Introduction
High-dose chemotherapy combined with autologous
hematopoietic stem cell transplantation (ASCT) has been widely used
in the treatment of hematologic malignancies, including multiple
myeloma (MM) and a number of different types of lymphoma. This
combination therapy has been proven to significantly improve the
progression-free survival (PFS) and overall survival (OS) times of
patients (1). Although since 2000, a
large number of targeted treatments have been used in clinical drug
therapy, ASCT still results in the most favorable patient outcome
(1–3). Efficient acquisition of autologous stem
cells is the premise of successful ASCT. Peripheral blood
hematopoietic stem cells (PBSCs) are the stem cell sources for
almost all ASCTs. Compared with ASCT with bone marrow hematopoietic
cells, it is generally accepted that ASCT with PBSCs requires fewer
cells for infusion; additionally, the collection procedure for
PBSCs is simpler, and patients recover more quickly, thus resulting
in a shorter period of hospitalization. During the PBSC
mobilization process, monitoring the mononuclear and CD34+ cell
count in the peripheral blood enables the prediction of acquisition
efficiency, and helps to determine the timing of acquisition. Hübel
et al (3) used peripheral
blood CD34+ cells as a routine predictive indicator. However, few
studies have reported precise changes in the CD34+ cell count
during the mobilization process.
MM and lymphoma are the major hematologic
malignancies treated with ASCT and further studies are required to
improve PBSC mobilization, as well as to accurately identify
patients at a high risk of mobilization failure. In the present
study, in order to identify the factors influencing hematopoietic
stem cell mobilization, the clinical data of 128 MM and lymphoma
patients who received ASCT were retrospectively analyzed.
Materials and methods
Patients
The present study was approved by the ethical
committee of the Bone Marrow Transplantation Center of the First
Affiliated Hospital, Zhejiang University, and all participants
provided written informed consent for participation. The clinical
data of MM and lymphoma patients admitted to the center between
April 2006 and October 2013 were retrospectively analyzed. All
participating patients were recruited following routine clinical
examinations, and were suitable for, and agreed to accept ASCT
therapy. A total of 128 patients (72 male and 56 female; mean age,
44 years; age range, 16–64 years) were studied. There were 53 cases
with MM and 75 cases with lymphoma; 7 of these were Hodgkin's
lymphoma (HL) and 68 were non-Hodgkin's lymphoma (NHL) based on the
World Health Organization diagnostic criteria (4). Most of the patients accepted PBSC
mobilization and collection for the first time, but three patients
underwent re-collection after first-time failure.
Treatment schemes and PBSC
mobilization protocol
All MM patients were treated with high-dose
cyclophosphamide (CTX; 3–4 g/m2 every two days). Of the
75 patients with lymphoma, 33 patients were treated with
chemotherapy adopting the CHOP scheme (cyclophosphamide,
epirubicin, vincristine and prednisone), five cases of which were
supplemented with rituximab, 20 cases with etoposide, and five
cases with rituximab and etoposide; 17 patients were treated with a
high-dose of CTX, two cases in which rituximab was additionally
included; nine patients underwent chemotherapy adopting the
HyperCVAD part A scheme (high-dose cyclophosphamide, doxorubicin,
dexamethasone and vincristine); another nine patients received
chemotherapy adopting the HyperCVAD part B scheme (high-dose
cytosine arabinoside and methotrexate), two cases in which
rituximab was additionally included; seven patients were treated
with chemotherapy adopting a MINE scheme (mitoxantraone, ifosfamide
and etoposide) and three cases in which rituximab was also
included.
Prior to stem cell collection, all 128 patients were
treated with granulocyte-colony stimulating factor (G-CSF; Kyowa
Hakko Kirin China Pharmaceutical Co., Ltd.) at the median dose of
5.10 µg/kg (range, 3.57–8.49) for the median period of 5 days
(range, 1–16) by subcutaneous injection. When the peripheral white
blood cell count decreased to 1.0–2.0×109/l after
chemotherapy, 300 µg/day G-CSF was administered to patients via two
consecutive subcutaneous injections. In the case of patients with a
weight >70 kg, 450 µg/day G-CSF was administered.
Stem cell collection
To collect cells, the COBE Spectra (Terumo BCT.
Inc.), continuous flow cell separator was used with a collecting
blood flow of 18–20 l. The collected mononuclear and CD34+ cells
were counted using a flow cytometer (BD FACSCanto™ II; BD
Biosciences). The efficiency of stem cell mobilization was defined
by the number of CD34+ cells and the patient's body weight:
≥2.0×106/kg was a successful mobilization;
<2.0×106/kg was a mobilization failure; and
>5.0×106/kg was an ideal mobilization (5,6). In
addition, the peripheral blood mononuclear cell (B-MNC) count, the
level of hemoglobin (Hb), the hematocrit level (Rct) and the blood
platelet (Plt) count were determined in peripheral blood cell
samples, using a Sysmex XN-9100™ Automated Hematology system
(Sysmex Corporation).
Statistical analysis
Continuous data that conform to the normal
distribution are presented as the mean ± standard deviation;
continuous data that are not normally distributed are presented as
the median (range), and classified data are presented as an actual
number (rate). The χ2 test was used for the comparison
of rate differences and the Mann-Whitney U test (two groups) or
Kruskal-Wallis test (multi groups) for the comparison of continuous
datasets. Multiple regression models were used to analyze factors
influencing stem cell mobilization and collection. When univariate
analysis produced statistical significance, further multivariate
analysis with a stepwise regression model was performed.
