Introduction
According to the latest cancer data, prostate cancer
(PCa) has the highest incidence of cancers among male tumors
worldwide. New cases in the United States in 2017 accounted for 19%
of total malignancies (1). Although
the treatment of PCa has achieved curative effect, and the 5-year
survival rate of PCa is relatively optimistic compared to other
malignant tumors (2–4), there are still many problems that need
to be solved in the diagnosis and treatment of PCa. Finding more
effective biological diagnostic and therapeutic targets remains a
hot topic in PCa research (5,6).
MicroRNAs are a type of specific RNA molecules of
20–22 nucleotides in length that can inhibit the expression of
target genes through specific binding to the 3′-untranslated region
(3′-UTR) of their target genes, thereby exerting their role in
regulating various molecular biological processes (7,8). In
tumors, miRNAs affect the biological behavior in many aspects such
as tumor occurrence, metastasis, invasion, microenvironment, and
autophagy (9–11). Several miRNAs have been identified to
be involved in the development and progression of PCa. miR-34a
could inhibit PCa stem cells and invasion directly through
repressing the expression of CD44, miR-195 targets RPS6KB1,
inhibits PCa proliferation and metastasis, and also, miR-409-3p/-5p
promotes the development, and metastasis to bone via
epithelial-to-mesenchymal transition (12–14). In
addition, miR-940 has been shown to suppress PCa cell invasion and
migration via inhibiting the expression of MIEN1 (15).
miR-1291 has been reported to be involved in the
regulation of multiple cancers. In different types of cancers, it
could regulate the growth and metastasis of tumor cells by
regulating different specific target genes. For example, in
pancreatic cancer, it suppresses tumorigenesis and cell growth via
targeting the FOXA2-AGR2 axis; in esophageal squamous cell cancer,
it inhibits cell proliferation and invasion through mucin 1 and
accelerates cell apoptosis; in renal cell carcinoma, it functions
as a tumor suppressor by regulating glucose transporter 1 (16–18). Also,
together with miR-133, miR-1291 acts as angio-miR to modulate HUVEC
angiogenesis (19). However, in PCa,
the expression and role of miR-1291 have not been studied yet.
In this study, we first detected the expression
level of miR-1291 in surgically removed PCa tissues compared to
adjacent normal tissues. Also, the expression of miR-1291 in PCa
cell lines was measured. With
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay and cell cycle detection, we demonstrated that miR-1291 could
affect the cell proliferation of PCa cells. Moreover, MED1 was
identified as a direct target for miR-1291 in PCa. Taken together,
miR-1291 was found to act as a tumor suppressor in PCa via MED1 and
has tpotential to be a diagnostic and therapeutic target.
Materials and methods
Patients and tissue samples
All 98 paired PCa tissues and adjacent normal
tissues were obtained from male patients (72.1±6.3 years of age)
who underwent surgical treatment in Tongde Hospital of Zhejiang
Province (Hangzhou, China) between June 2014 and August 2017. None
of the patients received preoperative radiotherapy or chemotherapy.
All samples were immediately stored in liquid nitrogen after
excision. The experiments were approved by the Ethics Committee of
Tongde Hospital of Zhejiang Province and all the patients or the
guardians signed an informed consent.
Cell lines and culture
The four cell lines, including three PCa-derived
cell lines DU-145 (cat. no. BNCC338240), PC3 (cat. no. BNCC337715),
LNCaP (cat. no. BNCC337702) and one normal prostate epithelial cell
line RWPE-1 (cat. no. BNCC100292), were obtained from BeNa Culture
Collection Co. (Beijing, China; http://www.bnbio.com/). Dulbecco's modified Eagles
medium (DMEM; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (FBS) and 1%
penicillin-streptomycin solution (both from Gibco; Thermo Fisher
Scientific, Inc., Rockville, MD, USA) was utilized to maintain the
cells. The cells were cultured in moist air at 37°C containing 5%
CO2.
RNA isolation and reverse transcription-quantitative
polymerase chain reaction (RT-qPCR). For PCa and normal tissues, a
total of 1 ml TRIzol solution (Invitrogen; Thermo Fisher
Scientific, Inc.) was added for lysis. For experimental cells,
3×105 cells were lysed by adding 1 ml TRIzol solution.
