Introduction
The incidence of gastric cancer (GC) varies
worldwide; the disease is four times more common in Japan than in
the UK and occurs in younger patients (1). In Japan, 50,000 people die of GC
annually. GC progression involves a variety of gene alterations and
a number of specific events (2), such
as overexpression of oncogenes and inactivation of tumor suppressor
genes. Helicobacter pylori (HP) infection and consequent
atrophic gastritis are regarded as risk factors for GC. Research
has shown that HP infection can cause GC via atrophic gastritis
(3,4).
Severe gastric atrophy (SA) and corpus-predominant gastritis,
intestinal metaplasia (IM), and dysplasia are well-known
predisposing factors for GC (5,6). Previous
research suggested that gastric carcinogenesis involves three
steps: HP infection, development of gastric precancerous
conditions, and carcinogenesis (7).
However, relatively few cases of HP infection progress to GC. This
discrepancy has prompted considerable research examining potential
associations between genetic polymorphisms and the risk of
progression from precancerous conditions to GC.
Methylation of several genes has been reported in
many cancers, including GC (8). The
DNA methyltransferase (DNMT) family includes three active mammalian
homologs: DNMT1, 3a, and 3b. DNMT3a and DNMT3b are considered de
novo enzymes that play critical roles in the dynamic DNA
methylation process during embryogenesis and pathogenesis (9). In both GC and para-cancerous tissues,
the expression of DNMTs is significantly higher than in normal
tissues (10), which suggests that
DNMT overexpression is involved in the development of gastric
mucosal atrophy and subsequent tumorigenesis. Many studies have
reported an association between rs1550117, a notable polymorphism
in the DNMT3A gene, and susceptibility to various cancers,
including GC (11,12). However, no significant association
between rs1550117 and GC susceptibility has been reported (13,14). Thus,
the influence of DNMT3A polymorphisms upon GC susceptibility
remains unclear, especially in the Japanese population.
In this study, we investigated potential
associations between SA and GC susceptibility and two DNMT3A
polymorphisms: rs6733868 C>G (in linkage with rs7605753,
rs13427202, rs7590760, rs6749992, and rs7586294) and rs13428812
A>G (in linkage with rs7583409 and rs7578575).
Materials and methods
Clinical samples
All subjects were enrolled at the Endoscopy Center
of Fujita Health University Hospital or Kanazawa Medical University
Hospital between April 2005 and March 2014. The study involved 343
patients with GC (GC group) and 708 subjects with no evidence of
gastric malignancies (non-GC group) on upper gastro-duodenal
endoscopy. In addition, 409 of 708 controls in which the degree of
histologic gastritis could be assessed according to the updated
Sydney system using biopsy specimens obtained from antrum (15) were classified into two groups: 99
patients in the SA group (atrophy score of 3 or metaplasia score
≥2) and 310 patients in the non-SA group. Diagnosis of all GCs was
made histologically at the Division of Pathology of each hospital.
Patients with severe systemic diseases, malignancies in other
organs, or who had received nonsteroidal anti-inflammatory drugs,
antibiotics, or HP eradication treatment were excluded. We judged
HP infection status as positive when the rapid urease test, urea
breath test, or histologic test was positive.
The Ethics Committees of Fujita Health University
and Kanazawa Medical University approved the protocol, and written
informed consent was obtained from all participating subjects.