Statistical analysis was conducted using SPSS (version 17.0; SPSS
Inc.), and P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient baseline status and PBSC
mobilization
Of the 53 patients with MM, the therapeutic outcome
of PBSC mobilization treatment was a partial response (PR) in 19
patients, and a complete response (CR) in 34 cases (Table I). In 75 patients with lymphoma, 40
(53.3%) achieved a CR (Table
II).
 | Table I.Baseline characteristics of MM
patients and the outcome of PBSCs mobilization. |
Table I.
Baseline characteristics of MM
patients and the outcome of PBSCs mobilization.
|
|
| Mobilization, n
(%) |
|
|---|
|
|
|
|
|
|---|
| Patient
characteristics | Patients, n
(%) | Success | Failure | P-value |
|---|
| Number | 53 (100) | 39 (74) | 14 (26) |
|
| Age,
yearsa | 55 (24–64) | 55 (24–64) | 55.5 (43–64) | 0.424 |
| Sexb |
|
|
| 0.832 |
|
Male | 29 (55) | 21 (54) | 8 (57) |
|
|
Female | 24 (45) | 18 (46) | 6 (43) |
|
| Status of
diseaseb |
|
|
| 0.524 |
|
PR/VGPR | 19 (36) | 13 (33) | 6 (43) |
|
|
CR/nCR | 34 (64) | 26 (67) | 8 (57) |
|
| Number of prior
linesb |
|
|
| 1.000 |
| 1 | 48 (91) | 35 (90) | 13 (93) |
|
| ≥2 | 5 (9) | 4 (10) | 1 (7) |
|
| Previous
lenalidomide treatmentb |
|
|
| 0.004 |
|
Yes | 4 (8) | 0 (0) | 4 (29) |
|
| No | 49 (92) | 39 (100) | 10 (71) |
|
| Previous
thalidomide treatmentb |
|
|
| 0.919 |
|
Yes | 9 (17) | 6 (15) | 3 (21) |
|
| No | 44 (83) | 33 (85) | 11 (79) |
|
| Previous velcade
treatmentb |
|
|
| 0.322 |
|
Yes | 41 (77) | 32 (82) | 9 (64) |
|
| No | 12 (23) | 7 (18) | 5 (36) |
|
| Treatment
coursesb |
|
|
| 0.075 |
|
1–4 | 22 (42) | 19 (49) | 3 (21) |
|
| ≥5 | 31 (58) | 20 (51) | 11 (79) |
|
| Isolation
timesb |
|
|
| 0.755 |
| 2 | 42 (79) | 30 (77) | 12 (86) |
|
|
>2 | 11 (21) | 9 (23) | 2 (14) |
|
| G-CSF dosage
(µg/kg)a | 5.0 (3.6–7.7) | 5.1 (3.6–7.7) | 5.0 (4.0–7.5) | 0.531 |
| G-CSF duration
(d)a |
|
|
| 0.682 |
|
1–5 | 40 (75) | 30 (77) | 10 (71) |
|
|
>5 | 13 (25) | 9 (23) | 4 (29) |
|
| Hematological
values |
|
|
|
|
| B-MNC
(×109/l)a | 2.1 (0.3–8.2) | 2.2 (0.4–8.2) | 1.8 (0.3–4.3) | 0.143 |
| Hb
(g/l)1 | 111 (66.3–142) | 112.2 (74–142) | 103.7
(66.3–122) | 0.083 |
| Rct
(%)1 | 33 (20.1–42.7) | 33.2
(21.8–42.7) | 31.4
(20.1–38.2) | 0.125 |
| Plt
(×109/l)1 | 90
(26.3–238.5) | 90
(26.3–238.5) | 89.5 (45–173) | 0.896 |
 | Table II.Baseline characteristics of patients
with lymphoma and the outcome of stem cell mobilization. |
Table II.
Baseline characteristics of patients
with lymphoma and the outcome of stem cell mobilization.
