Introduction
High mobility group box 1 (HMGB1) is a class of
non-histone chromatin protein that is constitutively expressed in
the nuclei of both cancerous and normal cells. It serves a
regulatory role in numerous cellular processes including DNA
repair, gene transcription, the construction and stability of
nucleosome structure, cell differentiation, proliferation,
migration and apoptosis, signal transduction, and tumour
occurrence, growth, metastasis and invasion (1,2). HMGB1
possesses two homologous DNA-binding motifs known as the HMG boxes
(A and B) and an acidic C-terminal tail. These motifs are able to
recognize and preferentially bind distorted DNA structures, such as
four-way junctions (4H) (3)
cisplatin-modified DNA (4),
hemi-catenated DNA loops (5) and
supercoiled DNA (6). The DNA-binding
capability of HMGB1 also suggests its involvement in DNA-dependent
nuclear processes, including transcription, DNA repair and
recombination.
HMGB1 has been indicated to be upregulated in
various solid tumour types, and high HMGB1 expression levels may
lead to the abnormal expression of certain genes associated with
tumorigenesis (7,8). Additionally, high HMGB1 expression
inhibits apoptotic signalling, which may promote tumour development
(9,10). Furthermore, extracellular HMGB1
promotes cancer cell proliferation, energy metabolism and
angiogenesis, as well as the synthesis of inflammatory factors,
whilst inhibiting the host anticancer immune response, and
contributing to cancer progression (11). Gliomas are the most common malignant
brain tumours and are associated with a high mortality rate
(12). Several studies have
investigated the association between HMGB1 and glioma, and
determined that HMGB1 mRNA and protein levels are significantly
higher in glioma tissues and glioma cell lines, compared with
adjacent normal tissues and immortalized human astrocytes (13,14).
Moreover, HMGB1 expression in glioblastoma multiforme (GBM) cell
lines can activate the AKT and ERK signalling pathways, promoting
GBM-cell invasion via an autocrine pathway (15). HMGB1 has also been reported to
function as a structural protein in nuclear chromatin, regulating
genome replication and recombination, mRNA transcription and DNA
repair (16). Previously, it was
revealed that proliferating glioma cells in GBM tissues exhibited
an upregulation in HMGB1 expression (17), suggesting that HMGB1 expression may
correlate with mitosis in glioma cells.
Paraformaldehyde (PFA) is a chemical fixative widely
used in immunohistochemistry (IHC) and immunocytochemistry (ICC),
with the ability to preserve cell morphology via the cross-linking
of different biomolecules. Nevertheless, PFA fixation has been
suggested to affect the interaction between HMGB1 and mitotic
chromosomes in both animal and plant cells (18,19). In
the present study, the effect of PFA fixation on this interaction
was analysed in human glioma cells; the localization of HMGB1 in
mitotic cells was investigated using enhanced green fluorescence
protein (EGFP)-tagged proteins and chromosome spreading. Chilled
methanol, supplemented with 5% (v/v) acetic acid (20), was used as an alternative fixative to
further determine the association between HMGB1 and mitotic
chromosomes in glioma cells.
Materials and methods
Glioma tissues and cell lines
The GBM tissue microarray (TMA) was obtained from
Wuhan Aiwei Biotechnology Co. Ltd., and comprised 60 GBMs in each
section. A total of 41 paraffin-embedded glioma tissue sections
(5-µm thick; 25 from males and 16 from females; median age: 47.7
years) were obtained between January 2017 and December 2018 from
the Department of Histology of the No. 988 Hospital of Joint
Logistic Support Force (Zhengzhou, China). All glioma samples were
fixed with 4% paraformaldehyde (PFA) for ~24 h at 4°C and evaluated
by two experienced pathologists according to the World Health
Organisation classification (2016) (21). The Research Ethics Committees of the
General Hospital of Chinese People's Liberation Army (Beijing,
China) and the No. 988 Hospital of Joint Logistic Support Force and
Zhengzhou University (Zhengzhou, China) reviewed and approved the
study according to the principles expressed in the Declaration of
Helsinki. Informed written consent was obtained from each patient,
prior to surgery.
The HA1800 astrocyte cell line was purchased from
Shanghai Ji Ning Industrial Co., Ltd., and three human glioma cell
lines [U251-MG, U87-MG (glioblastoma of unknown origin) and U118-MG
(glioblastoma of unknown origin)] were acquired from China
Infrastructure of Cell Line Resources (Institute of Basic Medical
Sciences, Chinese Academy of Medical Sciences). All four cell lines
were tested for mycoplasma contamination and the three glioma cell
lines were authenticated by STR profiling. The cells were
subsequently cultured in Dulbecco's modified Eagle's medium (DMEM;
cat. no. 04-052-1ACS) supplemented with 10% foetal bovine serum
(FBS; cat. no. 04-001-1ACS, both Biological Industries) and 1%
penicillin-streptomycin, and maintained at 37°C (5% CO2)
in a humidified incubator.
IHC
The paraffin-embedded glioma tissue sections were
deparaffnized with xylene (100%, twice for 5 min) and rehydrated by
gradient ethanol (100%, twice for 5 min; 95%, twice for 5 min; 90%
for 5 min; and 80% for 5 min) at room temperature (RT).
Subsequently, antigen retrieval was performed using citrate
solution (pH 6.0) at 95°C for 15 min. The slides were then treated
with methanol containing 3% H2O2 to quench
endogenous peroxidase activity, before being washed three times
with phosphate buffered saline (PBS) for 5 min each. The sections
were incubated in a blocking solution [5% bovine serum albumin
(BSA; Beijing Solarbio Science and Technology co. Ltd., cat. no.
