Introduction
The traditional magnetic resonance imaging (MRI)
scan can reveal the intracranial structure, intracranial tumor
size, location and surrounding edema; however, it cannot precisely
reveal the shape of the intracranial nerve fibers. With the
development of high-field MR and computer technology, diffusion
tensor imaging (DTI) has enabled non-invasive studies of the white
matter fiber fascicle (1). DT
tractography (DTT), which is developed from DTI, can reflect the
pathological state of the white matter fiber fascicle and the
anatomical connection with adjacent lesions, and assist the surgeon
to design a suitable surgical strategy prior to surgery (2).
DTI is a functional MRI technique that is commonly
used to evaluate the integrity of the white matter by measuring the
water diffusion (3). DTI has been
extensively applied to evaluate the specific white matter bands by
using fiber tracking and to examine the axonal impairment resulting
from the different disorders, such as corticospinal tract stroke,
impairment in chronic spinal cord injury and asymptomatic
neurocognitive impairment (4). The
corpus callosum, which is known to be involved in the process of
dichotic listening (5) and auditory
hallucinations (6), can be
visualized using DTI. Traditional MRI is limited by a resolution
that is 1to 2orders of magnitude greater compared with the
dimensions of cells and individual axons, therefore, revealing the
surrounding axonal structure is difficult (7). However, DTI could be used to
characterize the white matter fiber structure, integrity within the
brain and how the water within the brain is affected. Compared with
the traditional MRI technique, DTI is susceptible to numerous
detrimental artefacts that may impair the reliability and validity
of the obtained data (8). Meanwhile,
DTI is not typically applied to detect the changes in the auditory
processing. According to a previous study, the acquisition of DTI
usually requires a number of primary conditions (7), including i) behavior of all the
particles to be identical; ii) distribution of the displacements to
be a finite variance; and iii) the displacement of DTI in future
should be free of any influence that derives from the past DTI
application. If the aforementioned conditions are not met, this may
lead to a mono-exponential decay of MR signal (7). The purpose of the surgical strategy is
to guarantee the integrity of the functional nerves of patients
during surgery and to improve the quality of life of patients
following surgery. Therefore, DTT can be a reliable strategy for
doctors to improve surgical results and reduce the risk of surgery
(9,10).
In the present study, DTI scans were performed and
the generalized q-sampling imaging (GQI) methods were used to track
the function-related fiber fascicles (pyramidal fascicle,
associated with limb activity, and arcuate fascicle, associated
with language) on the basis of a conventional MRI scan, in the
trial group. The best safe surgical approach between the location
of the tumor and the fiber fascicle was subsequently designed, and
the results were compared with that in the patients of the control
group, who only underwent a preoperative conventional MRI scan.
Tumor resection rate, quality of life using Karnofsky performance
score (KPS) (10) and the
differences in postoperative functional improvement were compared
between the trial and control groups to explore the importance of
DTI in the protection of neurological function and the improvement
of prognosis in functional brain tumor surgery.
Materials and methods
Selection of subjects
A total of 42 patients, who were diagnosed with a
tumor located in the functional areas of the brain at The
Affiliated Hospital of Qingdao University (Qingdao, Shandong,
China) between June 2014 and June 2016, were randomly assigned to
either the trial group or the control group. A total of 21 patients
were recruited to the trial group where they underwent conventional
MRI and DTI scans (Fig. 1); this
group included 13 males and 8 females, with a mean age of 53.29
years. The tumors were located in 4 areas of the brain, including 8
tumors in the frontal lobe, 2 in the temporal lobe, 8 in the
precentral gyrus and 3 in the cerebral falx. The mean tumor volume
was 38,038.86±3,578.47 mm3. A total of 21 patients were
recruited to the control group where they underwent conventional
MRI scans; this group included 11 males and 10 females, with a mean
age of 48.24 years. The tumors were located in 4 areas of the
brain, including 6 tumors in the frontal lobe, 3 in the temporal
lobe, 7 in the precentral gyrus and 5 in the cerebral falx. The
mean tumor volume was 46,788.62±3,095.99 mm3. The
clinical symptoms of the patients included headaches, epilepsy and
limb hemiplegia. In addition, all patients underwent a craniotomy.
The KPS score was used to evaluate the quality of life and
functional status of the patients prior to and following surgery,
and the postoperative pathological examination findings were
considered as the final diagnosis basis. Briefly, the brain tissue
samples were fixed using 4% paraformaldehyde at room temperature
for 10 min, dehydrated using graded ethanol (85, 95 and 100%) (3
min per grade) and subsequently paraffin-embedded. Subsequently,
the paraffin blocks were sectioned (with thickness of 4 µm) and
stained using hematoxylin (cat. no. C0107) for 10 min at room
temperature and eosin (cat. no. C0109) for 5 min at room
temperature, according to the manufacturers protocols (both regents
were purchased from Beyotime Institute of Biotechnology). Finally,
the sections were sealed using the neutral-resin (Beyotime
Institute of Biotechnology) and observed using the light microscope
(model, BX-51; Olympus Corporation) at magnification of ×400.
