Introduction
Cervical cancer is one of the most common types of
cancer, and has a high mortality rate (1). Cervical cancer affects >1 out of
10,000 females and a mortality rate of 2.4 per 100,000 has been
reported in 2012 worldwide (1).
Cervical cancer primarily includes squamous cell carcinoma and
adenocarcinoma, and the former is responsible for >80% of cases
(2). Although various factors may
contribute to the development of cervical squamous cell carcinoma,
including an increase in the number of sexual partners and early
onset of sexual activity (3), human
papillomavirus (HPV) infection, mainly sexually transmitted, is
considered to be the principal cause of this disease (4). Over the last several decades, the rise
in HPV screening markedly reduced the incidence of cervical cancer,
although no further decrease has been evident more recently
(5). Therefore, development of novel
treatments remains critical for the improved prognosis of patients
with cervical squamous cell carcinoma.
The human genome transcribes not only messenger RNA
(mRNA) for protein translation, but also a large set of non-coding
RNAs (ncRNAs) that participate in physiological and pathological
processes (6). Long ncRNAs (lncRNAs)
are a group of ncRNAs that have been indicated in the pathogenesis
of numerous malignancies (7). HPV
infection and the development of cervical cancer are accompanied by
altered expression patterns of particular lncRNAs (8,9). However,
the specific involvement of lncRNAs in HPV-positive cervical cancer
is yet to be reported. lncRNA-oncogene-induced senescence 1
(lnRNA-OIS1) is a recently identified lncRNA that participates in
the regulation of cellular senescence (10). However, to the best of our knowledge,
the functionality of OIS1 in other human diseases has yet to be
reported. In view of this, the present study was carried out to
investigate the involvement of OIS1 in HPV-positive and
HPV-negative cervical squamous cell carcinoma. The findings of the
present study provided novel insight to the pathogenesis of
cervical squamous cell carcinoma and also suggested a novel
therapeutic target for this disease.
Materials and methods
Patients
The present study included 92 female patients with
cervical squamous cell carcinoma, diagnosed and treated in the
Department of Obstetrics and Gynecology, Beijing Hospital (Beijing,
China) between January 2015 and January 2017. The age range of the
92 patients with cervical squamous cell carcinoma was between 33
and 69 years (mean, 49.2±7.7 years). Inclusion criteria were as
follows: Patients with cervical squamous cell carcinoma and a
complete medical record (medical history within the past 5 years)
and patients willing to participate. Exclusion criteria were as
follows: Patients with other malignancies, severe diseases and
viral infections and patients who had been treated prior to
admission. All patients were screened for HPV infection using
polymerase chain reaction (PCR). A total of 22 patients were
diagnosed as HPV-negative; of the remaining HPV-positive patients,
19 were diagnosed as HPV-11-positive, 23 were HPV-16-positive and
28 were HPV-18-positive. Another 40 healthy females (mean, 49.8±7.2
years, range 31–70 years old) were included as healthy controls. No
significant differences in age, BMI or other basic information were
noted between the control group and the other patient groups. The
study was approved by the Ethics Committee of Beijing Hospital, and
all participants provided written informed consent.
Tissue collection
Tumor tissues and adjacent healthy tissues were
collected during surgical resection. Tissues were stored in liquid
nitrogen prior to being used. Only patients who were suitable for
surgical resection were enrolled. Additionally, whole blood (5 ml)
was extracted from the elbow vein of each participant on the day of
admission. Samples were prepared by incubating the blood samples at
room temperature for 2 h, followed by centrifugation at 1,000 × g
for 15 min for serum collection.
Cell lines and culture
The normal cervical HCvEpC (HPV-negative) and
Ect1/E6E7 (HPV-positive) cell lines were employed, in addition to
two human cervical squamous cell carcinoma cell lines, C33A
(HPV-negative) and SiHa (HPV-positive). Cells were purchased from
the American Type Culture Collection (Manassas, VA, USA) and
cultivated with ATCC-formulated Eagle's Minimum Essential Medium
containing 10% fetal bovine serum (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at 37°C with 5% CO2.
Transfection
OIS1 or MTK-1 full length cDNA fragments flanked by
EcoRI restriction sites (Sangon Biotech Co., Ltd., Shanghai,
China) were inserted into the pIRSE2-EGFP vector (Clontech
Laboratories, Inc., Mountain view, CA, USA). Cells were cultured
overnight to 80–90% confluence. Transfection of 4×105
cells/sample was conducted with 10 nM OIS1/MTK-1 expression vector
or empty vector (negative control) using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). OIS1 expression was detected by reverse
transcription-quantitative PCR (RT-qPCR) and an overexpression rate
of >150% compared with control cells was required prior to
subsequent experimentation. Cells were harvested for subsequent
experiments at 24 h following transfection.
Cell proliferation assay
A total of 5×103 cells in 100 µl
ATCC-formulated Eagle's Minimum Essential Medium containing 10%
fetal bovine serum were added to each well of a 96-well plate.