A8020) plus 0.3% Triton X-100 in PBS] for 1 h at RT, and then
incubated with a rabbit anti-human HMGB1 primary antibody (1:1,000;
cat. no. ab18256; Abcam) overnight at 4°C. The sections were then
rinsed with PBS and incubated with horseradish
peroxidase-conjugated goat anti-rabbit IgG polyclonal antibody
(1:1,000; OriGene Technologies, Inc.; cat. no. ZB-23) for 2 h at
RT. Subsequently, 3,3′-diaminobenzidine (DAB) staining was
performed; DAB was dropped onto the slides, which were then
incubated at 37°C for 3 min. The sections were then counterstained
using haematoxylin, washed with distilled water, differentiated
using 1% hydrochloric acid (in 70% ethanol) and mounted with
neutral resin. For the fluorescence-labelled reaction, the Alexa
Fluor 488 donkey anti-rabbit IgG secondary antibody (1:1,000;
Abcam; cat. no. ab150073) was used. The nuclei were stained with
DAPI (1:5,000; Beijing Solarbio Science & Technology Co. Ltd.;
cat. no. C0060) and mounted using 50% (v/v) glycerol with PBS.
Images were captured and analysed using a confocal microscope
(magnifications, ×40 and ×100; cat. no. U-TBI90; Olympus
Corporation).
ICC
The glioma and astrocyte cell lines were cultured
over sterile glass coverslips and then fixed for 20 min using: i)
4% PFA at RT; or ii) methanol with 5% (v/v) acetic acid at −20°C.
The cells were then rehydrated and permeabilized using 0.5% Triton
X-100 (in PBS) for 5 min at RT, prior to being blocked with 3% BSA
for 1 h at RT. The cells were then incubated with a HMGB1 primary
antibody (1:1,000 diluted in 1% BSA; Abcam; cat. no. ab18256)
overnight at 4°C, followed by subsequent incubation with Alexa
Fluor 488 donkey anti-rabbit IgG (1:1,000; Abcam; cat. no.
ab150073). The cells were then counterstained using DAPI.
Coverslips were mounted in 50% (v/v) glycerol with PBS, and
observed under an Olympus confocal microscope (magnification, ×100;
cat. no. U-TBI90; Olympus Corporation).
Cell transfection
Glioma and astrocyte cells were cultured to 80%
confluence in six-well culture plates, and then transfected with 2
µg pEGFP-C1 or pEGFP-HMGB1-C1 using Simple-Fect Transfection
Reagent (cat. no. profect-01; Zhengzhou Kebang Biological
Technology Co., Ltd.), according to the manufacturer's
protocol.
Live cell microscopy
Live cell microscopy was performed 48 h after
transfection. Briefly, the transfected cells were incubated for 10
min at 37°C in culture medium containing 0.2 µg/ml Hoechst 33342.
They were then washed before the sub-nuclear compartmentalization
and mitosis were evaluated under an Olympus confocal microscope
(magnification, ×100).
Chromosome spreading
The preparation of chromosome spreads was performed
according to pre-described methods (20). U87-MG, U118-MG and HA1800 cells were
incubated for 4 h at 37°C separately, in medium (DMEM; cat. no.
04-052-1ACS] supplemented with 10% FBS (cat. no. 04-001-1ACS), both
from Biological Industries, and 1% penicillin-streptomycin
containing 0.1 µg/ml nocodazole. They were subsequently dislodged
by swirling the media, centrifuged at 600 × g for 5 min at 4°C,
resuspended in hypotonic buffer [0.25% (w/v) sodium citrate and
37.5 mM KCl] and incubated for 20 min at 37°C. The cells were then
pelleted at 900 × g for 10 min. Following resuspension in 0.5 ml
hypotonic buffer, 5 ml ME buffer (methanol and glacial acetic acid,
4:1) was added and gently mixed. The cells were then re-pelleted
and resuspended in fresh ME buffer (methanol and glacial acetic
acid, 4:1). After 1 h incubation at RT, the cells were centrifuged
at 900 × g for 10 min, spread onto the pre-cooled (−20°C) and dry
coverslips (cat. no. EF.188105; Jiangsu Shitai Experimental
Equipment Co., Ltd.) and then air-dried. The chromosome spreads
were then incubated in blocking buffer and subjected to
immunodetection using an anti-HMGB1 antibody, according to the
methodology described above.
Western blotting
Cells were lysed using radioimmunoprecipitation
assay buffer and the protein concentration was determined using the
BCA Protein Assay kit (Beijing Solarbio Science & Technology
Co., Ltd., cat. no. PC0020). A total of 20 µg from each sample was
separated via SDS-PAGE on a 10% gel. The separated proteins were
then transferred onto PVDF membranes (EMD Millipore; cat. no.
IPVH00010), which were blocked for 1 h at RT in 5% non-fat dry milk
[in Tris-buffered saline wash buffer and Tween 20 (TBST)]. The
membranes were then incubated at 4°C overnight with the following
primary antibodies: anti-HMGB1 (Abcam; cat. no. ab18256; 1:2,000)
and anti-β-actin (OriGene Technologies, Inc.; cat. no. TA-09;
1:2,000). Following primary incubation, the membranes were
incubated at RT for 2 h with horseradish peroxidase-conjugated goat
anti-rabbit/mouse IgG polyclonal secondary antibodies (ZSGB-Bio;
cat. no. ZB-23; 1:5,000). Enhanced chemiluminescent substrate
(Dalian Meilun Biology Technology Co., Ltd.; cat no.
MA0186-Sep-25D) was used to conduct autoradiography and visualise
the protein bands. The signal intensity of each band was determined
using Image J software (National Institutes of Health; version
1.4.3.67).
