Introduction
As a highly invasive and destructive malignant tumor
of the digestive tract (1), gastric
cancer has a very high morbidity and mortality rate worldwide
(2) and greatly threatens human
health. The increase of pressure on life and work, changes in the
eating habits of individuals and the reoccurring food safety issues
have caused the incidence age of gastric cancer to be younger
(3). However, the potential incidence
of gastric cancer is often ignored by patients and medical staff
because the early stage of gastric cancer lacks specific symptoms
and is wrongly considered as chronic gastritis or gastric ulcer
(4). Consequently, most of the
clinically diagnosed gastric cancer patients are in advanced stage.
Studies have shown that the five-year survival of patients with
advanced gastric cancer is not optimistic (5), so how to diagnose gastric cancer in its
early stage has become the key to improving the prognosis of
gastric cancer patients.
At present, the common diagnostic methods for cases
of suspected gastric cancer are gastroscopy, CT and X-ray barium
meal (6,7), this involves pain and risks of which may
easily arouse patients' resistance and hinder clinical deployment.
However, the specificity and sensitivity of common serological
tumor markers such as carcinoembryonic antigen, cancer antigen 19-9
are not satisfactory when applied to the diagnosis of early gastric
cancer (8), so is pepsin PGI and PGII
which are related to the course of chronic atrophic gastritis
(9). Therefore, finding a
non-invasive serum biomarker that is easy to detect has become a
hot-spot in gastric cancer research due to a lack of
high-efficiency, non-invasive serum tumor markers for gastric
cancer in clinical practice (10).
Apolipoprotein (Apo), which can bind to free lipids to form
lipoproteins and transport lipids through the lymphatic and
circulatory systems to regulate lipid metabolism in serum and
plasma (11), is closely related to
the formation and progression of malignant tumors such as prostate
cancer and non-small cell lung cancer (12,13). It is
found that ApoC-I and ApoC-III show low expression in gastric
cancer (14). Transthyretin (TTR), a
liver-derived secreted protein also known as prealbumin, is known
for its role in the carrier of thyroid hormone, thyroxine, and
triiodothyronine (15), and is used
as a biomarker to reflect the nutritional status and disease
progression of patients with colorectal cancer (16,17). It is
currently recommended to screen for colorectal cancer in patients
with gastric cancer since the colorectal and stomach both belong to
the digestive system and share synchronized morbidity risk
(18). In this study, the expression
levels of ApoC-I, TTR and ApoC-III in the serum of patients with
gastric cancer were detected by enzyme-linked immunosorbent assay
(ELISA), and analysis and discussion were made to investigate the
value of ApoC-I, TTR and ApoC-III in diagnosing early gastric
cancer.
Patients and methods
Experimental subjects
Retrospective analysis was made of 60 patients with
gastric cancer first diagnosed in the First Affiliated Hospital of
Jiaxing University (Jiaxing, China) from March 2015 to April 2016.
They were enrolled into the gastric cancer group, 60 patients with
chronic atrophic gastritis first diagnosed in the same hospital
during the same period were enrolled into the benign lesion group,
and 60 healthy volunteers in the health service center of the First
Affiliated Hospital of Jiaxing University were enrolled into the
control group. The gastric cancer group consisted of 39 males and
21 females, aged 25–76 years, with a mean age of 62.58±10.47 years;
the benign lesion group consisted of 35 males and 25 females, aged
23–72 years, with a mean age of 41.83±10.84 years; the control
group consisted of 36 males and 24 females, aged 21–78 years, with
a mean age of 41.63±11.26 years. The general data of the three
groups were not statistically different (P>0.05).
Inclusion criteria: i) The gastric cancer group was
confirmed by clinical histopathology to meet the diagnostic
criteria for gastric cancer (19);
ii) the benign lesion group was diagnosed as chronic atrophic
gastritis by gastroscopy, CT and X-ray barium meal, and the
possibility of gastric cancer was excluded; and iii) the gastric
cancer group had not received any radiotherapy or chemotherapy for
nearly 6 months, the benign lesion group had not received
antibiotics or related treatment ever, and the control group was
healthy.