Cells were cultured in an incubator (37°C, 5% CO2), and
proliferation was assessed using Cell Counting Kit 8 (CCK-8) (Sigma
Aldrich; Merck KGaA). CCK-8 (10 µl) was added at 24, 48, 72 and 96
h. Cells were incubated for 3 h, and optical density was measured
at 450 nm.
RT-qPCR
Total RNA extraction was performed using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Tumor and adjacent healthy tissues were ground in liquid
nitrogen prior to the addition of TRIzol® for complete
cell lysis. cDNA was synthesized and qPCR conducted using the
SYBR® Green Real-Time PCR Master mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Primers were as
follows: OIS1 forward, 5′-GCAAGAGTTTCTACCCAAAG-3′ and reverse,
5′-CACATCTGCTGAGGACAGAG-3′; and β-actin forward,
5′-GACCTCTATGCCAACACAGT-3′ and reverse, 5′-AGTACTTGCGCTCAGGAGGA-3′.
Thermocycling conditions were as follow: 95°C for 55 sec, followed
by 40 cycles of 95°C for 10 sec and 57°C for 30 sec. OIS1
expression was normalized to β-actin using the 2−ΔΔCq
method (11).
Western blotting
Radioimmunoprecipitation assay buffer (Thermo Fisher
Scientific, Inc.) was used to extract total protein from in
vitro cultivated cells. A bicinchoninic acid assay was employed
for protein quantification. SDS-PAGE was conducted using a 10% gel,
with 30 µg protein per lane. Following transfer to polyvinylidene
difluoride membranes, 5% skimmed milk was applied for blocking for
1.5 h at room temperature. Incubation was then performed with the
following primary antibodies at 4°C overnight: Rabbit anti-MTK-1
antibody (1:1,200; cat. no. ab186125) and anti-GAPDH antibody
(1:1,200; cat. no. ab37168 (both Abcam, Cambridge, UK). Incubation
with an anti-rabbit IgG-HRP secondary antibody (1:1,000; cat. no.
MBS435036; MyBioSource. Inc., San Diego, CA, USA) was performed the
next day at room temperature for 2 h. An enhanced chemiluminescence
kit (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was used for
visualization, and Image J 1.46r software (National Institutes of