Statistical analysis
Data are presented as the mean ± standard deviation
(SD). Statistical analyses were performed using one-way ANOVA and
Tukey's multiple comparison test with GraphPad Prism 5 software
(GraphPad Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
HMGB1 combines with condensed
chromosomes in the proliferating cells of PFA-fixed glioma
tissues
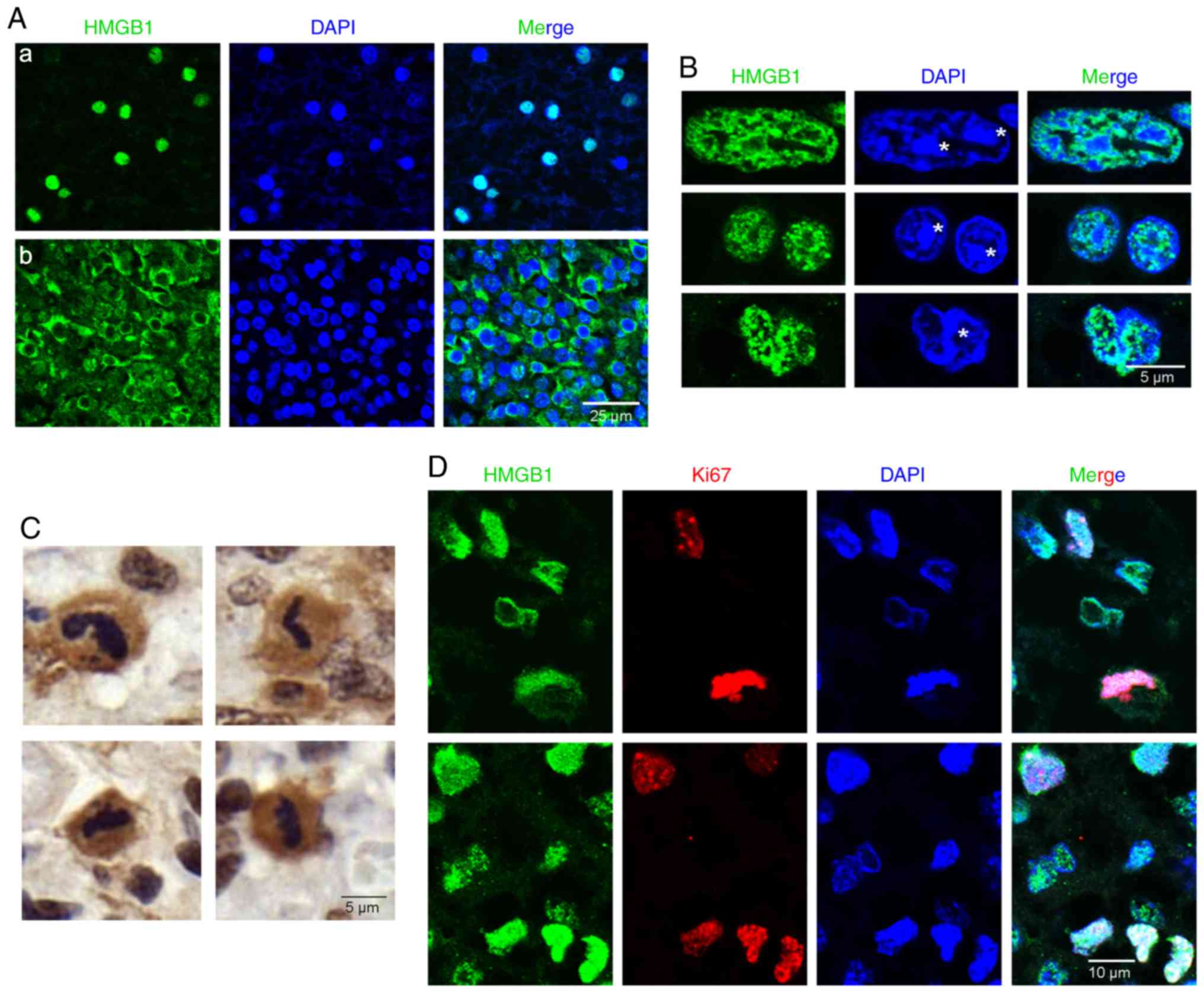
The cellular localization of HMGB1 in PFA-fixed
glioma tissues was examined, and HMGB1 was determined to localize
to both the nuclei (Fig. 1A-a) and
cytoplasm (Fig. 1A-b) of glioma
tissue cells. Within the nuclei in interphase (asterisks, Fig. 1B), HMGB1 was primarily aggregated in
close proximity to the chromatin blocks, with additional, diffuse
expression throughout these blocks. The HMGB1 expression pattern in
the TMA sections was then examined, indicating diffuse HMGB1
expression in the cytoplasm of some mitotic cells. However, DAB
staining could not determine whether HMGB1 was combined with the
condensed chromosomes (Fig. 1C). To
confirm localization, a co-staining IHC assay was performed for
HMGB1 and the proliferation marker Ki67. Consequently, HMGB1 was
discovered to combine with condensed chromosomes in Ki67-positive
proliferating glioma cells (Fig.
1D). Collectively, these results indicated that HMGB1 combined
with the chromosomes of proliferating cells of PFA-fixed glioma
tissues.
HMGB1 dissociates from mitotic
chromosomes in fixative-treated glioma cells
Ki67 is preferentially expressed during the late
G1, S, G2 and M phases of the cell cycle
(22). Therefore, Ki67-positive
cells may include not only mitotic cells, but also those in
interphase. To investigate the relationship between HMGB1 and
mitotic chromosomes in vitro, the intracellular localization
of HMGB1 was determined in glioma cells and HA1800 astrocytes.
HMGB1 protein expression levels were elucidated using western
blotting, and no significant difference was observed between HMGB1
expression in glioma cells and HA1800 astrocytes (Fig. S1).