Single nucleotide polymorphism
selection and detection
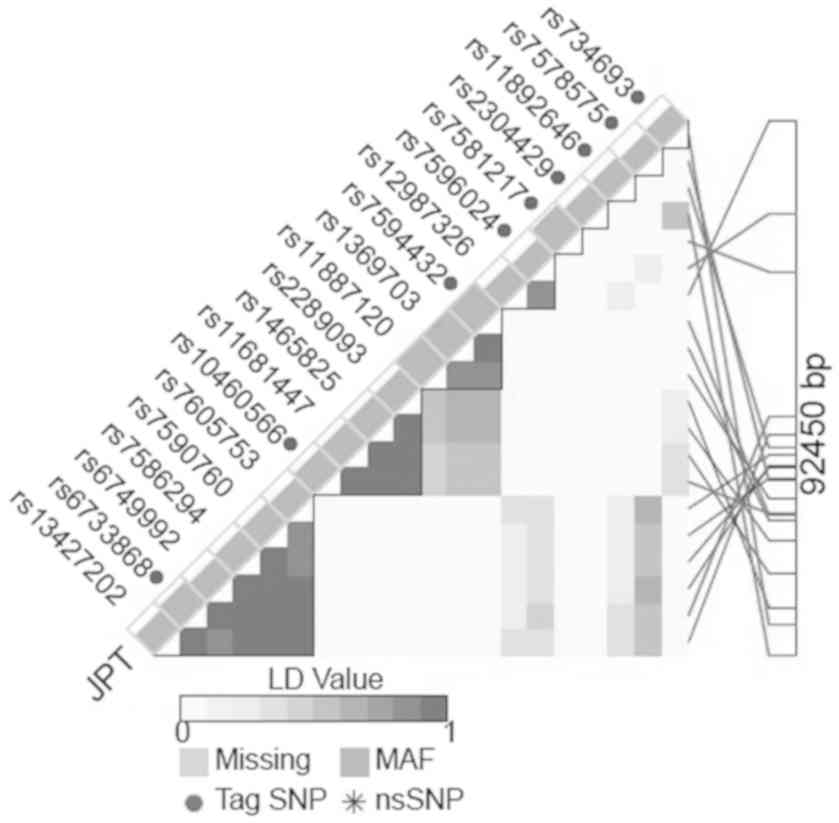
We selected the Tag polymorphism with high minor
allele frequency (MAF) in the DNMT3A gene region. We
selected rs6733868 C>G in a large linkage disequilibrium block
with a Hardy-Weinberg equilibrium (HWE) P-value >0.05 and MAF
>0.30 determined according to the LD TAG SNP selection database
(https://snpinfo.niehs.nih.gov/snpinfo/snptag.html,
Fig. 1). In addition, another SNP
with a high MAF (rs13428812 A>G) that had been investigated in a
previous study based on its association with GC susceptibility
(13) also was selected. This
polymorphism is in linkage with rs7578575 (Fig. 1).
Sample stocks of DNA isolated from peripheral blood
were used in the study. Genotyping of DNMT3A polymorphisms
was carried out using polymerase chain reaction (PCR)-single-strand
conformation polymorphism (SSCP) methods, as reported previously
(16,17). The rs6733868 and rs13428812 genotypes
were determined using the following primer pairs: for rs6733868,
forward 5′-ctagctagcgggagtcgctgtc-3′ and reverse
5′-ctcctggctgtgaagcggaag-3′; for rs13428812, forward
5′-ccccatcatgtcagataccctctg-3′ and reverse
5′-ccttcctaggggacacccttctatt-3′. PCR was carried out in a 20-µl
reaction volume containing 0.1 µg of genomic DNA. The DNA was
denatured at 95°C for 3 min, followed by 35 cycles at 96°C for 15
sec, 61°C for 30 sec, and 72°C for 30 sec, with final extension at
72°C for 5 min. PCR conditions for amplification of rs6733868 and
rs13428812 were the same. Thereafter, 2 µl of the PCR product was
denatured with 10 µl of formamide (Sigma-Aldrich Co., St. Louis,
MO, USA) at 90°C for 5 min. SSCP was carried out at 18°C. We used a
Gene Phor DNA separation system with Gene Gel Excel 12.5/24 (GE
Health Care Bio-Sciences AB, Stockholm, Sweden), after which the
denatured single-strand DNA bands were detected using a DNA silver
staining kit (GE Health Care Bio-Sciences AB).
Statistical analysis
The HWE of each allele was assessed using a
χ2 test. Data are expressed as mean ± SD. Differences in
mean age of patients in each group were evaluated using the
Student's t-test. Differences in ratios of HP infection status and
male to female patients were evaluated using Fisher's exact test.
Allele and genotype frequencies were determined by direct counting.
Differences in allele count also were evaluated using Fisher's
exact test. The strength of association between allele frequencies
and disease was assessed by calculating the odds ratio (OR) and 95%
confidence interval (CI) by logistic regression analysis. Adjusted
ORs considered age, gender, and HP infection status. An adjusted
analysis also was performed by logistic regression analysis after
adjustment for gender, age, and HP infection status. For all
analyses, the level of significance was set at P<0.05. Analyses
were performed using Stata software (version 13; StataCorp LP,
College Station, TX, USA).
