Introduction
Non-Hodgkin's lymphoma (NHL) is a group of malignant
tumors of lymphoid cells, which are classified as indolent or
aggressive, according to histopathological and biological
characteristics of carcinomatous cells (1–3). The
mortality rate of NHL was ranked in the top ten for
cancer-associated mortalities worldwide (4). Despite the important improvements in the
treatment of NHL over the past decade, to the best of our
knowledge, there has been little progress in improving survival for
patients with lymphoma (4). Drug
resistance remains an obstacle in achieving the optimal treatment
of lymphoma (3). Increasing evidence
has suggested that the bone marrow microenvironment protects
lymphoma cells from apoptosis induced by cytotoxic drugs, including
cell adhesion-mediated drug resistance (CAM-DR), leading to relapse
following chemotherapy (5–8). The emergence of CAM-DR remains a major
obstacle for the successful treatment of lymphoma. However, the
underlying molecular mechanisms involved in these processes require
further investigation.
LIM domains-containing protein 1 (LIMD1) is a tumor
suppressor gene located at chromosome 3p21.3, a region commonly
deleted in carcinomas, including gastric, renal, breast and ovarian
cancer (9–13). Loss or downregulation of LIMD1
expression has been reported to lead to abnormal cellular behavior,
including perturbation of cell cycle regulation and
hyper-proliferation of the cell (14). It has been previously reported that
LIMD1 may inhibit S-phase progression in mammalian cells, by
downregulating cellular factors necessary for transition from the
G0/G1 to S phase of the cell cycle (15). In hematologic malignancies,
accumulating data suggest that cell cycle arrest may contribute to
the CAM-DR phenotype (16–18). Therefore, the present study
investigated whether LIMD1 can also affect cell cycle progression
and CAM-DR in NHL.
The objective in the present study was to
investigate expression and function of LIMD1 in NHL and CAM-DR
phenotype. The findings of this study demonstrate for the first
time, to the best of our knowledge, that LIMD1 directly affects the
proliferation of NHL cells. Furthermore, it was indicated that
knockdown of LIMD1 may reverse the CAM-DR phenotype in NHL.
Therefore, these findings provide a potential novel therapeutic
target for NHL.
Materials and methods
Patients and specimens
The two cases of pathologically confirmed
extra-nodal lymphoma of mucosa-associated lymphoid tissues (MALT),
follicular lymphoma (FL), mantle cell lymphoma (MCL), diffuse large
B cell lymphoma (DLBCL), and reactive lymphoid (RL) were obtained
from 4 male and 6 female patients treated at the Second People's
Hospital of Nantong (Nantong, China) between April 2016 and
February 2017. The age of these patients ranged between 38 and 62
years (mean, 45 years). The collected specimens were subsequently
used in western blot analyses. The pathologists were blinded to the
study's aim. Written informed consent was obtained from each
patient prior to tissue acquisition. Institutional approval was
acquired from the Ethical Review Board of The Second People's
Hospital of Nantong prior to patient participation in the present
study.
Western blot analysis
Western blot analysis was carried out as previously
described (19). Tissue extracts were
made with a detergent lysis buffer [50 mM Tris (pH 7.4); 150 mM
NaCl; 1% NP-40; 0.5% sodium deoxycholate; 0.1% SDS; and the
protease inhibitor, 1 mmol/l phenylmethanesulfonyl fluoride]. Equal
amounts of protein (20 µg) was run on a 10% SDS-PAGE and
transferred to polyvinylidene difluoride filter (PVDF) membranes.
The membranes were blocked with 5% dried skim milk in TBST (20 mM
Tris, 150 mM NaCl, 0.05% Tween-20) at room temperature for 1 h and
subsequently incubated with primary antibody at 4°C overnight.
Subsequent to washing with TBST at room temperature three times,
each for 5 min, the membrane was incubated with HRP-labeled
secondary antibody for another 2 h at room temperature. The
membranes were detected with Phototope-HRP western blot detection
kit (Cell Signaling Technology, Inc., Danvers, MA, USA), according
to the manufacturer's protocols. The antibodies used in this study
include: Anti-LIMD1 antibody (dilution, 1:500; cat. no.,
sc-365050); anti-GAPDH antibody (dilution 1:500; cat. no.,
sc-25778); anti-proliferating cell nuclear antigen (PCNA) antibody
(dilution, 1:1,000; cat. no., sc-56); anti-cyclin E antibody
(dilution, 1:500; cat. no., sc-481); anti-cyclin dependent kinase 2
(CDK2) antibody (dilution 1:500; cat. no., sc-53219); and
HRP-labeled mouse anti-goat secondary antibody (dilution 1:2,000;
cat. no., sc-2354) all purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA).
Cell cultures and stimulation
The DLBCL cell line, OCI-Ly8, and the human mantle
cell lymphoma (MCL) cell line Jeko-1 were obtained from Fudan
University (Shanghai, China). These cells were all grown in
RPMI-1640 medium (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany),
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The human bone marrow stromal
cell line HS-5, was obtained from the Cell Library, Chinese Academy
of Sciences (Shanghai, China) and cultured in F12 medium
(Sigma-Aldrich; Merck KGaA) with 10% FBS. All cell lines were grown
at 37°C with 5% CO2.
