Introduction
Breast cancer is one of the most malignant cancer
types and the global leading cause of cancer-associated mortality
in women (1). The etiology of breast
cancer is multi-factorial and there are various associated risk
factors, including high breast density, a late first birth and
education-associated risk factors (2). In recent years, the incidence of breast
cancer in women has consistently increased (3). The primary causes of mortality in
patients with breast cancer result from excessive proliferation and
metastasis of cancer cells (4). Thus,
a greater comprehension of the mechanisms underlying breast cancer
has become a matter of urgency.
Milk fat globule-EGF factor 8 (MFG-E8), also known
as lactadherin (5), is a 46 kDa
glycoprotein originally found in milk and mammary epithelial cells
(6). The protein contains a signal
sequence for secretion, two N-terminal epidermal growth factor
domains, and two C-terminal discoidin domains with homology to the
C1 and C2 domains found in blood clotting factors V and VIII
(7,8).
MFG-E8 is primarily produced by macrophages and dendritic cells
(9), but is expressed in several cell
types, including mammary epithelial, myoepithelial (10), macrophage (11), dendritic (12), endothelial (13), intestinal and retinal epithelial
(14) cells. The protein exerts
various effects on cellular proliferation, differentiation,
apoptosis, migration and invasion (15). BA46, also known as MFG-E8, has been
studied as a potential marker for breast cancer as it was
identified in the circulation of patients with breast cancer, but
not in healthy subjects (7,16,17). There
is also considerable interest in BA46 as a potential target for
breast cancer therapy, because it is expressed in human breast
carcinoma (7,18,19), and
radioconjugates of monoclonal antibodies that specifically
recognize BA46 have successfully targeted human breast tumors
transplanted into mice (20–22).
In recent decades, numerous studies have examined
the mechanisms of MFG-E8 and breast cancer. These studies
determined that MFG-E8 was significantly expressed in systemic
lupus erythematosus (23), lung
fibrosis (24), breast cancer
(19) and melanoma (25,26).
Several different functions of MFG-E8 have been proposed in breast
cancer cell lines. Yang et al (27) identified the expression and function
of MFG-E8 in different breast cancer subtypes using a microarray
analysis of laser capture-microdissected tissues and in situ
analysis. As MFG-E8 expression levels were decreased in estrogen
receptor (ER)-positive and receptor tyrosine-protein kinase erbB-2
(erbB2)-positive human breast cancer, it was concluded that MFG-E8
may exert an inhibitory function in these cancer types (27). In contrast, MFG-E8 was identified to
be highly expressed in triple-negative [ER−/progesterone
receptor (PgR)−/erbB2−] breast cancer (TNBC)
cell lines and patient sera compared with non-triple-negative cell
lines including T47D, ZR75, MCF7, BT474 and SKBR3 and compared with
basal-like human breast cancer, respectively (27,28). These
findings underscore the putative value of MFG-E8 as a potential
biomarker and therapeutic target for breast carcinoma, although
further research is required to understand the functional
properties of MFG-E8 in breast carcinoma (15). In the present study, to determine the
effect of MFG-E8 on the malignant and metastatic potential of TNBC
cells, biological methods were used to investigate the function of
MFG-E8 in MDA-MB-231 cells in vitro. Cell viability,
migration, invasion and apoptosis were affected by the
downregulation of MFG-E8 in human breast cancer cells.
Materials and methods
Cell culture
The human breast carcinoma cell line lines Hs578Bst
(non-breast cancer cell line) (29),
Hs 578T, MCF-7, ZR-75-30, T47D and MDA-MB-231 were purchased from
the American Type Culture Collection (ATCC, Manassas, VA, USA). All
cell lines were cultured in Dulbecco's modified Eagle's medium
(DMEM) supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin/streptomycin at 37°C in an atmosphere with 5%
CO2, as recommended by ATCC.
RNA isolation and RT-qPCR
analysis
RNA extraction was performed using TRIzol reagent
(Life Technologies; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and reverse transcribed to complementary DNA (cDNA) using the
PrimeScript™ RT reagent kit (Takara Bio, Inc., Otsu, Japan). For
RT-qPCR, cDNA was mixed with the appropriate primers and the
SYBR-Green Super mix (Kapa Biosystems, Inc., Wilmington, MA, USA)
and run on the CFX96 Real-Time system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). All mRNA data were normalized to the expression
of GAPDH. The primers used in the present study were as follows:
Human MFG-E8 forward, 5′-GTAACTTTGGCTCTGTCC-3′ and reverse,
5′-GTTCTTCTTGTGGGAGTG-3′; human GAPDH forward,
5′-CCACTCCTCCACCTTTG-3′ and reverse, 5′-CACCACCCTGTTGCTGT-3′. The
procedure for RT-qPCR included 5 min at 99°C, followed by 40 cycles
of 15 sec at 94°C, 30 sec at 59°C and 45 sec at 72°C. The
2−∆∆Cq method was used to calculate the relative
expression of MGF-E8 (30).
Lentivirus production and
oligonucleotide transfection
Lentivirus containing MFG-E8 short hairpin RNA1 and
RNA2 (shRNA1, shRNA2) or scrambled oligonucleotides were obtained
from Wuhan Hualian Branch Biotechnology Co., Ltd. (Wuchang, Wuhan,
China). The cells were divided into four groups including the blank
control group (Control), the scramble group (pSi-Scramble), the
shRNA1 group, and the shRNA2 group. The target sequences for the
MFG-E8 shRNA were as follows: MFG-E8 shRNA2, forward,
5′-tatgGGACTGGCAGCAGTAAGATCTTTCAAGAGAAGATCTTACTGCTGCCAGTCCTTTTT-3′
and reverse,
5′-aattAAAAAGGACTGGCAGCAGTAAGATCTTCTCTTGAAAGATCTTACTGCTGCCAGTCC-3′.
The cells were inoculated into 6-well plates at a concentration of
3×105 cells/ml (1.2×106 per well), 4 ml of
complete medium was added, mixed well, and the plates were placed
in a CO2 incubator overnight at 37°C. Two µg of the
plasmid (pGMLV-SC5RNAi; Genomeditech, Shanghai, China) to be
transfected was diluted in 100 µl of serum-free medium to make
solution A and 25 µl of Lipofectamine® 2000 was diluted
in 100 µl of serum-free medium to make solution B. Solutions A and
B were mixed and agitated for 30 min at 18–21°C (room temperature),
then added to the cells for incubation in 6-well plates at 37°C for
6 h. Following transfection, the cells were washed twice with
serum-free medium and cultured for 3 days, after which the protein
expression in cells was evaluated. A total of 50 nM pSi-MFG-E8 and
20 nM pSi-Scramble (Wuhan Hualian Branch Biotechnology Co., Ltd.)
were diluted in Opti-L-15 reduced serum medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA); the diluted plasmid DNA
and Lipofectamine 2000 were then mixed at a ratio of 1:2.5 and
added to MDA-MB-231 cells.
MTT assay
Cell viability was measured using a MTT assay.
MDA-MB-231 cells (2×105 cells per well) were plated in
96-well plates, allowed to adhere overnight and transfected with
pSi-Scramble or pSi-MFG-E8 as aforementioned. Untreated MDA-MB-231
cells were used as the control. After transfection for 24, 48 and
72 h, 20 µl MTT (5 mg/ml) was added and the plates were incubated
for a further 4 h at 37°C. Subsequently, 150 µl DMSO was added to
dissolve the formazan crystals. The optical density (OD) was
detected at 490 nm using a microplate spectrophotometer. The cell
survival percentage was calculated as follows: (OD sample/OD
control) ×100%.