Results
Characteristics of subjects and the
frequencies of genotypes
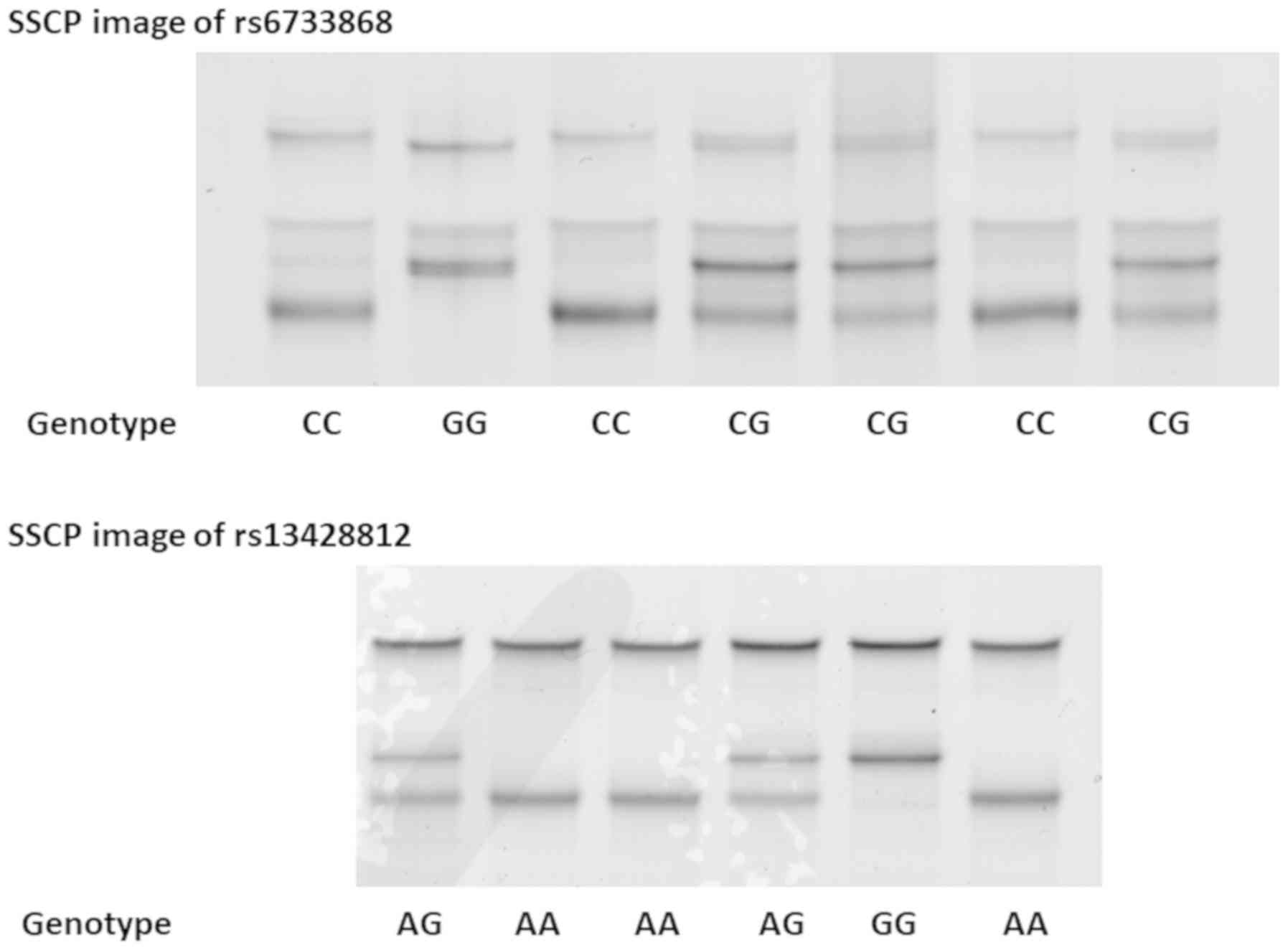
Single-strand DNAs of rs6733868 and rs13428812 were
clearly separated by SSCP (Fig. 2).
The characteristics of subjects in this study are summarized in
Table I. The mean age, male to female
ratio, and HP positivity ratio were significantly higher in the GC
group than in the non-GC group. The distribution of the rs6733868
C>G genotype in the GC group was 130CC, 181CG, and 32GG. The
distribution of the rs6733868 C>G genotype in the non-GC group
was 253CC, 338CG, and 117GG (HWE P=0.82). The rs6733868G allele
frequency in the GC and non-GC groups was 35.7 and 40.4%,
respectively (P=0.04). In addition, the frequency of the rs6733868
GG homozygote differed significantly between the GC and non-GC
groups (P=0.0018). The distribution of the rs13428812 A>G
genotype in the non-GC group was 389AA, 276AG, and 43GG (HWE
P=0.69). There was no significant difference in either the minor
allele frequency or distribution of the rs13428812 genotype between
the GC and non-GC groups.
 | Table I.Subject characteristics and genotype
frequencies. |
Table I.