The tumors were classified according to the 2007 the
World Health Organization classification of tumors of central
nervous system (11).
The present study was approved by the Ethics
Committee of The Affiliated Hospital of Qingdao University and all
patients provided written informed consent.
MRI
MRI scans were performed using a 3.0T GE MR machine
(GE Medical Systems, Milwaukee, WI, USA). The images were
constructed by using the image-processing Living Image v3.2
software (Caliper Life Sciences; PerkinElmer, Inc.). Conventional
MRI scans included the T1-weighted imaging (WI) sequence, T2WI
sequence and fluid-attenuated inversion recovery sequence.
Gadopentetate dimeglumine was used as the enhanced contrast agent,
scanning 24 layers with a layer thickness of 5.0 mm and a layer
spacing of 1.5 mm. DTI scans were performed using a 32-channel head
coil in which the patient's head was held in place securely to
prevent movement during the scan. The scan sequence was a single
shot spin-echo planar image, and the number of the
diffusion-sensitive gradient direction was 30. The following
parameters were used: b value, 1,000 sec/m2; repetition
time (TR), 7,700 msec; echo time (TE), 77 msec; vision fields of
view (FOV), 230×230 mm2; voxel size, 2×2×2
mm3; layer thickness, 2.0 mm; the number of excitations,
1; and 40 layers were continuously scanned without interval. The
scan time was 286 sec. To distinguish between the anatomical
regions, 3-dimensional T1WI scans were used with the sequence of a
double flash using the following parameters: TR, 1,900 msec; TE,
2.99 msec; reversal time, 900 msec; layer thickness, 0.9 mm; flip
angle, 9°; number of excitations, 1; vision FOV, 230×230
mm2; and voxel size, 0.9×0.9×0.9 mm3. The
scan time was 344 sec.
Image processing and analysis
The diffusion data in the Digital Imaging and
Communications in Medicine format generated by the scan were
imported into the DSI studio (http://dsi-studio.labsolver.org/) and processed with
the GQI data reconstruction method. The fibers were tracked using
the Trackvis software v0.6.1 (http://trackvis.org/), in which the commissural tracts
were depicted in red, the association fibers were presented in
green and the super-to-inferior running projection fibers were
depicted in blue. The quality assurance (QA) threshold was set to
0.03574, the maximum angle to 7°, the step length to 0.469 and the
total number of brain fibers to 20,000 in the DSI studio software
to reconstruct the GQI. The region of interest (ROI) method was
used to separate the pyramidal fascicle and arcuate fascicle. For
the pyramidal fascicle, the ROI was placed in one-third of the
bilateral internal capsule of the posterior limb and in the lateral
three-fifths of the bottom of the brain using the ‘AND’ logic to
track. For the arcuate fascicle, the seeding area was placed in the
bilateral superior temporal gyrus, middle temporal gyrus and
inferior temporal gyrus, and a mask area of interest was then
placed on the coronal position in the central posterior position
for fiber tracking. At the same time, the QA values of the
functional fiber fascicle were measured prior to and following
surgery, and the changes were observed.
Surgical management
All the patients underwent craniotomy of the brain
resection, which was completed by the same neurosurgeon using a
microscope. In the trial group, the design of the skin flap and the
surgical approach, the shape of the functional fascicle around the
tumor and the spatial association with the tumor according to the
preoperative DTT results were taken into consideration to avoid
damage to the functional conducting fascicle. In the control group,
the location of the tumor prior to surgery, as provided by the MRI
scan and the natural fissures, including the sulcus and lateral
fissure (which were close to the tumor), was selected for entry
into the brain to avoid damage to the important cortex, nerves and
blood vessels.
Postoperative evaluation
The postoperative tumor resection rate, the
postoperative functional changing status and the behavior status
(KPS score) were compared between the trial and control groups to
evaluate any differences. The fractional anisotrophy (FA) value is
the most commonly used quantitative analysis parameter of DTI,
which reflects the percentage of water molecule anisotropy to the
whole dispersion movement. The FA value ranges from 0 to 1, where 0
represents the isotropic dispersion of the water molecule, which
means that the probability and distance of dispersion in all
directions are equal, and 1 represents a large degree of
directional dependence of the dispersion motion of the water
molecules.
Statistical analysis
SPSS (v17.0; SPSS, Inc., Chicago, IL, USA) was used
for the statistical analysis. All of the data analyzed were
obtained from 6 repeat experiments, and 2 experts evaluated or
analyzed the imaging data. The χ2 test was used for
count data. Analysis of variance (ANOVA) was used to compare the
differences among multiple groups followed by Tukey's post-hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Comparison of the preoperative data
between the trial and control groups
There were no significant differences with regard to
gender (data not shown; χ2=0.389; P=0.533) or the
distribution of age (data not shown; χ2=0.452; P=0.414)
between the trial and control groups. There were also no
significant differences with regard to the lesion sites (data not
shown; χ2=4.057; P=0.908) or the volume of the lesion
(χ2=2.272; P=0.140). There were no statistically
significant differences with regard to lesion location
(χ2=2.682; P=0.139) and tumor volume
(χ2=2.469; P=0.158) between the trial and control
groups. There was also no significant difference with regard to the
preoperative muscle strength (χ2=0.138; P=0.864) and
aphasia symptoms (χ2=0.125; P=0.989) between the 2
groups.
