:1569–1574.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abdulkarim BS, Cuartero J, Hanson J,
Deschênes J, Lesniak D and Sabri S: Increased risk of locoregional
recurrence for women with T1-2N0 triple-negative breast cancer
treated with modified radical mastectomy without adjuvant radiation
therapy compared with breast-conserving therapy. J Clin Oncol.
29:2852–2858. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nguyen PL, Taghian AG, Katz MS, Niemierko
A, Abi Raad RF, Boon WL, Bellon JR, Wong JS, Smith BL and Harris
JR: Breast cancer subtype approximated by estrogen receptor,
progesterone receptor, and HER-2 is associated with local and
distant recurrence after breast-conserving therapy. J Clin Oncol.
26:2373–2378. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sjöström M, Lundstedt D, Hartman L,
Holmberg E, Killander F, Kovács A, Malmström P, Niméus E, Werner
Rönnerman E, Fernö M and Karlsson P: Response to radiotherapy after
breast-conserving surgery in different breast cancer subtypes in
the swedish breast cancer group 91 radiotherapy randomized clinical
trial. J Clin Oncol. 35:3222–3229. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cleator S, Heller W and Coombes RC:
Triple-negative breast cancer: Therapeutic options. Lancet Oncol.
8:235–244. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu T and Di G: Role of tumor
microenvironment in triple-negative breast cancer and its
prognostic significance. Chin J Cancer Res. 29:237–252. 2017.
View Article : Google Scholar : PubMed/NCBI
|