Subject characteristics and genotype
frequencies.
| Characteristics | Non-GC group | GC group | P-value |
|---|
| Number of
subjects | 708 | 343 |
|
| Mean age ± standard
deviation (years) | 61.0±13.7 | 65.3±11.4 |
<0.0001a |
| Male:female | 405:303 | 239:104 | 0.0001b |
| HP positive
rate | 435/708 | 296/343 |
<0.0001b |
| rs6733868 C>G |
|
|
|
| CC | 253 | 130 |
|
| CG | 338 | 181 |
|
| GG | 117 | 32 | 0.0018b |
| G
allele frequency | 40.4% | 35.7% | 0.04b |
| rs13428812
A>G |
|
|
|
| AA | 389 | 204 |
|
| AG | 276 | 128 |
|
| GG | 43 | 11 |
|
| G
allele frequency | 25.6% | 21.9% |
|
Association between GC susceptibility
and DNMT3A polymorphisms
Overall, patients homozygous for the rs6733868 G
allele had a significantly decreased risk for gastric
carcinogenesis as determined by logistic regression analysis after
adjustment for age, gender, and HP infection status (OR, 0.621; 95%
CI, 0.402–0.958; P=0.031, Table II).
When assessed by GC subtype, patients who were rs6733868 GG
homozygotes had a significantly decreased risk for the development
of intestinal cancers (OR, 0.494; 95% CI, 0.274–0.890; P=0.019,
Table II), whereas no significant
association was found between this genotype and diffuse types of
cancer. No significant association was found between GC
susceptibility and rs13428812 (Table
II).
 | Table II.Association between DNMT3A
polymorphisms and gastric cancer. |
Table II.
Association between DNMT3A
polymorphisms and gastric cancer.
| A, GG vs.
CC+CG |
|---|
|
|---|
| rs6733868
C>Ga, (n) | CC | CG | GG | OR (95% CI) | P-value |
|---|
| Non-GC (708) | 253 | 338 | 117 | Reference | – |
| Overall GC
(343) | 130 | 181 | 32 | 0.621
(0.402–0.958) | 0.031 |
| Intestinal
(195) | 84 | 103 | 15 | 0.494
(0.274–0.890) | 0.019 |
| Diffuse (133) | 44 | 78 | 16 | 0.769
(0.435–1.36) | 0.36 |
| (Unknown) | 2 | 0 | 1 | – | – |
|
| B, GG vs.
AA+AG |
|
| rs13428812
A>Ga, (n) | AA | AG | GG | OR (95%
CI) | P-value |
|
| Non-GC (708) | 389 | 276 | 43 | Reference | – |
| Overall GC
(343) | 204 | 128 | 11 | 0.664
(0.328–1.34) | 0.25 |
| Intestinal
(195) | 122 | 74 | 6 | 0.649
(0.260–1.62) | 0.35 |
| Diffuse (133) | 79 | 54 | 5 | 0.707
(0.271–1.85) | 0.48 |
| (Unknown) | 3 | 0 | 0 | – | – |
Association between DNMT3A
polymorphisms and GC susceptibility in subjects younger or older
than 60 years
Patients who were rs6733868 GG homozygotes exhibited
a significantly decreased risk for gastric carcinogenesis by
logistic regression analysis after adjustment for age, gender, and
HP infection status (OR, 0.534; 95% CI, 0.319–0.922; P=0.024,
Table III). On the other hand, a
significant association between rs13428812 and GC susceptibility
was not seen in subjects classified based on age (whether younger
or older than 60 years).
 | Table III.Association between DNMT3A
polymorphisms and GC susceptibility in subjects younger or older
than 60 years. |
Table III.
Association between DNMT3A
polymorphisms and GC susceptibility in subjects younger or older
than 60 years.