Health, Bethesda, MD, USA) was used to normalize the expression
level of MTK-1 to GAPDH.
Statistical analysis
All statistical analyses were performed using SPSS
19.0 (IBM Corp., Armonk, NY, USA). Comparisons between two groups
were performed by t-test, while comparisons between multiple groups
were performed by one way analysis of variance followed by the
least significant difference test. Patients were divided into high
and low expression groups according to the median serum level of
OIS1 (1.72). The χ2 test was conducted to determine the
association between serum levels of OIS1 and the
clinicopathological data of HPV-positive patients.. Data are
presented as the mean ± standard deviation. P<0.05 is considered
to indicate a statistically significant difference.
Results
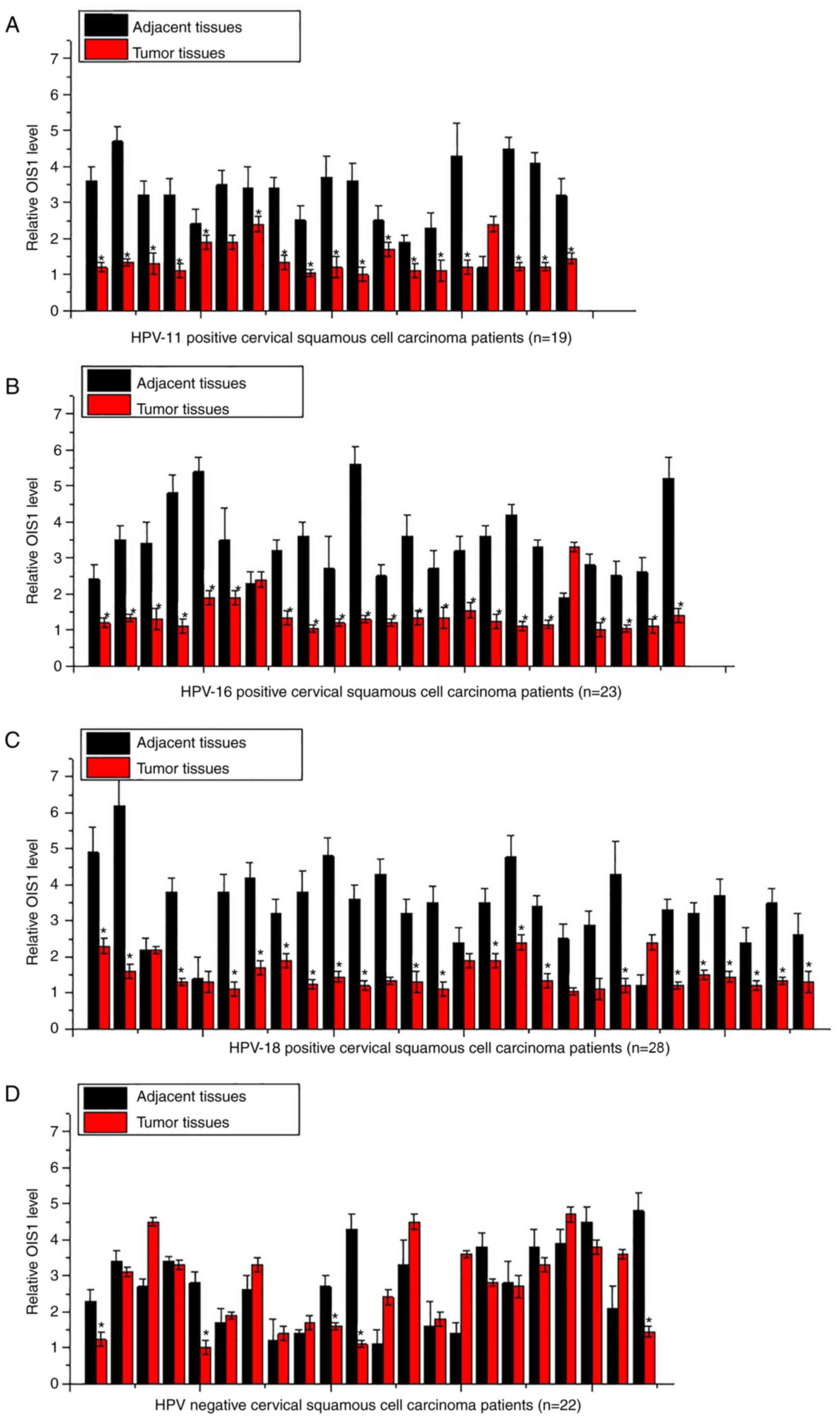
Expression of OIS1 in tumor tissues
and adjacent tissues
Expression of OIS1 in tumor tissues and adjacent
tissues of 90 patients with cervical squamous cell carcinoma was
detected by RT-qPCR. As illustrated in Fig. 1, 18 of the 19 HPV-11-positive patients
(Fig. 1A), 21 of the 23
HPV-16-positive patients (Fig. 1B)
and 21 of the 28 HPV-18-positive patients (Fig. 1C) displayed a significant lower
expression level of OIS1 in tumor tissues compared with that in the
adjacent tissues (P<0.05). By contrast, only five of the 22
HPV-negative patients displayed a lower expression level of OIS1 in
tumor tissues compared with adjacent tissues (Fig. 1D).
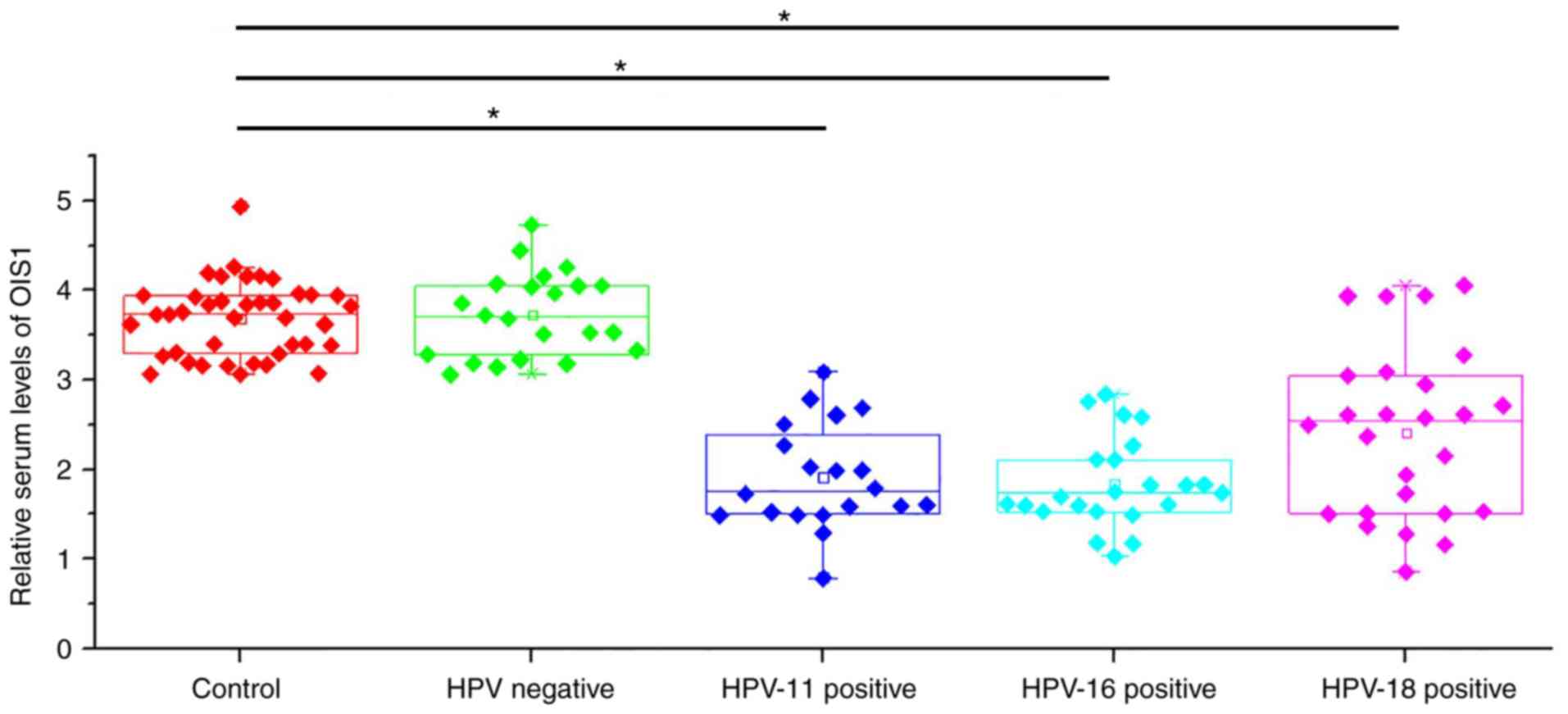
Serum levels of OIS1 in patients with
cervical squamous cell carcinoma and in healthy controls
Serum levels of OIS1 in patients with cervical
squamous cell carcinoma and in healthy controls were also
determined by RT-qPCR. As illustrated in Fig. 2, serum levels of OIS1 were
significantly lower in HPV-11, −16 and −18-positive patients
compared with those in the healthy controls (P<0.05), while no
significant differences were observed between HPV-negative patients
and healthy controls.