|
|
| Mobilization, n
(%) |
|
|---|
|
|
|
|
|
|---|
| Patient
characteristics | Patients, n
(%) | Success | Failure | P-value |
|---|
| Number | 75 (100) | 44 (59) | 31 (41) |
|
| Age,
yearsa | 38 (16–61) | 37.5 (17–57) | 39 (16–61) | 0.477 |
| Sexb |
|
|
| 0.699 |
|
Male | 44 (59) | 25 (57) | 19 (61) |
|
|
Female | 31 (41) | 19 (43) | 12 (39) |
|
| Histopathology
(WHO)c |
|
|
| 0.297 |
| Hodgkin
lymphoma | 7 (9) | 6 (14) | 1 (3) |
|
|
Lymphoblast lymphoma | 15 (20) | 5 (11) | 10 (32) |
|
|
DLBCL | 31 (42) | 20 (45) | 11 (36) |
|
|
PTCL | 15 (20) | 7 (16) | 8 (26) |
|
| Small B
lymphoma | 7 (9) | 6 (14) | 1 (3) |
|
| Status of
diseaseb |
|
|
| 0.009 |
| CR | 40 (53) | 29 (66) | 11 (35) |
|
| Not
CR | 35 (47) | 15 (34) | 20 (65) |
|
| Number of prior
treatments linesb |
|
|
| 0.046 |
| 1 | 44 (59) | 30 (68) | 14 (45) |
|
| ≥2 | 31 (41) | 14 (32) | 17 (55) |
|
| High dose
MTX/Ara-cb |
|
|
| 0.026 |
|
Yes | 17 (23) | 6 (14) | 11 (35) |
|
| No | 58 (77) | 38 (86) | 20 (65) |
|
| Treatment
coursesb |
|
|
| 0.012 |
|
1–8 | 53 (71) | 36 (82) | 17 (55) |
|
| ≥9 | 22 (29) | 8 (18) | 14 (45) |
|
| Separation
timesb |
|
|
| 1.000 |
| 2 | 71 (95) | 42 (95) | 29 (94) |
|
|
>2 | 4 (5) | 2 (5) | 2 (6) |
|
| G-CSF duration
(d)b |
|
|
| 0.001 |
|
1–5 | 60 (80) | 41 (93) | 19 (61) |
|
|
>5 | 15 (20) | 3 (7) | 12 (39) |
|
| Hematological
values |
|
|
|
|
| B-MNC
(×109/l)a | 1.9 (0.1–7.9) | 2.4 (0.6–7.9) | 1.4 (0.1–3.0) | <0.001 |
| Hb
(g/l)a | 98.3 (66–135) | 106.8 (68–135) | 91.5 (66–116) | <0.001 |
| Rct
(%)a | 28.7
(19.5–39.9) | 31.6
(20.7–39.9) | 26.8 (19.5–34) | <0.001 |
| Plt
(×109/l)a | 76.5 (19–250) | 109.5 (25–250) | 53 (19–176.5) | 0.001 |
| Treatment
regimesc |
|
|
| 0.052 |
| CHOP
like | 33 (44) | 21 (47) | 12 (39) |
|
|
CTX | 17 (23) | 12 (27) | 5 (16) |
|
|
HyperCVAD part A | 9 (12) | 3 (7) | 6 (19) |
|
|
HyperCVAD part B | 9 (12) | 2 (5) | 7 (23) |
|
|
MINE | 7 (9) | 6 (14) | 1 (3) |
|
Parameters of stem cell
collection
The patients (113 of 128 cases, 88.3%) were
subjected to stem cell collection for two consecutive days, while
four patients were subjected to collection only once, and 15
patients three times. The first and second acquisition of CD34+
cells represented 54.0 and 42.2% of the total CD34+ cells
collected, respectively. In the cell samples, the peripheral blood
mononuclear cell (B-MNC) count was 1.9×109/l (range,
0.1–8.2), the level of hemoglobin (Hb) was 104.2 g/l (range,
66.0–142.0), the hematocrit level (Rct) was 30.8% (range,
19.5–42.7), and the blood platelet (Plt) count was
88.0×109/l (range, 19.0–250.0).
Collection of CD34+ cells
The median CD34+ cell count collected from all
patients was 3.12×106/kg (range, 0.03–24.07). The median
CD34+ counts in MM and lymphoma patients were
4.16×106/kg (range, 0.10–19.02) and
2.40×106/kg (range, 0.03–24.07), respectively. Greater
numbers of CD34+ cells were collected from patients with MM
compared with patients with lymphoma; however, the difference was
not statistically significant (P=0.064). In addition,
>2.0×106/kg CD34+ cells were obtained in 83 of the
128 patients (64.8%), and >5.0×106/kg cells were
successfully collected from 45 patients (35.2%). The success rate
of CD34+ cell collection was 73.6% (39/53) in patients with MM and
58.7% (44/75) in patients with lymphoma, and the ideal rates were
43.4 (23/53) and 30.7% (23/75), respectively (Table I and II). This demonstrates that both the
success and ideal rates were greater in patients with MM compared
with patients with lymphoma. In 45 patients (35.2%), including 14
MM and 31 lymphoma cases, mobilization failed.
Stem cell mobilization and
univariate/multivariate analysis of stem cell mobilization
data
Univariate analysis demonstrated that the success
rate of mobilization was lower in patients MM who had previously
received lenalidomide, underwent >4 courses of treatment or had
a peripheral blood Hb level <100 g/l (Fig. 1A). These factors reduced the yield of
CD34+ cells, identifying them as negative factors that adversely
affect stem cell mobilization and collection. However, multivariate
analysis showed that only a low peripheral blood Hb level was
significantly associated with poor mobilization (P=0.041; OR,
1.040; 95% CI, 1.002–1.080; Table
III).