Cell co-culture
For adhesion assays, lymphoma cell lines OCL-Ly8 and
Jeko-1 (1×106/ml) were adhered to fibronectin-(FN;
Sigma-Aldrich; Merck KGaA) and HS-5 cells were plated as described
previously (20). Following three
washes with PBS to remove unattached cells, adherent cells were
used for the subsequent experiments. Lymphoma cells in suspension
(SUS) were used as negative control.
Transient transfection
The lymphoma cells lines OCL-Ly8 and Jeko-1 were
transfected with 50 nM small interfering RNA (si)-LIMD1 or
si-negative control. LIMD1 small interfering (si)RNA was designed
and synthesized by GeneChem Co. Ltd., (Shanghai, China). The
sequences used were: sense 5′-CTCACTCATGGAGACTATT-3′, and antisense
5′-AATAGTCTCCATGAGTGAGGC-3′. Full-length LIMD1 cDNA were generated
using polymerase chain reaction and cloned into pcDNA3.1 construct
(Invitrogen; Thermo Fisher Scientific, Inc.), generating the
myc-LIMD1 vector. Transfections were performed with the
transfection reagent Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. Following incubation for 6 h, the medium was replaced
with RPMI-1640 containing 10% FBS. Transfected cells were used for
subsequent experiments 48 h following transfection.
Cell counting assays of viable
cells
Lymphoma cells lines OCL-Ly8 and Jeko-1 were seeded
onto a 96-well cluster at a density of 1×106/well in a
volume of 90 µl, and then grown overnight with or without the
addition of 1 µm doxorubicin for 72 h (Sigma-Aldrich; Merck KGaA).
A total of 10 µl Cell Counting Kit-8 (CCK-8) reagent (Dojindo,
Molecular Technologies, Inc., Kumamoto, Japan) was added to the
different subset wells, including control-siRNA, LIMD1-siRNA,
myc-control and myc-LIMD1 transfected cells and subsequently
incubated for an additional 2 h at 37°C with 5 % CO2.
The absorbance of the cells was quantitated using a microplate
reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) at a
wavelength of 450 nm, with a reference wavelength of 630 nm.
Methylcellulose colony formation
assay
Lymphoma cells lines OCL-Ly8 and Jeko-1 were
resuspended at 1×103 cells/well in semisolid
methylcellulose medium (Stem Cell Technologies, Inc., Vancouver,
British Columbia, Canada) supplemented with 10% FBS in 24-well
plates. Plates were subsequently maintained for 7 days at 37°C with
5% CO2. Cells were counted using an inverted light
microscope (magnification, ×5). Data were obtained from three
independent experiments.
Flow cytometric analysis
For cell cycle analysis, lymphoma cells lines
OCL-Ly8 and Jeko-1 were fixed in 70% ethanol in PBS at −20°C
overnight, subsequently incubated with 1 mg/ml RNase A at 37°C for
30 min in PBS and then stained with 50 µg/ml propidium iodide at
4°C for 30 min in the dark. Cells were subsequently analyzed using
a FACScan™ flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA).
For cell apoptosis analysis, a flow cytometry assay
was performed using an Annexin-V fluorescein isothiocyanate
Apoptosis Detection Kit I (BD Biosciences), according to the
manufacturer's protocol. Subsequently, the results were quantified
using Cell Quest software v.0.9.13 (Becton, Dickinson and Company,
Franklin, Lakes, NJ, USA).
Statistical analysis
The data in the present study were analyzed using
SPSS v.19.0 software (IBM Corp., Armonk, NY, USA). Data are
presented as the mean ± standard error of the mean. For the
comparison of two conditions, the two-tailed t-test was
utilized. For testing of multiple groups, the one-way analysis of
variance test coupled with the Bonferroni post hoc test was
applied. All data shown represent the results of at least three
independent experiments. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of LIMD1 in NHL
Previous studies have suggested that LIMD1 is
downregulated in numerous solid malignancies, including lung,
breast and gastric cancer (10,14,21–23).
However, the expression of LIMD1 in NHL remains unclear. To examine
the potential role of LIMD1 in NHL, the expression of LIMD1 protein
was analyzed by western blotting in two cases of RL, MALT, FL, MCL
and DLBCL samples. It was indicated that the expression of LIMD1
was downregulated in progressive lymphoma, MCL and DLBCL, compared
with in indolent lymphoma, MALT and FL, and RL samples (Fig. 1A). Furthermore, it was indicated that
the expression of PCNA, a proliferative marker, was inversely
associated with LIMD1 expression (Fig.
1B).
