Introduction
According to statistical data from 2012, colorectal
cancer (CRC) is the third most popular cancer and the fourth most
common cause of cancer mortality globally (1). It is estimated that >1 million novel
cases of colorectal carcinoma are diagnosed annually (2,3). Despite
the improvement in surgical, radiotherapeutic and chemotherapeutic
regimens, ~50% of patients with CRC relapse within 5 years of
treatment. Evidence suggests that this is due to distant metastasis
(4). However, the responsiveness of
patients to treatment is diverse because of the accumulation of
multiple genetic mutations involving critical genes that govern
cell proliferation (5). Therefore, a
novel biomarker for CRC is urgently required to help diagnose the
disease at an early stage and determine the treatment response in
patients with CRC, thereby enabling an accurate prediction of the
prognosis of patients.
Lipid rafts, specialized domains in cell membranes,
are involved in a variety of cell signal transductions (6). The flotillin family of proteins,
including two isoforms flotillin-1 (FLOT1) and flotillin-2 (FLOT2),
are important markers of lipid rafts (7,8). It has
been reported that flotillin proteins ubiquitously express and
serve important functions in a number of biological processes,
including cell adhesion, endocytosis, actin reorganization,
signaling transduction, phagocytosis and actin cytoskeleton
dynamics (9–13). Furthermore, FLOT2 has been
demonstrated to be upregulated and involved in progression and
development in several types of cancer, including breast cancer and
melanoma (14–17). A previous microarray result has
identified that FLOT2 was upregulated in a cluster of breast tumors
(14). FLOT2 protein exhibited low
expression in non-tumorigenic cell lines, whereas high expression
was identified in certain metastatic melanoma cell lines in
vitro (15). Consistently, the
ectopic expression of FLOT2 was associated with the progression of
human melanoma in vivo (15).
A previous study revealed that knockdown of FLOT2 attenuated
the proliferation and metastasis of a human breast cancer cell line
(16). It also identified that the
increased FLOT2 protein expression was associated with poor
outcomes in patients with breast cancer and that it could be used
as a biomarker for breast cancer progression (17). Although the increase in FLOT2 is
associated with a number of types of tumor, to the best of our
knowledge, its function in regulating CRCs remains to be
determined.
To determine the association between FLOT2
expression and CRCs, cell lines and patient tissues were analyzed.
It was revealed that FLOT2 was apparently increased in CRC cell
lines and CRC tissues. The upregulated FLOT2 was associated with
invasion, lymph node metastasis, distant metastasis and patient
survival. Taken together, the results of the present study suggest
that FLOT2 acts as a biomarker for CRC and that FLOT2 may be a
potential drug target for the clinical treatment of CRC.
Materials and methods
Patient information and specimen
collection
A total of 8 CRC and corresponding normal mucosa
tissue samples (>10 cm away from the edge of the CRC) were
obtained from patients with CRC (age range, 56–72 years; sex, 5
male and 3 female) within 30 min of resection at the First
Affiliated Hospital of Nanchang University (Nanchang, China)
between April 2017 and June 2017, and then snap-frozen in liquid
nitrogen and stored at −80°C until use. Formaldehyde-fixed and
paraffin-embedded CRC tissue blocks (n=180) were obtained from the
stored files of the Department of General Surgery, The First
Affiliated Hospital of Nanchang University (Nanchang, China)
collected between January 2006 and December 2008. All patients had
undergone pre-operative clinical staging assessment with magnetic
resonance imaging. No patients had received chemotherapy or
radiotherapy prior to surgery. The various clinicopathological
parameters [age, sex, tumor size, tumor location, tumor
differentiation, histological types, depth of invasion, lymph node
metastasis and distant metastasis and American Joint Committee on
Cancer (AJCC) stage] were obtained from histopathology records. The
stage of colorectal cancer was described according to the 7th
edition of the AJCC Tumor-Node-Metastasis classification of
malignant tumors (18). Use of CRC
specimens and matched normal specimens for the present study was
approved by the Ethics Committee of the First Affiliated Hospital
of Nanchang University.
The 180 patients included 93 men and 87 women aged
between 21 and 92 years (mean, 61.5 years). The patients were
followed-up until mortality or to the end of the follow-up period
(30 November 2015). The follow-up durations, ranging between 2 and
92 months, were available for all patients and the median patient
survival time was 65 months. Distant metastasis occurred in 24
cases (13.4%), including 5 cases to the peritoneum, 17 cases to the
liver and 2 cases to the bone.
Cell culture
A total of seven CRC cell lines (HT-29, SW480, Lovo,
SW1116, SW620, Colo205 and DLD1) and one normal colon cell line
(FHC) were purchased from the American Type Culture Collection
(Manassas, VA, USA). Cells were cultured in RPMI-1640 medium
supplemented with 10% fetal bovine serum, 1% glutamine and 1%
penicillin/streptomycin (all from Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at 37°C in a humidified incubator containing an
atmosphere of 5% CO2.
RNA isolation, reverse transcription
and reverse-transcription-quantitative polymerase chain reaction
(RT-qPCR)
Cultured cells or tissues were lysed with
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) for RNA extraction, according to the manufacturer's protocol.