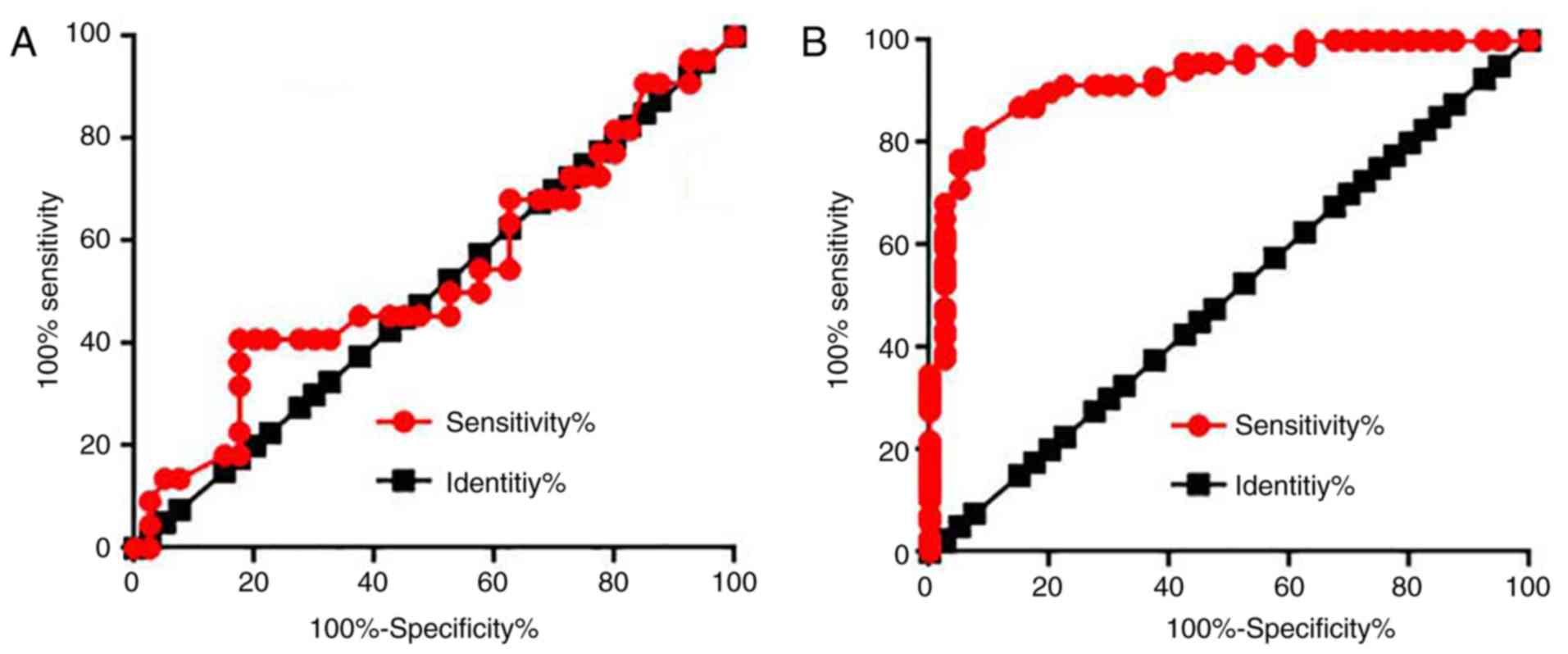
Diagnostic values of serum OIS1 for
HPV-negative and HPV-positive cervical squamous cell carcinoma
Receiver operating characteristic (ROC) curve
analysis was performed to evaluate the diagnostic values of serum
OIS1 for HPV-negative and -positive cervical squamous cell
carcinoma. For HPV-negative cervical squamous cell carcinoma, the
area under the curve was 0.5330, with a 95% confidence interval of
0.3757 to 0.6902 (P=0.6696) (Fig.