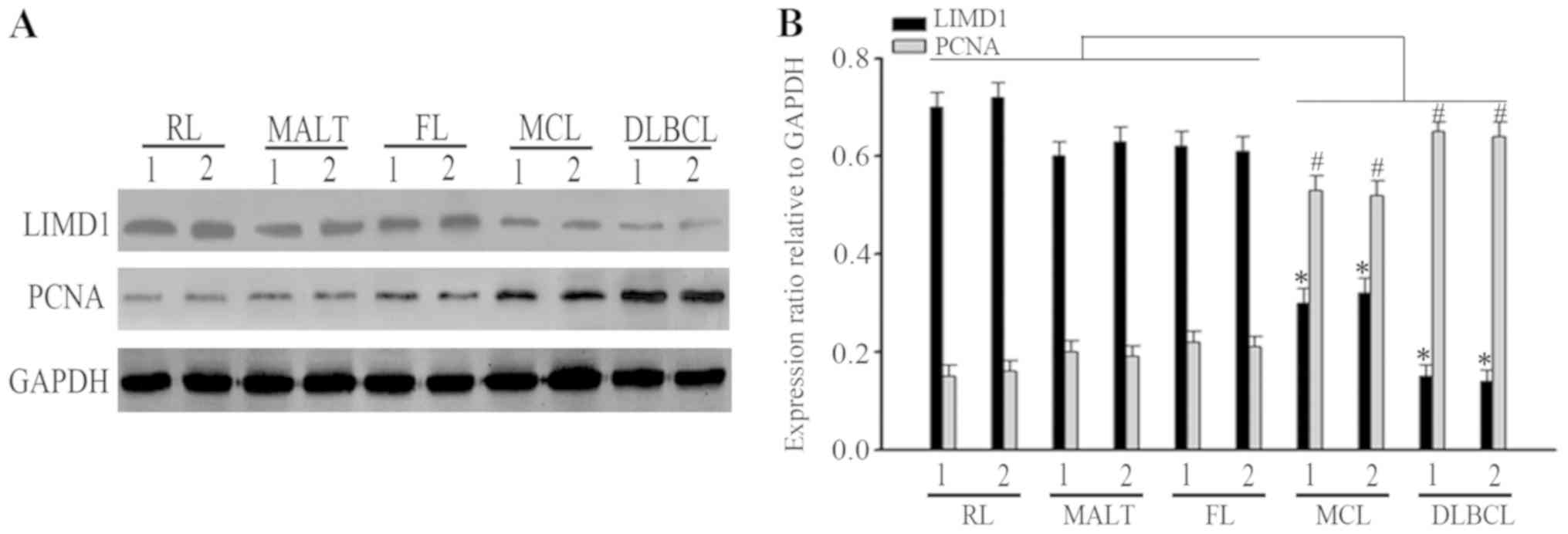 | Figure 1.Expression of LIMD1 in clinical
specimens. (A) Expression of LIMD1 protein in two cases of RL,
MALT, FL, MCL and DLBCL samples. (B) Bar chart of LIMD1 and PCNA
protein expression associated with the relative ratio of GAPDH
expression. *P<0.05; #P<0.05, compared with the
indolent lymphoma (MALT and FL) and RL samples. MALT,
mucosa-associated lymphoid tissues; FL, follicular lymphoma; MCL,
mantle cell lymphoma; DLBCL, diffuse large B-cell lymphoma; RL,
reactive lymphoid; LIMD1, LIM domain-containing protein 1; PCNA,
proliferating cell nuclear antigen. |
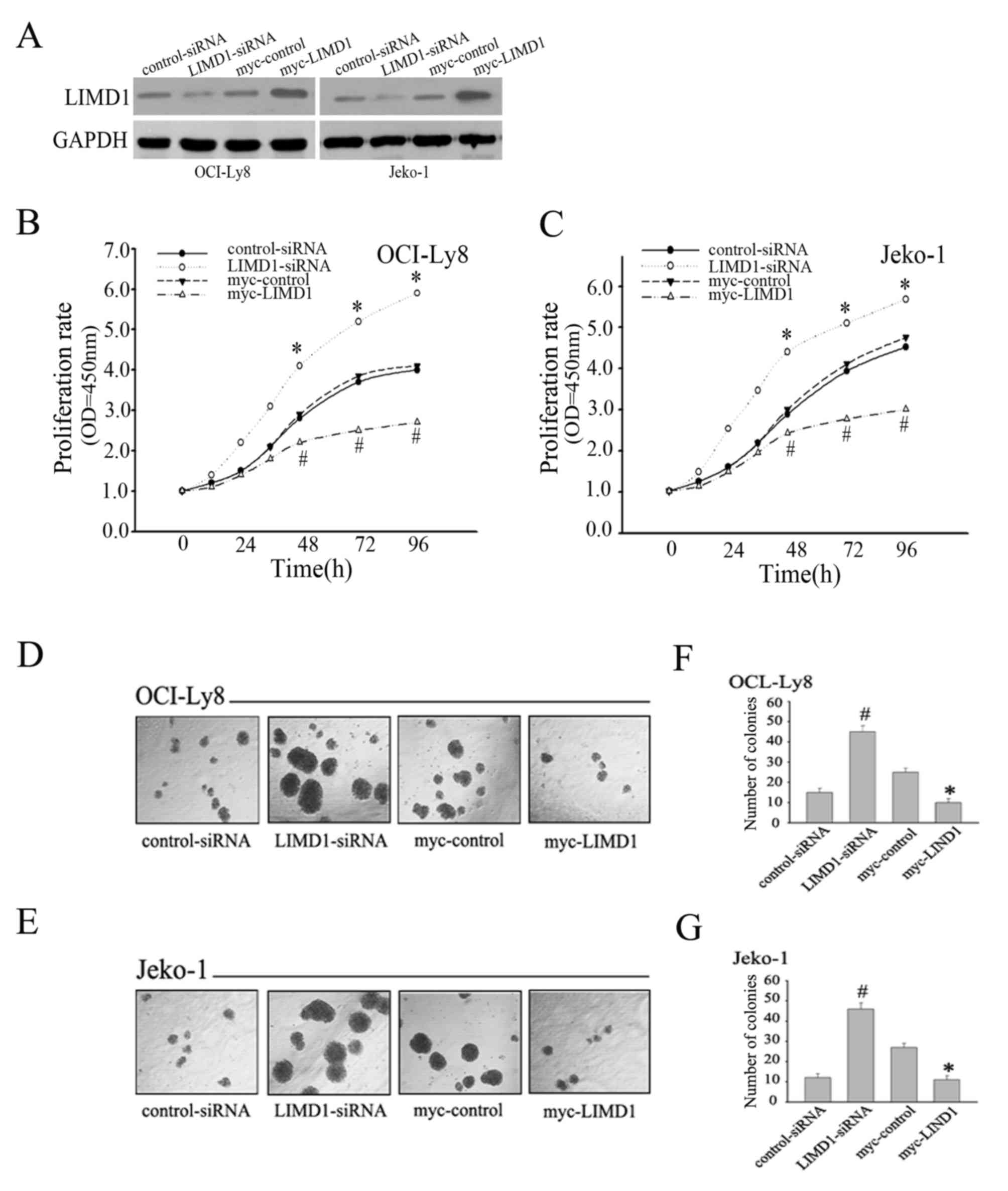
LIMD1 affects proliferation in
NHL
The present study demonstrated that LIMD1 was
inversely associated with PCNA expression. Therefore, it was
speculated that LIMD1 may serve a role in regulating the
proliferation of NHL cells. To validate this hypothesis, the NHL
cell lines OCI-Ly8 and Jeko-1 were used to examine the potential
effect of LIMD1 on proliferation. To investigate the effect of
LIMD1 on cell proliferation, transfection of LIMD1 in NHL cell
lines was performed and transfection efficiency was confirmed by
western blot analysis (Fig. 2A).