The extracted RNA was pretreated with RNase-free DNase, and 1 µg
RNA from each sample was reverse transcribed with TaqMan reverse
transcription reagents and random hexamer primers (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The RNA sample was
incubated in a thermocycler for 1 h at 40°C and then denatured at
95°C for 2 min prior to incubation on ice. For PCR-mediated
amplification of FLOT2 cDNA using FLOT2-specific primers, a
denaturation step at 95°C for 10 min was followed by 30 cycles
consisting of denaturation at 95°C for 60 sec, primer annealing at
55°C for 30 sec, and primer extension at 72°C for 30 sec. On
completion of the cycling, a final extension step at 72°C for 5 min
was performed before the reaction was stopped and stored at 4°C.
qPCR was performed on an ABI Fast 7500 instrument using Maxima SYBR
Green qPCR Master mix (Thermo Fisher Scientific, Inc.). The
2−ΔΔCq method was used for relative quantification
(19). The primers used in this study
were designed using Primer Express version 3.0 (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The primer pairs used were as
follows: FLOT2, 5′-CCCCAGATTGCTGCCAAA-3′ (forward) and
5′-TCCACTGAGGACCACAATCTCA-3′ (reverse); and GAPDH,
5′-ACCACAGTCCATGCCATCAC-3′ (forward) and 5′-TCCACCACCCTGTTGCTGTA-3′
(reverse).
Western blot analysis
Cells at 70–80% confluence were washed twice with
ice-cold PBS and lysed on ice in radioimmunoprecipitation assay
buffer (RIPA; Cell Signaling Technology, Inc., Danvers, MA, USA)
containing complete protease inhibitor cocktail (Roche Applied
Science, Mannheim, Germany). Fresh tissue samples were ground to
powder in liquid nitrogen and lysed with SDS-PAGE sample buffer.
Protein concentration was measured using a BCA protein assay kit
(Pierce; Thermo Fisher Scientific, Inc.). Equal protein samples (20
µg) were separated by SDS-PAGE (10.5% gels) and transferred to
polyvinylidene fluoride membranes (Merck KGaA, Darmstadt, Germany).
Membranes were blocked with 5% fat-free milk in Tris-buffered
saline containing 0.1% Tween-20 for 1 h at room temperature.
Membranes were incubated with rabbit anti-FLOT2 (1:1,000; cat. no.
ab96507; Abcam, Cambridge, UK) overnight at 4°C, and then with
horseradish peroxidase-conjugated rabbit anti-mouse secondary
antibodies (1:2,000, cat. no. ab6728; Abcam). FLOT2 expression was
detected using ECL prime western blotting detection reagent (GE
Healthcare, Chicago, IL, USA) according to the manufacturer's
instructions. GAPDH (mouse anti-GAPDH; 1:1,000; cat. no. ab9484;
Abcam) was used as a loading control.
Immunohistochemical (IHC)
staining
Samples were fixed in 4% formaldehyde solution,
embedded in paraffin blocks, cut into 4-µm-thick sections and
mounted on glass slides. Each slide was dewaxed in xylene and
rehydrated in a graded alcohol series, followed by boiling in 10
mmol/l citrate buffer (pH 6.0) for antigen retrieval. Following
inhibition of endogenous peroxidase activities for 30 min with
methanol containing 0.3% H2O2, the sections
were blocked with 2% bovine serum albumin for 30 min and incubated
overnight at 4°C with rabbit anti-FLOT2 antibody (1:100). Following
washing three times with PBS, the slides were incubated with
horseradish peroxidase-conjugated rabbit anti-mouse antibody
(1:400; cat. no. ab6728; Abcam) for 30 min, followed by reaction
with diaminobenzidine and counterstaining with Mayer hematoxylin.
The negative control consisted of non-specific mouse immunoglobulin
G rather than the primary antibody.
The results of immunostaining were evaluated by two
observers without prior knowledge of the clinical information of
the patients, on the basis of the proportion of positively stained
tumor cells and the intensity of staining (17). The scores attributed by the two
independent investigators were averaged. The proportion of tumor
cells was scored as follows: 1 (<10% positive tumor cells), 2
(10–50% positive tumor cells), 3 (50–75% positive tumor cells) and
4 (>75% positive tumor cells). The intensity of staining was
graded according to the following criteria: 0 (no staining), 1
(weak staining; light yellow), 2 (moderate staining; yellow brown)
and 3 (strong staining; brown). The staining index was calculated
as the product of the proportion of positive cells and the staining
intensity score. Threshold values for FLOT2 were chosen on the
basis of a measure of heterogeneity using the log-rank test with
respect to overall survival (OS). An optimal threshold value was
identified as follows: A staining index score of ≥6 was used to
define tumors with high FLOT2 expression and ≤4 indicated low FLOT2
expression.
Statistical analysis
All statistical analyses were performed using the
SPSS statistical software package (version 19.0; IBM Corp., Armonk,
NY, USA). Differences in FLOT2 expression between normal colon cell
and colorectal cancer cell lines were analyzed using an independent
t-test. Differences in FLOT2 expression between colorectal cancer
tissues and adjacent non-cancerous tissues in the same patient were
analyzed using a paired t-test. The associations between FLOT2
expression and clinicopathological characteristics were analyzed
using the Pearson χ2 test. Survival rates were
calculated according to the Kaplan-Meier method, and differences
were evaluated using the log-rank test. The Cox proportional
hazards regression model was used to determine the hazard ratio and
identify factors that independently predict survival. P<0.05 in
all cases was considered statistically to indicate a statistically
significant difference.