| A, rs6733868 |
|---|
|
|---|
| Age | CC | CG | GG | GG vs. CC+CG, OR
(95% CI) | P-value |
|---|
| <60 years |
| Non-GC
(278)a | 95 | 135 | 48 | Reference | – |
| GC
(105)a | 40 | 54 | 11 | 0.756
(0.360–1.59) | 0.46 |
| ≥60 years |
|
|
|
|
|
| Non-GC
(429)a | 158 | 202 | 69 | Reference | – |
| GC
(238)a | 90 | 127 | 21 | 0.534
(0.319–0.922) | 0.024 |
|
| B,
rs13428812 |
|
| Age | AA | AG | GG | GG vs. AA+AG, OR
(95% CI) | P-value |
|
| <60 years |
| Non-GC
(278)a | 146 | 114 | 18 | Reference | – |
| GC
(105)a | 62 | 41 | 2 | 0.344
(0.075–1.58) | 0.17 |
| ≥60 years |
| Non-GC
(429)a | 243 | 161 | 25 | Reference | – |
| GC
(238)a | 142 | 87 | 9 | 0.737
(0.329–1.65) | 0.46 |
Characteristics of subjects and
genotype frequencies in subjects in which gastric mucosal atrophy
was assessed
For 409 of 708 controls, the degree of histologic
gastritis could be assessed according to the updated Sydney system,
and the characteristics and genotype distributions for these
patients are shown in Table IV. In
all 409 such subjects, the genotype distribution of rs6733868 was
165CC, 186CG, and 58GG (HWE P=0.67), whereas that of rs13428812 was
249AA, 136AG, and 24GG (HWE P=0.39). The mean age, male to female
ratio, and HP positivity ratio were significantly higher in the SA
group than the non-SA group (Table
IV). The rs6733868G allele frequency in the SA and non-SA
groups was 29.3 and 39.4%, respectively (P=0.011). In addition, the
proportion of patients who were rs6733868 GG homozygotes differed
significantly between the SA and non-SA groups (P=0.0014). The
rs13428812G allele frequency was also significantly different
between the SA and non-SA groups (P=0.0082). In addition, the
proportion of the AA homozygotes was significantly higher and that
of the GG homozygotes significantly lower in the SA group than in
the non-SA group (P=0.044 and P=0.013, respectively, Table IV).
 | Table IV.Characteristics and genotype
frequencies of subjects in whom histological findings were
evaluated. |
Table IV.
Characteristics and genotype
frequencies of subjects in whom histological findings were
evaluated.
|
Characteristics | Total | Non-SA group | SA group |
P-valuec |
|---|
| Number of
subjects | 409 | 310 | 99 |
|
| Mean age ± SD
(years) | 59.9±13.3 | 58.4±13.7 | 64.4±11.1 | 0.0001a |
| Male:female | 240:169 | 168:142 | 72:27 | 0.0010b |
| H. pylori
positive rate | 262/409 | 169/310 | 93/99 |
<0.0001b |
| rs6733868
C>G |
|
|
|
|
| CC | 165 | 111 | 54 | 0.0014b |
| CG | 186 | 154 | 32 |
|
| GG | 58 | 45 | 13 |
|
| G
allele frequency (%) | 36.9 | 39.4 | 29.3 | 0.011b |
| rs13428812
A>G |
|
|
|
|
| AA | 249 | 180 | 69 | 0.044b |
| AG | 136 | 107 | 29 |
|
| GG | 24 | 23 | 1 | 0.013b |
| G
allele frequency (%) | 22.5 | 28.4 | 15.7 | 0.0082b |
Logistic regression analysis after adjustment for
age, gender, and HP infection status indicated that the rs6733868
CG+GG genotype was significantly associated with decreased severity
of gastric mucosal atrophy (OR, 0.495; 95% CI, 0.299–0.826;
P=0.0069, Table V), whereas
rs13428812 was not associated with mucosal atrophy.
 | Table V.Association between DNMT3A
polymorphisms and gastric mucosal atrophy. |
Table V.
Association between DNMT3A
polymorphisms and gastric mucosal atrophy.
| A, rs6733868
C>Ga |
|---|
|
|---|
| Atrophy status,
(n) | CC | CG | GG | OR (95% CI), CG+GG
vs. CC | P-value |
|---|
| Non-SA (310) | 111 | 154 | 45 | Reference |
|
| SA (99) | 54 | 32 | 13 | 0.495
(0.299–0.826) | 0.0069 |
|
| B, rs13428812
A>Ga |
|
| Atrophy status,
(n) | AA | AG | GG | OR (95% CI),
AG+GG vs. AA | P-value |
|
| Non-SA (310) | 180 | 107 | 23 | Reference |
|
| SA (99) | 69 | 29 | 1 | 0.909
(0.518–1.58) | 0.73 |
Association between HP infection
status and DNMT3A polymorphisms in control subjects
The mean age and male to female ratio were
significantly higher in HP-infected subjects than in uninfected
subjects (Table VI). The minor
allele frequency of both rs6733868 and rs13428812 was significantly
higher in uninfected subjects than in HP-infected subjects. The
proportions of patients who were rs6733868 GG and rs13428812 GG
homozygotes were also significantly higher in HP-infected subjects
than in uninfected subjects, whereas those of the rs6733868 CC and
rs13428812 AA homozygotes were significantly lower in HP-infected
than in uninfected subjects. The number of minor alleles of both
rs6733868 and rs13428812 was significantly correlated with the
frequency of HP infection (P=0.0070 and P=0.0050 by ANCOVA,
respectively).
 | Table VI.Association between HP infection
status and DNMT3A polymorphisms in control subjects. |
Table VI.