Subsequently, CCK-8 assays and Methylcellulose colony formation
assays were used to measure the effect of LIMD1 on cell growth. As
expected, the knockdown of LIMD1 resulted in a significant increase
in proliferation rate (Fig. 2B and C)
and colony formation ability (Fig.
2D-G) in OCI-Ly8 and Jeko-1 cells compared with the control
group (P<0.05). By contrast, overexpression of LIMD1 resulted in
a significant inhibition of OCI-Ly8 and Jeko-1 cell proliferation
rate (Fig. 2B-G) (P<0.05, compared
with the control group). Therefore, the aforementioned results
support the anti-proliferative role of LIMD1 in NHL cells.
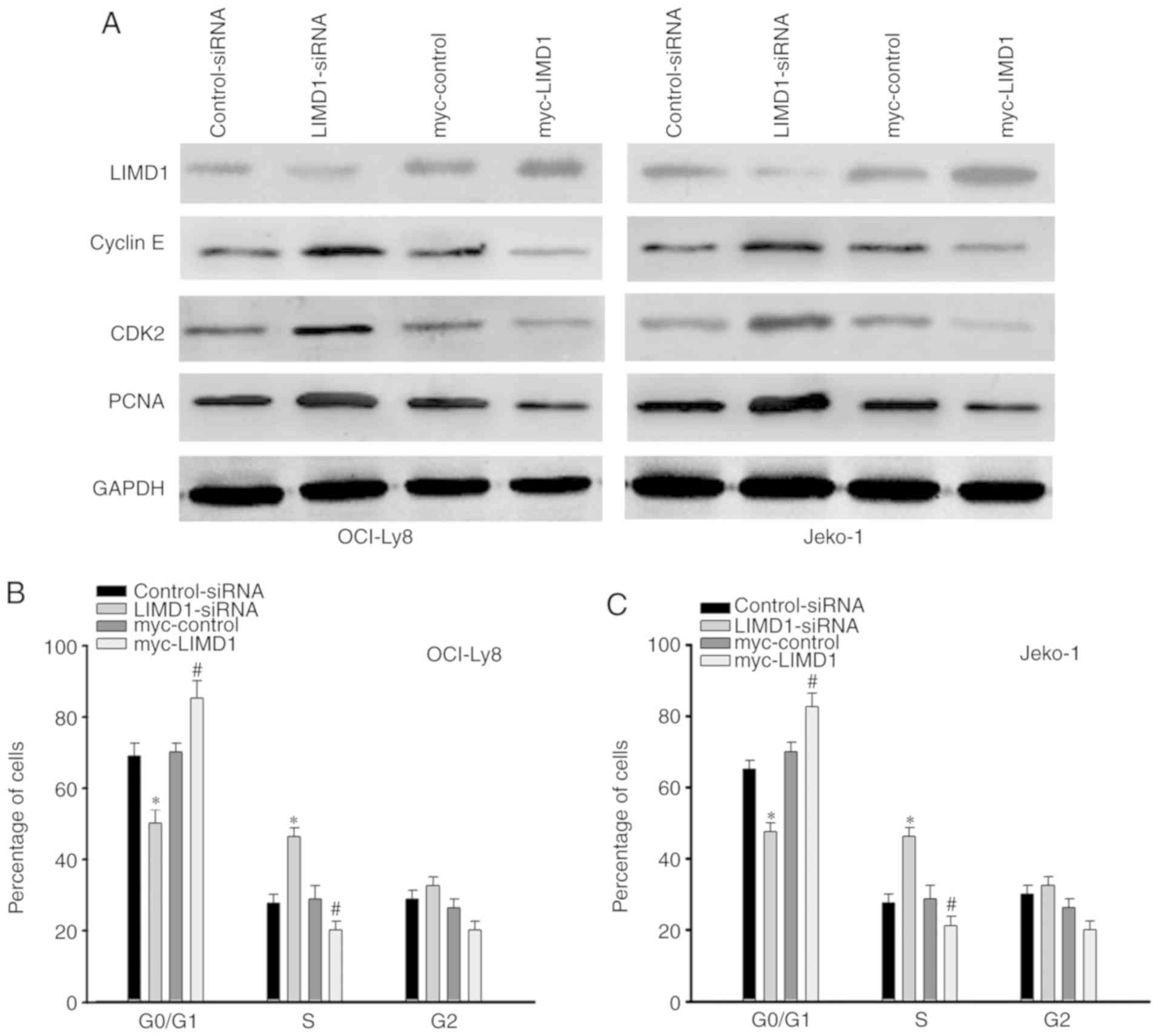
LIMD1 inhibits cell cycle
progression
Previous studies have indicated that LIMD1 serves a
crucial anti-proliferation role by downregulating cellular factors,
including PCNA and cyclin E, which transit cells from the
G0/G1 to the S phase of the cell cycle
(14,15). Therefore, the expression of PCNA and
cyclin E was evaluated in OCI-Ly8 and Jeko-1 cells. It was
indicated that knockdown of LIMD1 resulted in a significant
increase (P<0.05) of cyclin E, CDK2 and PCNA expression compared
with that in control-siRNA transfected cells (Fig. 3A). By contrast, overexpression of
LIMD1 resulted in a significant decrease (P<0.05, compared with
the control group) in the expression of the aforementioned proteins
(Fig. 3A). In addition, flow
cytometric analysis indicated that the knockdown of LIMD1 in NHL
cells accelerated transit from the G0/G1
phase to the S phase (Fig. 3B and C).