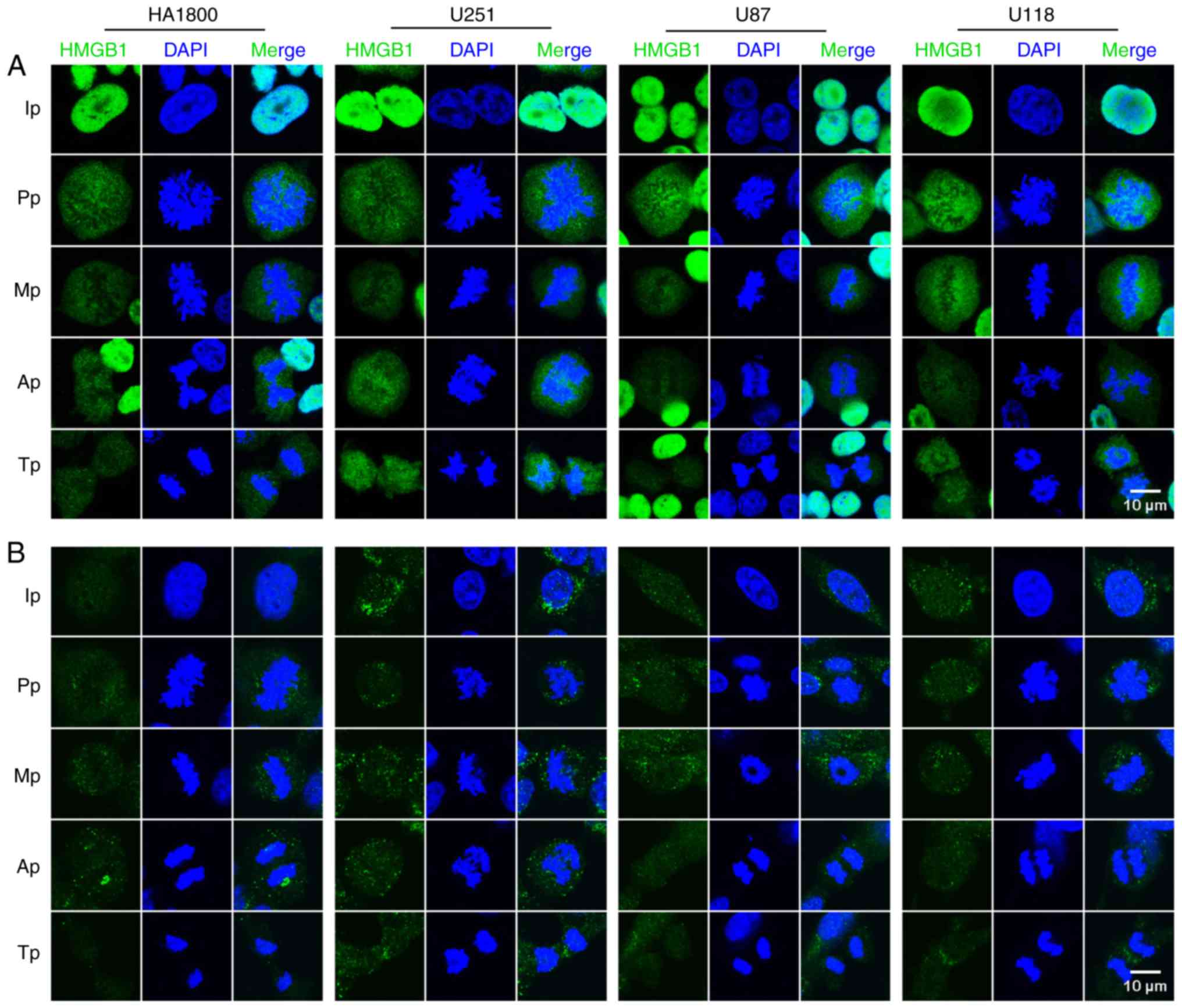
The localization of HMGB1 in HA1800 astrocytes and
three glioma cell lines (all fixed with PFA) was then investigated.
The results revealed that HMGB1 was detected in the nuclei, with
diffuse expression in the nucleoli (Fig.
2A). Notably, the majority of HMGB1 was found to be dissociated
from the mitotic chromosomes, with certain cells showing only a
minor fraction of HMGB1 expression with condensed chromatin in the
nuclei, at each phase of mitosis (Fig.
2A). Considering the potential limitations of PFA, which have
been highlighted in previous literature (18–20), an
alternative fixation procedure (chilled methanol with 5% (v/v)
acetic acid) (20) was conducted.
However, the staining results were similar to those obtained with
PFA fixation, implying that this was also an unsuitable method of
fixing glioma cells (Fig. 2B). In
summary, the results of the current study demonstrated that HMGB1
was dissociated from mitotic chromosomes in fixative-treated glioma
cells and astrocytes.