Association between HP infection
status and DNMT3A polymorphisms in control subjects.
| Variable | HP uninfected | HP infected | P-value |
|---|
| No. of
subjects | 273 | 435 |
|
| Mean age ± SD
(years) | 59.9±15.3 | 61.7±12.5 | 0.084a |
| Male:female | 133:140 | 272:163 | 0.0003b |
| rs6733868
C>G |
|
|
|
| CC | 88 | 165 | 0.018b |
| CG | 125 | 213 |
|
| GG | 60 | 57 | 0.0025b |
| G
allele frequency (%) | 44.9 | 33.0 | 0.0075b |
| rs13428812
A>G |
|
|
|
| AA | 135 | 254 | 0.024b |
| AG | 114 | 162 |
|
| GG | 24 | 19 | 0.023b |
| G
allele frequency (%) | 30.0 | 23.0 | 0.0059b |
Discussion
Global DNA methylation patterns reportedly alter the
hyper-methylation of CpG islands and the hypo-methylation of
non-CpG islands (18). The action of
de novo DNMTs, including DNMT3a, is responsible for this
alteration during early tumorigenesis (19). In gastric carcinogenesis,
overexpression of DNMT3a occurs in both cancerous and
para-cancerous tissues (10). In
addition, HP infection reportedly induces aberrant DNA methylation
of CpG islands, subsequently suppressing the function of tumor
suppressor genes in the gastric mucosa, and ultimately resulting in
carcinogenesis (20,21). HP infection reportedly does not
directly induce either the mRNA or protein expression of DNMT1,
DNMT3a, or DNMT3b in the gastric mucosa (22). However, the rs1550117 genetic
polymorphism results in increased transcription of the
DNMT3A gene, and an increase in the level of DNMT3A
mRNA associated with gastric carcinogenesis (23). Thus, polymorphisms in DNMT3A
are thought to play an important role in gastric carcinogenesis.
However, although many studies have examined the relationship
between rs1550117 and carcinogenesis, the potential association
remains unclear. Based on the hypothesis that the other
polymorphism in DNMT3A is more clearly associated with
gastric carcinogenesis, we investigated the potential associations
of other polymorphisms in DNMT3A that are not in strong
linkage with rs1550117.
Specifically, we investigated two allele sites of
DNMT3A (rs6733868 and rs13428812). The distributions of both
rs6733868 and 13428812 in our control group were in HWE (P=0.82 and
P=0.69, respectively), and were similar to those reported in the
Japanese population in the HapMap database (P=0.98 and P=0.99,
respectively). We found a decreased association between
DNMT3A homozygotes and gastric carcinogenesis, especially
for intestinal types of cancer. However, no association was
observed between rs13428812 and susceptibility to GC. HP infection
is known to cause chronic inflammation, which subsequently
progresses to atrophic gastritis, intestinal metaplasia, and
finally, GC (15). In our present
study, the rs6733868 minor allele was associated with a
significantly lower risk of gastric mucosal atrophy. These
observations suggest that in carriers of the rs6733868 minor
allele, progression to gastric mucosal atrophy and subsequent
development of GC may be inhibited in the homozygotes. In addition,
a stronger association was detected in older rather than younger
subjects, consistent with the expectation that intestinal types of
GC occur as a result of an extended period of chronic inflammation
and tissue rearrangement, including metaplastic change.
Previously, Cao et al (13) reported that rs1550117 is associated
with higher risk of HP infection but not of gastric atrophy or GC.
Those authors speculated that this DNMT3A polymorphism
facilitates HP infection by promoting methylation of the gene
encoding MUC-1, a membrane-bound mucin expressed on the surface of
gastric epithelial cells that normally provides a protective
barrier against HP infection (24).
Interestingly, our results showed that both rs6733868 and
rs13428812 were strongly associated with the risk of HP infection.