Conversely, overexpression of LIMD1 led to a significant increase
(P<0.05, compared with the control group) in the percentage of
NHL cells arrested in the G0/G1 phase
(Fig. 3B and C). Therefore, these
results indicate that LIMD1 may inhibit G0/G1
to S phase transition by inducing G0/G1
arrest.
Knockdown of LIMD1 reverses the CAM-DR
phenotype
Increasing evidence indicates that the adhesion of
lymphoma cells to extracellular matrices or stromal cells in the
bone marrow microenvironment induces cell cycle arrest and protects
cells from drug-induced apoptosis (16,24,25). Based
on the results, the present study proposed that the
anti-proliferation role of LIMD1 should be one of the major reasons
for CAM-DR in NHL cells. To investigate the role of LIMD1 in the
CAM-DR phenotype, cell adhesion assays were used to examine the
expression of LIMD1. It was indicated that adhesion of NHL cells to
FN and HS-5 cells significantly increased (P<0.05) LIMD1
expression, compared with their suspension (SUS) counterparts
(Fig. 4A and B). Subsequent cell
viability assays revealed that adhesion to FN and HS-5 cells
significantly protected (P<0.05) NHL cells from
doxorubicin-induced cytotoxicity compared with the same cell types
cultured in SUS, as these effects were attenuated by the
downregulation of LIMD1 (Fig. 4C).
Flow cytometry assays verified an increase in drug-induced
apoptotic cells among LIMD1-siRNA transfected cells compared with
the controls (Fig. 4D). Taking into
consideration all aforementioned findings, the present study data
indicate that knockdown of LIMD1 expression may reverse the CAM-DR
phenotype in NHL.
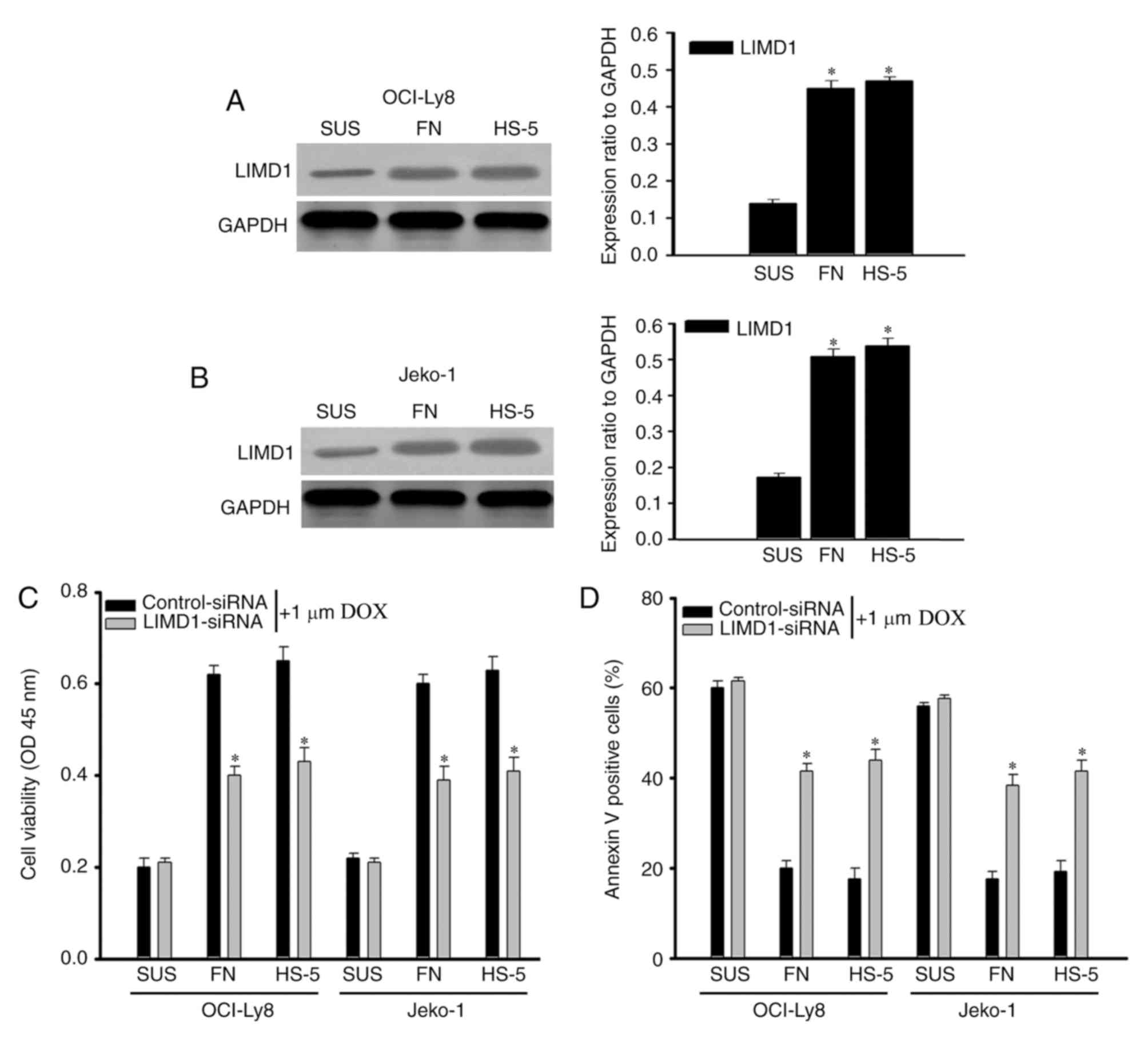 | Figure 4.Knockdown of LIMD1 reverses the
CAM-DR phenotype. (A) OCI-Ly8 and (B) Jeko-1 cells were adhered to
FN or HS-5 cells, or cultured SUS, to detect the expression of
LIMD1. The bar chart demonstrates the LIMD1 protein expression
ratio relative to GAPDH. *P<0.05, compared with cells in SUS.
(C) Viability of OCI-Ly8 and Jeko-1 cells transfected with
control-siRNA or LIMD1-siRNA and adhered to FN, HS-5 cells, or
cultured in SUS along with DOX treatment. (D) DOX-induced cell
apoptosis in OCI-Ly8 and Jeko-1 cells transfected with
control-siRNA or LIMD1-siRNA adhered to FN, HS-5 cells or cultured
SUS along with DOX treatment. *P<0.05, compared with cells in
SUS. CAM-DR, cell adhesion-mediated drug resistance; SUS,
suspension; si, small interfering; LIMD1, LIM domain-containing
protein 1; FN, fibronectin; DOX, doxorubicin; OD, optical
density. |
Discussion
NHL is a group of malignant neoplasms in the lymph
tissues, which includes DLBCL, MCL, FL and MALT (3,7). Although