Exclusion criteria: i) Individuals with heart,
brain, liver and kidney dysfunction; ii) individuals with
coagulation disorders or malignant tumor with the digestive tract
and/or other systems; and iii) individuals with severe mental
disorders, communication disorders or mental confusion.
The study was approved by the Ethics Committee of
the First Affiliated Hospital of Jiaxing University, and all the
examinees were explained the experiment contents in detail and then
they signed the informed consent.
Experimental methods
Experimental apparatus and
reagents
Human APO-C1 ELISA Kit (CSB-E13807h; Cusabio Biotech
Co., Ltd., Wuhan, China); Human APO-CIII ELISA Kit (SBJ-H1035;
Nanjing SenBeiJia Biological Technology Co., Ltd., Nanjing, China);
human TTR ELISA Kit (HZ-TTR-Hu; Shanghai Huzhen Industrial Co.,
Ltd., Shanghai, China); FLUOstar Omega automatic multi-function
microplate reader (FLUOstar Omega; Bio-Gene Technology Ltd.,
Melbourne, Australia); spectrophotometer (OD-1000+; Shanghai
Genesci Medical Technology Co., Ltd., Shanghai, China).
Determination of the concentration of
ApoC-I, TTR and ApoC-III in the serum by ELISA
After 8 h, blood samples (3 ml each) were taken in
the morning from the patients' elbow venous blood using a vacuum
lancet and were placed at room temperature for 2 h. The
agglutinated blood samples were then placed in a centrifuge at the
speed of 2,900 × g for 15 min. The supernatant was carefully
aspirated to obtain serum, which was dispensed and stored in a
−80°C medical refrigerator.
The concentration of ApoC-I, TTR and ApoC-III in the
serum samples to be tested was determined by ELISA. First, blank
control wells, sample wells and standard wells were set, adding 285
µl of the dilution to the sample wells, then adding 15 µl of the
sample, mixed gently, and set the standard wells according to the
instructions. The sample wells and standard wells were sealed with
the supplied tape and incubated for 2 h at 37°C. Second, the tape
was removed, the liquid was discarded from the reaction wells, and
100 µl of biological antibody (1X) was added to each well; the
wells were sealed again and incubated at 37°C for 1 h. Third, the
liquid was discarded from the wells, and then the wells were washed
with 200 µl of wash buffer and diluted with distilled water twice
for 3 min each time. Fourth, 100 µl of the enzyme labeling reagent
in the kit was added to each reaction well except the blank well,
and the wells were sealed and incubated at 37°C for 1 h. Then the
washing was repeated 5 times. Fifth, 50 µl of TMB developer was
added to each well and the wells were incubated at 37°C for 30 min
in the dark. Finally, 50 µl of the stop solution was added into
each well and the liquid in each well was gently mixed to terminate
the reaction. A blank well was used as a zero reference value, and
the optical density value (OD value) in each reaction well was
measured using a spectrophotometer with a wavelength of 450 nm.
Then the sample protein concentration was calculated according to
the standard curve.
Statistical analysis
The experimental data were statistically analyzed
using SPSS 19.0 statistical software (SPSS Inc., Chicago, IL, USA).
The comparison of the enumeration data (%) between groups was
performed using Chi-square test. The comparison of measurement data
(mean ± SD) between two groups was performed using the t-test, and
the comparison between multiple groups was performed using one-way
analysis of variance with Least Significant Difference test and the
receptor operating characteristic curve (ROC curve). Statistical
significance was set at P<0.05.
Results
Expression of ApoC-I, TTR and ApoC-III
in the serum of the gastric cancer, benign lesion and control
groups
The expression levels of ApoC-I, TTR and ApoC-III in
the gastric cancer group, benign lesion group and control group
were different from each other (P<0.01), with the expression
levels of ApoC-I, TTR and ApoC-III in the gastric cancer group
being lower than that of the benign lesion group (P<0.05), and
the expression levels of ApoC-I, TTR and ApoC-III in the benign
lesion group being lower than that of the control group
(P<0.05). Specific data are shown in Table I.
 | Table I.Comparison of the expression levels of
ApoC-I, TTR and ApoC-III in the serum in the three groups. |
Table I.