Results
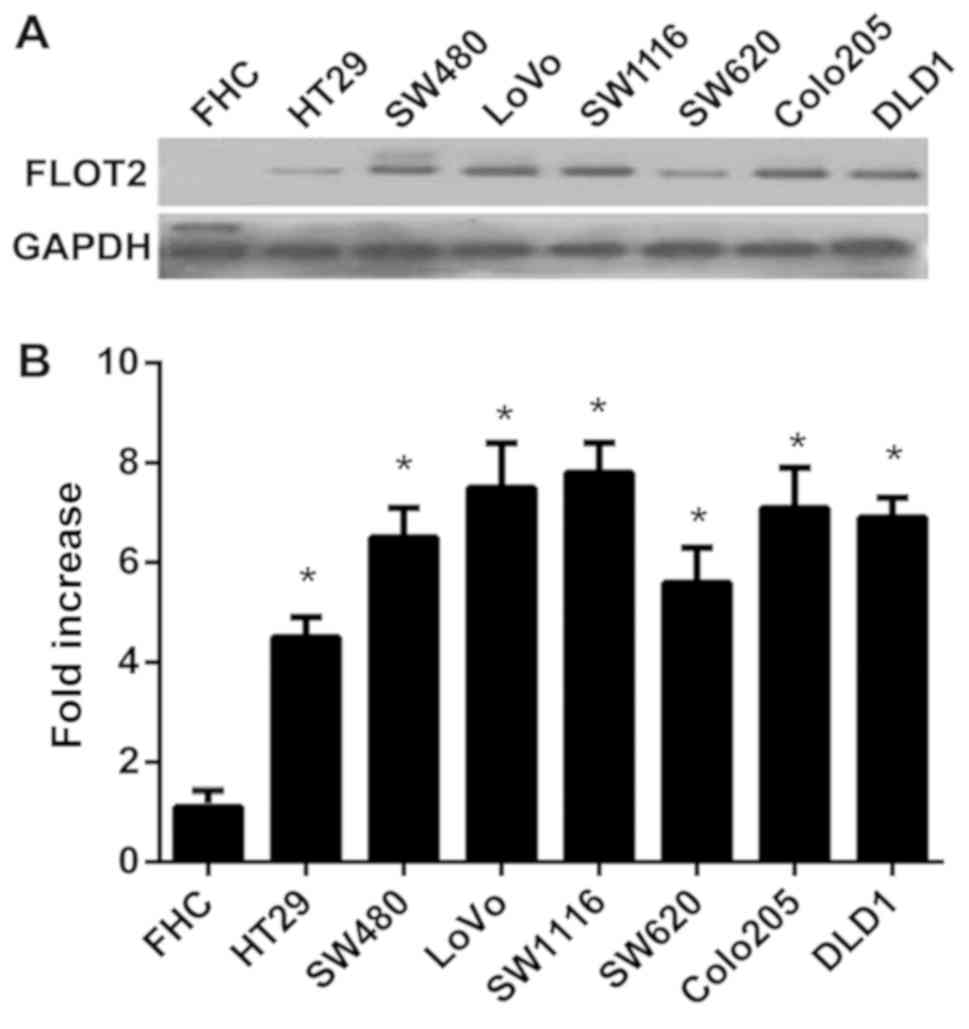
FLOT2 is highly expressed in seven CRC
cell lines
To investigate the function of FLOT2 in CRCs, the
expression of FLOT2 in CRC cell lines was determined. The
expression levels of FLOT2 mRNA and protein were compared in seven
CRC cell lines (HT-29, SW480, Lovo, SW1116, SW620, Colo205 and
DLD1) and one normal colon cell line (FHC). FLOT2 protein was
highly expressed in CRC cell lines and only weakly expressed in FHC
(Fig. 1A). In addition, FLOT2 mRNA
expression was at least 4-fold higher in CRC cell lines compared
with FHC cells (Fig. 1B). Taken
together, these results indicate that CRC cell lines exhibit high
FLOT2 expression, indicating that FLOT2 possibly serves a
function in CRCs.