standard systemic chemotherapy remains the main therapy option, the
emergence of clinical drug resistance usually leads to therapy
failure (4,25). It has been documented that the bone
marrow microenvironment may provide components necessary for cell
survival, growth and the development of acquired multidrug
resistance (26–29). This environment consists of
hematopoietic cells, stromal cells and the extracellular matrix. It
also indicates that the tumor cells that adhered to components of
the bone marrow microenvironment may block apoptosis induced by
chemotherapy agents, including CAM-DR (5,16,17). The emergence of multidrug resistance
remains a major obstacle for the successful treatment of NHL.
Previous studies have demonstrated that adhesion of
hematopoietic tumors to FN may block cell cycle progression, and
that this is associated with drug resistance (16,17).
Furthermore, cell cycle arrest has been reported to be associated
with the inhibition of CDK2 (3,4,25). It has been reported that LIMD1 may
prevent the entry of cells into the S phase by downregulating genes
involved in G1 to S phase transitions (14,15). Based
on these findings, the expression of LIMD1 in NHL cells and the
effect of LIMD1 on CAM-DR in OCI-Ly8 and Jeko-1 NHL cell lines were
investigated. In the present study, it was indicated that LIMD1
expression was downregulated in progressive lymphomas, namely MCL
and DLBCL, compared with in indolent lymphomas, namely MALT and FL
tissues. Subsequently, it was indicated that LIMD1 inhibited
proliferation by inducing cell cycle arrest and suppressing genes
involved in the G1/S transition. In addition, it was indicated that
LIMD1 participated in the process of CAM-DR in NHL. In the present
study it was demonstrated that adhesion of OCI-Ly8 and Jeko-1 cells
to FN or HS-5 cells significantly increased LIMD1 expression
compared with cells cultured in SUS. It was subsequently verified
that adhesion to FN or HS-5 cells may protect OCI-Ly8 and Jeko-1
cells from doxorubicin-induced cytotoxicity compared with their SUS
counterparts, while the protective effect was reversed when the
cells were transfected with LIMD1-siRNA.
LIMD1, a member of the ZYXIN family of proteins, is
widely expressed in human and mouse tissues (30,31).
Previous research has demonstrated that LIMD1 directly interacts
with retinoblastoma and inhibits E2F transcription factor
1-mediated transcription (13,23).
Furthermore, it has been reported that upregulation of LIMD1
inhibited tumor growth in 80% of lung cancers, breast cancers and
head squamous cell carcinomas, indicating possible
tumor-suppressing functions (30).