Cell cycle assay
The cell cycle distribution was determined using
flow cytometry. MDA-MB-231 cells were fixed in 70% cold ethanol at
4°C for 30 min. Following fixation, the cells were washed twice
with PBS and collected by centrifugation at 3,000 × g for 30 sec at
4°C. The collected pellets were suspended and incubated in PBS
containing 20 µl/ml of propidium iodide (PI), 0.2% Triton X-100,
and 40 µg/ml RNaseA at 4°C for at least 30 min. Finally, the cell
cycle phase distribution was assessed using a flow cytometer.
Tumor cell migration and invasion
assays
Transwell chambers (8-µm diameter, 24-well format)
(Corning Incorporated, Corning, NY, USA) were used in the assay.
For the cell invasion assay, the internal surface of each
polycarbonate membrane was coated with Matrigel™ (30 µg) for 30 min
at 37°C for gel formation (however, for the cell migration assay,
the polycarbonate membrane was not coated with Matrigel) and then
blocked with 500 µl serum-free DMEM media. MDA-MB-231 cells
(1×105 cells) were seeded into the upper chamber in 200
µl of serum-free medium and the lower compartment of the chamber
was filled with 600 µl DMEM supplemented with 0.2% bovine serum
albumin (Gibco; Thermo Fisher Scientific, Inc.) and 10% FBS. After
incubation for 24 h at 37°C, the cells on the upper surface of the
membrane were carefully removed using a cotton swab. The cells that
had migrated to or invaded the lower surface of the membrane were
fixed in 1 ml of 4% methanol for 10 min at 18–21°C (room
temperature) and stained with 0.1% crystal violet for 30 min at
37°C. A Nikon inverted microscope (TS100-F; Nikon Corporation,
Tokyo, Japan) used to observe migratory cells in lower chamber
(magnification, ×200).
Western blotting
MDA-MB-231 cell pellets were lysed in ice-cold
radioimmunoprecipitation assay buffer containing complete protease
inhibitor cocktail (Bio-Swamp, Shanghai, China) for 30 min on ice.
The protein concentration was determined using a BCA protein assay
kit. Equal amounts (20 µg) of proteins were fractionated on an
appropriate percentage (10%) SDS-polyacrylamide gel and transferred
onto a polyvinylidene difluoride membrane. After the transfer,
non-specific binding to the membrane was blocked by incubation with
5% nonfat dry milk in Tris-buffered saline and Tween 20 (TBST) at
room temperature for 2 h, which was followed by overnight
incubation with primary antibodies in TBST and 5% nonfat dry milk
on a shaker at 4°C. The primary antibodies were as follows:
Anti-MFG-E8 (1:1,000; cat. no. ab168733; rabbit); anti-E-cadherin
(1:1,000; cat. no. ab76055; mouse); anti-N-cadherin (1:500; cat.
no. ab18203; rabbit); anti-vimentin (1:500; cat. no. ab8978;
mouse); anti-caspase-3 (1:500; cat. no. ab4051; rabbit);
anti-caspase-9 (1:500; cat. no. ab69514; rabbit);
anti-Bcl-2-associated X protein (Bax; 1:1,000; cat. no. ab53154;
rabbit); anti-B-cell lymphoma 2 (Bcl-2; 1:500; cat. no. ab32124;
rabbit); anti-β-actin (1:10,000; cat. no. ab8227; rabbit) (all from
Abcam, Cambridge, UK); anti-cleaved caspase-3 (1:500; cat. no.
9664P; rabbit); anti-cleaved caspase-9 (1:800; cat. no. 9505P;
human) (both from Cell Signaling Technology, Inc., Danvers, MA,
USA); anti-matrix metalloproteinase (MMP)-9 (1:800; cat. no.
sc-21733; mouse) and anti-MMP-2 (1:500; cat. no. sc-13595; mouse)
(both from Santa Cruz Biotechnology, Inc., Dallas, TX, USA). The
membranes were incubated with horseradish peroxidase
(HRP)-conjugated secondary antibodies [Goat anti-rabbit
immunoglobulin (IgG) H&L, 1:5,000, cat. no. ab6721; goat
anti-mouse IgG, 1:5,000, cat. no. ab6789; goat anti-human IgG,
1:10,000, cat. no. ab6858; Abcam] at 4°C overnight and the protein
bands were visualized by using an Immobilon ECL Ultra Western HRP
substrate (EMD Millipore, Billerica, MA, USA) and using ImageJ 1.8
software (National Institutes of Health, Bethesda, MD, USA). The
levels of MMP-2 and MMP-9 were detected using an ELISA kit which
was purchased from AmyJet Scientific, Inc. (cat. no. KA0391; Wuhan,
China).
Flow cytometric analysis of
apoptosis
The analysis of apoptotic cells was conducted using
annexin V-fluorescein and PI staining. In accordance with the
manufacturer's protocol (Wuhan Hualian Branch Biotechnology Co.,
Ltd.), transfected or untransfected control MDA-MB-231 cells were
collected by trypsinization, washed with PBS, resuspended in 100 µl
annexin V FLUOS labeling solution and incubated for 10–15 min at
15–25°C. Cellular apoptosis was evaluated using flow cytometry.
Flow cytometric analysis clearly differentiated normal (living)
cells, which exhibit low annexin V and low PI staining, from
apoptotic cells (high annexin V and low PI staining) and necrotic
cells (high annexin V and high PI staining). The data were analyzed
by using CellQuest data acquisition and analysis software (version
5.1; BD Biosciences, Franklin Lakes, NJ, USA).
Statistical analysis
All data are presented as the mean ± standard error.
Statistical analysis for comparison between treated groups and
corresponding controls was performed using SPSS 19.0 software (IBM
Corp., Armonk, NY, USA), and the data were analyzed using a
two-sample Student's t-test or analysis of variance followed by the
Least Significant Difference post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
MFG-E8 expression levels in breast
carcinomas cell lines
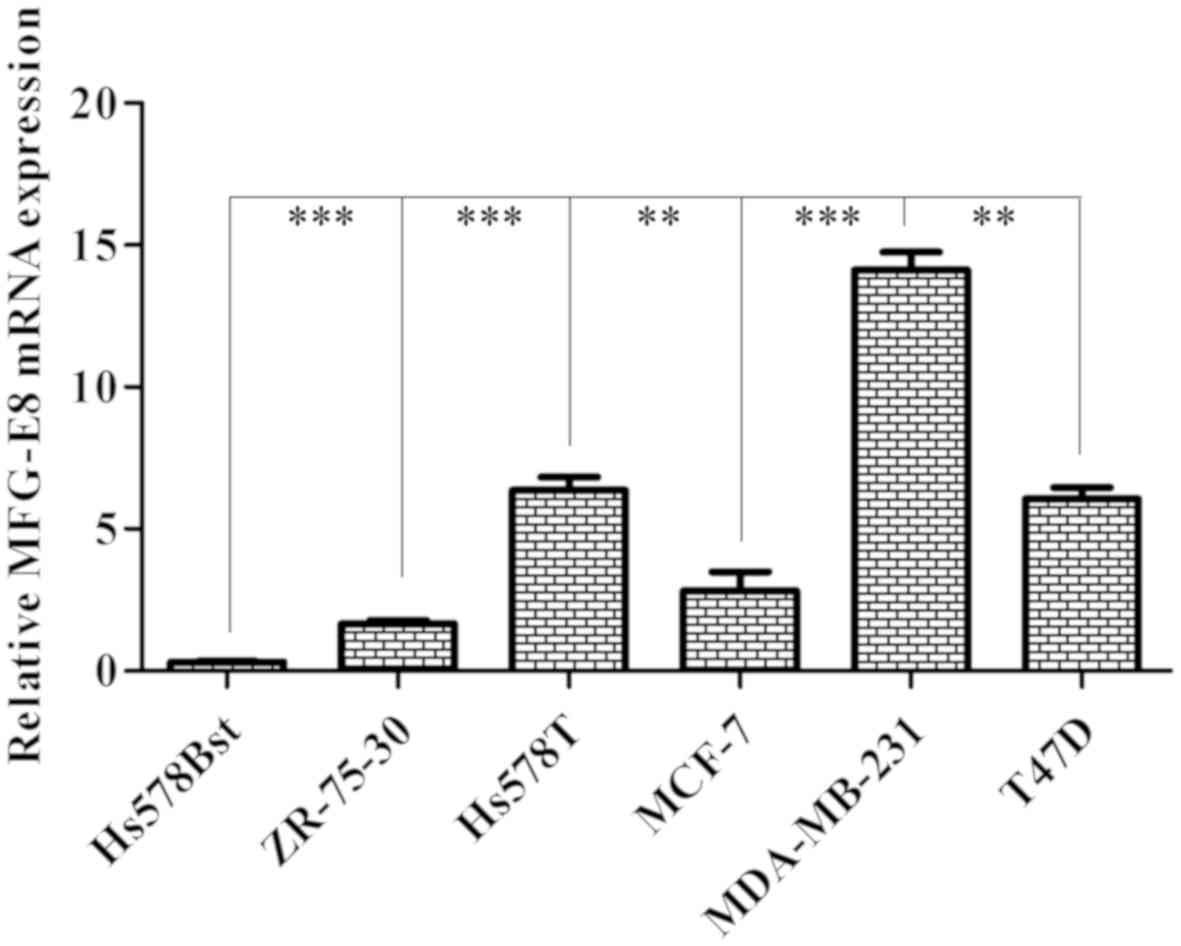
RT-qPCR was used to examine the expression level of
MFG-E8 in different breast carcinoma cell lines (Hs578Bst, Hs578T,
MCF-7, T47D, ZR-75-3, MDA-MB-231). According to the results, the
endogenous expression levels of MFG-E8 in MDA-MB-231 cells were
significantly higher compared with that in other cells (P<0.01;
Fig. 1). It was concluded that MFG-E8
was expressed in breast carcinoma cell lines and the expression
level differed across various breast cancer subtypes.