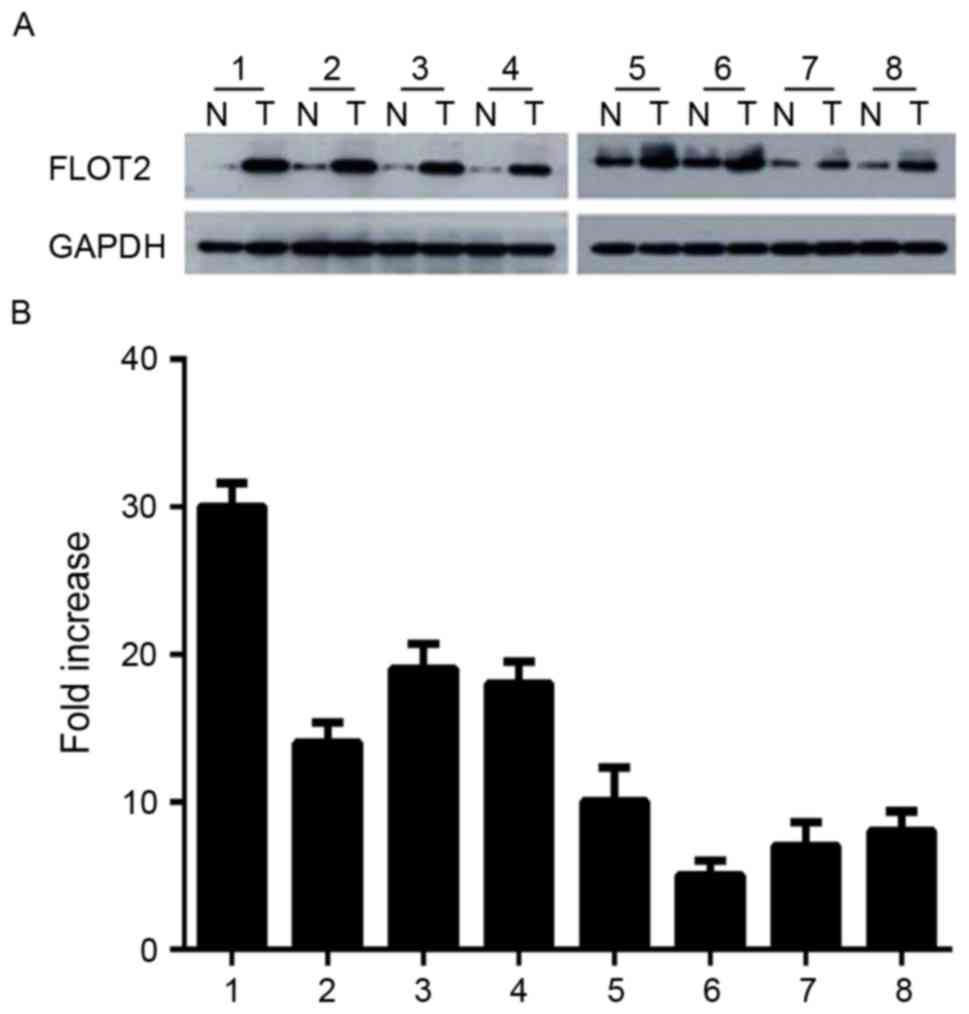
Expression of FLOT2 is increased in
CRC tissues
Since FLOT2 is increased in CRC cell lines, it was
investigated whether this was the case in vivo. CRC tissue
samples and adjacent non-cancerous tissues were obtained from 8
patients with CRC. FLOT2 protein was upregulated in CRC samples
compared with matched controls (Fig.
2A). Consistent with this data, FLOT2 mRNA was also expressed
at higher levels in all CRC tissue samples compared with adjacent
non-cancerous tissues, with a differential expression that ranged
from 6.3-fold to 30.2-fold (Fig. 2B).
These data indicated that FLOT2 is upregulated in vitro and
in vivo.
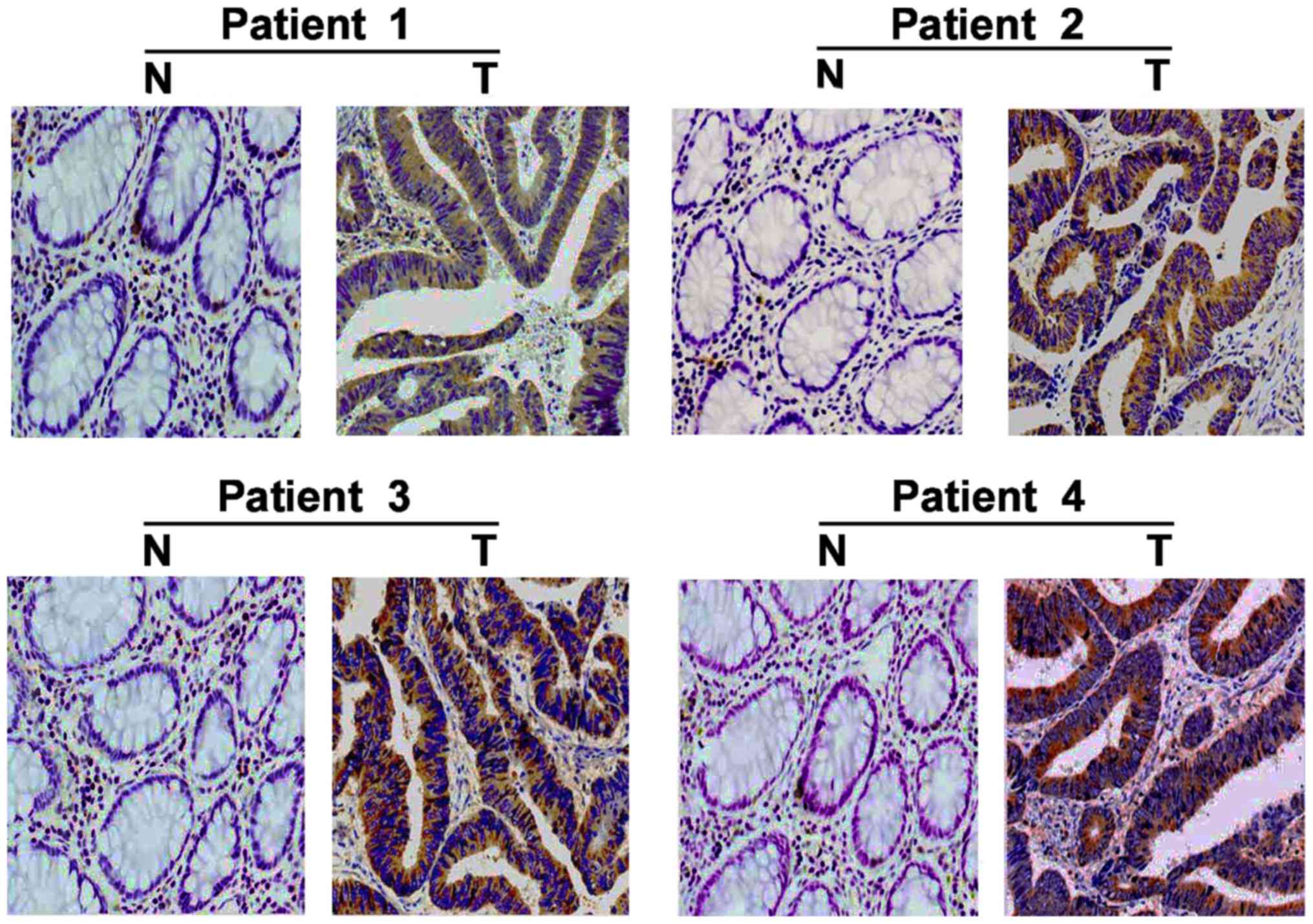
In addition, IHC staining was used to examine the
expression of FLOT2 in 180 paired paraffin-embedded CRC
tissues. FLOT2 protein was predominantly localized on the plasma
membrane (Fig. 3). In the 180
samples, a high level of FLOT2 protein was detected in 119 samples
(66.11%; Table I) and low or no
staining was observed in 61 tumor samples (33.89%; Table I). As presented in Fig. 3, no or weak signals were detected in
the adjacent non-cancerous tissues and normal colorectal tissues.
In contrast, FLOT2 was highly expressed in CRC tissues. Taken
together, these observations indicate that FLOT2 is overexpressed
in CRC tissues.
 | Table I.Association between FLOT2 expression
and other clinicopathological features in colorectal carcinoma. |
Table I.
Association between FLOT2 expression
and other clinicopathological features in colorectal carcinoma.
|
| FLOT2 expression |
|
|---|
|
|
|
|
|---|