3A). For HPV-positive cervical squamous cell carcinoma, the
area under the curve was 0.9207, with a 95% confidence interval of
0.9609 to 0.1020 (P=0.6696) (Fig.
3B). The data suggest that serum OIS1 may be used to diagnose
HPV-positive, but not HPV-negative cervical squamous cell
carcinoma.
Associations between serum levels of
OIS1 and the clinicopathological data of HPV-positive patients
Patients were divided into high and low expression
groups according to the median (1.72) serum level of OIS1. The
χ2 test was conducted to determine the association
between serum levels of OIS1 and the clinicopathological data of
HPV-positive patients. Serum levels of OIS1 revealed no significant
associations between patient age, distant tumor metastasis, or
smoking and alcohol consumption habits, although there was a
significant association with tumor size (Table I).
 | Table I.Associations between serum levels of
oncogene-induced senescence 1 and clinicopathological data of human
papilloma virus-positive patients. |
Table I.
Associations between serum levels of
oncogene-induced senescence 1 and clinicopathological data of human
papilloma virus-positive patients.
|
|
| Expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Variable | Cases, n | High, n | Low, n | χ2 | P-value |
|---|
| Age, years |
|
|
| 0.53 | 0.47 |
|
>45 | 33 | 15 | 18 |
|
|
| ≤45 | 35 | 19 | 16 |
|
|
| Primary tumor
diameter, cm |
|
|
| 18.53 | <0.001 |
|