Representative case reports
A 61-year-old male patient was admitted to the
Affiliated Hospital of Qingdao University (Shandong, China) with
progressive speech loss as the main complaint and included in the
present study. Conventional MRI revealed lesions in the left
temporal lobe space and a tumor located in the left temporal
linguistic function area. Preoperative DTI examination revealed the
location of the tumor and the arcuate fascicle and was used to
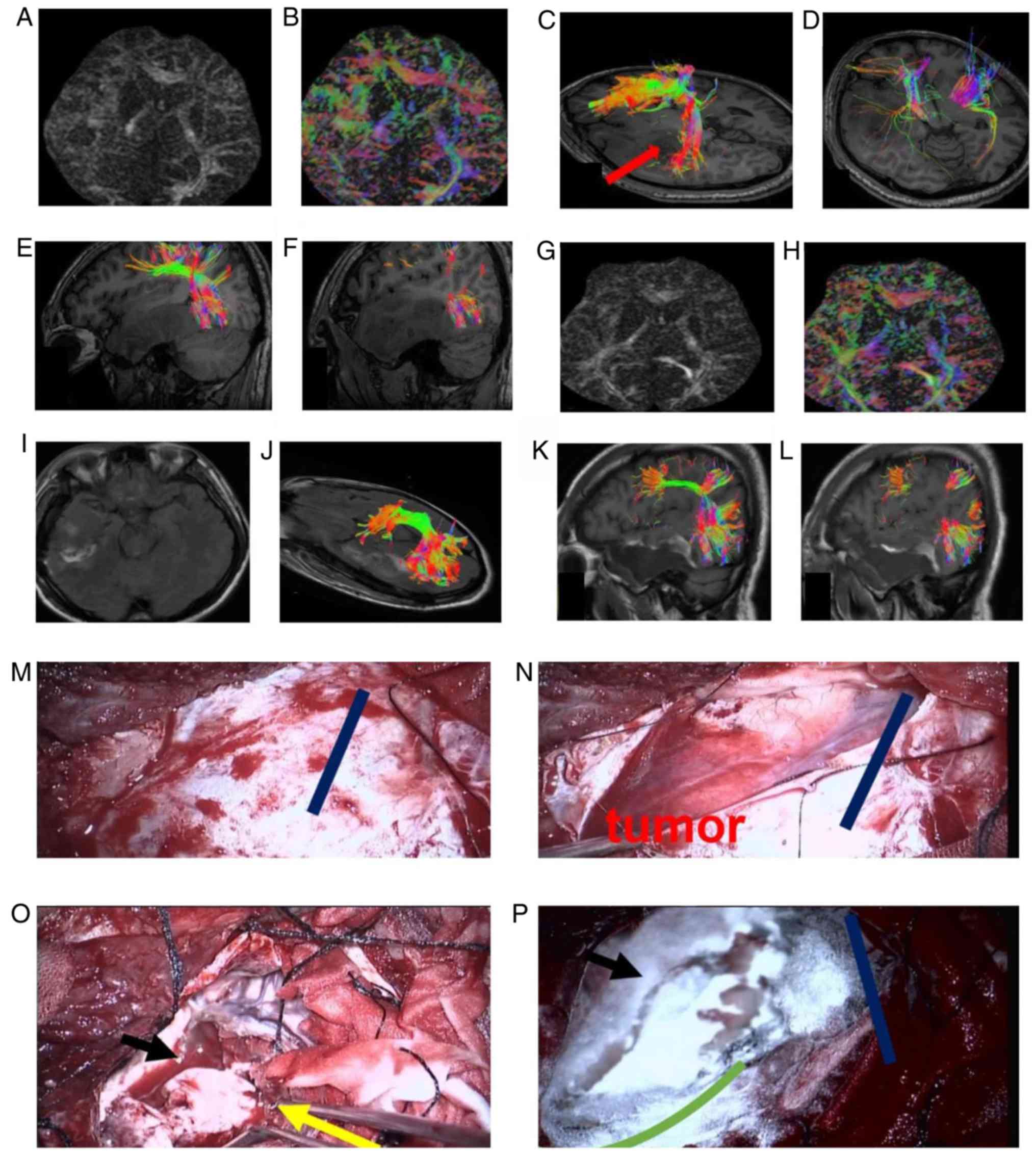
assist with the surgery. The DTI management (GA) graph (Fig. 1A and B) illustrates the DTI images.
The different colors reveal the shape of the fiber fascicle. Red
represents the horizontal direction, green represents the front and
rear direction, and blue represents the vertical direction. The GA
graph reveals the reconstructed arcuate fascicle (Fig. 1C). The tumor was in the ventral
temporal section of the arcuate fascicle and the tail part of the
arcuate fascicle was pushed back. The red arrow reveals the safe
surgical approach to avoid the destruction of the arcuate fascicle.