Downregulation of MFG-E8 in MDA-MB-231
cells
MFG-E8 is markedly upregulated in certain human
breast carcinoma cell lines (26);
however, little is known about the biological function of MFG-E8 in
these cell lines. We hypothesized that MFG-E8 may play a role in
breast cancer. To verify the hypothesis regarding the function of
MFG-E8 in breast cancer cells, MDAMB-231 cells were selected as a
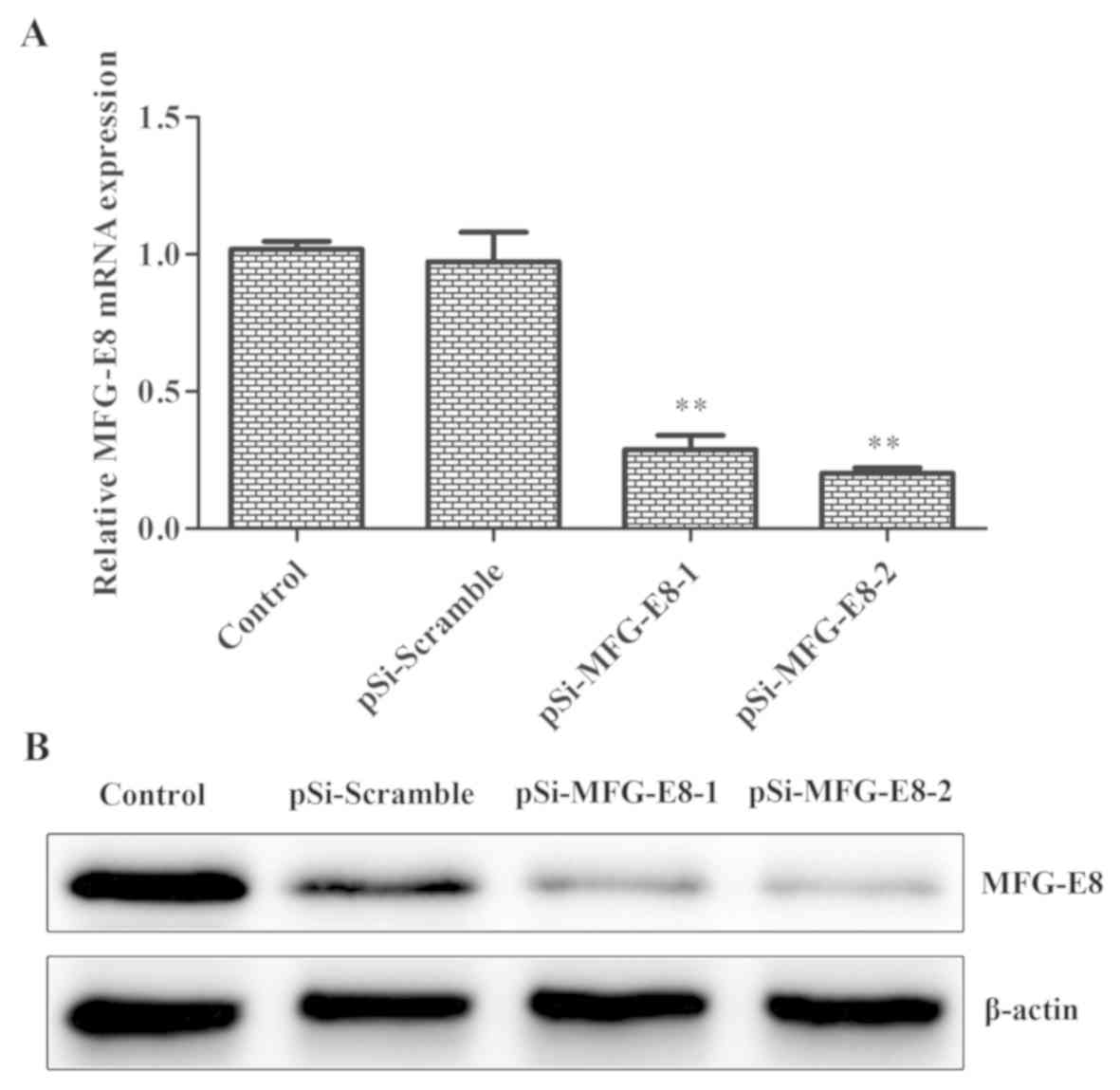
model for further studies. Downregulated expression of MFG-E8 in
MDA-MB-231 cells occurred following infection with lentivirus
containing shRNAs, including shRNA1 and shRNA2 for MFG-E8. The mRNA
expression level of MFG-E8 was reduced by 5.06 times and protein
expression was reduced by 7.37 times following transfection with
the lentivirus small interfering (si)RNA. RT-qPCR were used to
detect the optimal shRNA to target MFG-E8. As presented in Fig. 2A, the mRNA levels of MFG-E8 were
significantly decreased in the siRNA-transfected cells compared
with the blank control (MDA-MB-231 untreated) and scramble control.
shRNA2 was used for subsequent experiments. Western blotting, which
was used to further validate the interference efficiency, revealed
that the protein expression of MFG-E8 was also markedly decreased
in the siRNA-transfected cells compared with the blank
control-untransfected MDA-MB-231 cells and scramble control groups
(Fig. 2B). These results indicated
that lentivirus siRNA successfully downregulated the mRNA and
protein expression of MFG-E8 in TNBC cells.