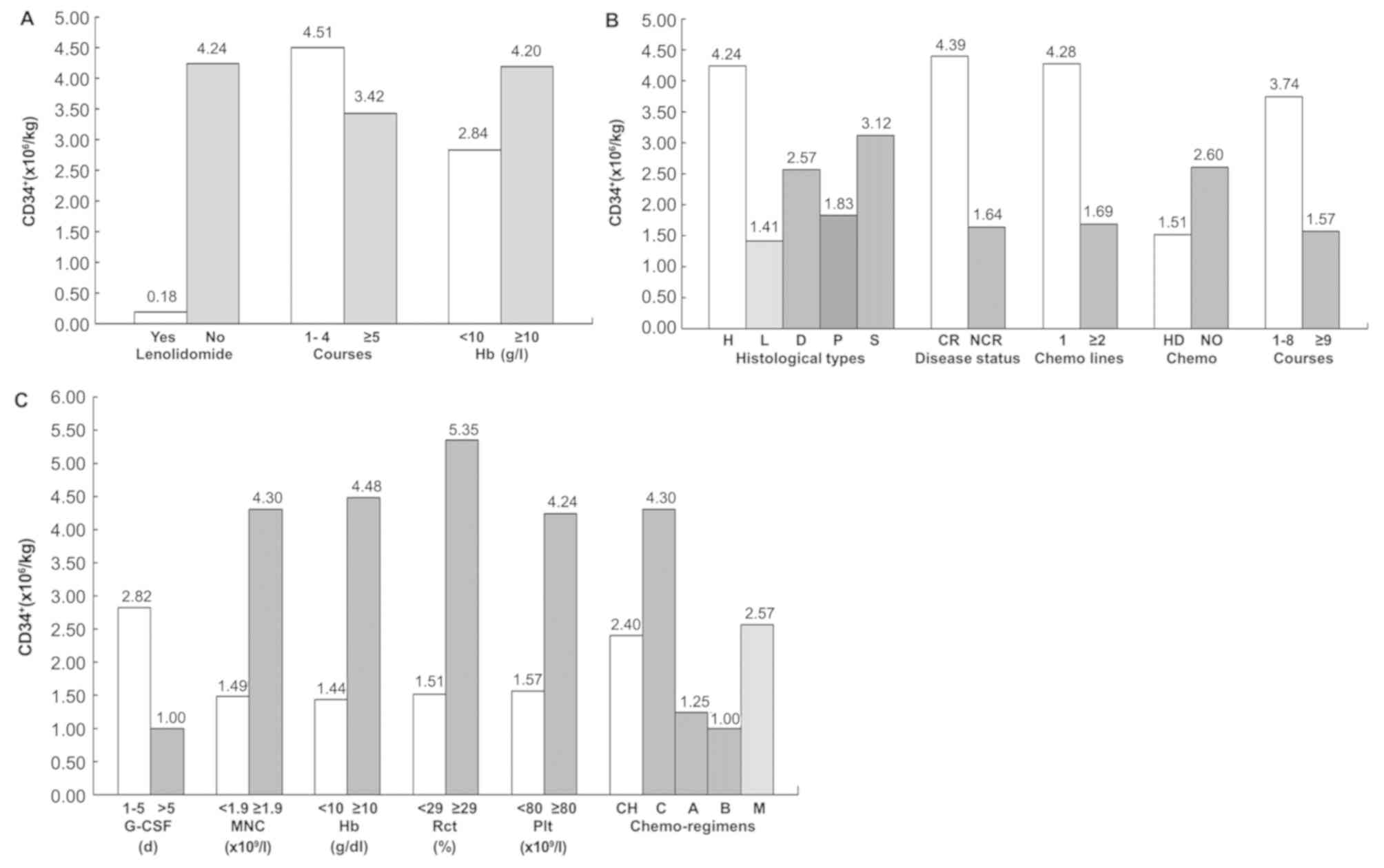 | Figure 1.Effect of baseline status on the
mobilization of CD34+ cells. (A) Effects of lenalidomide
application, chemotherapy course number and hemoglobin level on
CD34+ cell mobilization in patients with multiple myeloma. (B)
Effects of pathological type, disease status, the number of
chemotherapy lines, high dose chemotherapy and chemotherapy course
number on CD34+ cell mobilization in patients with lymphoma. (C)
Effects of G-CSF administration duration, MNC count, the hemoglobin
(Hb) level, hematocrit (Rct: Reticulocyte), platelet (Plt) count
and chemotherapy scheme on CD34+ cell mobilization in lymphoma
patients. CR, complete response; NCR, near CR; H, Hodgkin's
lymphoma; L, Lymphoblast lymphoma; D, Diffuse larger B cell
lymphoma; P, Peripheral T-cell lymphoma; S, Small B cell lymphoma;
HD, high dose cytosine arabinoside/methotrexate, CH, CHOP regime;
C, CTX regime; A, HyperCVAD-A regime; B HyperCVAD-B regime; M, MINE
regime. |
 | Table III.Univariate and multivariate
statistical analysis of factors influencing mobilization in 53
patients with MM. |
Table III.
Univariate and multivariate
statistical analysis of factors influencing mobilization in 53
patients with MM.
|
| Successful
mobilization |
|---|
|
|
|
|---|
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Prognostic
factors | P-value | OR | 95% CI | P-value | OR | 95% CI |
|---|
| Without treatment
of lenalidomide | 0.027 | 1.400 | 1.005–1.950 | NS |
|
|
| Treatment courses
(≤4) | 0.024 | 3.483 | 0.840–14.449 | NS |
|
|
| Hb (≥100 g/l) | 0.014 | 2.500 | 0.685–9.121 | 0.041 | 1.040 | 1.002–1.080 |
The analysis showed that the factors negatively
affecting stem cell mobilization and collection were disease
without complete remission, previous chemotherapy beyond the
first-line, >8 courses chemotherapy treatment, previous
treatment with high dose MTX/Ara-c, application of the HyperCVAD-B
mobilization scheme, administration of G-CSF for >5 days, and
collected B-MNC, Hb, Rct and Plt values below 1.9×109/l,
100 g/l, 29% and 80×109/l, respectively (Table II). The association between these
influencing factors and the yield of CD34+ cells is shown in
Fig. 1B. Multivariate regression
analysis revealed that the disease remission status, usage of
MTX/Ara-c, and the Rct value significantly affected the success
rate of mobilization and acquisition of CD34+ cells. However,
HyperCVAD-B mobilization scheme and Plt value below
80×109/l were negative affecting factors (Table IV and Fig. 1C). In 20 of 35 patients (57.1%) who
did not achieve CR, stem cell collection failed. The failure rate
of patients who were previously treated with high dose MTX/Ara-c
was 64.7%. The failure rates of patients whose Rct was <29% or
Plt was <80×109/l, were 61.5 and 57.9% respectively,
and those patients who adopted the HyperCVAD scheme plan A or B
were 67.7 (6/9) and 77.8% (7/9), respectively (data not shown).
 | Table IV.Univariate and multivariate
statistical analysis of factors influencing mobilization in 75
lymphoma patients. |
Table IV.