The pyramidal fascicle on the left and right side is shown in
Fig. 1D. Due to the large tumor and
peritumoral edema, the pyramidal fascicle should be protected
during the removal process. The vector phase reveals the shape of
the arcuate fascicle and the locational association between the
cortical end point and the tumor (Fig.
1E and F). The postoperative GA graph, the postoperative GA
color graph, complete tumor resection are shown in Fig. 1G, H and I, respectively. The shape of
the arcuate fascicle following the removal of the tumor in the
axial and sagittal positions is shown in Fig. 1J-L. These images reveal that the
temporal segment of the arcuate fascicle was integrate, and the
fiber fascicle was thicker, indicating that there was a possibility
to restore the function of the fiber fascicle following the removal
of the tumor and the edema compression. The status prior to the
opening of the dura mater (Fig. 1M)
was evaluated and the appropriate tumor-removal angle was designed
according to the preoperative DTI. The dark blue, straight line
represents the lateral cut. The opening of the dura mater and the
exposure of the tumor (Fig. 1N) were
also observed. The careful removal of the tumor is shown in
Fig. 1O; the black arrow reveals
that the resection boundary of the tumor was deep into the middle
cranial fossa, and the yellow arrows reveals the posterior temporal
cut to the edema. In Fig. 1P, the
completed tumor resection is shown and the green arc reveals the
normal brain tissue boundary. The patient improved with regard to
sensory aphasia following surgery. Postoperative pathological
analysis revealed that the tumor was an astrocytoma (using World
Health Organisation grade II) (12).
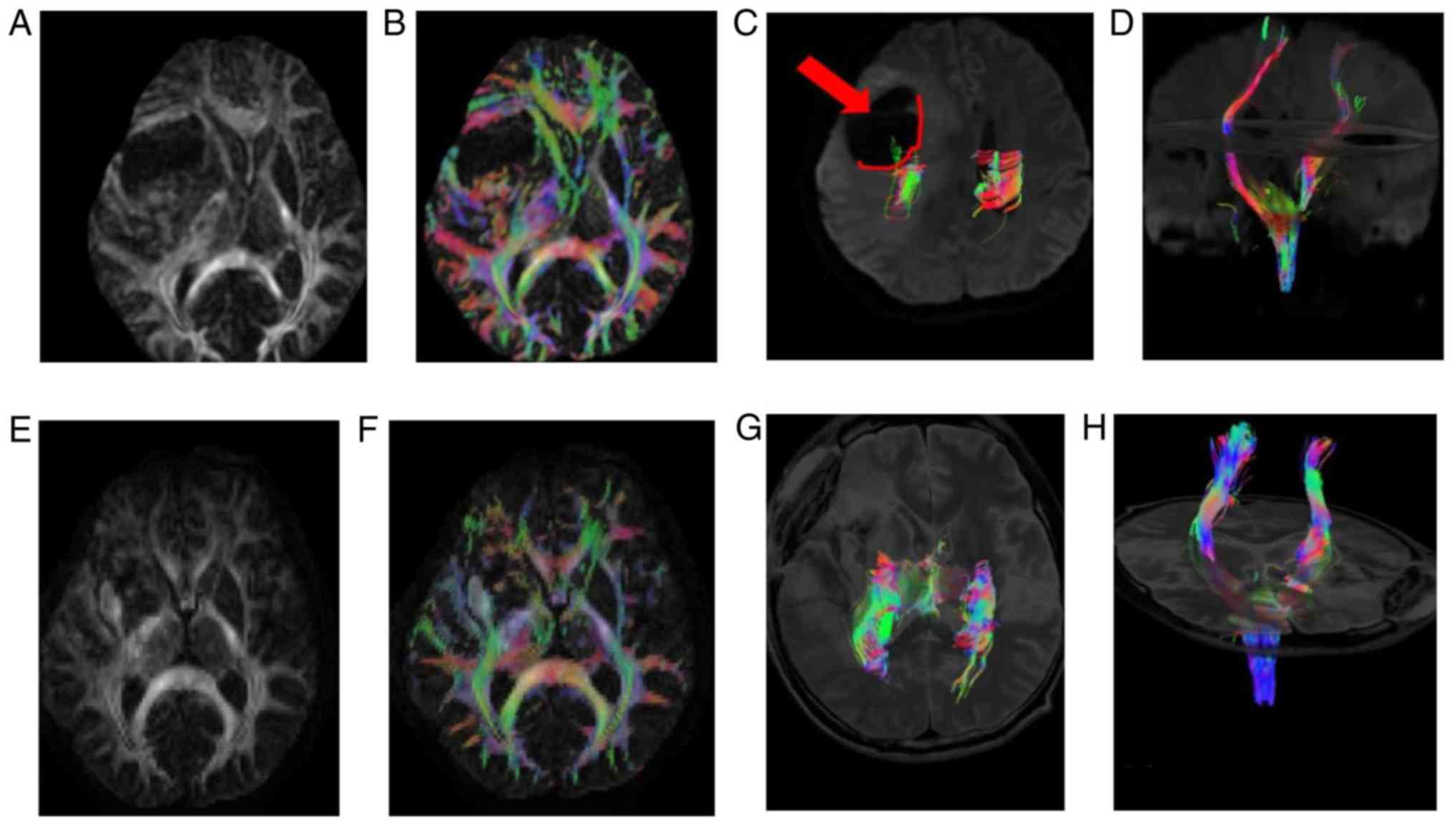
A 44-year-old female suffering from headaches and
clumsiness in the right limb was admitted to the Affiliated
Hospital of Qingdao University (Shandong, China) and recruited to
the present study. MRI revealed lesions occupying the left temporal
pelvic area and edema. Due to the limitation of limb activity, DTI
examination was performed prior to surgery. The preoperative video
material was collected (Fig. 2A-D)
and the tumor was shown to compress the pyramidal fascicle from the
posterior internal side. The pyramidal fascicle from the coronal
display of the tumor side is significantly squeezed to the vicinity
of the center line compared with the pyramidal fascicle of the
uninjured side. The postoperative DTI review data were also
collected (Fig. 2E-H). Following the
complete resection of the tumor from axial and coronal phase, the
pyramidal fascicle of the injured side was reset, and the
postoperative limb activity of the patient was improved.