>5 | 20 | 16 | 4 |
|
|
| 3–5 | 24 | 14 | 10 |
|
|
| 1–3 | 24 | 4 | 20 |
|
|
| Tumor distant
metastasis |
|
|
| 0.97 | 0.32 |
| Yes | 28 | 12 | 16 |
|
|
| No | 40 | 22 | 18 |
|
|
| Smoking |
|
|
| 1.08 | 0.30 |
| Yes | 22 | 9 | 13 |
|
|
| No | 46 | 25 | 21 |
|
|
| Alcohol
consumption |
|
|
| 0.30 | 0.58 |
| Yes | 18 | 8 | 10 |
|
|
| No | 50 | 26 | 24 |
|
|
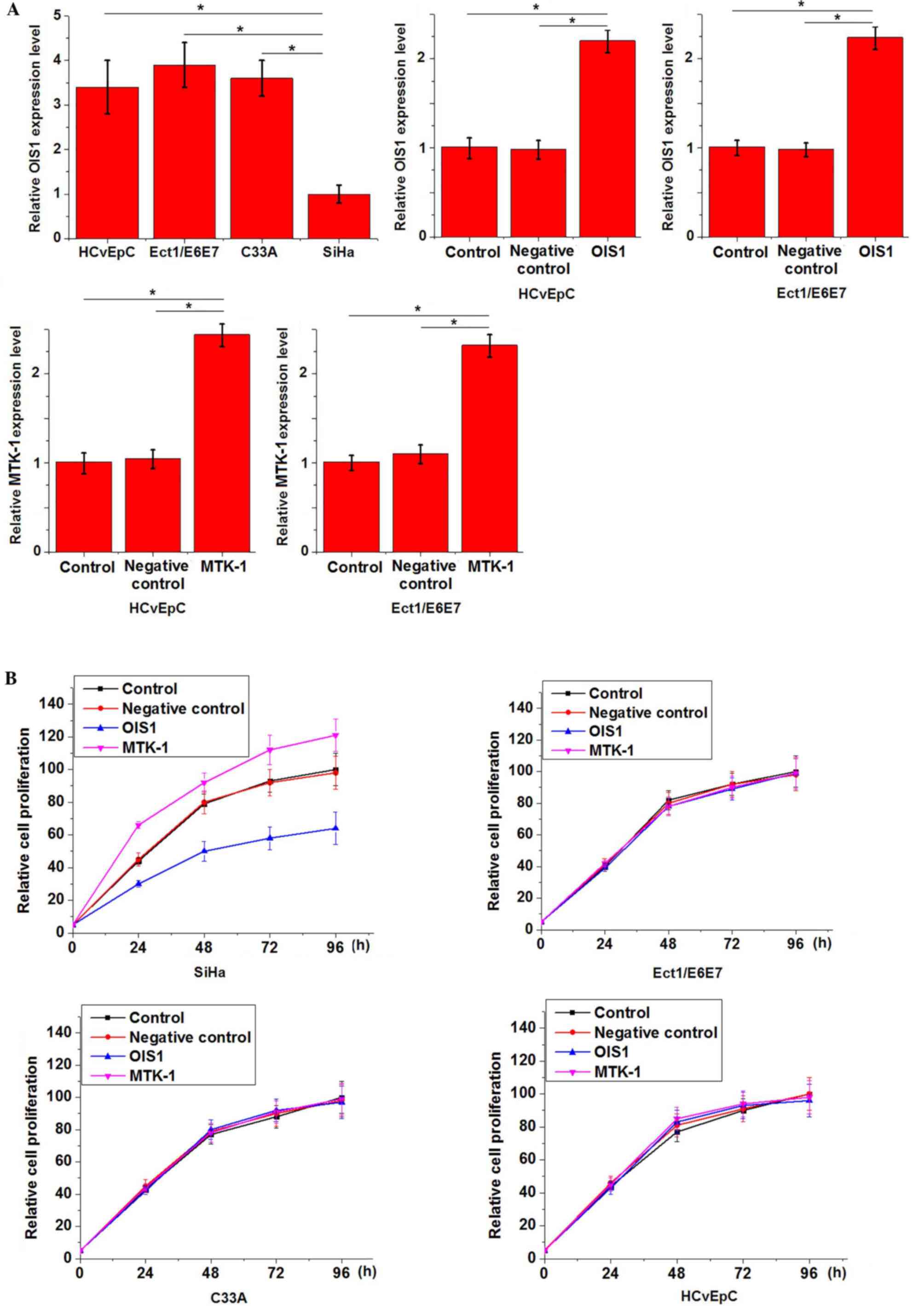
Effects of OIS1 and MTK-1
overexpression on cell proliferation
The two normal cervical cell lines HCvEpC
(HPV-negative) and Ect1/E6E7 (HPV-positive), and two human cervical
squamous cell carcinoma cell lines, C33A (HPV-negative) and SiHa
(HPV-positive), were employed. Expression of OIS1 in these cell
lines was detected by RT-qPCR. Fig.
4A illustrates that the expression level of OIS1 was
significantly lower in the SiHa cells compared with that in the
other three cell lines, and that overexpression of OIS1 and MTK-1
was successfully achieved via transfection. OIS1 overexpression
inhibited, while MTK-1 overexpression promoted the cell
proliferation of SiHa cells, but not the other three cell lines
(Fig. 4B; P<0.05).
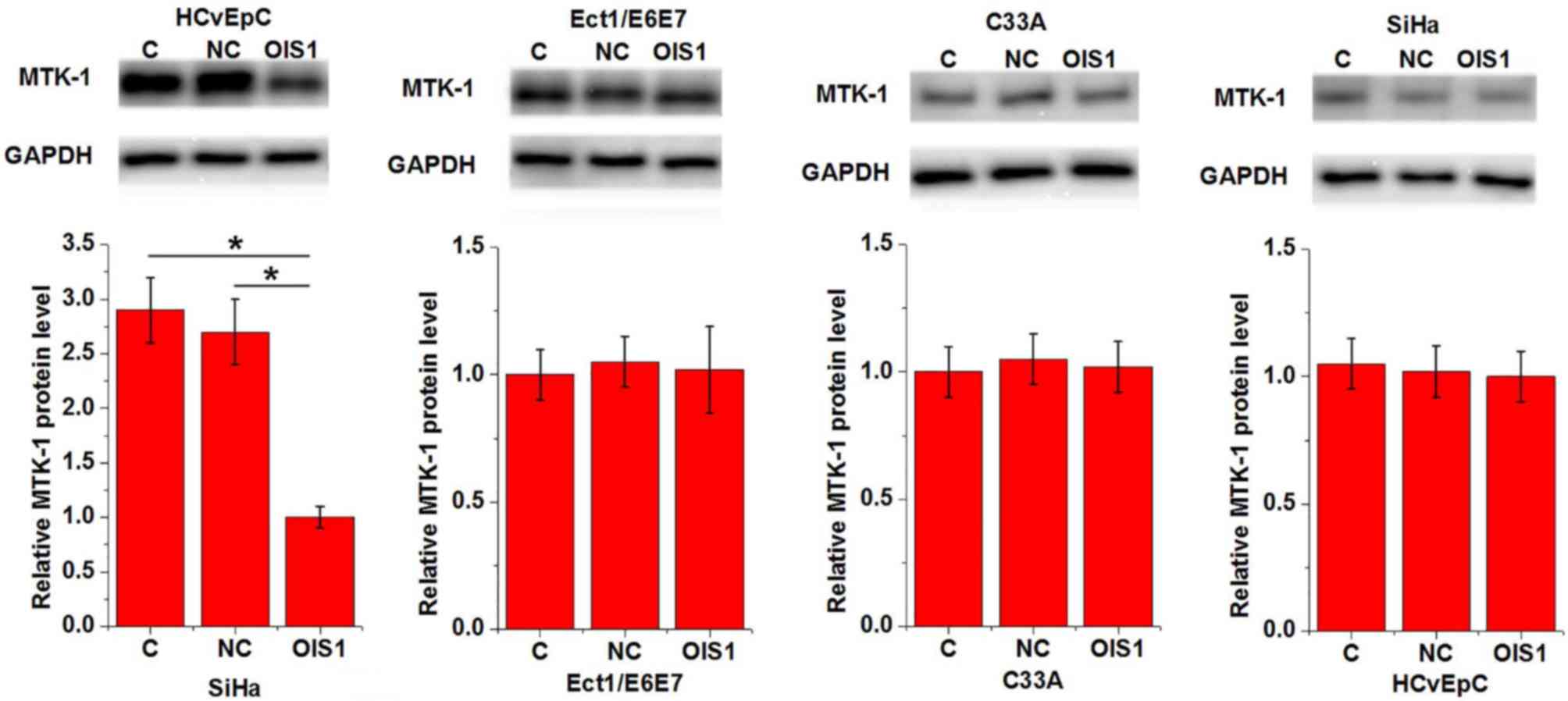
Effects of OIS1 overexpression on
MTK-1 expression
The effects of OIS1 overexpression on MTK-1
expression were detected by western blotting. As illustrated in
Fig. 5, OIS1 overexpression markedly
downregulated the expression of MTK-1 in SiHa cells, but not in any
of the other cell lines.