Univariate and multivariate
statistical analysis of factors influencing mobilization in 75
lymphoma patients.
|
| Successful
mobilization |
|---|
|
|
|
|---|
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Prognostic
factors | P-value | OR | 95% CI | P-value | OR | 95% CI |
|---|
| Histopathology
(WHO) | 0.049 | – | – | NS |
|
|
| Disease status (Not
CR) | 0.002 | 0.284 | 0.108–0.746 | 0.024 | 0.212 | 0.055–0.813 |
| Number of prior
lines (1) | 0.014 | 2.602 | 1.006–6.729 | NS |
|
|
| G-CSF
administration duration (1–5 d) | 0.010 | 8.632 | 2.178–34.211 | NS |
|
|
| High dose MTX/Ara-c
(none) | 0.003 | 0.287 | 0.093–0.891 | 0.045 | 0.197 | 0.040–0.963 |
| Treatment courses
(1–8) | 0.021 | 3.706 | 1.306–10.513 | NS |
|
|
| HyperCVAD-B
mobilization scheme | 0.029 | – | – | 0.030 | 1.975 | 1.070–3.646 |
| B-MNC ≥1.9 | <0.001 | 4.500 | 1.681–12.046 | NS |
|
|
| Hb ≥100 | <0.001 | 5.558 | 2.009–15.379 | NS |
|
|
| Rct (≥29%) | <0.001 | 8.176 | 2.826–23.650 | 0.008 | 1.229 | 1.056–1.431 |
| Plt
≥80×109/l | <0.001 | 4.278 | 1.591–11.505 | 0.032 | 1.017 | 1.001–1.034 |
Adverse events
The main adverse event in patients was
agranulocytosis and the median duration time of neutrophil
granulocyte deficiency was 3 days (range, 0–15 days). In 27
patients including 12 MM and 15 lymphoma patients, the deficiency
lasted >5 days, and in two lymphoma patients the duration was
> 10 days; 13 patients were free from agranulocytosis. Other
adverse events included gastrointestinal reactions during
chemotherapy, fever due to granulocyte deficiency, hypokalemia,
fever associated with G-CSF treatment, and lower limb, waist or
back pain, which did not exceed level 2 and were completely
resolved following treatment (Table
V). No enlargement of the spleen or spleen rupture, and no
other serious adverse events were observed.
 | Table V.Adverse events. |
Table V.
Adverse events.
| Adverse events, n
(%) | Total (n=128) | Multiple myeloma
(n=53) | Lymphoma
(n=75) | P-value |
|---|
| Hematologic events
(3/4 grade) |
|
|
|
|
|
Neutropeniaa | 115 (89.8) | 49 (92.5) | 66 (88.0) | 0.850 |
|
Thrombocytopeniaa | 40 (31.3) | 14 (26.4) | 26 (34.7) | 0.470 |
|
Anemiaa | 12 (9.4) | 3 (5.7) | 9 (12.0) | 0.267 |
| Non-hematological
events (all grades) |
|
|
|
|
| Nausea
and vomitinga | 59 (46.1) | 28 (52.8) | 31 (41.3) | 0.438 |
|
Infectiona | 66 (51.6) | 25 (47.2) | 41 (54.7) | 0.635 |
|
Fatiguea | 21 (16.4) | 6 (11.3) | 15 (20.0) | 0.265 |
|
Kaliopeniaa | 21 (16.4) | 9 (17.0) | 12 (16.0) | 0.901 |
|
Diarrheaa | 9 (7.0) | 3 (5.7) | 6 (8.0) | 0.634 |
| GPT
elevationa | 5 (3.9) | 2 (3.8) | 3 (4.0) | 0.950 |
|
Osteodynia | 2 (1.6) | 1 (1.9) | 1 (1.3) | 0.807 |
|
Rasha | 3 (2.3) | 1 (1.9) | 2 (2.7) | 0.779 |
|
Coagulation
functiona | 1 (0.8) | 0 | 1 (1.3) | 0.402 |
Discussion
A key factor in successful ASCT is efficient PBSC
acquisition. The general requirement of CD34+ cell numbers for
ensuring successful hematopoietic functional reconstruction is
2.0×106 cells/kg of patient weight. The acquisition of
5×106 cells/kg of CD34+ cells is considered to be ideal
and this number is sufficient for quick reconstruction of
hematopoiesis (7–9). G-CSF has been widely used as a PBSC
mobilization stimulator. Early studies found that G-CSF alone could
increase PBSCs by 10 to 100 times, with the peak of the drug
concentration in plasma occurring on the fifth day (10). G-CSF may interfere with the
interaction between stromal cell-derived factor-1 (SDF1) and the
CXC chemokine type 4 receptor (CXCR4), and affect the expression of
bone marrow stromal cell related adhesion molecules (11,12).