| Characteristic | Low/no (n=61) | High (n=119) | P-value |
|---|
| Sex |
|
| 0.979 |
| Male | 32 | 61 |
|
|
Female | 29 | 58 |
|
| Age, years |
|
| 0.829 |
|
<60 | 17 | 35 |
|
| ≥60 | 44 | 84 |
|
| Tumor size, cm |
|
| 0.730 |
|
<5 | 25 | 50 |
|
| ≥5 | 36 | 69 |
|
| Tumor location |
|
| 0.497 |
|
Colon | 20 | 30 |
|
|
Rectum | 41 | 89 |
|
| Histological
type |
|
| 0.189 |
| NMC | 58 | 94 |
|
| MC | 3 | 25 |
|
| Differentiation |
|
| 0.517 |
| Well | 16 | 25 |
|
|
Moderate | 22 | 35 |
|
| Poor | 23 | 59 |
|
| Depth of
invasion |
|
| 0.024 |
| T1 | 18 | 19 |
|
| T2 | 19 | 26 |
|
| T3 | 14 | 34 |
|
| T4 | 10 | 40 |
|
| Lymph node
metastasis |
|
| 0.005 |
| N0 | 33 | 41 |
|
|
N1-2 | 28 | 78 |
|
| Distant
metastasis |
|
| 0.008 |
| M0 | 55 | 101 |
|
| M1 | 6 | 18 |
|
| AJCC stage |
|
| 0.011 |
|
I+II | 30 | 46 |
|
|
III+IV | 31 | 73 |
|
Association of FLOT2 expression and
clinicopathological features of patients with CRC
FLOT2 expression was identified to be increased in
CRC tissues, suggesting that certain connections exist between
FLOT2 levels and CRC. To investigate this possibility, statistical
analysis was performed. Associations between FLOT2 expression and
the clinicopathological characteristics of CRC were identified,
including depth of invasion (P=0.024), lymph node metastasis
(P=0.005), distant metastasis (P=0.008) and AJCC stage (P=0.011)
(Table I). In contrast, no apparent
associations were identified between FLOT2 expression and other
factors, including sex, age, tumor size, tumor location,
histological types and tumor differentiation (Table I). These results indicate that FLOT2
may serve as a biomarker for the diagnosis of CRC.