The total RNA was measured for purity concentration by a UV
spectrophotometer (Hitachi, Ltd., Tokyo, Japan) and stored at
−80°C.
The PrimeScript RT reagent (Takara Bio, Inc.,
Kusatsu, Japan) was used to perform reverse transcription according
to the manufacturer's instructions. The SYBR-Green Master Mix I
(Takara Bio, Inc.) was employed to perform the RT-qPCR using the
ABI 7500 Fast Real-Time PCR System (Applied Biosystems, Inc.;
Thermo Fisher Scientific, Inc., Foster City, CA, USA). U6 was
applied as internal control for all miRNA samples and GAPDH for all
mRNA samples. The reaction steps were as follows: pre-denaturation
for 30 sec at 95°C; followed by 45 cycles of 5 sec at 95°C and 30
sec at 60°C per cycle, and finally a dissolution medium was added.
The relative expression levels were measured using the
2−ΔΔCq method (20). All
primer probes were designed by Guangzhou RiboBio Co., Ltd.
(Guangzhou, China). Each experiment was performed in triplicate.
The primers sequences used were as follows: MED1 forward,
5′-CCTTTAGAAAGGCAGAACTCCTCTTCCGGATCACCCCGG-3′ and reverse,
5′-CCGGGGTGATCCGGAAGAGGAGTTCTGCCTTTCTAAAGG-3′; miR-1291 forward,
5′-ACACTCCAGCTGGGTGGCCCTGACTGAAGACC-3′ and reverse,
5′-TGGTGTCGTGGAGTCG-3′; GAPDH forward, 5′-TGACTTCAACAGCGACACCCA-3′
and reverse, 5′-GGAGTGTTGGAGAAGTCATATTAC-3′; U6 forward,
5′-CTCGCTTCGGCAGCACATAT-3′ and reverse,
5′-TTGCGTGTCATCCTTGCG-3′.
Cell transfection of miR-1291 and
pcDNA-MED1
miR-1291 mimics, negative control (NC), inhibitors,
inhibitors negative control (INC) and pcDNA-MED1 were designed and
synthesized by the Guangzhou RiboBio Co., Ltd. For transfection,
appropriate amount of cells was planted in a 6-well plate. When the
confluence reached 50–60%, the appropriate amount of miR-1291
mimics, NC, inhibitors, INC or pcDNA-MED1, were added, using
Lipofectamine (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. The efficiency of
transfection was determined using RT-qPCR.
MTT assay
MTT assay was applied to detect cell proliferation.
A total of 3.5×103 cells were seeded into 96-well plates
per well after treatment with miR-1291 mimics, NC, inhibitors or
INC. At 0, 24, 48 and 72 h, 0.5 mg/ml MTT buffer (Thermo Fisher
Scientific, Inc.) was added per well and cells were cultured for 2
h in darkness. Next, the absorbance at 490 nm was detected using a
spectrophotometer (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The experiment was repeated 3 times.
Cell cycle detection
Cell cycle distribution was detected using a flow
cytometer (FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA).
DU-145 and LNCaP cells after miR-1291 mimics or inhibitor treatment
were harvested and washed with phosphate-buffered saline (PBS)
buffer. After centrifugation, at 20°C for 5 min at 1,500 × g, cells
were re-suspended in 500 µl binding buffer containing 1% propidium
iodide (PI; Vazyme, Nanjing, China). Human TruStain FcX™ (cat. no.
422301; BioLegend, Inc., San Diego, CA, USA), used as the blocking
solution, was added to the cells for incubation at room temperature
for 5 min. Then, the cell cycle distribution was measured and
recorded. Data were analyzed using the CellQuest Pro software
(version 3.3; BD Biosciences).
Luciferase assay
The constructed 3′-UTR sequence containing wild-type
or mutated miR-1291 binding sequence of MED1 was inserted into the
pLG3 promoter vector (Promega Corpo., Madison, WI, USA),
respectively (pLG3-MED1-WT or pLG3-MED1-Mutant). DU-145 cells were
seeded in 6-well plates and transfected with pLG3-MED1-WT or
pLG3-MED1-Mutant, miR-1291 mimics and NC using Lipofectamine 3000
(Invitrogen; Thermo Fisher Scientific, Inc.). After 48 h, the
relative luciferase activity was measured using the Luciferase
assay kit (Promega Corp.).