Cell viability is affected by MFG-E8
in MDA-MB-231 cells
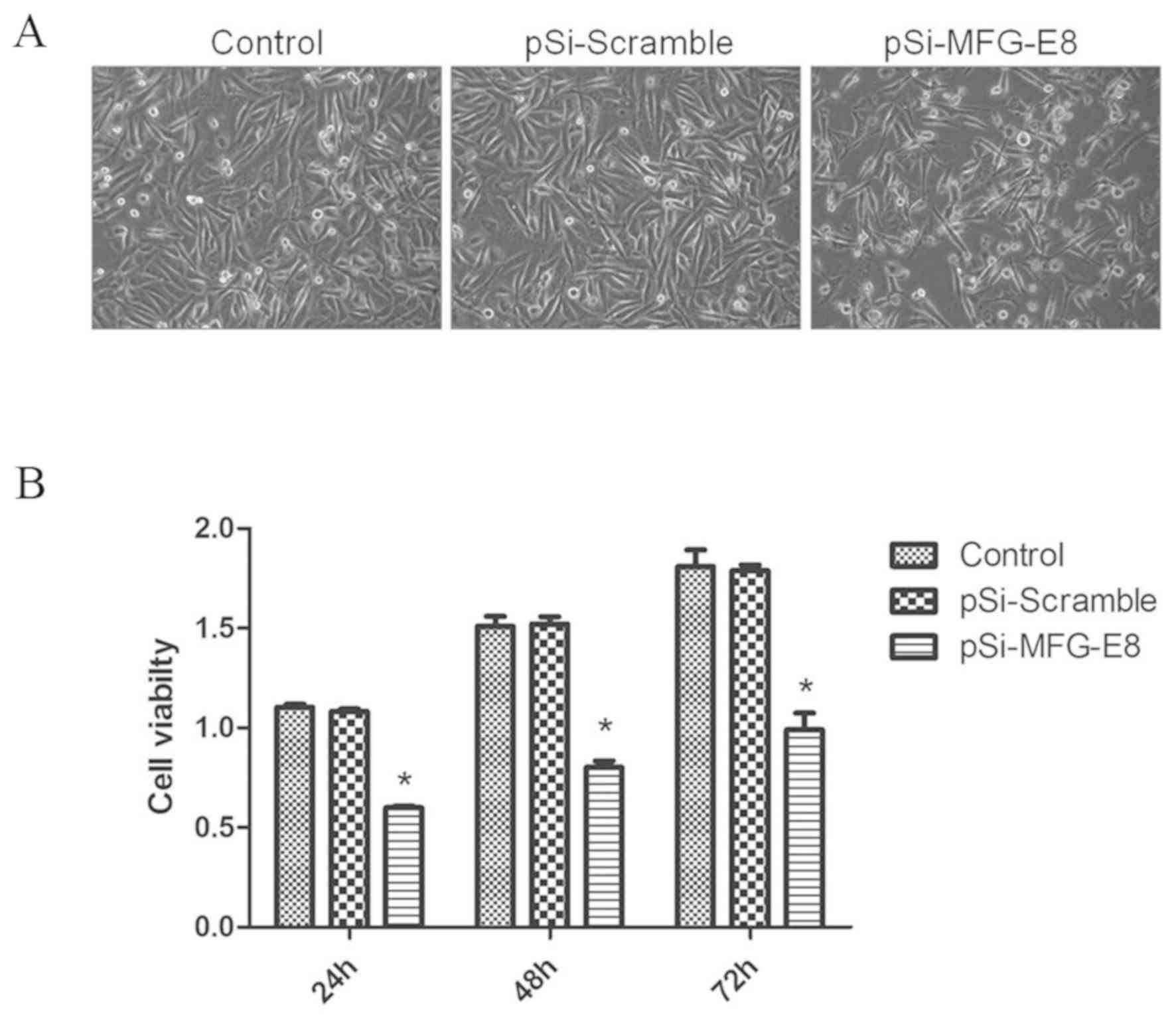
To explore the effect of MFG-E8 knockdown in
MDAMB-231 cells, the morphological changes of MDA-MB-231 cells were
observed under an inverted microscope. Morphological observation
revealed that the control cells in the pSi-Scramble and untreated
groups exhibited normal morphology with polygonal shapes, firmly
adherent growth, distinct cell borders and intercellular tight
junction. However, the MDA-MB-231 cells transfected with pSi-MFG-E8
exhibited abnormal morphology, with small and round in shape
characteristics, detachment from the cell culture wells, cell
debris and an increase in intercellular particles in a
time-dependent manner following transfection, which are all
indicative of apoptosis (Fig. 3A).
These results indicated that high levels of MFG-E8 in MDAMB-231
cells is important for maintaining normal cell morphology and
growth in vitro. To understand the effect of MGF-E8
knockdown on the cell viability, MDA-MB-231 cells were transfected
with pSi-MFG-E8 for 24 to 72 h, and evaluated using a MTT assay. As
shown in Fig. 3B, MDA-MB-231 cell
viability was significantly decreased in the siRNA-transfected
groups compared with the blank control (MDA-MB-231 untreated) and
scramble control 24, 48 and 72 h following transfection, while
there were no significant differences between the pSi-Scramble and
control groups. Taken together, the aforementioned results
suggested that MGF-E8 lentivirus siRNA significantly suppressed the
viability of MDA-MB-231 cells.
MFG-E8 expression affects breast
cancer cell cycle
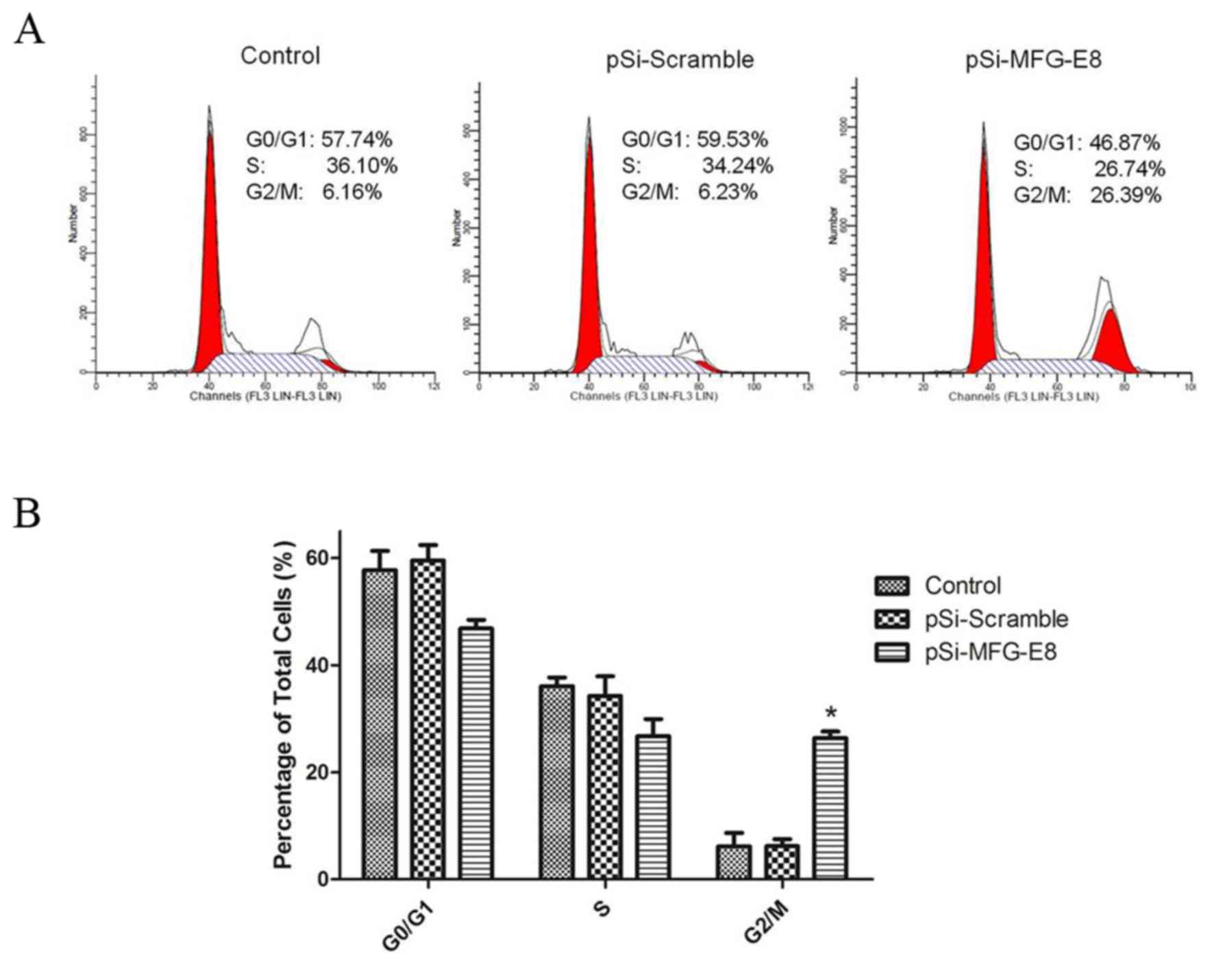
To determine whether MGF-E8 downregulation induced
growth inhibition of MDA-MB-231 cells, the cell cycle distribution
was evaluated by flow cytometry. The results confirmed that the
cell cycles of MDA-MB-231 cells were affected by inhibition of
MGF-E8, with the cell cycles being arrested at the G2/M
phase. As shown in Fig. 4, the
proportion of MDA-MB-231 cells that accumulated in the
G2/M phase were significantly increased from 6.59±1.3%
in the pSi-Scramble group and 6.21±1.9% in the blank control
(MDA-MB-231 untreated) group to 26.48±1.2% in the pSi-MFG-E8 group,
and reduced from 59.64±2.9 and 57.35±3.2%, respectively, to
46.42±1.6% in the G0/G1 phase 24 h after
transfection. These results revealed that MDA-MB-231 cell growth
inhibition were mediated by G2/M cell-cycle arrest
following MGF-E8 downregulation.