Comparison of the expression levels of
ApoC-I, TTR and ApoC-III in the serum in the three groups.
| Items | Gastric cancer group
(n=60) | Benign lesion group
(n=60) | Control group
(n=60) | F-value | P-value |
|---|
| ApoC-I (g/l) | 9.53±2.57 |
12.83±2.52a |
17.39±6.23a,b | 54.160 | <0.001 |
| TTR (mg/l) | 168.34±26.34 |
204.48±52.73a |
253.37±36.473a,b | 68.230 | <0.001 |
| ApoC-III (g/l) | 17.35±3.57 |
22.72±5.34a |
30.17±6.273a,b | 92.600 | <0.001 |
Relationship between the three factors
and clinicopathological features of gastric cancer
The expression levels of ApoC-I, TTR and ApoC-III in
the gastric cancer group had certain correlation with the clinical
stage, lymph node metastasis and differentiation of patients in the
gastric cancer group (P<0.05), and no association with patients'
sex or age (P>0.05). Specific data are shown in Tables II–IV.
 | Table II.Relationship between ApoC-I and
clinicopathological features of gastric cancer (mean ± SD). |
Table II.
Relationship between ApoC-I and
clinicopathological features of gastric cancer (mean ± SD).
| Variables | Case | ApoC-I (g/l) | t-test | P-value |
|---|
| Sex |
|
| 0.340 | 0.735 |
| Male | 39 | 9.56±2.38 |
|
|
|
Female | 21 | 9.34±2.42 |
|
|
| Age |
|
| 0.223 | 0.825 |
| ≤60
years | 32 | 9.46±2.72 |
|
|
| >60
years | 28 | 9.62±2.84 |
|
|
| Clinical stage |
|
| 2.331 | 0.023 |
| I+II
stage | 36 | 9.92±3.14 |
|
|
| III+IV
stage | 24 | 8.21±2.13 |
|
|
| Lymph node
metastasis |
|
| 2.556 | 0.013 |
| Without
metastasis | 42 | 9.72±3.81 |
|
|
| With
metastasis | 18 | 7.28±2.04 |
|
|
| Degree of
differentiation |
|
| 2.440 | 0.018 |
|
High/moderate level | 43 | 10.34±3.73 |
|
|
| Low
level | 17 | 8.34±2.45 |
|
|
 | Table IV.Relationship between ApoC-III and
clinicopathological features of gastric cancer (mean ± SD). |
Table IV.
Relationship between ApoC-III and
clinicopathological features of gastric cancer (mean ± SD).
| Variables | Cases | ApoC-III (g/l) | t-test | P-value |
|---|
| Sex |
|
| 0.427 | 0.671 |
|
Male | 39 | 17.63±3.23 |
|
|
|
Female | 21 | 17.24±3.63 |
|
|
| Age |
|
| 0.210 | 0.835 |
| ≤60
years | 32 | 17.42±3.12 |
|
|
| >60
years | 28 | 17.25±3.14 |
|
|
| Clinical stage |
|
| 2.459 | 0.017 |
| I–II
stage | 36 | 18.72±4.13 |
|
|
| III–IV
stage | 24 | 16.25±3.27 |
|
|
| Lymph node
metastasis |
|
| 2.391 | 0.020 |
| Without
metastasis | 42 | 18.54±3.82 |
|
|
| With
metastasis | 18 | 16.03±3.49 |
|
|
| Degree of
differentiation |
|
| 2.088 | 0.041 |
|
High/moderate level | 43 | 18.39±3.32 |
|
|
| Low
level | 17 | 16.62±2.81 |
|
|
Comparison of diagnostic value
According to the ROC curves of the expression levels
of ApoC-I, TTR and ApoC-III in the serum of patients from the
gastric cancer group and benign lesion group, the AUC areas of
ApoC-I, TTR and ApoC-III were 0.844 (0.775–0.914), 0.743
(0.651–0.836) and 0.814 (0.738–0.891), respectively. The diagnostic
critical values were 11.75 g/l, 189.50 mg/l and 21.19 g/l
(P<0.001), as shown in Fig. 1. The
diagnostic critical value was used as a positive indicator to
calculate the sensitivity, specificity, diagnostic coincidence
rate, positive predictive value and negative predictive value of
ApoC-I, TTR, ApoC-III and the combined detection (any of the three
indexes at the diagnostic critical value was regarded as positive
for the combined detection). The specificity and negative
predictive value of combined detection were proven to be higher
than the separate detection of the three factors (P<0.05)
(Table V).