Association between FLOT2 expression
and prognosis in patients with CRC
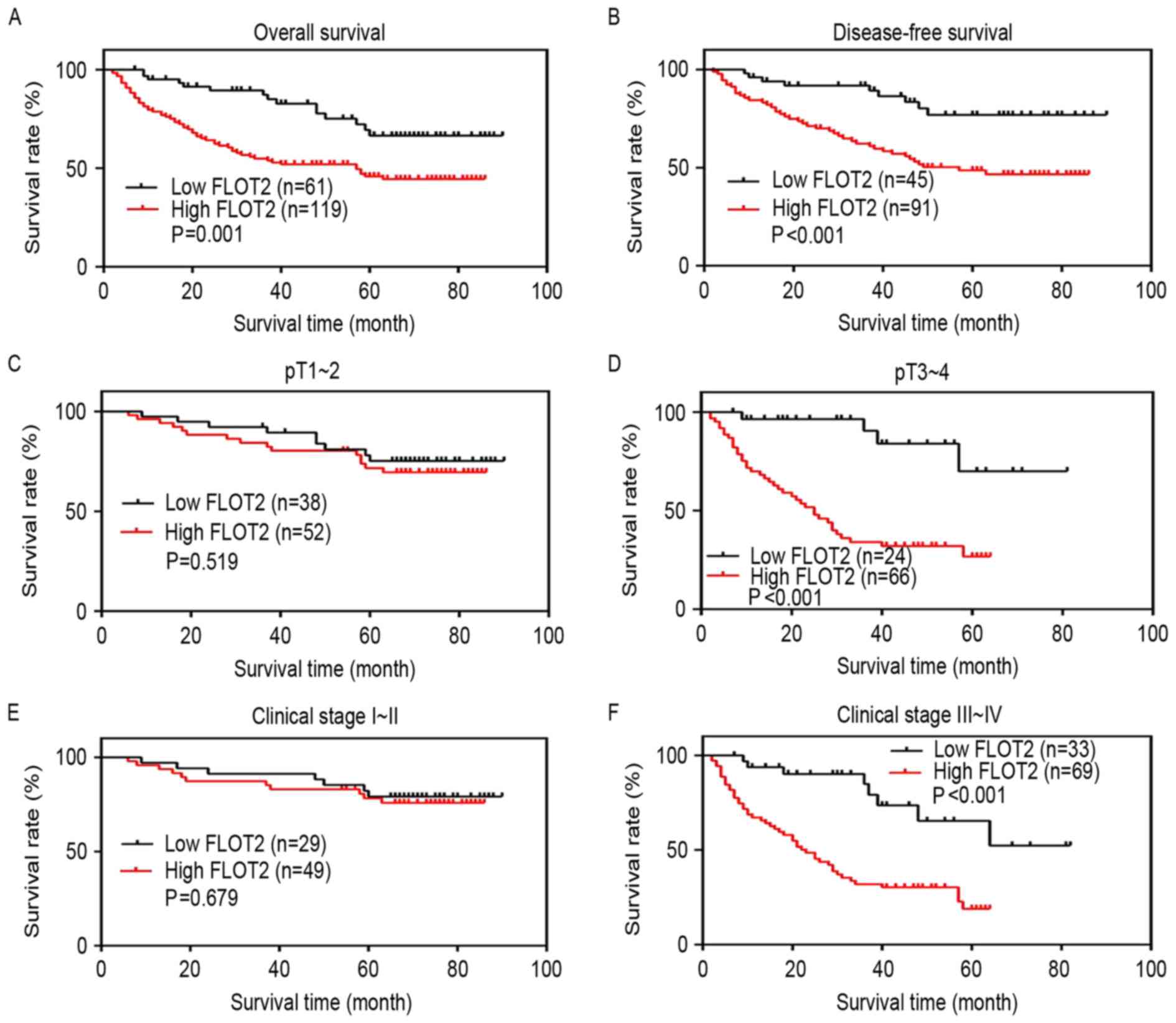
Next, whether the expression of FLOT2 regulates the
survival of patients was investigated. The Kaplan-Meier analysis
and the log-rank test were used to analyze the effect of FLOT2
expression on survival time. Survival curves revealed that the
patients with CRC with high FLOT2 expression had worse OS and
disease-free survival (DFS) compared with those with low FLOT2
expression (P=0.001 and P<0.001) (Fig.
4A and B). The survival rate of patients with CRC with high
FLOT2 expression decreased in the pT3-4 subgroup, but not in the
pT1-2 subgroup (P<0.001 and P=0.519) (Fig. 4C and D). Similar results were
identified in the AJCC stage I–II and III–IV subgroups (P<0.001
and P=0.679) (Fig. 4E and F). As
presented in Table II, the
univariate survival analysis indicated that the OS was markedly
associated with FLOT2 expression, depth of invasion, lymph node
metastasis, distant metastasis and AJCC stage. Furthermore,
multivariate survival analysis was performed using Cox proportional
hazard model to confirm that FLOT2 expression level, lymph node
metastasis, distant metastasis and AJCC stage were independent poor
prognostic factors for OS of CRC.
 | Table II.Cox regression analysis of prognostic
factors for overall survival in patients with colorectal
cancer. |
Table II.
Cox regression analysis of prognostic
factors for overall survival in patients with colorectal
cancer.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | HR | CI (95%) | P-value | HR | CI (95%) | P-value |
|---|
| Sex | 1.137 | 0.713–1.936 | 0.372 |
|
|
|
| Age | 0.765 | 0.536–1.821 | 0.427 |
|
|
|
| Tumor size | 0.634 | 0.345–1.176 | 0.425 |
|
|
|
| Tumor location | 0.213 | 0.819–2.778 | 0.719 |
|
|
|
|
Differentiation | 0.729 | 0.467–1.306 | 0.116 |
|
|
|
| Depth of
invasion | 3.231 | 1.312–5.832 | 0.034 | 1.442 | 0.845–1.886 | 0.676 |
| Lymph node
metastasis | 3.509 | 2.399–5.442 | 0.004 | 2.123 | 1.371–4.734 | 0.002 |
| Distant
metastasis | 5.738 | 3.548–8.536 | 0.002 | 3.631 | 2.514–6.722 | 0.001 |
| AJCC stage | 3.448 | 2.928–6.335 | 0.021 | 1.693 | 1.021–4.561 | 0.027 |
| FLOT2
expression | 4.485 | 2.375–7.488 | 0.001 | 2.987 | 0.962–5.135 | 0.013 |
Discussion
Nearly 50% of patients with CRC succumb owing to
distant metastasis, particularly to the liver. The patients with
liver metastasis are not suitable for surgical treatment, thus the
survival rate of patients with liver metastasis is <10%
(20). Conversely, the prognosis of
patients with CRC remains poor. Therefore, there is an urgent
requirement to identify a novel biomarker for CRC.