LIMD1 is a tumor suppressor gene encoded at chromosome 3p21.3, a
region usually lost in a number of solid tumors, including lung,
breast, gastric, ovarian and renal cancer (9–12,21). It has been reported that loss or
downregulation of LIMD1 expression may led to a cancerous
phenotype, due to its anti-proliferative properties (15). However, the function of LIMD1 in the
development of NHL remains unclear. In the present study, it was
demonstrated that LIMD1 may inhibit the proliferation of NHL cells
by inducing cell cycle arrest, and that knockdown of LIMD1 may
reverse the CAM-DR phenotype in NHL cells.
In summary, the present study demonstrated for the
first time, to the best of our knowledge, that LIMD1 may block cell
cycle progression and downregulate genes involved in the G1 to S
phase transition in NHL. Silencing of LIMD1 could reverse the
CAM-DR phenotype in NHL. Therefore, based on the data, it is
proposed that the inhibition of LIMD1 expression may be a novel
effective strategy for the treatment of NHL.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key Research
Project of Liyang Science and technology Bureau (grant no. LC
2016009), the National Natural Science Foundation of China (grant
no. 81600158), the Six Talent Peaks Project in Jiangsu Province
(grant no. 2015-SWYY-021), the Natural Science Foundation of
Jiangsu province Grants (grant nos. BK2012231, BK20160060), the
Major Research of Natural Science Foundation for Colleges and
Universities in Jiangsu Province (grant no. 16KJ320004) and the
Innovative Training Program for Undergraduates in Nantong
University and Nantong Science and Technology Projects (grant nos.
QYZ15069, MS22016059 and YYZ16026).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YH, BS, SZ, LG, JZ, MD, JHZ and HX were responsible
for the completion of experiments. JT, LZ, YW and CW undertook
study design and wrote the manuscript All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from each
patient prior to tissue acquisition. Institutional approval was
acquired from the Ethical Review Board of The Second People's
Hospital of Nantong prior to patient participation in the present
study.
Patient consent for publication
All the patients provided written informed consent
for the publication of any associated data and accompanying
images.
Competing interests
All the authors declare no competing interests.
References
|
1
|
Gayle S, Landrette S, Beeharry N, Conrad
C, Hernandez M, Beckett P, Ferguson SM, Mandelkern T, Zheng M, Xu
T, et al: Identification of apilimod as a first-in-class PIKfyve
kinase inhibitor for treatment of B-cell non-hodgkin lymphoma.
Blood. 129:1768–1778. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Riley SN: Investigating the multivariate
nature of NHL player performance with structural equation modeling.
PLoS one. 12:e01843462017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yin H, Miao X, Wu Y, Wei Y, Zong G, Yang
S, Chen X, Zheng G, Zhu X, Guo Y, et al: The role of the chaperonin
containing t-complex polypeptide 1, subunit 8 (CCT8) in B-cell
non-hodgkin's lymphoma. Leuk Res. 45:59–67. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu X, Miao X, Wu Y, Li C, Guo Y, Liu Y,
Chen Y, Lu X, Wang Y and He S: ENO1 promotes tumor proliferation
and cell adhesion mediated drug resistance (CAM-DR) in
non-hodgkin's lymphomas. Exp Cell Res. 335:216–223. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hazlehurst LA and Dalton WS: Mechanisms
associated with cell adhesion mediated drug resistance (CAM-DR) in
hematopoietic malignancies. Cancer Metastasis Rev. 20:43–50. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Landowski TH, Olashaw NE, Agrawal D and
Dalton WS: Cell adhesion-mediated drug resistance (CAM-DR) is
associated with activation of NF-kappa B (RelB/p50) in myeloma
cells. Oncogene. 22:2417–2421. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miao X, Xu X, Wu Y, Zhu X, Chen X, Li C,
Lu X, Chen Y, Liu Y, Huang J, et al: Overexpression of TRIP6
promotes tumor proliferation and reverses cell adhesion-mediated
drug resistance (CAM-DR) via regulating nuclear p27(Kip1)
expression in non-hodgkin's lymphoma. Tumour Biol. 37:1369–1378.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakagawa Y, Nakayama H, Nagata M, Yoshida
R, Kawahara K, Hirosue A, Tanaka T, Yuno A, Matsuoka Y, Kojima T,
et al: Overexpression of fibronectin confers cell adhesion-mediated
drug resistance (CAM-DR) against 5-FU in oral squamous cell
carcinoma cells. Int J Oncol. 44:1376–1384. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hou X, Li T, Ren Z and Liu Y: Novel
BRCA2-interacting protein, LIMD1, is essential for the centrosome
localization of BRCA2 in esophageal cancer cell. Oncol Res.
24:247–253. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sharp TV, Al-Attar A, Foxler DE, Ding L,
de A Vallim TQ, Zhang Y, Nijmeh HS, Webb TM, Nicholson AG, Zhang Q,
et al: The chromosome 3p21.3-encoded gene, LIMD1, is a critical
tumor suppressor involved in human lung cancer development. Proc
Natl Acad Sci USA. 105:19932–19937. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Spendlove I, Al-Attar A, Watherstone O,
Webb TM, Ellis IO, Longmore GD and Sharp TV: Differential
subcellular localisation of the tumour suppressor protein LIMD1 in
breast cancer correlates with patient survival. Int J Cancer.
123:2247–2253. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huggins CJ, Gill M and Andrulis IL:
Identification of rare variants in the hLIMD1 gene in breast
cancer. Cancer Genet Cytogenet. 178:36–41. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kiss H, Kedra D, Yang Y, Kost-Alimova M,
Kiss C, O'Brien KP, Fransson I, Klein G, Imreh S and Dumanski JP: A
novel gene containing LIM domains (LIMD1) is located within the
common eliminated region 1 (C3CER1) in 3p21.3. Hum Genet.