MFG-E8 expression regulates breast
cancer cell migration and invasion
To examine the function of MFG-E8 in cell migration
and invasion, migration and invasion assays were performed in 8-µm
diameter pore size Transwell chambers in 24-well plates. In the
Transwell migration assay, it was demonstrated that the number of
cells that had migrated to the lower chambers was significantly
reduced following MFG-E8 downregulation compared with the blank
control (MDA-MB-231 untreated) and scramble control groups
(Fig. 5A and B). Furthermore, the
cell invasion assays revealed that knockdown of MFG-E8 by siRNA
transfection significantly inhibited the invasion of MDA-MB-231
cells compared with the blank control (MDA-MB-231 untreated) and
scramble control groups (Fig. 5A and
B). To investigate the underlying mechanism of the inhibition
of cell migration and invasion by MFG-E8 downregulation, western
blot analysis was used to detect the expression of associated
proteins. Compared with the blank control (MDA-MB-231 untreated)
and scramble control groups, the expression levels of N-cadherin
and vimentin in the MFG-E8 downregulation group were markedly
decreased, and the level of E-cadherin was markedly increased
(Fig. 5C). In addition, the
expression of MMP-9 and MMP-2 was markedly decreased, which was
confirmed by the results of the ELISA, whereby MMP-2 and MMP-9
levels were significantly decreased compared with the blank control
(MDA-MB-231 untreated) and scramble control groups (Fig. 5D).
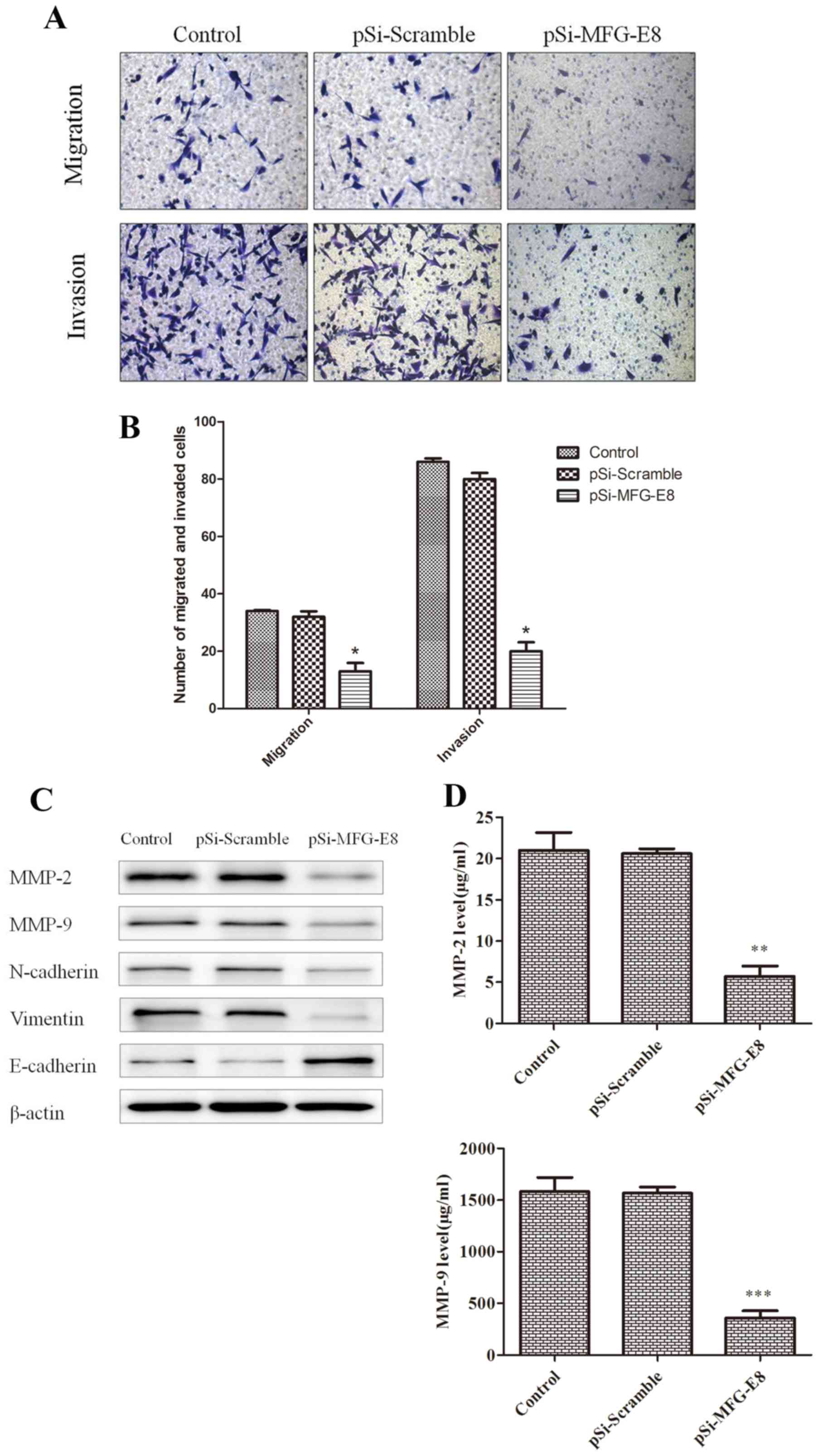 | Figure 5.MFG-E8 expression regulates breast
cancer cell migration and invasion. (A) Light microscopic
examination following Transwell assays revealed the effects of
MFG-E8 on the migration and invasion of MDA-MB-231 cells
(magnification, ×200). (B) The migration and invasion rate of
MDA-MB-231 cells that migrated/invaded through the membrane were
counted in five random fields per group. (C) Expression levels of
MMP-2, MMP-9, N-cadherin, Vimentin and E-cadherin in MDA-MB-231
cells following transfection with pSi-Scramble or pSi-MFG-E8 for 48
h were analyzed by western blotting. (D) Levels of MMP-2, MMP-9 in
MDA-MB-231 cells were analyzed by ELISA. *P<0.05, **P<0.01,
***P<0.001, pSi-MFG-E8 group vs. pSi-Scramble group. β-actin was
used as a loading control. Control, non-transfected cells;
pSi-Scramble, cells transfected with control shRNA lentiviral
vector; pSi-MFG-E8, cells transfected with control shRNA-MFG-E8
lentiviral vector; MFG-E8, Milk fat globule-EGF factor 8; shRNA,
short hairpin RNA; MMP, matrix metalloproteinase. |
Knockdown of MFG-E8 in MDA-MB-231
cells induces cell apoptosis
According to the aforementioned results, it was
demonstrated that the MFG-E8 knockdown cells exhibited the typical
morphology of apoptosis. Next, the proportion of apoptotic
MDA-MB-231 cells under each condition was examined using flow
cytometry. MGF-E8 knockdown significantly reduced the proportion of
viable MDA-MB-231 cells from 94.32±2.13% and 95.41±1.44% to
69.85±3.61% 48 h following transfection compared with the blank
control (MDA-MB-231 untreated) and pSi-Scramble groups (Fig. 6A and B). At 48 h post-transfection,