HMGB1 combines with mitotic
chromosomes in both live glioma cells and mitotic chromosome
spreads
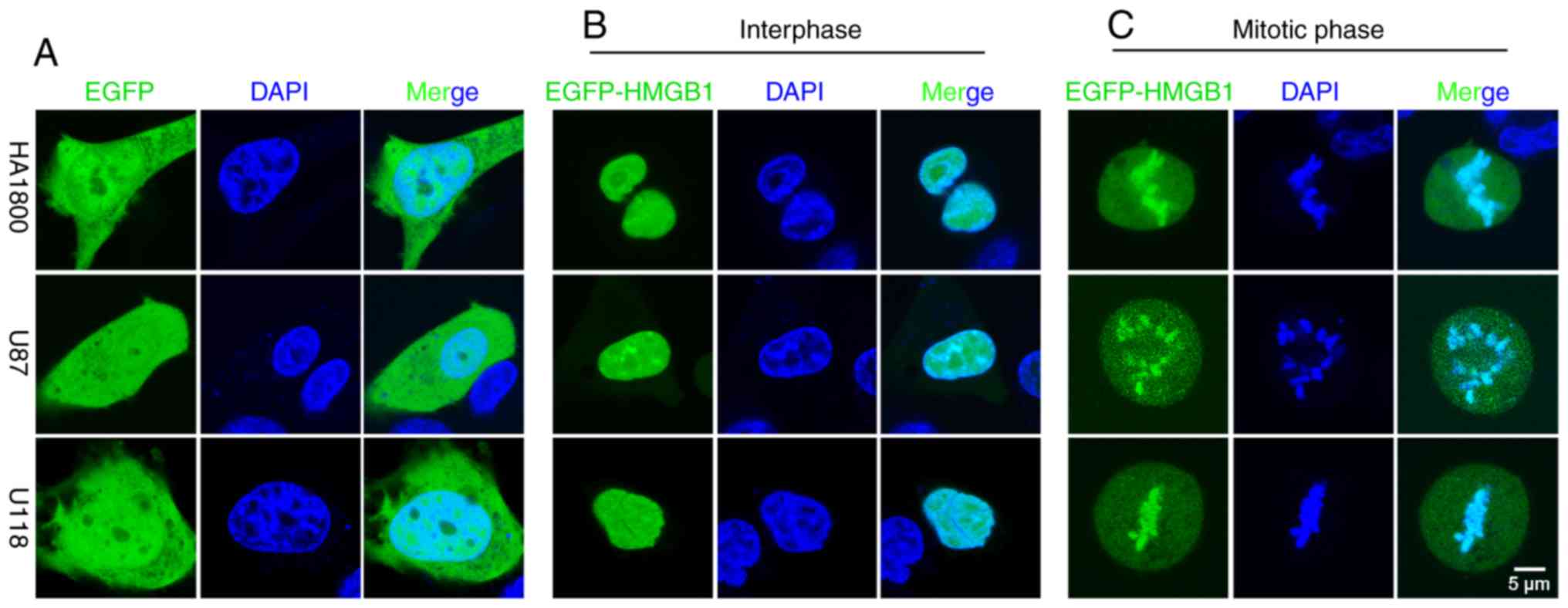
To avoid artefacts that may have been introduced by
the fixation procedures, an EGFP-tagged human HMGB1 (EGFP-HMGB1)
plasmid was constructed and transfected into the glioma (U87-MG and
U118-MG) and astrocyte (HA1800) cell lines; EGFP localization was
subsequently determined. In the live cells, EGFP localized to both
the cytoplasm and nuclei during interphase (Fig. 3A). In EGFP-HMGB1 transfected cells,
there was no significant difference between the localization of
HMGB1 between fixed and unfixed cells in interphase. In both cases,
HMGB1 localized to the nuclei and diffused into the nucleoli
(Figs. 2A and 3B). The present results were consistent
with previously reported studies (16). However, there was a discrepancy in
the results between fixed and unfixed mitotic cells; diffuse
expression was detected in the cytoplasm, but compacted EGFP
expression was detected along the metaphase plate (Fig. 3C), indicating an association between
HMGB1 and the mitotic chromosomes in glioma cells and
astrocytes.
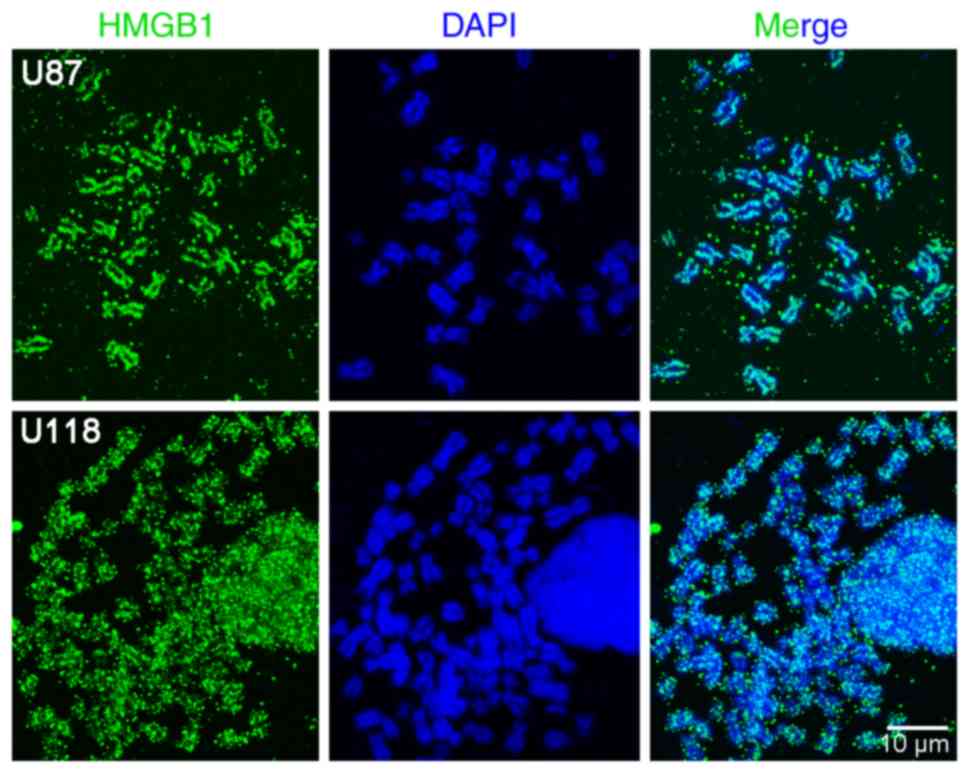
Chromosomal details can be resolved more accurately
using chromosome spreading, compared with a whole-cell
visualisation techniques; thus, chromosome spreads were prepared
from U87-MG and U118-MG glioma cells treated with nocodazole to
initiate cell cycle arrest. HMGB1 was found to be significantly
associated with mitotic chromosomes, and distributed non-uniformly
along the condensed chromatin (Fig.
4). However, multiple HMGB1-positive areas were detected in
close proximity to the mitotic chromosome. This may be attributable
to the use of methanol/glacial acetic acid during fixation (for the
preparation of the chromosome spreads). Although the reason for the
uneven distribution of HMGB1 in the mitotic chromosomes requires
further investigation, the results using live cells and mitotic
chromosome spreading techniques revealed that HMGB1 combines with
mitotic chromatin in all of the cell lines analysed.