105:552–559. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huggins CJ and Andrulis IL: Cell cycle
regulated phosphorylation of LIMD1 in cell lines and expression in
human breast cancers. Cancer Lett. 267:55–66. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mayank AK, Sharma S, Deshwal RK and Lal
SK: LIMD1 antagonizes E2F1 activity and cell cycle progression by
enhancing Rb function in cancer cells. Cell Biol Int. 38:809–817.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hang Q, Fei M, Hou S, Ni Q, Lu C, Zhang G,
Gong P, Guan C, Huang X and He S: Expression of Spy1 protein in
human non-Hodgkin's lymphomas is correlated with phosphorylation of
p27 Kip1 on Thr187 and cell proliferation. Med Oncol. 29:3504–3514.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hazlehurst LA, Damiano JS, Buyuksal I,
Pledger WJ and Dalton WS: Adhesion to fibronectin via beta1
integrins regulates p27kip1 levels and contributes to cell adhesion
mediated drug resistance (CAM-DR). Oncogene. 19:4319–4327. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sarkar S, Maiti GP, Jha J, Biswas J, Roy
A, Roychoudhury S, Sharp T and Panda CK: Reduction of proliferation
and induction of apoptosis are associated with shrinkage of head
and neck squamous cell carcinoma due to neoadjuvant chemotherapy.
Asian Pac J Cancer Prev. 14:6419–6425. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang X, Wang Y, Nan X, He S, Xu X, Zhu X,
Tang J, Yang X, Yao L, Wang X and Cheng C: The role of the orphan G
protein-coupled receptor 37 (GPR37) in multiple myeloma cells. Leuk
Res. 38:225–235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Damiano JS, Cress AE, Hazlehurst LA, Shtil
AA and Dalton WS: Cell adhesion mediated drug resistance (CAM-DR):
Role of integrins and resistance to apoptosis in human myeloma cell
lines. Blood. 93:1658–1667. 1999.PubMed/NCBI
|
|
21
|
Chen Z, Zhu X, Xie T, Xie J, Quo K and Liu
X: Drug resistance reversed by silencing LIM domain-containing
protein 1 expression in colorectal carcinoma. Oncol Lett.
8:795–798. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ghosh S, Ghosh A, Maiti GP, Alam N, Roy A,
Roy B, Roychoudhury S and Panda CK: Alterations of 3p21.31 tumor
suppressor genes in head and neck squamous cell carcinoma:
Correlation with progression and prognosis. Int J Cancer.
123:2594–2604. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ghosh S, Ghosh A, Maiti GP, Mukherjee N,
Dutta S, Roy A, Roychoudhury S and Panda CK: LIMD1 is more
frequently altered than RB1 in head and neck squamous cell
carcinoma: Clinical and prognostic implications. Mol Cancer.
9:582010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang J, Ji L, Wang Y, Huang Y, Yin H, He
Y, Liu J, Miao X, Wu Y, Xu X, et al: Cell adhesion down-regulates
the expression of vacuolar protein sorting 4B (VPS4B) and
contributes to drug resistance in multiple myeloma cells. Int J
Hematol. 102:25–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ouyang Y, Zhong F, Wang Q, Ding L, Zhang
P, Chen L, Wang Y and Cheng C: DIXDC1 promotes tumor proliferation
and cell adhesion mediated drug resistance (CAM-DR) via enhancing
p-Akt in Non-Hodgkin's lymphomas. Leuk Res. 50:104–111. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Balsas P, Palomero J, Eguileor Á,
Rodríguez ML, Vegliante MC, Planas-Rigol E, Sureda-Gómez M, Cid MC,
Campo E and Amador V: SOX11 promotes tumor protective
microenvironment interactions through CXCR4 and FAK regulation in
mantle cell lymphoma. Blood. 130:501–513. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bjorklund CC, Baladandayuthapani V, Lin
HY, Jones RJ, Kuiatse I, Wang H, Yang J, Shah JJ, Thomas SK, Wang
M, et al: Evidence of a role for CD44 and cell adhesion in
mediating resistance to lenalidomide in multiple myeloma:
Therapeutic implications. Leukemia. 28:373–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsubaki M, Takeda T, Yoshizumi M, Ueda E,
Itoh T, Imano M, Satou T and Nishida S: RANK-RANKL interactions are
involved in cell adhesion-mediated drug resistance in multiple
myeloma cell lines. Tumour Biol. 37:9099–9110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Waldschmidt JM, Simon A, Wider D, Müller
SJ, Follo M, Ihorst G, Decker S, Lorenz J, Chatterjee M, Azab AK,
et al: CXCL12 and CXCR7 are relevant targets to reverse cell
adhesion-mediated drug resistance in multiple myeloma. Br J
Haematol. 179:36–49. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Foxler DE, James V, Shelton SJ, Vallim TQ,
Shaw PE and Sharp TV: PU.1 is a major transcriptional activator of
the tumour suppressor gene LIMD1. FEBS Lett. 585:1089–1096. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao MK, Wang Y, Murphy K, Yi J, Beckerle
MC and Gilmore TD: LIM domain-containing protein trip6 can act as a
coactivator for the v-Rel transcription factor. Gene Exp.
8:207–217. 1999.
|