the percentage of apoptotic MDA-MB-231 cells transfected with
pSi-MGF-E8 was 24.20±1.5%, significantly higher compared with that
of the pSi-Scramble group (4.11±3.9%) and the blank control
(MDA-MB-231 untreated) group (3.76±2.2%). To confirm whether MFG-E8
knockdown affected the expression level of proteins associated with
apoptosis, the protein expression levels of caspase-3, cleaved
caspase-3, caspase-9, cleaved caspase-9, Bax and Bcl-2 were
investigated. After MDA-MB-231 cells were transfected with pSi-
MFG-E8 or pSi-Scramble for 48 h, the expression levels of cleaved
caspase-3, and cleaved caspase-9 were markedly increased in the
pSiRNA-MFG-E8 group compared with blank control (MDA-MB-231
untreated) and pSi-Scramble groups (Fig.
6C). Conversely, the expression levels of the precursor
protein, caspase-3 and caspase-9 were decreased. In addition,
compared with the blank control (MDA-MB-231 untreated) and
pSi-Scramble groups, MFG-E8 downregulation markedly decreased the
level of Bcl-2 and increased the level of Bax resulting in an
increase in the Bax/Bcl-2 ratio.
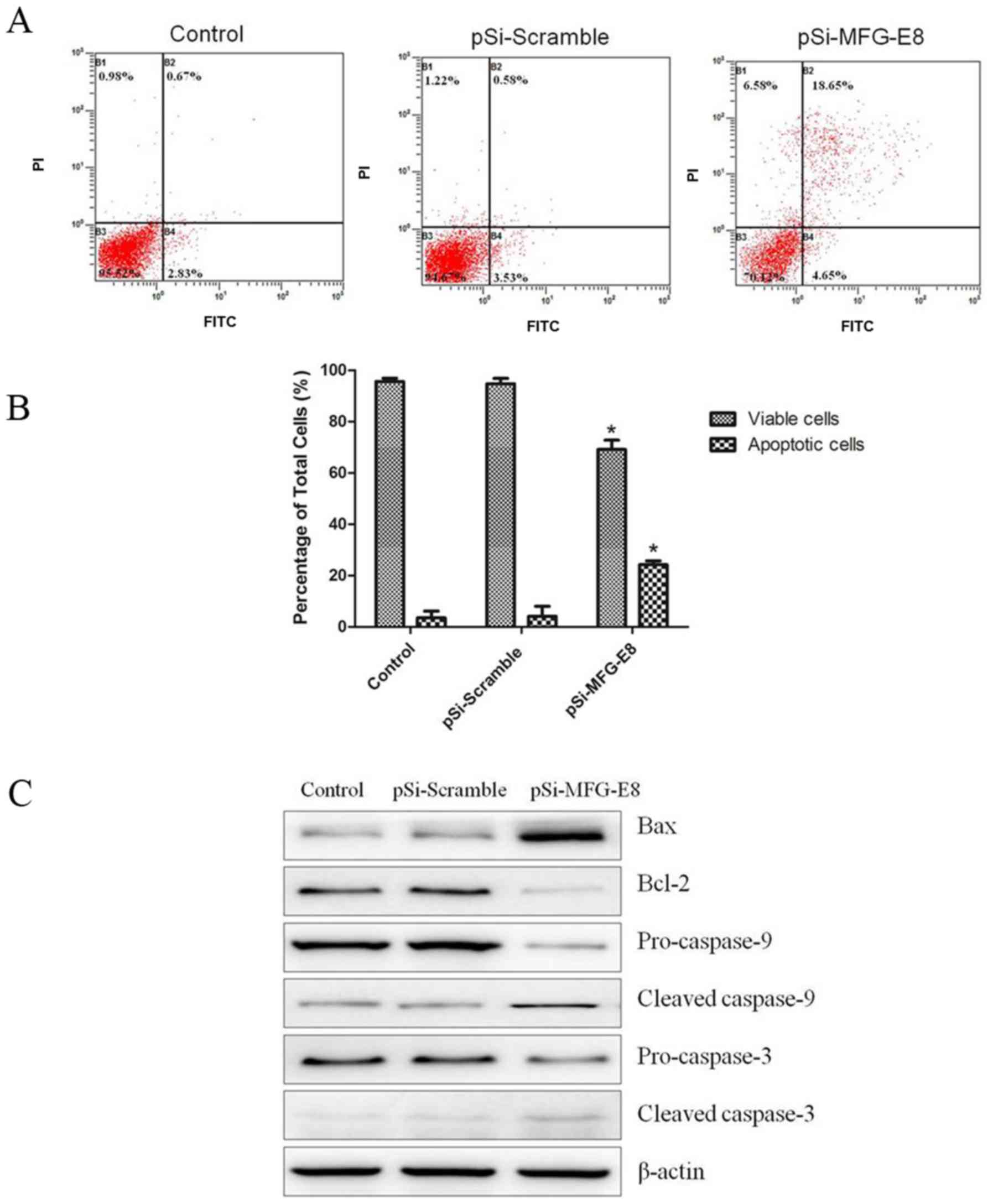 | Figure 6.Knockdown of MFG-E8 in MDA-MB-231
cells induces cell apoptosis. (A) Representative plots of MFG-E8
knockdown-induced apoptosis in MDA-MB-231 cells as determined using
flow cytometry. MDA-MB-231 cells were transfected with pSi-Scramble
or pSi-MFG-E8, and apoptosis were evaluated by flow cytometry using
FITC and PI staining 48 h after transfection. (B) The percentage of
viable and apoptotic cells as presented as the mean ± standard
error. *P<0.05, pSi-MFG-E8 group vs. pSi-Scramble group. (C)
Expression levels of caspase-9, cleaved caspase-9, caspase-3,
cleaved caspase-3, Bcl-2 and Bax in MDA-MB-231 cells after
transfection with pSi-Scramble or pSi-MFG-E8 for 48 h were analyzed
by western blotting. β-actin was used as loading control. Control,
non-transfected cells; pSi-Scramble, cells transfected with control
shRNA lentiviral vector; pSi-MFG-E8, cells transfected with control
shRNA-MFG-E8 lentiviral vector; MFG-E8, Milk fat globule-EGF factor
8; shRNA, short hairpin RNA; Bcl-2, B-cell lymphoma 2; Bax,
Bcl-2-associated X protein; FITC, fluorescein isothiocyanate; PI,
propidium iodide. |
Discussion
Breast cancer is the most frequently diagnosed
cancer and the global leading cause of cancer-associated mortality
in women (1). Furthermore, it
accounts for 23% of the total number of new cancer cases and 14% of
the total number of cancer mortalities in the USA (31). In the past, numerous studies have
examined the cellular changes in the different types of breast
cancer cells. MFG-E8 is a glycoprotein that is expressed in several
cell types and human malignancies (32). Previous studies have indicated that
the function and expression of MFG-E8 depends on the subtype of
human breast carcinoma. MFG-E8 expression is decreased in
ER-positive and erbB2-positive human cancer, and may serve a
suppressive function in these types. In contrast, MFG-E8 is highly
expressed in TNBC cell lines and patient sera (15). However, as the expression profiles and
functions of MFG-E8 in TNBC cells have not yet been thoroughly
analyzed, further studies are required (33).