Discussion
HMGB1 is a non-histone chromatin-binding protein,
which has been proven to regulate the tumorigenesis and progression
of multiple cancer types, including mesothelioma, gastric and
colorectal cancer and hepatocellular carcinoma (23,24). The
results of the present study, and those of a study conducted by
Wang et al (25), indicated
that HMGB1 expression was upregulated in glioma tissues. HMGB1 is
typically expressed in the nucleus of normal cells. However, in
tumour cells it may be localized to the nucleus, cytoplasm or
extracellular space, regulating gene transcription and the
autophagic and inflammatory pathways associated with tumour cell
proliferation (25,26). Consequently, the detection of both
nuclear and cytoplasmic HMGB1 in the glioma tissues used in the
present study was unsurprising.
In interphase nuclei, HMGB1 exhibits a differential
distribution pattern between cells from glioma tissues and cultured
glioma cells; HMGB1 accumulated in the vicinity of, or distributed
diffusely on the chromatin blocks in cells from glioma tissues.
Whereas in cultured glioma cells, the distribution of HMGB1 almost
entirely overlapped with DAPI or Hoechst staining, confirming that
the protein is distributed throughout the entire nucleus in glioma
cells, in vitro. It is speculated that this discrepancy is
due to the different distribution patterns of chromatin in glioma
tissues, compared with those in cultured glioma cells. For example,
in glioma tissues, chromatin is unevenly distributed and packaged
in the interphase nuclei. Conversely, in the nuclei of glioma cells
in vitro, it is more evenly distributed. The diffuse HMGB1
expression observed in the chromatin blocks may represent an
artefact of PFA fixation, in accordance with the results of the
present study, which indicated that treatment with PFA may lead to
the dissociation of HMGB1 from compacted chromatin. The
dissociation of HMGB1 from the compacted chromosomes suggests a
weak affinity of HMGB1 for chromatin, such that the transition from
the bound to unbound state is rapid and transient. This
status-change feature of HMGB1 may result in the ability of glioma
cells to adapt rapidly to various environmental stressors. It has
been demonstrated that the acetylation of HMGB1 weakens its binding
affinity to DNA, promoting its migration to various tissues and
resulting in the secretion of proinflammatory cytokines (27).
In the present study, although the majority of
HMGB1-positive nuclei were at the interphase stage in glioma
tissues, a proportion of proliferating cells also expressed HMGB1.
Moreover, HMGB1 was localized in the condensed chromosomes of
certain Ki67-positive proliferating cells in PFA-fixed glioma
tissue sections. However, this result was not consistent in
PFA-fixed glioma cells in vitro.
PFA is a frequently-used fixative in microscopic
studies. However, it has been discovered to create artefacts due to
the disruption of cellular structures and lowered protein
antigenicity (28). In fact, certain
studies have suggested that PFA fixation may affect the interaction
between mammalian HMGB proteins and mitotic chromosomes (18,19).
Kumar et al (20) proposed
that chilled methanol (−20°C) with 5% (v/v) acetic acid was a
suitable alternative fixative for mitotic chromatin. Therefore,
this fixative was applied to re-investigate the binding of HMGB1 to
the mitotic chromosomes in glioma cells. Counterintuitively, HMGB1
failed to bind the mitotic chromosomes. This may be because this
fixation method was also unsuitable for the observation of glioma
cells; it was thus hypothesized that that live-cell imaging of
fluorescently-tagged proteins may represent an improved method for
the observation of HMGB1-chromatin interactions, as it would bypass
any potential artefacts caused by the fixation process (18,29).
Therefore, EGFP-tagged hHMGB1 plasmids were transfected into live
astrocyte and glioma cells, and binding of HMGB1 to the mitotic
chromosomes was observed. Moreover, a chromosomal spread assay
confirmed the binding of HMGB1 to the mitotic chromosomes. Thus,
the results of the present study suggest that HMGB1 is a component
of the mitotic chromosome, and that the use of fixatives may
disrupt its affinity for mitotic chromosomes in glioma cells.
In the present study, it was observed that HMGB1 was
bound to the condensed chromosomes of proliferating glioma cells
in vivo fixed with PFA, and it is hypothesized that this
result was due to the possible manipulation of cells by fixation.
HMGB1 protein in cultured cells may be more accessible to
manipulation by fixatives, compared with those in vivo.
Moreover, Ki67 is present during all active phases of the cell
cycle (G1, S, G2 and M) (22); therefore, the HMGB1 and Ki67
double-positively stained cells may not have been in the mitotic
stage of the cell cycle. Both PFA and chilled methanol with 5%
acetic acid can create artefacts during fixative cross-linking,
which may explain the dissociation of HMGB1 from condensed mitotic
chromosomes in glioma cells. Thus, novel fixation procedures are
required to accurately determine the association between HMGB1 and
mitotic chromosomes. Applying high pressure freezing or embedding
cells in resin may represent improved methods for preserving
cellular structure (30,31). Moreover, chromosome spreading is a
more accurate method of resolving chromosomal details (20). Thus, this method was used in the
present study to observe the uneven distribution of HMGB1 on
mitotic chromosomes. However, only the combination of HMGB1 with
mitotic chromosomes in cultured glioma cells was elucidated. The
histological effect on mitotic chromosomes in glioma tissues should
be investigated in future studies.
Chromosomal spreading is an alternative technique
used to assess protein binding to mitotic chromosomes in the
tissues. However, it is first necessary to sort glioma cells from
glioma tissues via flow cytometry, after which the subsequent
procedures are similar to those used for cultured cells. Notably,
the treatment of chromosomes with fixatives (methanol/glacial
acetic acid) is a routine step during chromosomal spread assays.
This treatment may lead to the dissociation of protein from the
mitotic chromosomes, as demonstrated in the present study. Hence,
the identification of novel fixation procedures would be beneficial
in the preparation of chromosomal spread specimens.
During mitosis, transcription is halted, chromatin
condenses and the majority of basal transcription factors (TFs) are
reportedly excluded from the chromosomes. However, a class of TFs
was discovered that are continuously bound to mitotic chromosomes,
suggesting a potential mechanism for the maintenance of certain
transcriptional programmes throughout the cell cycle, termed
mitotic bookmarking (32).