Western blot analysis
Protein was extracted using the RIPA solution
containing 0.5 M EDTA, protease inhibitors and phosphatase
inhibitors (both from Beyotime Institute of Biotechnology,
Shanghai, China). Then, the protein sample was mixed with SDS-PAGE
protein loading buffer (Beyotime Institute of Biotechnology) in a
ratio of 1:4. The sample was then placed in boiling water for 5
min. A total of 30 µg of protein were loaded per lane for the
electrophoresis. Proteins were separated by 10% SDS-PAGE and
transferred to polyvinylidene fluoride (PVDF) membranes (EMD
Millipore, Billerica, MA, USA). After being blocked with 5% BSA for
2 h, the membranes were incubated at 4°C with specific primary
antibodies. Rabbit polyclonal MED1 antibody (dilution, 1:1,000;
cat. no. ab64965) and rabbit polyclonal GAPDH antibody (dilution,
1:2,000; cat. no. ab37168) were purchased from Abcam (Cambridge,
MA, USA). Membranes were then incubated with secondary goat
anti-rabbit (HRP) IgG antibody (dilution, 1:2,000; cat. no. ab6721;
Abcam) for 2 h at room temperature and then washed 3 times with
TBST (Beyotime Institute of Biotechnology). The secondary antibody
was detected with an enhanced chemiluminescence (ECL) system
(Pierce Biotechnology, Inc.; Thermo Fisher Scientific, Inc.,
Rockford, IL, USA). The experiments were performed in triplicate.
The gray value was analyzed using ImageJ software (version 1.38;
National Institutes of Health, Bethesda, MD, USA).
Xenograft assay
Thirty nude male mice, weighing 18–22 g, were
purchased from the Beijing Weitong Lihua Experimental Animal
Technology Co., Ltd. (Beijing, China) to be used in the xenograft
model. The mice were housed in a temperature controlled room
(21±2°C), on a 12:12-h light/dark cycle (lights on at 06:00), and
had free access to water and food. The experiment was approved by
the Ethics Committee of Tongde Hospital of Zhejiang Province. The
5–6 weeks-old mice were injected with 1×106 miR-1291
mimics or NC treated DU-145 cells. The cells were re-suspended in
100 µl PBS and injected subcutaneously on the flank of the mouse.
Every week, the length and width of the xenograft tumors were
measured and their volume was calculated using the formula 0.5 ×
Length × Width2. After 5 weeks, mice were sacrificed and
the tumors were removed and weighed. Then, the mRNA and protein of
xenografts were extracted, and immunohistochemistry (IHC) was used
to detect the expression of MED1.
IHC analyses of MED1
Xenograft tissues were formalin fixed and paraffin
embedded, and then, 4-mm sections were mounted on slides. After
antigen retrieval and non-specific serum closure, the slides were
attained with rabbit polyclonal MED1 antibody (dilution, 1:100;
cat. no. ab64965; Abcam) and then incubated for 2 h at 37°C. After
incubation with secondary goat anti-rabbit (HRP) IgG antibody
(dilution, 1:500; cat. no. ab6721; Abcam) for 1 h at 37°C, DAB
reagents (Guangzhou RiboBio Co., Ltd.) and hematoxylin were used
for color development. The expression of MED1 in xenograft tumor
sections was described and photographed using light microscopy
(BX-42; Olympus Corp., Tokyo, Japan) with a magnification of
×400.
Statistical analysis
t-test and ANOVA test, followed by the Least
Significant Difference post hoc test, were realized by Statistical
Product and Service Solutions (SPSS) 18.0 version software (SPSS,
Inc., Chicago, IL, USA) and GraphPad prism version 6.0 software
(GraphPad Software, Inc., La Jolla, CA, USA) to analyze the
differences. Pearson's correlation test was used to investigate the
correlation between miR-1291 expression and the MED1 mRNA level.