Even though its precise mechanism is not well understood, G-CSF
treatment resulted in a higher yield of PBSCs, even when
hematopoiesis was inhibited during chemotherapy. Therefore G-CSF
has been widely used clinically (7–9,13–16);
however, no consensus recommendation for the selection of
chemotherapy schemes has been established.
In our center, all PBSC mobilization was performed
by chemotherapy combined with G-CSF. In accordance with other
centers, patients with MM received chemotherapy with high-dose CTX
(8,17–19),
while patients with lymphoma underwent chemotherapy with various
regimens. The present study revealed that adverse events due to
treatment were at acceptable levels, but there was a significant
difference in the success rates of stem cell mobilization. The
combined success rate of CTX, CHOP and MINE therapeutic schemes was
68.4%, while that of HyperCVAD scheme plans A or B was only 27.8%,
suggesting that HyperCVAD might not be a suitable treatment option
for patients with lymphoma. Other studies recommended various
schemes with a success rate >70%, which included high-dose CTX,
intermediate dose cytarabine, cytarabine combined with etoposide,
ESHAP/DSHAP and ICE/RICE (8,20–23).
In the present study, the overall mobilization
failure rate was 35.2% (45 of 128 patients), the failure rate of MM
patients was 26.4% (14 of 53 patients) and that of lymphoma
patients was 41% (31 of 75 patients). The mobilization failure rate
appeared to be relatively higher than previously reported rates of
5–30% (8,18,19,21–26). The
reasons for this discrepancy are unknown; however, some invariable
or variable factors might have played a role, such as mobilization
timing, mobilization schemes, and various parameters set by the
separator. Further investigation is required to elucidate whether
altering these factors could optimize PBSC mobilization in
lymphoma, as well as in MM patients. Lymphoma and MM share the same
malignant tumor cell origin and their therapeutic strategies are
similar. However, in the present study the failure rate of lymphoma
patient treatment was two times greater than that of MM patients.
It has been reported that in NHL and HL, both G-CSF mobilization
alone and G-CSF combined with chemotherapy resulted in similar
failure rates, and these rates were four times greater than those
of MM (8,9). It may be that previous high-dose
chemotherapy in lymphoma patients affected stem cell mobilization
and collection. In MM patients, treatment with multiple courses of
lenalidomide (27), high-dose
chemotherapy, administration of purine analogues, and 1–3 lines of
chemotherapy were reported to be adverse factors for mobilization
(21,25). In accordance with this, lenalidomide
treatment, multiple courses of chemotherapy, incomplete alleviation
of disease, large-doses of cytarabine or MTX, and previous
chemotherapy of more than 2 lines, were found to be unfavorable
factors for successful mobilization in MM patients. These factors
may have negatively affected patient's bone marrow function, and
more effective mobilization strategies should be considered.
Additionally, the efficiency of stem cell collection
after the second round of mobilization was evaluated; three of 45
patients who failed the first round of mobilization underwent a
second round. However, the yield of CD34+ cells was insufficient,
which confirmed the general concept that if the first mobilization
failed, despite the type of disease and mobilization scheme, the
success rate of a second mobilization was also low (8,23,25,28).
Therefore, it should be emphasized that the optimal yield of CD34+
cells is achieved following the first mobilization. However,
several randomized clinical trials have suggested that a new
mobilization agent, plerixafor, which inhibits the binding of
chemokine SDF-1 to its receptor CXCR4, is ideal for the
mobilization of PBSCs, either used alone or in combination with
G-CSF (13,14,17,18,24,26,29–32).
Plerixafor successfully mobilized PBSCs even in patients who
received high-doses of chemotherapy or who failed the first
mobilization (14,17,30–32).
However, it is not widely used in Chinese clinical practice.
It has been reported that specific parameters prior
to PBSC collection, such as the duration time of G-CSF
administration and B-MNC, Plt and CD34+ cell counts, may predict
mobilization success (15,23,25,26,33,34).
However, when used alone, none of these factors were able to
predict mobilization outcome. The present study revealed that a
higher hemoglobin level was a significant factor for successful
mobilization both in MM and lymphoma patients. Conversely, a G-CSF
administration time >5 days, and lower numbers of B-MNC and Plt
were associated with mobilization failure in lymphoma, but not MM
patients. Regardless of the disease and the mobilization scheme
applied, the peripheral blood CD34+ cell count measured between
mobilization and collection have been reported to correlate with
the first day acquisition rate. Hence, the peripheral blood CD34+
cell count appears to be the only factor that could be used to
determine the optimal timing of PBSC collection (8,16,23,25).
However, due to the retrospective nature of the present study, data
on these CD34+ cell counts were unavailable.
In summary, the present study revealed that
chemotherapy combined with G-CSF in MM and lymphoma patients
yielded CD34+ cells; however, the failure rate was relatively high.
Multivariate analyses revealed that Hb values <100 g/l in MM
patients who did not achieve CR, high dose MTX/Ara-c medication,
HyperCVAD-B mobilization scheme, as well as low Rct (≤29%) and Plt
counts (≤80×109/l) in lymphoma patients were significant
factors for insufficient PBSC mobilization. It is anticipated that
the introduction of the mobilization agent plerixafor to the scheme
may increase the success rate of mobilization in patients with poor
bone marrow function. Therefore, it is important to further
investigate the factors associated with successful stem cell
mobilization and collection for the establishment of a more
efficient and cost-effective ideal protocol, especially for
patients with a high risk of failure. However, since the study was
limited to a relatively small number of patients, a prospective
study with a larger cohort will be required for further
confirmation of these findings.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81471532), the
National Science and Technology Support Program (grant no.