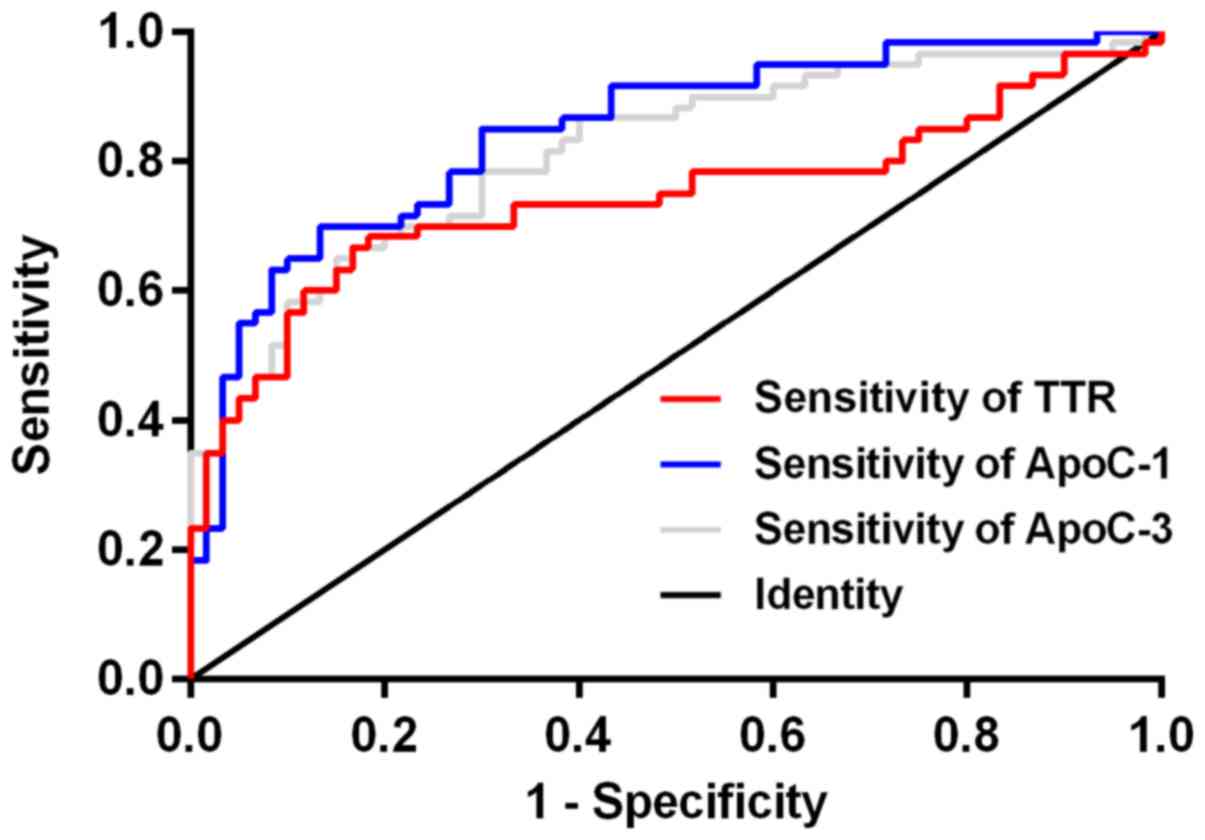 | Figure 1.ROC curve of ApoC-I, TTR and ApoC-III
in diagnosing gastric cancer. According to the ROC curves of the
expression levels of ApoC-I, TTR and ApoC-III in the serum of
patients from the gastric cancer group and benign lesion group, the
AUC areas of ApoC-I, TTR and ApoC-III were 0.844 (0.775–0.914),
0.743 (0.651–0.836), and 0.814 (0.738–0.891), respectively, the
diagnostic critical values were 11.75 g/l, 189.50 mg/l and 21.19
g/l. ApoC-I, apolipoprotein C-I; TTR, transthyretin; ROC, receptor
operating characteristic. |
 | Table V.Comparison of the diagnostic values
of ApoC-I, TTR and ApoC-III [n(%)]. |
Table V.