All the results are expressed as mean ± SD. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-1291 is downregulated in PCa
tissues and cell lines
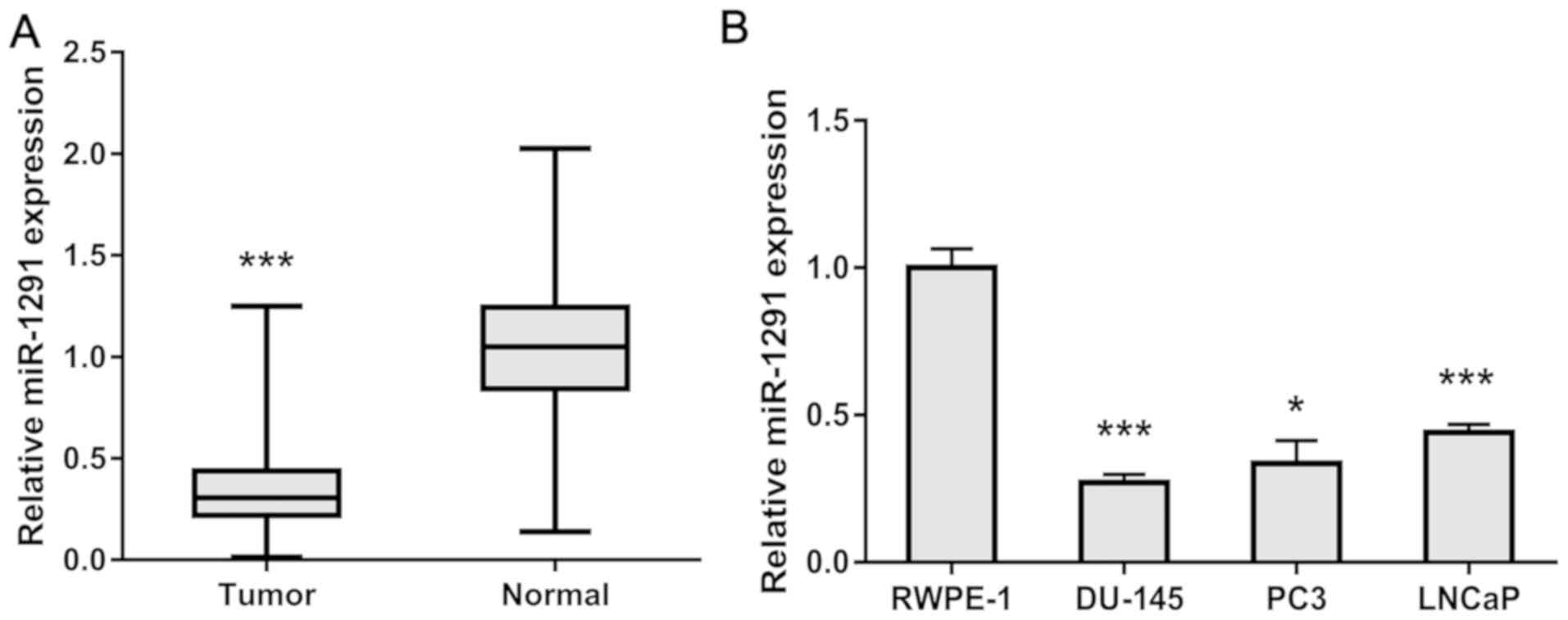
To study the relationship of miR-1291 and PCa, we
detected the expression level of miR-1291 in 98 paired PCa tissues
and adjacent normal tissues. As shown in Fig. 1A, the expression level of miR-1291 in
PCa tissues was significantly lower than that in adjacent normal
prostate tissues. Also, we obtained three PCa-derived cell lines
and measured their miR-1291 level compared to the normal prostate
epithelial cell line RWPE-1. The expression of miR-1291 in PCa
cells was obviously lower than RWPE-1 cells (Fig. 1B). These results indicate that
miR-1291 might act as a tumor suppressor in PCa.