Accordingly, HMGB1 (a nuclear protein involved in the
transcription-level regulation of various genes) may serve as a
mitotic bookmarker for daughter glioma cells, enabling the
re-establishment of the original transcription programme in glioma
cells. Nevertheless, the combination of HMGB1 with mitotic
chromosomes in astrocytes was also detected. Therefore, the
discrepancy of mitotic chromosomal binding to HMGB1 between normal
glial and glioma cells should be investigated in future studies,
using techniques such as chromatin immunoprecipitation. In
addition, determination of the role of HMGB1 binding to enable
glioma cells to ‘remember’ their identity would increase our
understanding of glioma development and progression.
In the present study, HMGB1 was typically expressed
in the nuclei during interphase, whilst it dissociated from the
condensed chromosomes during the mitotic phase of all four cell
lines, following PFA treatment. It was observed that, although the
majority of HMGB1 had dispersed into the cytoplasm, a minor
fraction of HMGB1 remained in the nucleus, where it combined with
the condensed chromosomes of all four cell types (Fig. 2A). It is possible that PFA did not
comprehensively disrupt the interaction of HMGB1 with all of the
condensed chromosomes. However, the difference in the binding
affinity of HMGB1 to mitotic chromosomes, between normal astrocytes
and glioma cell lines, is yet to be elucidated. Moreover, no
significant difference was observed between the total expression
level of HMGB1 in the four cell lines (Fig. S1). This was inconsistent with the
result in glioma tissues, in which HMGB1 expression was upregulated
(17). The presence of alternative
stimulating factors in vivo may provide a possible
explanation for this difference.
The present study revealed that HMGB1 was
constitutively expressed in the nuclei of four cell lines under
non-stimulating conditions, which differed from the diffuse
expression (in the nuclei, cytoplasm and extracellular space)
observed in glioma tissues (17). It
has been revealed that glioma cells secrete numerous chemokines,
cytokines and growth factors that promote the infiltration of
non-neoplastic cells, creating a specific tumor microenvironment
that influences the biological properties of glioma cells (33). As a highly conserved nuclear protein,
HMGB1 is a chromatin-binding factor that is able to alter DNA
structure and promote access to transcriptional protein assemblies
on specific DNA targets (1,34,35).
Therefore, the difference in HMGB1 function between the nuclei of
normal astrocytes and glioma cells should be investigated in future
studies.
In conclusion, the results of the present study
suggest that HMGB1 combines with mitotic chromosomes in glioma
cells. However, the use of fixatives leads to the dissociation of
HMGB1 from mitotic chromosomes. Additionally, EGFP-tagged HMGB1
proteins in live glioma cells imitated the localization of
endogenous HMGB1 protein at different mitotic stages. Chromosome
spreading is a technique that may also be applied to investigate
the combination of HMGB1 with mitotic chromosomes. A proportion of
studies on glioma have used fixatives to treat tissues or cells.
Considering the artefacts induced by fixatives, the biological
function of HMGB1, especially with regard to its sub-cellular
localization, should be carefully reconsidered.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81402455) and the
Key Scientific Research Projects of Higher Education Institutions
in Henan Province (grant no. 20A310020). The funding sources had no
influence on the study design or the collection, analysis and
interpretation of data, or manuscript writing.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
Study concept and design was conceived by LJ, YH and
AY. Acquisition of data was performed by LJ, HS, YS, WF, GW and XL.
LJ, HS and YS analysed and interpreted the data. LJ, HS and YS
conducted the statistical analyses. Writing of the manuscript was
conducted by AY, LJ and YH. All authors approved the final version
of the manuscript.
Ethics approval and consent to
participate
The Research Ethics Committees of the General
Hospital of Chinese People's Liberation Army (Beijing, China), No.
988 Hospital of Joint Logistic Support Force; and Zhengzhou
University (Zhengzhou, Henan Province, China) reviewed and approved
the study according to the principles expressed in the Declaration
of Helsinki. Written informed consent was obtained from each
participant.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tang D, Kang R, Zeh HJ 3rd and Lotze MT:
High-mobility group box 1 and cancer. Biochim Biophys Acta.
1799:131–140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Belgrano FS, de Abreu da Silva IC, Bastos
de Oliveira FM, Fantappie MR and Mohana-Borges R: Role of the
acidic tail of high mobility group protein B1 (HMGB1) in protein
stability and DNA bending. PLoS One. 8:e795722013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bianchi ME, Beltrame M and Paonessa G:
Specific recognition of cruciform DNA by nuclear protein HMG1.
Science. 243:1056–1059. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park S and Lippard SJ: Redox
state-dependent interaction of HMGB1 and cisplatin-modified DNA.
Biochemistry. 50:2567–2574. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jaouen S, de Koning L, Gaillard C,
Muselikova-Polanska E, Stros M and Strauss F: Determinants of
specific binding of HMGB1 protein to hemicatenated DNA loops. J Mol
Biol. 353:822–837. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ugrinova I, Pashev IG and Pasheva EA:
Post-synthetic acetylation of HMGB1 protein modulates its
interactions with supercoiled DNA. Mol Biol Rep. 36:1399–1404.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Völp K, Brezniceanu ML, Bösser S, Brabletz
T, Kirchner T, Göttel D, Joos S and Zörnig M: Increased expression
of high mobility group box 1 (HMGB1) is associated with an elevated
level of the antiapoptotic c-IAP2 protein in human colon
carcinomas. Gut. 55:234–242. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choi YR and Kim H, Kang HJ, Kim NG, Kim
JJ, Park KS, Paik YK, Kim HO and Kim H: Overexpression of high
mobility group box 1 in gastrointestinal stromal tumors with KIT
mutation. Cancer Res. 63:2188–2193. 2003.PubMed/NCBI
|
|
9
|
Wagner KU and Schmidt JW: The two faces of
Janus kinases and their respective STATs in mammary gland
development and cancer. J Carcinog. 10:322011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guerriero JL, Ditsworth D, Catanzaro JM,
Sabino G, Furie MB, Kew RR, Crawford HC and Zong WX: DNA alkylating
therapy induces tumor regression through an HMGB1-mediated
activation of innate immunity. J Immunol. 186:3517–3526. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang R, Zhang Q, Zeh HJ 3rd, Lotze MT and
Tang D: HMGB1 in cancer: Good, bad, or both? Clin Cancer Res.