Postoperative pathological analysis revealed that the tumor was a
glioblastoma (World Health Organization grade IV) (12).
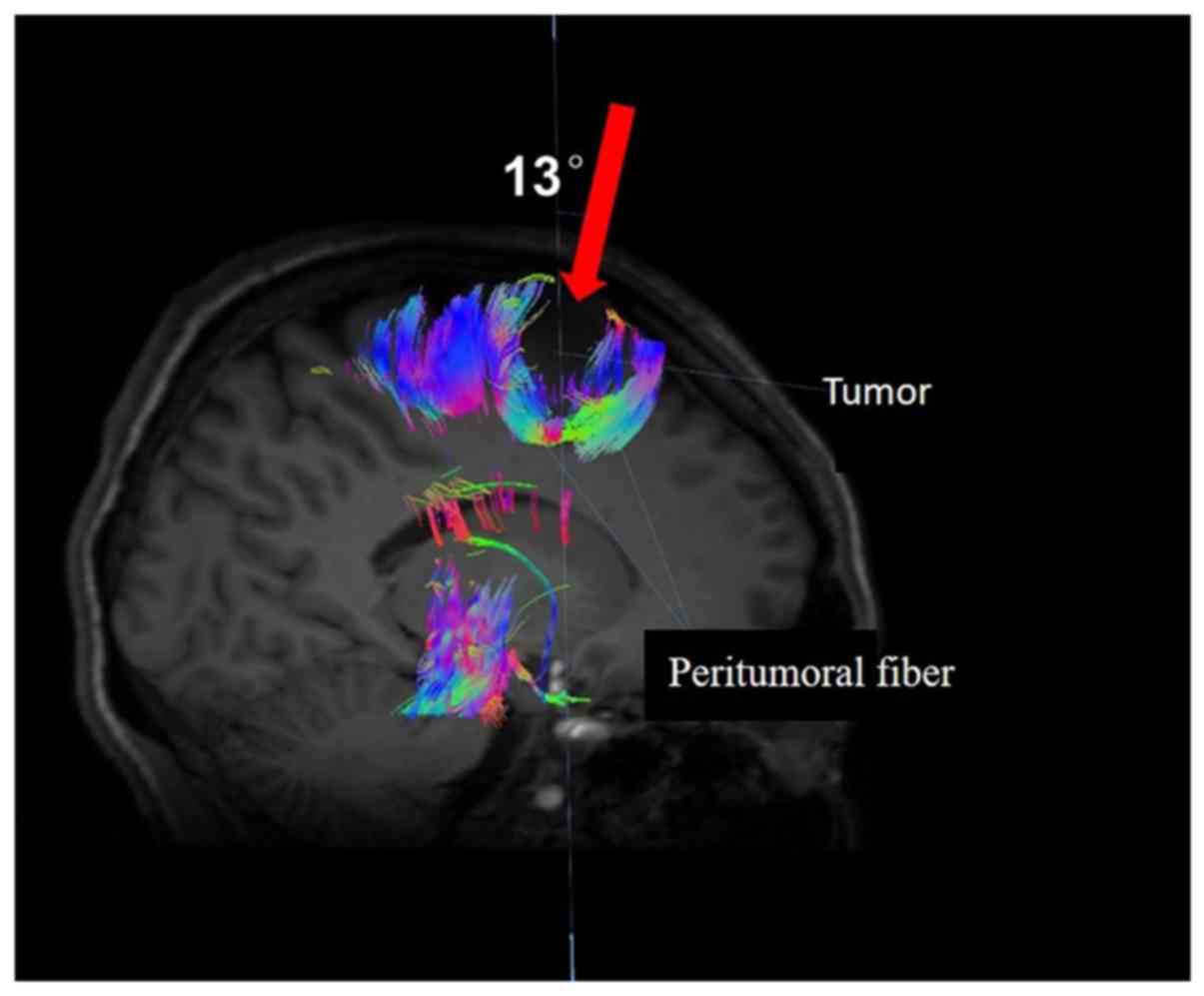
A third patient, a 51-year-old female suffering from
a laborious right foot raise was also admitted to the Affiliated
Hospital of Qingdao University (Shandong, China) and subsequently
recruited in to the present study. The MRI revealed lesions on the
left side of the brain (Fig. 3).