In the present study, it was identified that the
expression of FLOT2 was apparently upregulated in CRC cell lines
compared with in a normal colon cell line. Furthermore, using an
in vivo assay, it was revealed that the CRC tissues
exhibited high FLOT2 expression, whereas adjacent normal tissues
exhibited low FLOT2 expression. These results clearly demonstrated
that FLOT2 was markedly overexpressed in human CRC tissues compared
with in normal colorectal epithelium. Although the underlying
molecular mechanisms leading to FLOT2 overexpression in human CRC
tissues are unclear, FLOT2 is proposed as a potential biomarker for
the diagnosis of CRC. Given that the mRNA levels of FLOT2
were also increased in CRC tissues, we hypothesize that gene
amplification may account for FLOT2 overexpression. This
possibility requires investigation in future studies using
techniques including next-generation sequencing and genome-wide
association studies.
The association between the expression of FLOT2 and
the clinical characteristics of patients with CRC was investigated.
An association between FLOT2 expression and the depth of invasion,
lymph node metastasis, distant metastasis and AJCC stage was
identified. FLOT2 expression may also be associated with the
histological type in CRC. It was identified that FLOT2 expression
was high in the majority of the mucinous adenocarcinoma of CRC
(25/28). In contrast, the rate of low FLOT2 expression (58/152) did
not differ from the rate of high expression (94/152) in
non-mucinous adenocarcinoma. However, because only a small number
of mucinous adenocarcinomas samples were obtained, it was not
possible to identify a clear association between the FLOT2
expression and the histological types in CRCs (P=0.189). The
inclusion of a greater number of mucinous adenocarcinomas samples
may solve the problem.
A number of previous studies have suggested that
FLOT2 serves critical functions in the progression and metastasis
of several human malignant tumors, including nasopharyngeal
carcinoma, gastric cancer, cervical carcinoma, non-small cell lung
cancer, melanoma and breast cancer (15–17,21–24).
For instance, Zhao et al (24)
identified that FLOT2 is an indispensable member for transforming
growth factor β signaling, which is essential for the
epithelial-mesenchymal transition (EMT) process in nasopharyngeal
carcinoma metastasis. In cultured AGS and SGC7901 cells, knockdown
of FLOT2 expression with specific siRNA resulted in an
evident inhibition of the proliferative, migratory and invasive
capabilities of the cells compared with those of control cells
(21). In the present study, it was
identified that the expression of FLOT2 in CRC tissues was markedly
increased compared with the adjacent non-cancerous counterparts.
Furthermore, correlation analysis indicated that the increased
FLOT2 expression was associated with certain clinicopathological
properties, including the depth of invasion, lymph node metastasis,
distant metastasis and AJCC stage. Collectively, these results
suggest that increased FLOT2 expression may promote CRC cell
proliferation and metastasis. Further studies are required to
investigate whether FLOT2 indeed modulates the proliferation and
migration of CRC cells.
Furthermore, the OS and DFS rates were determined in
patients with CRC with a low or high level of FLOT2, and it was
identified that there was an inverse association between the level
of FLOT2 and prognosis of patients. Similarly, it was identified
that the patients with a high level of FLOT2 had a poorer outcome
compared with those with a low expression level of FLOT2 in the
pT3-4 subgroup and the AJCC stage III–IV subgroup. Nevertheless, no
statistical association between the high FLOT2 expression and the
shorter OS time was identified in either the pT1-2 subgroup or AJCC
stage I–II subgroup. We hypothesize that this is due to the good
prognosis of patients with CRC at an early stage and limited number
of the clinical cases. In addition, Cox proportional hazards models
revealed that high FLOT2 expression maintained its independent
prognostic impact on OS. The results of the present study indicate
that FLOT2 may be a predictor of poor prognosis for CRC.
It was identified that upregulation of FLOT2 was
associated with poor prognosis and decreased survival of patients
with CRC. Multivariate analysis indicated that the FLOT2 protein
expression level could be used as an independent prognostic
predictor for patients with CRC. Thus, the FLOT2 expression level
may be useful for determining the prognosis and guiding the
follow-up schedule in patients with CRC. FLOT2 may be useful as a
biomarker for CRC diagnosis and a drug target for CRC
treatment.
Acknowledgements
The authors thank Professor Sheng Liu and Professor
Li Xia for providing help with pathological diagnosis and
immunohistochemistry experiments.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 201581402401).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
TL participated in the study design, literature
research, data analysis and interpretation, and approved the final
version of the manuscript. CC took part in the experiments, data
acquisition, analysis and interpretation, and manuscript
preparation and editing. QX participated in the experiments, data
acquisition, analysis and interpretation. DL took part in the study
concept and design, literature research, experimental procedures,
data acquisition, analysis and interpretation, statistical
analysis, and manuscript preparation, editing, review and final
approval.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Nanchang University
(Nanchang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cunningham D, Atkin W, Lenz HJ, Lynch HT,
Minsky B, Nordlinger B and Starling N: Colorectal cancer. Lancet.