Specifically, rs6733868 was associated with higher HP infection
risk, severe gastric mucosal atrophy, and susceptibility to GC,
especially intestinal types of cancer, whereas rs13428812 was
associated only with HP infection risk. Potential associations of
rs6733868 with clinical disorders have not been reported. In
addition, there are no data available regarding the influence of
either polymorphism on the expression or function of DNMT3a.
However, we hypothesize that these polymorphisms affect the
expression of DNMT3a to varying degrees, as many studies suggest.
If so, the difference in risk association between rs6733868 and
rs13428812 may depend on the difference in the number of affected
genes and/or the degree of methylation. Of course, the expression
of DNMT3a is not regulated only by DNMT3A polymorphisms. Kim
et al (25) reported that
DNMT3A mutations and allelic losses, which decrease the
enzymatic activity of the protein product, are observed in many
solid cancers, suggesting that abnormal expression or accumulation
of DNMT3a in cancer tissues may be due to defects in the
degradation of mutant products rather than to the polymorphisms
themselves.
There are some clinical limitations to our study.
The first limitation is that our subjects were patients who visited
our hospital for either endoscopic examinations due to specific
symptoms or further follow-up after health examinations. Subjects
reporting no symptoms are essential for control groups. In
addition, we could not confirm whether very small histologic
neoplasia was present in any of the control group subjects. A
second limitation is that we assessed histologic gastritis using
biopsy samples only from the antrum, because the antral mucosa is
affected by HP infection for the longest period. Clearer results
might have been obtained if the degree of gastritis in the corpus
was assessed at the same time. A third limitation is that ethnicity
is an important factor affecting heterogeneity. Although different
countries and populations have different dietary and lifestyle
habits (26), this retrospective
research was performed at a single Japanese center.
In conclusion, regarding the influence of rs6733868,
our results suggest that the risk of HP infection decreases
depending on the number of minor alleles, the risk of severe
gastric mucosal atrophy decreases in minor allele carriers, and the
risk of developing GC decreases in minor allele homozygotes. In
contrast, rs13428812 is associated only with HP infection and not
with severe gastric mucosal atrophy or gastric carcinogenesis.
Thus, the data suggest that both DNMT3A polymorphisms
participate in the progression from HP to gastric mucosal atrophy
and ultimately to gastric carcinogenesis in both degree and
manner.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WJ analyzed the data and wrote the paper. TO, MN,
NS, HT, TH, MO, TN and RH obtained the samples and performed the
experiments to obtain the data. TaS determined the genotype. TT and
ToS obtained the samples and participated in the design of the
study. TA was responsible for the conception and design of the
study. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committees of Fujita Health University
and Kanazawa Medical University approved the protocol, and written
informed consent was obtained from all participating subjects.
Patient consent for publication
All patients gave informed consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Neugut AI, Hayek H and Howe G:
Epidemiology of gastric cancer. Semin Oncol. 23:281–291.
1996.PubMed/NCBI
|
|
2
|
Yasui W, Sentani K, Motoshita J and
Nakayama H: Molecular pathobiology of gastric cancer. Scand J Surg.
95:225–231. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Watanabe Y, Kurata JH, Mizuno S, Mukai M,
Inokuchi H, Miki K, Ozasa K and Kawai K: Helicobacter pylori
infection and gastric cancer. A nested case-control study in a
rural area of Japan. Dig Dis Sci. 42:1383–1387. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ozasa K, Kurata JH, Higashi A, Hayashi K,
Inokuchi H, Miki K, Tada M, Kawai K and Watanabe Y: Helicobacter
pylori infection and atrophic gastritis: A nested case-control
study in a rural town in Japan. Dig Dis Sci. 44:253–256. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Correa P: A human model of gastric
carcinogenesis. Cancer Res. 48:3554–3560. 1988.PubMed/NCBI
|
|
6
|
Uemura N, Okamoto S, Yamamoto S, Matsumura
N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N and Schlemper RJ:
Helicobacter pylori infection and the development of gastric
cancer. N Engl J Med. 345:784–789. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hamajima N, Naito M, Kondo T and Goto Y:
Genetic factors involved in the development of Helicobacter
pylori-related gastric cancer. Cancer Sci. 97:1129–1138. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sapari NS, Loh M, Vaithilingam A and Soong
R: Clinical potential of DNA methylation in gastric cancer: A
meta-analysis. PLoS One. 7:e362752012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li E: Chromatin modification and
epigenetic reprogramming in mammalian development. Nat Rev Genet.