The aim of the present study was to explore the role
of MFG-E8 in TNBC cells and examine the underlying molecular
mechanisms. Western blotting and RT-qPCR were used to detect the
expression levels of MFG-E8 in different human breast carcinoma
cell lines. The gene expression data revealed that MFG-E8 was
highly expressed in TNBC cells, including MDA-MB-231 (34,35),
compared with in other cell lines. A MFG-E8 siRNA lentiviral vector
was constructed and transfected it into MDA-MB-231 cells. It was
confirmed that the expression of MFG-E8 mRNA and protein was
effectively downregulated in MDA-MB-231 cells following
transfection. Biological methods were used to evaluate the
consequences of MFG-E8 downregulation in breast cancer cells. The
morphological changes were observed using an inverted microscope
that demonstrated cell shrinkage and deformation in the breast
cancer MDA-MB-231 cells transfected with pSi-MFG-E8, with a
time-dependent increase in the number of round, and detached cells.
This was consistent with the features of apoptosis and suggested
that the transfection of pSi-MFG-E8 was effective in the
downregulation of MFG-E8 expression, and the induction of breast
cancer cell death. The viability of MFG-E8 siRNA-transduced cells
was significantly inhibited, which indicated that MFG-E8
downregulation impaired cell growth in vitro. The inhibition
of MFG-E8 induced G2/M cell cycle arrest. The effect of MGF-E8 on
MDA-MB-231 cell migration and invasion was assessed using a
Transwell assay, and the results revealed that knockdown of MFG-E8
significantly inhibited the migration and invasion of MDA-MB-231
cells compared with the control groups. To further establish the
effect of MFG-E8 on cell migration and invasion, western blotting
and ELISA were used to detect the expression of migration and
invasion-associated proteins. The expression of N-cadherin,
vimentin, MMP-2 and MMP-9 was markedly downregulated in the MFG-E8
downregulation group, and the expression of E-cadherin was markedly
upregulated compared with the control groups. In addition, the
levels of MMP-9 and MMP-2 was markedly decreased, which was
confirmed by the results of the ELISA compared with the control
groups. The results demonstrated that the repression of MFG-E8 by
siRNA significantly affected MDAMB-231 cell cycle progression and
cell invasion activity via key proteins in the cell cycle and
invasion associated pathway.
Apoptosis is an active process of cellular
self-destruction, to explore the mechanism underlying MDA-MB-231
cell apoptosis induced by MFG-E8 downregulation, the expression of
apoptosis-associated proteins were analyzed by western blotting.
After MFG-E8 knockdown in MDA-MB-231 cells, the proportion
apoptotic cells were significantly higher in the pSi-MFG-E8 group
compared with in the pSi-Scramble and control groups. After MFG-E8
silencing, the expression of Bax, cleaved caspase-3, and cleaved
caspase-9 in the pSi-MFG-E8 group was markedly upregulated, whereas
the protein expression of Bcl-2, caspase-3, and capase-9 was
markedly reduced. The downregulation of caspase-3 and the
upregulation of cleaved caspase-3 provided supporting evidence of
apoptosis, thus suggesting that the knockdown of MFG-E8 induced
cell apoptosis through an increase in the expression of
apoptosis-associated proteins. These results indicated that the
MFG-E8 lentivirus siRNA decreased the viability of MDA-MB-231 cells
through the induction of apoptosis. This study explored the effect
of MFG-E8 on TNBC cell viability, invasion, migration and apoptosis
in only one TNBC cell line, MDA-MB-231. Thus, we will further
investigate the effects of MFG-E8 on proliferation, invasion,
migration and apoptosis in other TNBC cell lines in subsequent
studies to confirm the results of the present study.
In conclusion, MFG-E8 interference significantly
suppressed the viability, migration, invasion of MDA-MB-231cells,
and caused cell cycle arrest at the G2/M phase, and
ultimately leading to apoptosis. The data indicated that MFG-E8
expression was significantly associated with the viability and
invasive potential of TNBC. Furthermore, the inhibition of MFG-E8
may provide a novel target for the prevention and treatment of
human breast carcinoma. In this study, the biological consequences
of MFG-E8 downregulation in breast cancer cells were focused upon,
but further in vivo and in vitro experiments are
required to uncover the mechanisms of differential gene regulation
in the pathogenesis of human breast carcinoma and provided
potential targets associated with MFG-E8 for novel strategies for
clinical treatment with human breast carcinoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Key Scientific Research Project of Wuhan City Health and Family
Planning Commission (grant no. WX16B05).
Availability of data and materials
All datasets used during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
YY performed the lentivirus production,
oligonucleotide transfection and assessed the proliferation of
cells using an MTT assay and was a major contributor in writing the
manuscript. JL analyzed the data regarding cell proliferation,
expression of associated mRNA and proteins, cell cycle, apoptosis
and cell invasion activity. QS conducted the cell experiments
including the expression of associated mRNA and proteins using
RT-qPCR and western blotting. KZ performed cell cycle and apoptosis
analysis using flow cytometry. XY performed the cell migration and
invasion analysis using Transwell assay. YT contributed the
conception and design of the present study. JZ was involved in
designing the experiment protocol, all data analysis, drafting the
manuscript and revising it critically for important intellectual
content, giving final approval of the version to be published and
was responsible for the acquisition of funding. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ren J, Li J, Pfeiffer RM, Wang Y,
Guida JL, Fang Y, Shi J, Zhang K, Li N, et al: Breast cancer risk
factors and mammographic density among high-risk women in urban
China. NPJ Breast Cancer. 4:32018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carey LA, Metzger R, Dees EC, Collichio F,
Sartor CI, Ollila DW, Klauber-DeMore N, Halle J, Sawyer L, Moore DT
and Graham ML: American Joint Committee on cancer
tumor-node-metastasis stage after neoadjuvant chemotherapy and
breast cancer outcome. J Natl Cancer Inst. 97:1137–1142. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Steeg PS: Tumor metastasis: Mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ceriani RL, Peterson JA, Lee JY, Moncada R
and Blank EW: Characterization of cell surface antigens of human
mammary epithelial cells with monoclonal antibodies prepared
against human milk fat globule. Somatic Cell Genet. 9:415–427.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Raymond A, Ensslin MA and Shur BD:
SED1/MFG-E8: A bi-motif protein that orchestrates diverse cellular
interactions. J Cell Biochem. 106:957–966. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Taylor MR, Couto JR, Scallan CD, Ceriani
RL and Peterson JA: Lactadherin (formerly BA46), a
membrane-associated glycoprotein expressed in human milk and breast
carcinomas, promotes Arg-Gly-Asp (RGD)-dependent cell adhesion. DNA
Cell Biol. 16:861–869. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ogura K, Nara K, Watanabe Y, Kohno K, Tai
T and Sanai Y: Cloning and expression of cDNA for O-acetylation of
GD3 ganglioside. Biochem Biophys Res Commun. 225:932–938. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fens MH, Mastrobattista E, de Graaff AM,
Flesch FM, Ultee A, Rasmussen JT, Molema G, Storm G and Schiffelers
RM: Angiogenic endothelium shows lactadherin-dependent phagocytosis
of aged erythrocytes and apoptotic cells. Blood. 111:4542–4550.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Atabai K, Fernandez R, Huang X, Ueki I,
Kline A, Li Y, Sadatmansoori S, Smith-Steinhart C, Zhu W, Pytela R,
et al: Mfge8 is critical for mammary gland remodeling during
involution. Mol Biol Cell. 16:5528–5537. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leonardi-Essmann F, Emig M, Kitamura Y,
Spanagel R and Gebicke-Haerter PJ: Fractalkine-upregulated milk-fat
globule EGF factor-8 protein in cultured rat microglia. J
Neuroimmunol. 160:92–101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Asano K, Miwa M, Miwa K, Hanayama R,
Nagase H, Nagata S and Tanaka M: Masking of phosphatidylserine
inhibits apoptotic cell engulfment and induces autoantibody
production in mice. J Exp Med. 200:459–467. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bu HF, Zuo XL, Wang X, Ensslin MA, Koti V,
Hsueh W, Raymond AS, Shur BD and Tan XD: Milk fat globule-EGF
factor 8/lactadherin plays a crucial role in maintenance and repair
of murine intestinal epithelium. J Clin Invest. 117:3673–3683.