2014BAI09B12) and the Natural Science Foundation of Zhejiang
Province (grant no. LY16H080001).
Availability of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GZ, JH, ZC and DH were responsible for the
conception and design of the study. All authors were responsible
for the data acquisition and analysis. GZ was responsible for
statistical analysis. GZ drafted the manuscript. GZ and JH revised
and commented on the draft. YL, JS, GW, JS and WZ were responsible
for primary data collection. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethical
committee of the Bone Marrow Transplantation Center of the First
Affiliated Hospital, Zhejiang University, and all participants
signed informed consent forms.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hamadani M: Autologous hematopoietic cell
transplantation: An update for clinicians. Ann Med. 46:619–632.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vincent Rajkumar S: Multiple myeloma: 2014
Update on diagnosis, risk-stratification, and management. Am J
Hematol. 89:999–1009. 2014.PubMed/NCBI
|
|
3
|
Hubel K, de la Rubia J, Azar N and
Corradini P: Current status of haematopoietic autologous stem cell
transplantation in lymphoid malignancies: A european perspective.
Eur J Haematol. 94:12–22. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Swerdlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H and Thiele J: WHO classification of tumours of
haematopoietic and lymphoid tissues. Lyon, France: IARC Press;
2008
|
|
5
|
Gasová Z, Marinov I, Hrubá A, Benesová K
and Turek P: The efficiency of PBPC collections and the
relationship to the precollection concentration of CD 34+ cells in
blood. Transfus Sci. 20:181–188. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pozotrigo M, Adel N, Landau H, Lesokhin A,
Lendvai N, Chung DJ, Chimento D, Riedel E, Chen X, Reich L, et al:
Factors impacting stem cell mobilization failure rate and
efficiency in multiple myeloma in the era of novel therapies:
Experience at memorial sloan kettering cancer center. Bone Marrow
Transplant. 48:1033–1039. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hoggatt J, Speth JM and Pelus LM: Concise
review: Sowing the seeds of a fruitful harvest: Hematopoietic stem
cell mobilization. Stem cells. 31:2599–2606. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pusic I, Jiang SY, Landua S, Uy GL, Rettig
MP, Cashen AF, Westervelt P, Vij R, Abboud CN, Stockerl-Goldstein
KE, et al: Impact of mobilization and remobilization strategies on
achieving sufficient stem cell yields for autologous
transplantation. Biol Blood Marrow Transplant. 14:1045–1056. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gertz MA, Wolf RC, Micallef IN and
Gastineau DA: Clinical impact and resource utilization after stem
cell mobilization failure in patients with multiple myeloma and
lymphoma. Bone Marrow Transplant. 45:1396–1403. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dreger P, Haferlach T, Eckstein V, Jacobs
S, Suttorp M, Löffler H, Müller-Ruchholtz W and Schmitz N:
G-CSF-mobilized peripheral blood progenitor cells for allogeneic
transplantation: Safety, kinetics of mobilization, and composition
of the graft. Br J Haematol. 87:609–613. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Petit I, Szyper-Kravitz M, Nagler A, Lahav
M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos
F, Fujii N, et al: G-CSF induces stem cell mobilization by
decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol.
3:687–694. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Salvucci O, Jiang K, Gasperini P, Maric D,
Zhu J, Sakakibara S, Espigol-Frigole G, Wang S and Tosato G:
MicroRNA126 contributes to granulocyte colony-stimulating
factor-induced hematopoietic progenitor cell mobilization by
reducing the expression of vascular cell adhesion molecule 1.
Haematologica. 97:818–826. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shaughnessy P, Uberti J, Devine S, Maziarz
RT, Vose J, Micallef I, Jacobsen E, McCarty J, Stiff P, Artz A, et
al: Plerixafor and G-CSF for autologous stem cell mobilization in
patients with NHL, Hodgkin's lymphoma and multiple myeloma: Results
from the expanded access program. Bone Marrow Transplant.
48:777–781. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abhyankar S, DeJarnette S, Aljitawi O,
Ganguly S, Merkel D and McGuirk J: A risk-based approach to
optimize autologous hematopoietic stem cell (HSC) collection with
the use of plerixafor. Bone Marrow Transplant. 47:483–487. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sugrue MW, Williams K, Pollock BH, Khan S,
Peracha S, Wingard JR and Moreb JS: Characterization and outcome of
‘hard to mobilize’ lymphoma patients undergoing autologous stem
cell transplantation. Leuk Lymphoma. 39:509–519. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rossi G, Skert C, Morello E, Almici C,
Arcaini L, Basilico C, Cavalli L, Botto B, Castelli A, Pica G, et
al: PBSC mobilization in lymphoma patients: Analysis of risk
factors for collection failure and development of a predictive
score based on the kinetics of circulating CD34+ cells and WBC
after chemotherapy and G-CSF mobilization. Hematol Oncol.