Diffusion tensor tractography was performed to reconstruct the
peritumoral fiber and indicated that the clear midline 13° angle
was the safe surgical approach, with no damage to the peritumoral
fiber. Following surgery, the muscle strength of the patient
improved with no secondary neurological dysfunction. Postoperative
pathological analysis revealed that the tumor was a central
neurocytoma (using World Health Organization grade II) (12).
Postoperative pathological types and
number of cases
To compare the clinical outcomes effectively, the
postoperative pathological types were determined. There were no
significant differences with regard to the postoperative
histopathological types (including meningioma, oligodendroglioma,
astrocytoma, glioblastoma, metastatic carcinoma, cavernous
hemangioma and central neurocytoma) and the number of cases between
the trial and control groups (χ2=2.500; P=0.927;
Table I).
 | Table I.Postoperative tumor pathological types
and the number of cases. |
Table I.
Postoperative tumor pathological types
and the number of cases.
| Pathological
types | Trial group, n | Control group, n | χ2 | P-value |
|---|
| Meningioma | 5 | 6 | 2.5 | 0.927 |
|
Oligodendroglioma | 6 | 5 |
|
|
| Astrocytoma | 4 | 5 |
|
|
| Glioblastoma | 3 | 2 |
|
|
| Metastatic
carcinoma | 1 | 1 |
|
|
| Cavernous
hemangioma | 1 | 2 |
|
|
| Central
neurocytoma | 1 | 0 |
|
|
Resection numbers do not significantly
differ
In the trial group, 18 patients underwent total
resection, while 3 patients underwent subtotal resection; the total
resection rate was 85.71%. In the control group, 15 patients
underwent total resection, while 6 patients underwent subtotal
resection; the total resection rate was 71.43%. The χ2
test revealed no significant differences between the trial and
control groups for the total number of resections
(χ2=1.273; P=0.259; Table
II).
 | Table II.Comparison of the resection rates
between the trial and control groups. |
Table II.
Comparison of the resection rates
between the trial and control groups.
| Group name | Number of cases | Subtotal resection,
n | Total resection,
n | χ2 | P-value |
|---|
| Trial group | 21 | 3 | 18 | 1.273 | 0.259 |
| Control group | 21 | 6 | 15 |
|
|
Symptom improvement rate is
significantly higher in the trial group
The changes in the postoperative symptoms were
classified into 3 types (worse, no change or better) in terms of
functional impairment (hemiplegia or aphasia) compared with that in
the preoperative condition. The symptom improvement rate was 85.71%
in the trial group and 47.62% in the control group. The
χ2 test revealed that the symptom improvement rate was
significantly higher in the trial group compared with that in the
control group (χ2=6.952; P=0.031; Table III).
 | Table III.Changes in the postoperative
functional impairment symptoms compared with the preoperative
symptoms. |
Table III.
Changes in the postoperative
functional impairment symptoms compared with the preoperative
symptoms.
|
|
| Functional
impairment, n/total n |
|
|
|---|
|
|
|
|
|
|
|---|
| Group name | Number of cases | Worse | No change | Better | χ2 | P-value |
|---|
| Trial group | 21 | 1/21 | 2/21 | 18/21 | 6.952 | 0.031 |
| Control group | 21 | 5/21 | 6/21 | 10/21 |
|
|
KPS value of the patients is
significantly increased in the trial group
The KPS value of the patients in the trial group was
68.59±6.73 preoperatively and 79.06±7.40 postoperatively. ANOVA
revealed that the KPS value postoperatively was significantly
higher compared with the value preoperatively (P<0.001; Table IV). The KPS value of the patients in
the control group was 65.84±9.05 preoperatively and 73.45±6.18
postoperatively. ANOVA revealed that the KPS value was
significantly higher postoperatively compared with the value
preoperatively (P<0.001; Table
IV). ANOVA also revealed that the KPS value of the trial group
was significantly higher compared with that in the control group
(P=0.039; Table IV).
 | Table IV.Changes in the KPS values prior to
and following surgery between the trial and control groups. |
Table IV.
Changes in the KPS values prior to
and following surgery between the trial and control groups.
| KPS value | Prior to
surgery | Following
surgery |
|---|
| Trial group | 68.59±6.73 |
79.06±7.40a,b |
| Control group | 65.84±9.05 |
73.45±6.18c |
Discussion
DTI is a novel technique of signal acquisition and
image contrast based on traditional MRI (12,13). The
technique utilizes the difference in the diffusion of water
molecules in the brain for imaging (14). Clinically, DTT can be a reliable
strategy for doctors to improve surgical results and reduce the
risk of surgery (15,16).