2007.PubMed/NCBI
|
|
14
|
Nandrot EF, Anand M, Almeida D, Atabai K,
Sheppard D and Finnemann SC: Essential role for MFG-E8 as ligand
for alphavbeta5 integrin in diurnal retinal phagocytosis. Proc Natl
Acad Sci USA. 104:12005–12010. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carrascosa C, Obula RG, Missiaglia E, Lehr
HA, Delorenzi M, Frattini M, Rüegg C and Mariotti A:
MFG-E8/lactadherin regulates cyclins D1/D3 expression and enhances
the tumorigenic potential of mammary epithelial cells. Oncogene.
31:1521–1532. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ceriani RL, Sasaki M, Sussman H, Wara WM
and Blank EW: Circulating human mammary epithelial antigens in
breast cancer. Proc Natl Acad Sci USA. 79:5420–5424. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ceriani RL, Thompson K, Peterson JA and
Abraham S: Surface differentiation antigens of human mammary
epithelial cells carried on the human milk fat globule. Proc Natl
Acad Sci USA. 74:582–586. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peterson JA, Zava DT, Duwe AK, Blank EW,
Battifora H and Ceriani RL: Biochemical and histological
characterization of antigens preferentially expressed on the
surface and cytoplasm of breast carcinoma cells identified by
monoclonal antibodies against the human milk fat globule.
Hybridoma. 9:221–235. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Larocca D, Peterson JA, Urrea R, Kuniyoshi
J, Bistrain AM and Ceriani RL: A Mr 46,000 human milk fat globule
protein that is highly expressed in human breast tumors contains
factor VIII-like domains. Cancer Res. 51:4994–4998. 1991.PubMed/NCBI
|
|
20
|
Couto JR, Blank EW, Peterson JA and
Ceriani RL: Anti-BA46 monoclonal antibody Mc3: Humanization using a
novel positional consensus and in vivo and in vitro
characterization. Cancer Res. 55:1717–1722. 1995.PubMed/NCBI
|
|
21
|
Ceriani RL and Blank EW: Experimental
therapy of human breast tumors with 131I-labeled monoclonal
antibodies prepared against the human milk fat globule. Cancer Res.
48:4664–4672. 1988.PubMed/NCBI
|
|
22
|
Ceriani RL, Blank EW, Couto JR and
Peterson JA: Biological activity of two humanized antibodies
against two different breast cancer antigens and comparison to
their original murine forms. Cancer Res. 55 Suppl 23:S5852–S5856.
1995.
|
|
23
|
Yamaguchi H, Takagi J, Miyamae T, Yokota
S, Fujimoto T, Nakamura S, Ohshima S, Naka T and Nagata S: Milk fat
globule EGF factor 8 in the serum of human patients of systemic
lupus erythematosus. J Leukoc Biol. 83:1300–1307. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Atabai K, Jame S, Azhar N, Kuo A, Lam M,
McKleroy W, Dehart G, Rahman S, Xia DD, Melton AC, et al: Mfge8
diminishes the severity of tissue fibrosis in mice by binding and
targeting collagen for uptake by macrophages. J Clin Invest.
119:3713–3722. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jinushi M, Nakazaki Y, Carrasco DR,
Draganov D, Souders N, Johnson M, Mihm MC and Dranoff G: Milk fat
globule EGF-8 promotes melanoma progression through coordinated Akt
and twist signaling in the tumor microenvironment. Cancer Res.
68:8889–8898. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Matsuda A, Jacob A, Wu R, Zhou M, Nicastro
JM, Coppa GF and Wang P: Milk fat globule-EGF factor VIII in sepsis
and ischemia-reperfusion injury. Mol Med. 17:126–133. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang C, Hayashida T, Forster N, Li C, Shen
D, Maheswaran S, Chen L, Anderson KS, Ellisen LW, Sgroi D and
Schmidt EV: The integrin alpha(v)beta(3–5) ligand MFG-E8 is a
p63/p73 target gene in triple-negative breast cancers but exhibits
suppressive functions in ER(+) and erbB2(+) breast cancers. Cancer
Res. 71:937–945. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Richardson AL, Wang ZC, De Nicolo A, Lu X,
Brown M, Miron A, Liao X, Iglehart JD, Livingston DM and Ganesan S:
X chromosomal abnormalities in basal-like human breast cancer.
Cancer Cell. 9:121–132. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang X, Sang X, Diorio C, Lin SX and
Doillon CJ: In vitro interactions between mammary fibroblasts (Hs
578Bst) and cancer epithelial cells (MCF-7) modulate aromatase,
steroid sulfatase and 17β-hydroxysteroid dehydrogenases. Mol Cell
Endocrinol. 412:339–348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hou L, Chen M, Zhao X, Li J, Deng S, Hu J,
Yang H and Jiang J: FAT4 functions as a tumor suppressor in
triple-negative breast cancer. Tumour Biol. Nov 28–2016.(Epub ahead
of print). View Article : Google Scholar
|
|
33
|
Tomao F, Papa A, Zaccarelli E, Rossi L,
Caruso D, Minozzi M, Vici P, Frati L and Tomao S: Triple-negative
breast cancer: New perspectives for targeted therapies. OncoTargets
Ther. 8:177–193. 2015. View Article : Google Scholar
|
|
34
|
Rahman NA, Yazan LS, Wibowo A, Ahmat N,
Foo JB, Tor YS, Yeap SK, Razali ZA, Ong YS and Fakurazi S:
Induction of apoptosis and G2/M arrest by ampelopsin E from
Dryobalanops towards triple negative breast cancer cells,
MDA-MB-231. BMC Complement Alternat Med. 16:3542016. View Article : Google Scholar
|
|
35
|
Furuya K, Sasaki A, Tsunoda Y, Tsuji M,
Udaka Y, Oyamada H, Tsuchiya H and Oguchi K: Eribulin upregulates
miR-195 expression and downregulates Wnt3a expression in
non-basal-like type of triple-negative breast cancer cell
MDA-MB-231. Hum Cell. 29:76–82. 2016. View Article : Google Scholar : PubMed/NCBI
|