Comparison of the diagnostic values
of ApoC-I, TTR and ApoC-III [n(%)].
| Items | Sensitivity | Specificity | Diagnostic
coincidence rate | Positive predictive
value | Negative predictive
value |
|---|
| ApoC-I | 86.67
(52/60)a | 70.00 (42/60) | 78.33 (94/120) | 74.29 (52/70) | 84.00
(42/50)b |
| TTR | 81.67
(49/60)a | 68.33 (41/60) | 75.00 (90/120) | 72.06 (49/68) | 78.85
(41/52)b |
| ApoC-III | 83.33
(50/60)a | 66.67 (40/60) | 75.00 (90/120) | 71.43 (50/70) | 80.00
(40/50)b |
| Combined
detection | 100.00 (60/60) | 53.33 (32/60) | 76.67 (92/120) | 68.18 (60/88) | 100.00 (32/32) |
Discussion
As one of the most common malignant tumors in
developing countries, gastric cancer poses a serious threat to
human health (20). The survival of
patients with gastric cancer at an advanced and moderate stage is
generally frustrating and the pain induced by cancer causes a heavy
physical and mental burden to patients and their families (21), whereas gastric cancer at an early
stage can achieve better long-term clinical efficacy by endoscopic
resection (22). So early diagnosis
and effective treatment are essential for the survival of patients
with gastric cancer.
In recent years, biological markers have played an
increasingly important role in the discovery of gastrointestinal
malignancies (23). Tumor markers can
reflect tumorigenesis or tumor progression, with more than one kind
of tumor marker being able to mark one certain tumor. Early
detection of tumor markers in serum has important reference
significance for early diagnosis, timely treatment and prognosis of
the disease (24).
In this study, the expression levels of ApoC-I, TTR
and ApoC-III in the serum of patients with gastric cancer were
analyzed. The results showed that the expression levels of serum
ApoC-I, TTR and ApoC-III in the gastric cancer group were lower
than those in the benign lesion group, and levels of serum ApoC-I,
TTR and ApoC-III in the benign lesion group were lower than those
in the control group, which indicated that ApoC-I, TTR and ApoC-III
showed low expression in gastric cancer. After analyzing the serum
samples of 103 gastric cancer patients and cancer-free individuals
by mass spectrometry, Cohen et al (14) found that the identificated peptides
were fragments of ApoC-I and ApoC-III, which could be used as the
basis for the diagnosis of gastric cancer patients when combined
with other clinical indicators. TTR (25) is not only a key indicator for
assessing nutritional status, but also a sign of good prognosis for
patients with malnutrition. Recent research findings (26) indicated that TTR could be used as an
independent prognostic risk factor for gastric cancer, which can
now provide side verification for the results of this study. The
expression levels of ApoC-I, TTR and ApoC-III in the serum of
patients with gastric cancer at clinical III/IV stage, lymph node
metastasis and high/medium differentiation were higher than those
with poorly differentiated gastric cancer at clinical I/II stage
and without lymph node metastasis. On the one hand, APOC-I can
mediate the proliferation and apoptosis of the cancer cells and
regulate the cell cycle by controlling the signal pathways for
Survivin, p21 and caspase-3 (27). On
the other hand, as important regulators of lipoprotein metabolism
in humans, ApoC-I and ApoC-III can delay the clearance of
triglycerides in many aspects: ApoC-I can inhibit the binding of
lipoproteins to LDL receptors to directly interfere with the uptake
of fatty acids; ApoC-III inhibits fat degradation by interfering
with the binding of lipoproteins to glycosaminoglycan on the cell
surface (28). Literature has shown
that patients with gastric cancer have lower serum lipid levels
than normal individuals and disordered lipoprotein metabolism.