375:1030–1047. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jary M, Lecomte T, Bouché O, Kim S, Dobi
E, Queiroz L, Ghiringhelli F, Etienne H, Leger J, Godet Y, et al:
Prognostic value of baseline seric Syndecan-1 in initially
unresectable metastatic colorectal cancer patients: A simple
biological score. Int J Cancer. 139:2325–2335. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barkhatov L, Fretland AA, Kazaryan AM,
Rosok BI, Brudvik KW, Waage A, Bjornbeth BA, Sahakyan MA and Edwin
B: Validation of clinical risk scores for laparoscopic liver
resections of colorectal liver metastases: A 10-year observed
follow-up study. J Surg Oncol. 114:757–763. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pritchard CC and Grady WM: Colorectal
cancer molecular biology moves into clinical practice. Gut.
60:116–129. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Simons K and Toomre D: Lipid rafts and
signal transduction. Nat Rev Mol Cell Biol. 1:31–39. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Babuke T and Tikkanen R: Dissecting the
molecular function of reggie/flotillin proteins. Eur J Cell Biol.
86:525–532. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bickel PE, Scherer PE, Schnitzer JE, Oh P,
Lisanti MP and Lodish HF: Flotillin and epidermal surface antigen
define a new family of caveolae-associated integral membrane
proteins. J Biol Chem. 272:13793–13802. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Banning A, Tomasovic A and Tikkanen R:
Functional aspects of membrane association of reggie/flotillin
proteins. Curr Protein Pept Sci. 12:725–735. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lang DM, Lommel S, Jung M, Ankerhold R,
Petrausch B, Laessing U, Wiechers MF, Plattner H and Stuermer CA:
Identification of reggie-1 and reggie-2 as
plasmamembrane-associated proteins which cocluster with activated
GPI-anchored cell adhesion molecules in non-caveolar micropatches
in neurons. J Neurobiol. 37:502–523. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Langhorst MF, Reuter A and Stuermer CA:
Scaffolding microdomains and beyond: The function of
reggie/flotillin proteins. Cell Mol Life Sci. 62:2228–2240. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schulte T, Paschke KA, Laessing U,
Lottspeich F and Stuermer CA: Reggie-1 and reggie-2, two cell
surface proteins expressed by retinal ganglion cells during axon
regeneration. Development. 124:577–587. 1997.PubMed/NCBI
|
|
13
|
Stuermer CA, Lang DM, Kirsch F, Wiechers
M, Deininger SO and Plattner H: Glycosylphosphatidyl
inositol-anchored proteins and fyn kinase assemble in noncaveolar
plasma membrane microdomains defined by reggie-1 and −2. Mol Biol
Cell. 12:3031–3045. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hazarika P, McCarty MF, Prieto VG, George
S, Babu D, Koul D, Bar-Eli M and Duvic M: Up-regulation of
Flotillin-2 is associated with melanoma progression and modulates
expression of the thrombin receptor protease activated receptor 1.
Cancer Res. 64:7361–7369. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Berger T, Ueda T, Arpaia E, Chio II,
Shirdel EA, Jurisica I, Hamada K, You-Ten A, Haight J, Wakeham A,
et al: Flotillin-2 deficiency leads to reduced lung metastases in a
mouse breast cancer model. Oncogene. 32:4989–4994. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X, Yang Q, Guo L, Li XH, Zhao XH,
Song LB and Lin HX: Flotillin-2 is associated with breast cancer
progression and poor survival outcomes. J Transl Med. 11:1902013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee KW, Lee SS, Kim SB, Sohn BH, Lee HS,
Jang HJ, Park YY, Kopetz S, Kim SS, Oh SC and Lee JS: Significant
association of oncogene YAP1 with poor prognosis and cetuximab
resistance in colorectal cancer patients. Clin Cancer Res.
21:357–364. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun L, Hu S, Yu L, Guo C, Sun L, Yang Z,
Qi J and Ran Y: Serum haptoglobin as a novel molecular biomarker
predicting colorectal cancer hepatic metastasis. Int J Cancer.
138:2724–2731. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cao K, Xie D, Cao P, Zou Q, Lu C, Xiao S,
Zhou J and Peng X: SiRNA-mediated flotillin-2 (Flot2)
downregulation inhibits cell proliferation, migration, and invasion
in gastric carcinoma cells. Oncol Res. 21:271–279. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Lin L, Huang Z, Ji B, Mei S, Lin Y
and Shen Z: High expression of flotillin-2 is associated with poor
clinical survival in cervical carcinoma. Int J Clin Exp Pathol.
8:622–628. 2015.PubMed/NCBI
|
|
23
|
Wang YL, Yao WJ, Guo L, Xi HF, Li SY and
Wang ZM: Expression of flotillin-2 in human non-small cell lung
cancer and its correlation with tumor progression and patient
survival. Int J Clin Exp Pathol. 8:601–607. 2015.PubMed/NCBI
|
|
24
|
Zhao L, Lin L, Pan C, Shi M, Liao Y, Bin J
and Liao W: Flotillin-2 promotes nasopharyngeal carcinoma
metastasis and is necessary for the epithelial-mesenchymal
transition induced by transforming growth factor-β. Oncotarget.
6:9781–9793. 2015.PubMed/NCBI
|