Discussion
HPV infection is the primary cause of cervical
cancer (4). The present study
reported lncRNA-OIS1 is specifically involved in the pathogenesis
of HPV-associated cervical cancer, which is likely due to its
interaction with MTK-1. It was also reported that MTK-1 is involved
in the regulation of tumor growth.
HPV infection results in alterations in the
expression of a number of lncRNAs (12), and lncRNAs act as intermediates in
HPV-mediated pathogenesis connecting HPV infection and downstream
signaling pathways (13). During the
development of cervical cancer, numerous lncRNAs display altered
expression patterns, and serve different roles to promote or
inhibit tumor progression (14,15). In
the present study, OIS1 was significantly downregulated in tumor
tissues compared with adjacent healthy tissues in the majority of
HPV-11, −16 and −18-positive, but not HPV-negative cervical
squamous cell carcinoma patients. Furthermore, serum levels of OIS1
were significantly lower in HPV-11, −16 and −18-positive cervical
squamous cell carcinoma patients compared with healthy controls,
while no significant differences in serum levels of OIS1 were
observed between healthy controls and HPV-negative patients. The
data suggest that downregulation of OIS1 specifically influences
the pathogenesis of HPV-positive cervical squamous cell
carcinoma.
Early diagnosis is key to the improved survival of
patients with cancer (16). In the
present study, ROC curve analysis revealed that OIS1 may be used to
effectively distinguish cases of HPV-positive cervical squamous
cell carcinoma from healthy controls. However, the value of serum
OIS1 in the diagnosis of HPV-negative cervical squamous cell
carcinoma is questionable. The present data suggest that serum OIS1
may serve as an effective diagnostic biomarker in patients with
HPV-positive cervical squamous cell carcinoma. Expression of
lncRNAs is regulated by various factors, including aging (17), tobacco consumption (18) and alcohol abuse (19). In the present study, serum levels of
OIS1 exhibited no significant association with patient age, smoking
or alcohol consumption. This suggests that serum OIS1 may be a
potential diagnostic marker for HPV-positive cervical squamous cell
carcinoma.
It was also distinguished that in patients with
HPV-positive cervical squamous cell carcinoma, serum levels of OIS1
were significantly associated with tumor size, but not distant
tumor metastasis. OIS1 also inhibited the proliferation of
HPV-positive, but not HPV-negative cervical squamous cell carcinoma
cell lines. Notably, OIS1 overexpression exhibited no significant
effects on the proliferation of HPV-negative or -positive normal
cervical cell lines. The regulation of cell migration by MTK-1 has
been demonstrated (20). In the
present study, MTK-1 positively regulated the proliferation of
HPV-positive cervical squamous cell carcinoma cell lines, but not
any other cell lines. It was also observed that OIS1 overexpression
significantly inhibited the expression of MTK-1 in HPV-positive
cervical squamous cell carcinoma cell lines, but not any of the
other cell lines used. This suggests that OIS1 is a tumor
suppressor in HPV-positive cervical squamous cell carcinoma,
potentially by inhibiting the expression of MTK-1. Notably, OIS1
only influenced HPV-positive, but not HPV-negative cervical
squamous cell carcinoma cells or normal cervical cells. Therefore,
OIS1 may be considered a safe target for the treatment of
HPV-positive cervical squamous cell carcinoma.
In conclusion, OIS1 expression was specifically
downregulated in HPV-positive cervical squamous cell carcinoma.
OIS1 inhibits the proliferation of HPV-positive cervical squamous
cell carcinoma cells by inhibiting the expression of MTK-1.
Acknowledgements
Not applicable.
Funding
The authors would like to acknowledge the financial
support of the Doctor Starting Fund of Beijing Hospital (grant no.
BJ-2014-023).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DZ and QL designed experiments. DZ, FWu and YC
performed experiments. FWe and QM analyzed data. QL interpreted
data and drafted the manuscript. All authors reviewed and approved
the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Beijing Hospital, and all participants provided
written informed consent.