33:125–132. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cooper DL, Medoff E, Patel N, Baker J,
Pratt K, Foss F, Seropian SE, Perreault S and Wu Y: Autologous stem
cell mobilization in the age of plerixafor. Clin Lymphoma Myeloma
Leuk. 16:411–416. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Teusink A, Vinks A, Zhang K, Davies S,
Fukuda T, Lane A, Nortman S, Kissell D, Dell S, Filipovich A and
Mehta P: Genotype-directed dosing leads to optimized voriconazole
levels in pediatric patients receiving hematopoietic stem cell
transplantation. Biol Blood Marrow Transplant. 22:482–486. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tuchman SA, Bacon WA, Huang LW, Long G,
Rizzieri D, Horwitz M, Chute JP, Sullivan K, Morris Engemann A,
Yopp A, et al: Cyclophosphamide-based hematopoietic stem cell
mobilization before autologous stem cell transplantation in newly
diagnosed multiple myeloma. J Clin Apher. 30:176–182. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Calderon-Cabrera C, Carmona Gonzalez M,
Martin J, Ríos Herranz E, Noguerol P, De la Cruz F, Carrillo E,
Falantes JF, Parody R, Espigado I, et al: Intermediate doses of
cytarabine plus granulocyte-colony-stimulating factor as an
effective and safe regimen for hematopoietic stem cell collection
in lymphoma patients with prior mobilization failure. Transfusion.
55:875–879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xia W, Ma CK, Reid C, Bai L, Wong K,
Kerridge I, Ward C and Greenwood M: Factors determining pbsc
mobilization efficiency and nonmobilization following ICE with or
without rituximab (R-ICE) salvage therapy for refractory or
relapsed lymphoma prior to autologous transplantation. J Clin
Apher. 29:322–330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ozkan HA, Bal C and Gulbas Z:
Chemomobilization with high-dose etoposide and G-CSF results in
effective and safe stem cell collection in heavily pretreated
lymphoma patients: Report from a single institution study and
review. Eu J Haematol. 92:390–397. 2014. View Article : Google Scholar
|
|
23
|
Re A, Cattaneo C, Skert C, Balsalobre P,
Michieli M, Bower M, Ferreri AJ, Hentrich M, Ribera JM, Allione B,
et al: Stem cell mobilization in HIV seropositive patients with
lymphoma. Haematologica. 98:1762–1768. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Clark RE, Bell J, Clark JO, Braithwaite B,
Vithanarachchi U, McGinnity N, Callaghan T, Francis S and Salim R:
Plerixafor is superior to conventional chemotherapy for first-line
stem cell mobilisation, and is effective even in heavily pretreated
patients. Blood Cancer J. 4:e2552014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sancho JM, Morgades M, Grifols JR, Juncà
J, Guardia R, Vives S, Ferrà C, Batlle M, Ester A, Gallardo D, et
al: Predictive factors for poor peripheral blood stem cell
mobilization and peak CD34(+) cell count to guide pre-emptive or
immediate rescue mobilization. Cytotherapy. 14:823–829. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee KH, Jung SK, Kim SJ, Jang JH, Kim K,
Kim WS, Jung CW, Kim DW and Kang ES: Incidence and risk factors of
poor mobilization in adult autologous peripheral blood stem cell
transplantation: A single-centre experience. Vox Sang. 107:407–415.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumar S, Dispenzieri A, Lacy MQ, Hayman
SR, Buadi FK, Gastineau DA, Litzow MR, Fonseca R, Roy V, Rajkumar
SV and Gertz MA: Impact of lenalidomide therapy on stem cell
mobilization and engraftment post-peripheral blood stem cell
transplantation in patients with newly diagnosed myeloma. Leukemia.
21:2035–2042. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Watts MJ, Ings SJ, Flynn M, Dodds D,
Goldstone AH and Linch DC: Remobilization of patients who fail to
achieve minimal progenitor thresholds at the first attempt is
clinically worthwhile. Br J Haematol. 111:287–291. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Russell N, Douglas K, Ho AD, Mohty M,
Carlson K, Ossenkoppele GJ, Milone G, Pareja MO, Shaheen D,
Willemsen A, et al: Plerixafor and granulocyte colony-stimulating
factor for first-line steady-state autologous peripheral blood stem
cell mobilization in lymphoma and multiple myeloma: Results of the
prospective PREDICT trial. Haematologica. 98:172–178. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Duarte RF, Shaw BE, Marin P, Kottaridis P,
Ortiz M, Morante C, Delgado J, Gayoso J, Goterriz R,
Martínez-Chamorro C, et al: Plerixafor plus granulocyte CSF can
mobilize hematopoietic stem cells from multiple myeloma and
lymphoma patients failing previous mobilization attempts: EU
compassionate use data. Bone Marrow Transplant. 46:52–58. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Arcaini L, Laszlo D, Rizzi S, Balzarotti
M, Antoniazzi F, Zilioli VR, Guggiari E, Farina L, Todisco E,
Bonfichi M, et al: Plerixafor and G-CSF for PBSC mobilization in
patients with lymphoma who failed previous attempts with G-CSF and
chemotherapy: A REL (Rete Ematologica Lombarda) experience.
Leukemia Res. 35:712–714. 2011. View Article : Google Scholar
|
|
32
|
Keating GM: Plerixafor: A review of its
use in stem-cell mobilization in patients with lymphoma or multiple
myeloma. Drugs. 71:1623–1647. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ikeda K, Kozuka T and Harada M: Factors
for PBPC collection efficiency and collection predictors. Transfus
Apher Sci. 31:245–259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Perea G, Sureda A, Martino R, Altés A,
Martínez C, Cabezudo E, Amill B, Martín-Henao GA, González Y, Muñoz
L, et al: Predictive factors for a successful mobilization of
peripheral blood CD34+ cells in multiple myeloma. Annals Hematol.
80:592–597. 2001. View Article : Google Scholar
|