3:662–673. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ding WJ, Fang JY, Chen XY and Peng YS: The
expression and clinical significance of DNA methyltransferase
proteins in human gastric cancer. Dig Dis Sci. 53:2083–2089. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang W, Xu Y, Ma G, Qi W, Gu H and Jiang
P: Genetic polymorphism of DNA methyltransferase 3A rs1550117
A>G and risk of cancer: A meta-analysis. J Invest Surg.
28:346–353. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li H, Li W, Liu S, Zong S, Wang W, Ren J,
Li Q, Hou F and Shi Q: DNMT1, DNMT3A and DNMT3B polymorphisms
associated with gastric cancer risk: A systematic review and
meta-analysis. EBioMedicine. 13:125–131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao XY, Jia ZF, Cao DH, Kong F, Jin MS,
Suo J and Jiang J: DNMT3a rs1550117 polymorphism association with
increased risk of Helicobacter pylori infection. Asian Pac J
Cancer Prev. 14:5713–5718. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang XX, He XQ, Li FX, Wu YS, Gao Y and Li
M: Risk-association of DNA methyltransferases polymorphisms with
gastric cancer in the Southern Chinese population. Int J Mol Sci.
13:8364–8378. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Correa P: Human gastric carcinogenesis: A
multistep and multifactorial process-First American cancer society
award lecture on cancer epidemiology and prevention. Cancer Res.
52:6735–6740. 1992.PubMed/NCBI
|
|
16
|
Arisawa T, Nakamura M, Otsuka T, Jing W,
Sakurai N, Takano H, Hayashi T, Ota M, Nomura T, Hayashi R, et al:
Genetic polymorphisms of MAFK, encoding a small Maf protein,
are associated with susceptibility to ulcerative colitis in Japan.
World J Gastroenterol. 23:5364–5370. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ota M, Tahara T, Otsuka T, Jing W, Nomura
T, Hayashi R, Shimasaki T, Nakamura M, Shibata T and Arisawa T:
Association between receptor interacting serine/threonine kinase 2
polymorphisms and gastric cancer susceptibility. Oncol Lett.
15:3772–3778. 2018.PubMed/NCBI
|
|
18
|
Li W and Chen BF: Aberrant DNA methylation
in human cancers. J Huazhong Univ Sci Technolog Med Sci.
33:798–804. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fernandez AF, Assenov Y, Martin-Subero JI,
Balint B, Siebert R, Taniguchi H, Yamamoto H, Hidalgo M, Tan AC,
Galm O, et al: A DNA methylation fingerprint of 1628 human samples.
Genome Res. 22:407–419. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maekita T, Nakazawa K, Mihara M, Nakajima
T, Yanaoka K, Iguchi M, Arii K, Kaneda A, Tsukamoto T, Tatematsu M,
et al: High levels of aberrant DNA methylation in Helicobacter
pylori-infected gastric mucosae and its possible association
with gastric cancer risk. Clin Cancer Res. 12:989–995. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tahara T, Arisawa T, Shibata T, Wang FY,
Nakamura M, Sakata M, Nagasaka M, Takagi T, Kamiya Y, Fujita H, et
al: Risk prediction of gastric cancer by analysis of aberrant DNA
methylation in non-neoplastic gastric epithelium. Digestion.
75:54–61. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakajima T, Maekita T, Oda I, Gotoda T,
Yamamoto S, Umemura S, Ichinose M, Sugimura T, Ushijima T and Saito
D: Higher methylation levels in gastric mucosae significantly
correlate with higher risk of gastric cancers. Cancer Epidemiol
Biomarkers Prev. 15:2317–2321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan H, Liu D, Qiu X, Qiao F, Wu Q, Su X,
Zhang F, Song Y, Zhao Z and Xie W: A functional polymorphism in the
DNA methyltransferase-3A promoter modifies the susceptibility in
gastric cancer but not in esophageal carcinoma. BMC Med. 8:122010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamada N, Nishida Y, Tsutsumida H, Hamada
T, Goto M, Higashi M, Nomoto M and Yonezawa S: MUC1 expression is
regulated by DNA methylation and histone H3 lysine 9 modification
in cancer cells. Cancer Res. 68:2708–2716. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim MS, Kim YR, Yoo NJ and Lee SH:
Mutational analysis of DNMT3A gene in acute leukemias and common
solid cancers. APMIS. 121:85–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Menotti A and Puddu PE: How the seven
Countries study contributed to the definition and development of
the Mediterranean diet concept: A 50-year journey. Nutr Metab
Cardiovasc Dis. 25:245–252. 2015. View Article : Google Scholar : PubMed/NCBI
|