Numerous reports exist in the literature on the
application of DTI and its guidance for craniotomy with functional
MRI and intraoperative navigation. Previous studies reported that
the preoperative DTI can affect the choice of surgical strategy,
and with a combination of intra-operative DTI and navigation,
surgeon scan better resect tumors and protect neurological function
(17,18). Kuhnt et al (19) performed preoperative and
intraoperative MRI scanning and reconstruction of language-related
fibers for 32 patients diagnosed with glioma in the linguistic
area, and suggested that the application of intra-operative MRI is
essential for tumor resection and functional protection. D'Andrea
et al (20) used preoperative
DTI for patients with glioma near the right lateral temporal lobe
to illustrate the tumor and the optic fascicle spatial shape. The
through-temporal safety surgery approach was designed to avoid the
destruction of the optic fascicle and included preoperative DTI and
reconstruction of the optic fascicle to reduce the impact of
cerebrospinal fluid caused by the release of brain drift. Thus, the
complete removal of the tumor and the integrity of the optic
fascicle was confirmed (20,21). Hayashi et al (22) performed preoperative and
postoperative DTI for patients with brain tumors and found that in
5/7 patients, the arcuate fascicles following surgery were more
clearly visible compared with those prior to surgery. In addition,
in 6 patients, the language function score increased compared with
that prior to surgery, and the difference was statistically
significant (P=0.004). This study not only validates the important
role of the arcuate fascicle in language function, but also reveals
the positive effect of preoperative DTI-guided surgery on
functional protection (22).
In the present study, the arcuate fascicle and the
pyramidal fascicle, which are the 2 fiber fascicles associated with
language and limb movements, respectively, were selected as the
fiber-tracking objects, and conventional MRI and DTI scans were
performed for the 21 patients in the trial group. According to DTI
tracking of the fiber fascicles, the designated surgical strategy
was chosen. The control group, with 21 patients, underwent a
conventional MRI scan only, and the surgeon chose the surgical
approach nearest to the cortex and away from the functional area.
In the trial group, 18 patients underwent total tumor resection and
3 patients underwent subtotal resection (85.71% total resection
rate). In the control group, 15 patients underwent total resection
and 6 patients underwent subtotal resection (71.43% total resection
rate). There were no statistically significant differences in the
total resection rate between the trial group and the control group.
Although preoperative DTI can indicate the positional association
between the functional fiber and the tumor, and guide the design of
the surgical approach, the tumor resection rate was not
significantly improved, as without intra-operative navigation and
intra-operative MRI, the tumor resection rate and resection scope
are mainly associated with the experience of the surgeon. Following
surgery, the symptom improvement rate was compared between the
trial and control groups. The trial group had an improvement rate
of 85.71%, while the control group had an improvement rate of
47.62%. The symptom improvement rates were statistically
significant when comparing between the trial and control groups.
The KPS improvement values between the trial and control groups
were compared prior to and following surgery, and it was revealed
that the postoperative KPS values for the trial and control groups
were increased compared with the preoperative KPS values; the KPS
improvement rate for the trial group was higher compared with that
of the control group. This suggests that preoperative DTI can guide
a safer surgical approach to improve the symptoms of the patients
and to avoid the occurrence of iatrogenic dysfunction with a
surgeon's limited operation experience. In summary, the present
study revealed that for patients with tumors in the functional
areas of the brain, preoperative DTI can 3-dimensionally reveal the
tumor and its location with regard to the peripheral nerve, and
this can guide the surgical approach and removal of the scope of
assessment. However, the use of preoperative DTI cannot
significantly improve the rate of total resection of the tumor.
Meanwhile, only 3 glioblastoma cases were discovered in the trial
group and 2 in the control group, therefore, it is difficult to
improve the prognosis among these patients, which is important to
avoid neurological function of iatrogenic injury. The effects of
preoperative DTI on the improvement of the prognosis of patients
would require evaluation in future investigations. Moreover, the
present study was not restricted to primary brain tumors, such as
metastatic carcinoma and meningioma, which would also require
specific investigation in further studies.
In conclusion, the use of DTT is an effective
supplement to traditional MRI; it has particular relevance in
preoperative planning, particularly in functional areas of brain
tumors, and can significantly improve the prognostic function of
patients.
Acknowledgements
Not applicable.
Funding
This study was supported by the Shandong Natural
Science Foundation (grant no. ZR2016HB64) and the Funding of
Applied Research Project for postdoctoral researchers in Qingdao
(grant no. 40518060079).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ and HJ designed the study. HZ, YF, LC and JL
performed the imaging analysis and associated tests or experiments.
HL reviewed the literature. JL and HL conducted the statistical
analysis. HZ wrote the manuscript. HJ critical corrected and final
reviewed the manuscript for publication. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Affiliated Hospital of Qingdao University
(Qingdao, China) and the patients provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tian W, Zhang J, Tian F, Shen J, Niu T, He
G and Yu H: Correlation of diffusion tensor imaging parameters and
Gleason scores of prostate cancer. Exp Ther Med. 15:351–356.
2018.PubMed/NCBI
|
|
2
|
Castellano A, Bello L, Michelozzi C,
Gallucci M, Fava E, Iadanza A, Riva M, Casaceli G and Falini A:
Role of diffusion tensor magnetic resonance tractography in
predicting the extent of resection in glioma surgery. Neuro Oncol.