19:4046–4057. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seidu RA, Wu M, Su Z and Xu H: Paradoxical
role of high mobility group Box 1 in glioma: A suppressor or a
promoter? Oncol Rev. 11:3252017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang J, Liu C and Hou R: Knockdown of
HMGB1 improves apoptosis and suppresses proliferation and invasion
of glioma cells. Chin J Cancer Res. 26:658–668. 2014.PubMed/NCBI
|
|
14
|
Gupta P, Ghosh S, Nagarajan A, Mehta VS
and Sen E: β-defensin-3 negatively regulates TLR4-HMGB1 axis
mediated HLA-G expression in IL-1β treated glioma cells. Cell
Signal. 25:682–689. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng P, Ma Y, Gao Z and Duan L: High
mobility group box 1 (HMGB1) predicts invasion and poor prognosis
of glioblastoma multiforme via activating AKT signaling in an
autocrine pathway. Med Sci Monit. 24:8916–8924. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Prasad R, Liu Y, Deterding LJ, Poltoratsky
VP, Kedar PS, Horton JK, Kanno S, Asagoshi K, Hou EW, Khodyreva SN,
et al: HMGB1 is a cofactor in mammalian base excision repair. Mol
Cell. 27:829–841. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jia L, Song Y, Song H, Wang G, Fan W, Li
X, Zheng H and Yao A: Overexpression of high mobility group box 1
(HMGB1) has no correlation with the prognosis in glioma. Biomark
Med. 13:851–863. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li MW, Zhou L and Lam HM: Paraformaldehyde
fixation may lead to misinterpretation of the subcellular
localization of plant high mobility group box proteins. PLoS One.
10:e1350332015.
|
|
19
|
Pallier C, Scaffidi P, Chopineau-Proust S,
Agresti A, Nordmann P, Bianchi ME and Marechal V: Association of
chromatin proteins high mobility group box (HMGB) 1 and HMGB2 with
mitotic chromosomes. Mol Biol Cell. 14:3414–3426. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kumar S, Chaturvedi NK, Kumar S and Tyagi
RK: Agonist-mediated docking of androgen receptor onto the mitotic
chromatin platform discriminates intrinsic mode of action of
prostate cancer drugs. Biochim Biophys Acta. 1783:59–73. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 world health organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bruno S and Darzynkiewicz Z: Cell cycle
dependent expression and stability of the nuclear protein detected
by Ki-67 antibody in HL-60 cells. Cell Prolif. 25:31–40. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Jiang Z, Yan J and Ying S: HMGB1
as a potential biomarker and therapeutic target for malignant
mesothelioma. Dis Markers. 2019:41831572019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu T, Zhang W, Yang G, Li H, Chen Q, Song
R and Zhao L: HMGB1 overexpression as a prognostic factor for
survival in cancer: A meta-analysis and systematic review.
Oncotarget. 7:50417–50427. 2016.PubMed/NCBI
|
|
25
|
Wang XJ, Zhou SL, Fu XD, Zhang YY, Liang
B, Shou JX, Wang JY and Ma J: Clinical and prognostic significance
of high-mobility group box-1 in human gliomas. Exp Ther Med.
9:513–518. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He SJ, Cheng J, Feng X, Yu Y, Tian L and
Huang Q: The dual role and therapeutic potential of high-mobility
group box 1 in cancer. Oncotarget. 8:64534–64550. 2017.PubMed/NCBI
|
|
27
|
Guo ZS, Liu Z, Bartlett DL, Tang D and
Lotze MT: Life after death: Targeting high mobility group box 1 in
emergent cancer therapies. Am J Cancer Res. 3:1–20. 2013.PubMed/NCBI
|
|
28
|
Wilson SM and Bacic A: Preparation of
plant cells for transmission electron microscopy to optimize
immunogold labeling of carbohydrate and protein epitopes. Nat
Protoc. 7:1716–1727. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Raccaud M, Friman ET, Alber AB, Agarwal H,
Deluz C, Kuhn T, Gebhardt JCM and Suter DM: Mitotic chromosome
binding predicts transcription factor properties in interphase. Nat
Commun. 10:4872019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
McDonald KL and Auer M: High-pressure
freezing, cellular tomography, and structural cell biology.
Biotechniques. 41:137–141. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xiong H, Zhou Z, Zhu M, Lv X, Li A, Li S,
Li L, Yang T, Wang S, Yang Z, et al: Chemical reactivation of
quenched fluorescent protein molecules enables resin-embedded
fluorescence microimaging. Nat Commun. 5:39922014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Teves SS, An L, Hansen AS, Xie L, Darzacq
X and Tjian R: A dynamic mode of mitotic bookmarking by
transcription factors. Elife. 5(pii): e222802016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gieryng A, Pszczolkowska D, Walentynowicz
KA, Rajan WD and Kaminska B: Immune microenvironment of gliomas.
Lab Invest. 97:498–518. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lotze MT and Tracey KJ: Tracey
High-mobility group box 1 protein (HMGB1): Nuclear weapon in the
immune arsenal. Nat Rev Immunol. 5:331–342. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Müller S, Scaffidi P, Degryse B, Bonaldi
T, Ronfani L, Agresti A, Beltrame M and Bianchi ME: New EMBO
members' review: The double life of HMGB1 chromatin protein:
architectural factor and extracellular signal. EMBO J.
20:4337–4340. 2001. View Article : Google Scholar : PubMed/NCBI
|