Blood lipids and lipoprotein levels can be used as important
indicators to reflect the progression and prognosis of gastric
cancer (29), so constant monitoring
of the blood lipid levels of gastric cancer patients is of great
guiding significance to the prognosis of patients (30). The low expression of ApoC-I and
ApoC-III indicates the decrease of the body's ability to degrade
triglyceride, the timely degradation of the serum and the decreased
blood lipid level, which may reflect the fact that the higher
severity of the disease causes a worse prognosis. As mentioned
above, TTR is important for the nutritional status of patients. Low
expression of TTR often reflects malnutrition in patients (25), which seriously affects the overall
survival of patients with gastric cancer (31). The lower the expression level of TTR
is, the poorer nutritional status the gastric cancer patient is in,
so the low expression of TTR may indicate the severity of the
disease progression of gastric cancer. The specificity and negative
predictive value of combined detection were proven to be higher
than the separate detection of the three factors. It was suggested
that ApoC-I, TTR and ApoC-III may be potential biomarkers of
gastric cancer, enjoying higher diagnostic value when used for
combined detection of gastric cancer. Closely related to the
occurrence and development of various malignant tumors such as
breast cancer, ApoC-I has a certain anticancer effect on breast
tumor cells, and has the ability to inhibit the expression of PCNA,
Ki-67 and Bcl-2 proteins, enhance Bax protein expression, and
inhibit cell proliferation (32).
ApoC-III has been found to be a potential target for the diagnosis
and treatment of hepatocellular carcinoma (33), and also a potential biomarker for
pancreatic cancer (34). Previous
studies have shown that TTR combined with other factors can
diagnose the severity of gastric cancer, and can make a
supplementary diagnosis of patients with gastroscopy evaluation
(35). The prognosis of patients with
high expression of TTR in serum is better than the prognosis of
patients with low expression of TTR (26). These studies showed that ApoC-I, TTR,
ApoC-III were closely related to the occurrence of many malignant
tumors, and could be used as potential biomarkers for several
tumors, having great reference value in the early diagnosis and
treatment of gastric cancer when used in combined detection.
However, this study has shortcomings: the sample
size is too small due to the limited experimental conditions, and
needs expanding or multi-center research.
In summary, the expression levels of ApoC-I, TTR and
ApoC-III in the serum of patients in the gastric cancer group were
lower than those of patients in the benign lesion group and healthy
volunteers from the control group. The combined detection of serum
ApoC-I, TTR and ApoC-III of gastric cancer deserves further study
as it can improve the specificity and the positive predictive value
of diagnosis, having great significance in diagnosing gastric
cancer and concluding the pathological pattern.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MW and JW performed ELISA. MW and HJ collected and
analyzed the general data of the patients. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The First Affiliated Hospital of Jiaxing University (Jiaxing,
China) and written informed consents were signed by the patients
and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu X, Li Z, Song Y, Wang R, Han L, Wang
Q, Jiang K, Kang C and Zhang Q: AURKA induces EMT by regulating
histone modification through Wnt/β-catenin and PI3K/Akt signaling
pathway in gastric cancer. Oncotarget. 7:33152–33164.
2016.PubMed/NCBI
|
|
2
|
Yoon H and Kim N: Diagnosis and management
of high risk group for gastric cancer. Gut Liver. 9:5–17. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takatsu Y, Hiki N, Nunobe S, Ohashi M,
Honda M, Yamaguchi T, Nakajima T and Sano T: Clinicopathological
features of gastric cancer in young patients. Gastric Cancer.