14:192–202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stadlbauer A, Nimsky C, Buslei R,
Salomonowitz E, Hammen T, Buchfelder M, Moser E, Ernst-Stecken A
and Ganslandt O: Diffusion tensor imaging and optimized fiber
tracking in glioma patients: Histopathologic evaluation of
tumor-invaded white matter structures. Neuroimage. 34:949–956.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee JS, Han MK, Kim SH, Kwon OK and Kim
JH: Fiber tracking by diffusion tensor imaging in corticospinal
tract stroke: Topographical correlation with clinical symptoms.
Neuroimage. 26:771–776. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Musiek FE and Weihing J: Perspectives on
dichotic listening and the corpus callosum. Brain Cogn. 76:225–232.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seok JH, Park HJ, Chun JW, Lee SK, Cho HS,
Kwon JS and Kim JJ: White matter abnormalities associated with
auditory hallucinations in schizophrenia: A combined study of
voxel-based analyses of diffusion tensor imaging and structural
magnetic resonance imaging. Psychiatry Res. 156:93–104. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ye AQ, Hubbard Cristinacce PL, Zhou FL,
Yin Z, Parker GJ and Magin RL: Diffusion tensor MRI phantom
exhibits anomalous diffusion. Conf Proc IEEE Eng Med Biol Soc.
2014:746–749. 2014.PubMed/NCBI
|
|
8
|
Liu B, Zhu T and Zhong J: Comparison of
quality control software tools for diffusion tensor imaging. Magn
Reson Imaging. 33:276–285. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Savardekar AR, Patra DP, Thakur JD,
Narayan V, Mohammed N, Bollam P and Nanda A: Preoperative diffusion
tensor imaging-fiber tracking for facial nerve identification in
vestibular schwannoma: A systematic review on its evolution and
current status with a pooled data analysis of surgical concordance
rates. Neurosurg Focus. 44:E52018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rades D, Bolm L, Kaesmann L and Bartscht
T: Karnofsky performance score is predictive of survival after
palliative irradiation of metastatic bile duct cancer. Anticancer
Res. 37:949–951. 2017.PubMed/NCBI
|
|
11
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hattori N, Hirose Y, Sasaki H, Nakae S,
Hayashi S, Ohba S, Adachi K, Hayashi T, Nishiyama Y, Hasegawa M and
Abe M: World Health Organization grade II–III astrocytomas consist
of genetically distinct tumor lineages. Cancer Sci. 107:1159–1164.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ouyang A, Jeon T, Sunkin SM, Pletikos M,
Sedmak G, Sestan N, Lein ES and Huang H: Spatial mapping of
structural and connectional imaging data for the developing human
brain with diffusion tensor imaging. Methods. 73:27–37. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jang SH and Shin SM: The usefulness of
diffusion tensor tractography for estimating the state of
corticobulbar tract in stroke patients. Clin Neurophysiol.
127:2708–2709. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Landi A, Innocenzi G, Grasso G, Meschini
A, Fabbiano F, Castri P and Delfini R: Diagnostic potential of the
diffusion tensor tractography with fractional anisotropy in the
diagnosis and treatment of cervical spondylotic and posttraumatic
myelopathy. Surg Neurol Int. 7 (Suppl 25):S705–S707. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Singh S, Trivedi R, Singh K, Kumar P,
Shankar LR and Khushu S: Diffusion tensor tractography in
hypothyroidism and its correlation with memory function. J
Neuroendocrinol. 26:825–833. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun GC, Chen XL, Zhao Y, Wang F, Hou BK,
Wang YB, Song ZJ, Wang D and Xu BN: Intraoperative high-field
magnetic resonance imaging combined with fiber tract
neuronavigation-guided resection of cerebral lesions involving
optic radiation. Neurosurgery. 69:1070–1084. 2011.PubMed/NCBI
|
|
18
|
Muthusami P, James J, Thomas B,
Kapilamoorthy TR and Kesavadas C: Diffusion tensor imaging and
tractography of the human language pathways: Moving into the
clinical realm. J Magn Reson Imaging. 40:1041–1053. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuhnt D, Bauer MH and Nimsky C: Brain
shift compensation and neurosurgical image fusion using
intraoperative MRI: Current status and future challenges. Crit Rev
Biomed Eng. 40:175–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
D'Andrea G, Familiari P, Di Lauro A,
Angelini A and Sessa G: Safe resection of gliomas of the dominant
angular gyrus availing of preoperative FMRI and intraoperative DTI:
Preliminary series and surgical technique. World Neurosurg.
87:627–639. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kis D, Mate A, Kincses ZT, Voros E and
Barzo P: The role of probabilistic tractography in the surgical
treatment of thalamic gliomas. Neurosurgery. 10 (Suppl
2):S262–S272. 2014. View Article : Google Scholar
|
|
22
|
Hayashi Y, Kinoshita M, Nakada M and
Hamada J: Correlation between language function and the left
arcuate fasciculus detected by diffusion tensor imaging
tractography after brain tumor surgery. J Neurosurg. 117:839–843.
2012. View Article : Google Scholar : PubMed/NCBI
|