Patient consent for publication
All patients provided written informed consent for
the publication of this manuscript.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Koh WJ, Greer BE, Abu-Rustum NR, Apte SM,
Campos SM, Cho KR, Chu C, Cohn D, Crispens MA, Dorigo O, et al:
Cervical cancer, version 2.2015. J Natl Compr Canc Netw.
13:395–404. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang PM, Chou CJ, Tseng SH and Hung CF:
Bioinformatics and in vitro experimental analyses identify the
selective therapeutic potential of interferon gamma and apigenin
against cervical squamous cell carcinoma and adenocarcinoma.
Oncotarget. 8:46145–46162. 2017.PubMed/NCBI
|
|
3
|
Maqbool DJ, George MP, Kenduiwa SC,
Rocelyn S, Carisse MT, Varghese SP, Avednigo CC, Honrado VG and
Naidoo SR: Cervical cancer risk factors among female high school
students in Baguio city. World Sci Res. 4:1–9. 2017. View Article : Google Scholar
|
|
4
|
Crosbie EJ, Einstein MH, Franceschi S and
Kitchener HC: Human papillomavirus and cervical cancer. Lancet.
382:889–899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hildesheim A, Gonzalez P, Kreimer AR,
Wacholder S, Schussler J, Rodriguez AC, Porras C, Schiffman M,
Sidawy M, Schiller JT, et al: Impact of human papillomavirus (HPV)
16 and 18 vaccination on prevalent infections and rates of cervical
lesions after excisional treatment. Am J Obstet Gynecol.
215:212.e1–212.e15. 2016. View Article : Google Scholar
|
|
6
|
Eddy SR: Non-coding RNA genes and the
modern RNA world. Nat Rev Genet. 2:919–929. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gutschner T and Diederichs S: The
hallmarks of cancer: A long non-coding RNA point of view. RNA Bio.
9:703–719. 2012. View Article : Google Scholar
|
|
8
|
Nohata N, Abba MC and Gutkind JS:
Unraveling the oral cancer lncRNAome: Identification of novel
lncRNAs associated with malignant progression and HPV infection.
Oral Oncol. 59:58–66. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng L, Yuan X, Jiang B, Tang Z and Li GC:
LncRNAs: Key players and novel insights into cervical cancer.
Tumour Biol. 37:2779–2788. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li L, van Breugel PC, Loayza-Puch F,
Ugalde AP, Korkmaz G, Messika-Gold N, Han R, Lopes R, Barbera EP,
Teunissen H, et al: LncRNA-OIS1 regulates DPP4 activation to
modulate senescence induced by RAS. Nucleic Acids Res.
46:4213–4227. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goedert L, Plaça JR, Nunes EM, Debom GN
and Espreafico EM: Long noncoding RNAs in HPV-induced oncogenesis.
Adv Tumor Virol. 2016:1–9. 2016.
|
|
13
|
Ma X, Sheng S, Wu J, Jiang Y, Gao X, Cen
X, Wu J, Wang S, Tang Y, Tang Y and Liang X: LncRNAs as an
intermediate in HPV16 promoting myeloid-derived suppressor cell
recruitment of head and neck squamous cell carcinoma. Oncotarget.
8:42061–42075. 2017.PubMed/NCBI
|
|
14
|
Cao S, Liu W, Li F, Zhao W and Qin C:
Decreased expression of lncRNA GAS5 predicts a poor prognosis in
cervical cancer. Int J Clin Exp Pathol. 7:6776–6783.
2014.PubMed/NCBI
|
|
15
|
Jiang S, Wang HL and Yang J: Low
expression of long non-coding RNA LET inhibits carcinogenesis of
cervical cancer. Int J Clin Exp Pathol. 8:806–811. 2015.PubMed/NCBI
|
|
16
|
Haresaku S, Makino M, Sugiyama S, Naito T
and Mariño RJ: Comparison of practices, knowledge, confidence, and
attitude toward oral cancer among oral health professionals between
Japan and Australia. J Cancer Edu. 33:429–435. 2018. View Article : Google Scholar
|
|
17
|
Neppl RL, Wu CL and Walsh K: lncRNA
Chronos is an aging-induced inhibitor of muscle hypertrophy. J Cell
Biol. 216:3497–3507. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Qiu M, Xu Y, Li M, Dong G, Mao Q,
Yin R and Xu L: Long noncoding RNA CCAT2 correlates with smoking in
esophageal squamous cell carcinoma. Tumour Biol. 36:5523–5528.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zheng H, Li P, Kwok JG, Li WT, Qu Y, Wang
XQ, Kisseleva T, Wang-Rodriguez J and Ongkeko WM: Alcohol and
hepatitis virus-dysregulated lncRNAs as potential biomarkers for
hepatocellular carcinoma. Oncotarget. 9:224–235. 2017.PubMed/NCBI
|
|
20
|
Sollome JJ, Thavathiru E, Camenisch TD and
Vaillancourt RR: HER2/HER3 regulates extracellular acidification
and cell migration through MTK1 (MEKK4). Cell Signal. 26:70–82.
2014. View Article : Google Scholar : PubMed/NCBI
|