19:472–478. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shimada H, Noie T, Ohashi M, Oba K and
Takahashi Y: Clinical significance of serum tumor markers for
gastric cancer: A systematic review of literature by the Task Force
of the Japanese Gastric Cancer Association. Gastric Cancer.
17:26–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang C, Zhang J, Cai M, Zhu Z, Gu W, Yu Y
and Zhang X: DBGC: A database of human gastric cancer. PLoS One.
10:e01425912015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim T, Chung H, Yu W, Kim JY, Kim GC and
Choi J: Localization of gastric cancer by CT gastrography: A
prospective study. Hepatogastroenterology. 56:1580–1584.
2009.PubMed/NCBI
|
|
7
|
Yanai H, Matsubara Y, Kawano T, Okamoto T,
Hirano A, Nakamura Y, Nakamura H, Nishikawa J and Okita K: Clinical
impact of strip biopsy for early gastric cancer. Gastrointest
Endosc. 60:771–777. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanda M and Kodera Y: Recent advances in
the molecular diagnostics of gastric cancer. World J Gastroenterol.
21:9838–9852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang YK, Yu JC, Kang WM, Ma ZQ, Ye X,
Tian SB and Yan C: Significance of serum pepsinogens as a biomarker
for gastric cancer and atrophic gastritis screening: A Systematic
Review and Meta-Analysis. PLoS One. 10:e01420802015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shin VY, Ng EK, Chan VW, Kwong A and Chu
KM: A three-miRNA signature as promising non-invasive diagnostic
marker for gastric cancer. Mol Cancer. 14:2022015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song D, Yue L, Zhang J, Ma S, Zhao W, Guo
F, Fan Y, Yang H, Liu Q, Zhang D, et al: Diagnostic and prognostic
significance of serum apolipoprotein C-I in triple-negative breast
cancer based on mass spectrometry. Cancer Biol Ther. 17:635–647.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang X, Han J, Hardie DB, Yang J and
Borchers CH: The use of matrix coating assisted by an electric
field (MCAEF) to enhance mass spectrometric imaging of human
prostate cancer biomarkers. J Mass Spectrom. 51:86–95. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi J, Yang H, Duan X, Li L, Sun L, Li Q
and Zhang J: Apolipoproteins as differentiating and predictive
markers for assessing clinical outcomes in patients with small cell
lung cancer. Yonsei Med J. 57:549–556. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cohen M, Yossef R, Erez T, Kugel A, Welt
M, Karpasas MM, Bones J, Rudd PM, Taieb J, Boissin H, et al: Serum
apolipoproteins C-I and C-III are reduced in stomach cancer
patients: Results from MALDI-based peptidome and immuno-based
clinical assays. PLoS One. 6:e145402011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vieira M and Saraiva MJ: Transthyretin: A
multifaceted protein. Biomol Concepts. 5:45–54. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fentz AK, Spörl M, Spangenberg J, List HJ,
Zornig C, Dörner A, Layer P, Juhl H and David KA: Detection of
colorectal adenoma and cancer based on transthyretin and C3a-desArg
serum levels. Proteomics Clin Appl. 1:536–544. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Helgason HH, Engwegen JY, Zapatka M,
Vincent A, Cats A, Boot H, Beijnen JH and Schellens JH:
Identification of serum proteins as prognostic and predictive
markers of colorectal cancer using surface enhanced laser
desorption ionization-time of flight mass spectrometry. Oncol Rep.
24:57–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi BW, Kim HW, Won KS, Song BI, Cho KB
and Bae SU: Diagnostic accuracy of 18F-FDG PET/CT for detecting
synchronous advanced colorectal neoplasia in patients with gastric
cancer. Medicine (Baltimore). 95:e47412016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smyth EC, Verheij M, Allum W, Cunningham
D, Cervantes A and Arnold D; ESMO Guidelines Committee, : Gastric
cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 27 Suppl 5:v38–v49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rahman R, Asombang AW and Ibdah JA:
Characteristics of gastric cancer in Asia. World J Gastroenterol.
20:4483–4490. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kang JI, Chung HC, Jeung HC, Kim SJ, An SK
and Namkoong K: FKBP5 polymorphisms as vulnerability to anxiety and
depression in patients with advanced gastric cancer: A controlled
and prospective study. Psychoneuroendocrinology. 37:1569–1576.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choi J, Kim SG, Im JP, Kim JS and Jung HC:
Long-term clinical outcomes of endoscopic resection for early
gastric cancer. Surg Endosc. 29:1223–1230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Duffy MJ, Lamerz R, Haglund C, Nicolini A,
Kalousová M, Holubec L and Sturgeon C: Tumor markers in colorectal
cancer, gastric cancer and gastrointestinal stromal cancers:
European group on tumor markers 2014 guidelines update. Int J
Cancer. 134:2513–2522. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jin Z, Jiang W and Wang L: Biomarkers for
gastric cancer: Progression in early diagnosis and prognosis
(Review). Oncol Lett. 9:1502–1508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dellière S and Cynober L: Is transthyretin
a good marker of nutritional status? Clin Nutr. 36:364–370. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shimura T, Shibata M, Gonda K, Okayama H,
Saito M, Momma T, Ohki S and Kono K: Serum transthyretin level is
associated with prognosis of patients with gastric cancer. J Surg
Res. 227:145–150. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Su WP, Sun LN, Yang SL, Zhao H, Zeng TY,
Wu WZ and Wang D: Apolipoprotein C1 promotes prostate cancer cell
proliferation in vitro. J Biochem Mol Toxicol. 32:e221582018.
View Article : Google Scholar
|
|
28
|
Shachter NS: Apolipoproteins C-I and C-III
as important modulators of lipoprotein metabolism. Curr Opin
Lipidol. 12:297–304. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ghahremanfard F, Mirmohammadkhani M,
Shahnazari B, Gholami G and Mehdizadeh J: The valuable role of
measuring serum lipid profile in cancer progression. Oman Med J.
30:353–357. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang T, Cui G, Feng WM, Shi QL, Cui J, Li
XN, Wang QC and Shen H: Correlation analysis between glycolipids
metabolism and clinicopathologic fe atures in patients with gastric
cancer. Zhonghua Yi Xue Za Zhi. 96:2545–2547. 2016.(In Chinese).
PubMed/NCBI
|
|
31
|
Fujiya K, Kawamura T, Omae K, Makuuchi R,
Irino T, Tokunaga M, Tanizawa Y, Bando E and Terashima M: Impact of
malnutrition after gastrectomy for gastric cancer on long-term
survival. Ann Surg Oncol. 25:974–983. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun Y, Zhang J, Guo F, Zhao W, Zhan Y, Liu
C, Fan Y and Wang J: Identification of apolipoprotein C-I peptides
as a potential biomarker and its biological roles in breast cancer.
Med Sci Monit. 22:1152–1160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang D, Yuan W, Li H, Li S, Chen Z and
Yang H: Identification of key pathways and biomarkers in
sorafenib-resistant hepatocellular carcinoma using bioinformatics
analysis. Exp Ther Med. 16:1850–1858. 2018.PubMed/NCBI
|
|
34
|
Park J, Lee E, Park KJ, Park HD, Kim JW,
Woo HI, Lee KH, Lee KT, Lee JK, Park JO, et al: Large-scale
clinical validation of biomarkers for pancreatic cancer using a
mass spectrometry-based proteomics approach. Oncotarget.
8:42761–42771. 2017.PubMed/NCBI
|
|
35
|
Ahn HS, Shin YS, Park PJ, Kang KN, Kim Y,
Lee HJ, Yang HK and Kim CW: Serum biomarker panels for the
diagnosis of gastric adenocarcinoma. Br J Cancer. 106:733–739.
2012. View Article : Google Scholar : PubMed/NCBI
|