Ectopic miR-1291 effect on cell
proliferation and cell cycle of PCa
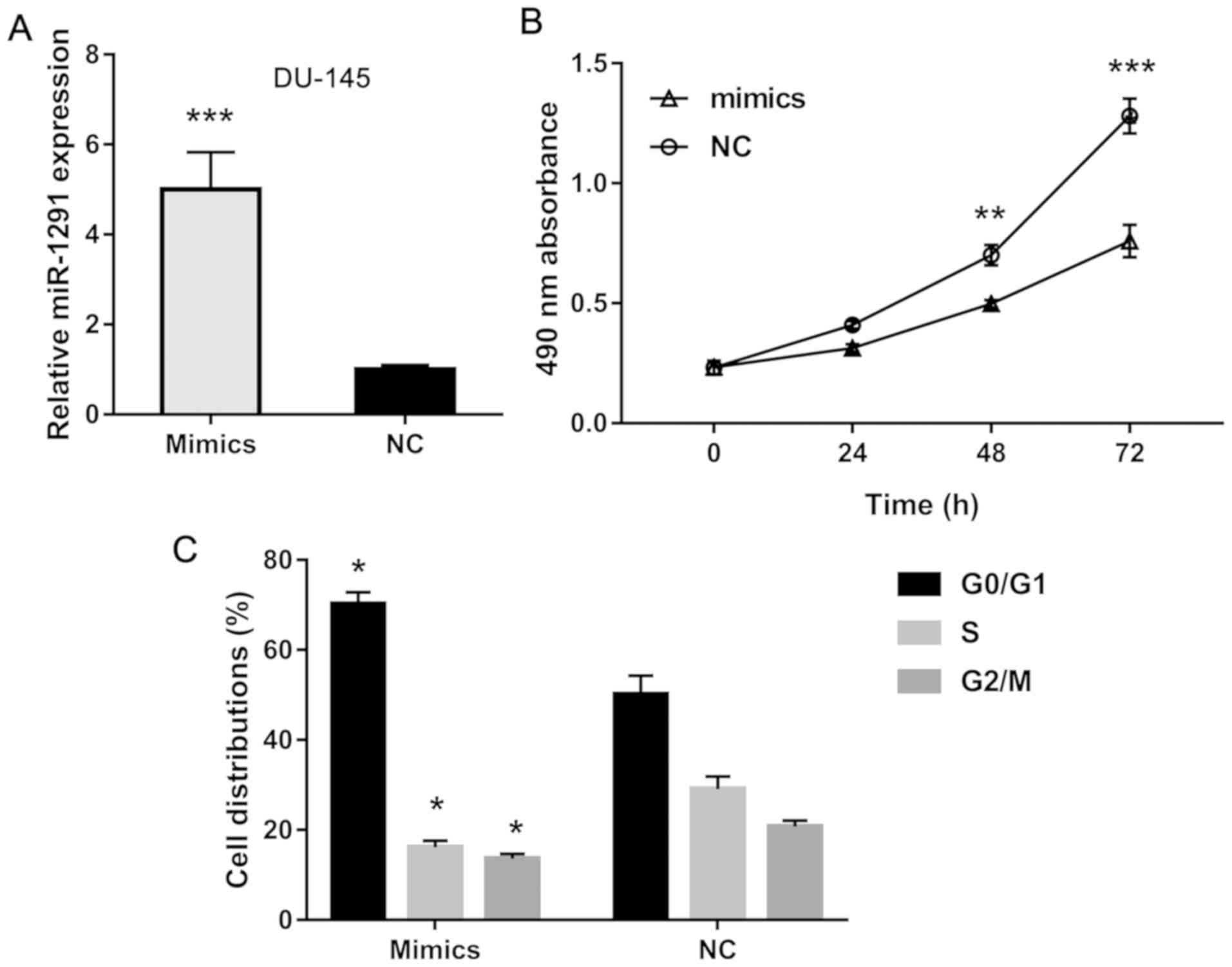
To further study the effects of miR-1291 on PCa
cells, we established miR-1291 up- and downregulated cells using
miR-1291 mimics and inhibitors. DU-145 cells transfected with
miR-1291 mimics showed an increased miR-1291 level compared to the
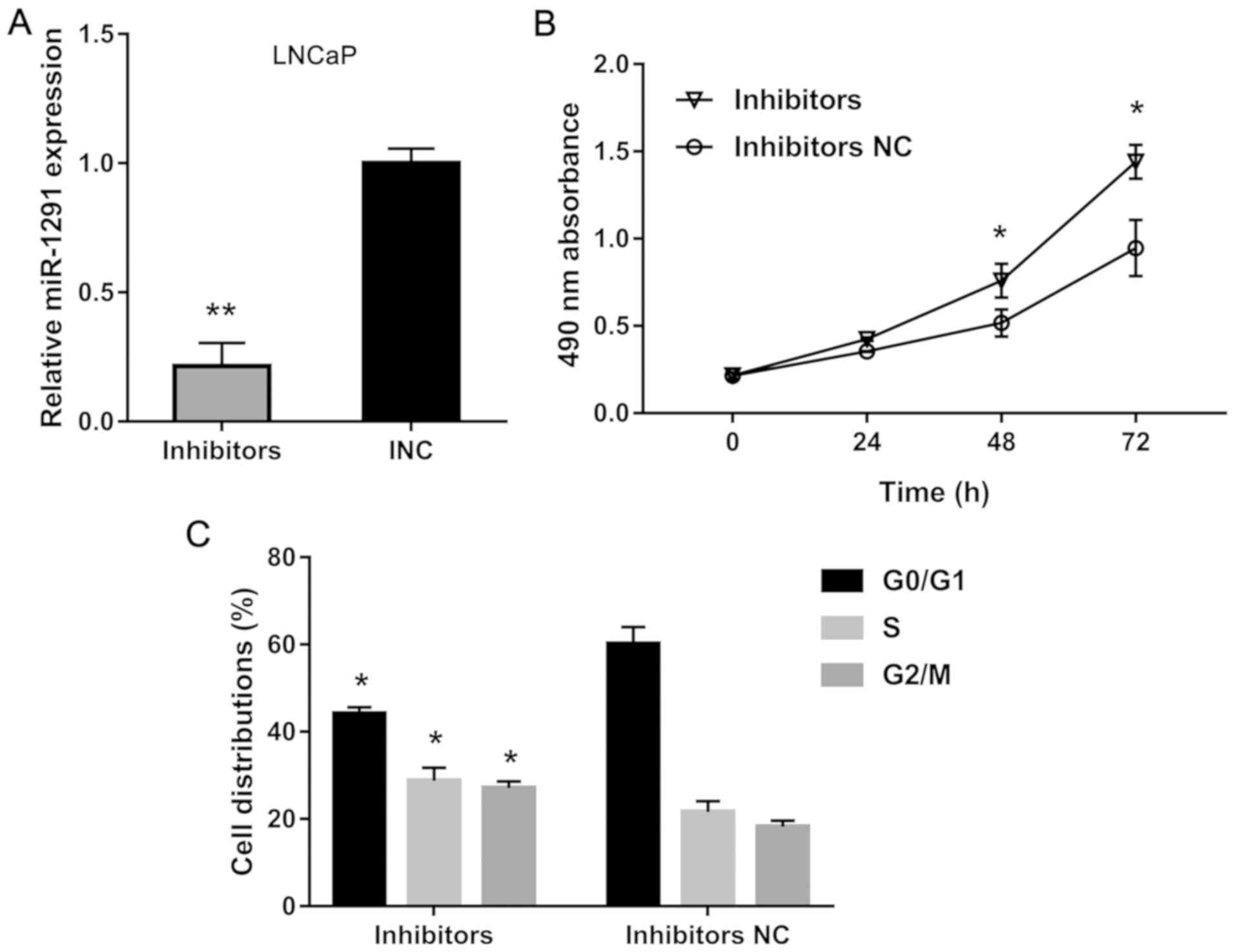
NC group (Fig. 2A), while LNCaP cells
transfected with miR-1291 inhibitors showed a decreased miR-1291
level compared to the INC group (Fig.
3A). Using MTT assay, we explored the cell proliferation
ability and found that overexpression of miR-1291 obviously
inhibits cell growth of DU-145 cells (Fig. 2B), but knockdown of miR-1291 promotes
the proliferation of LNCaP cells (Fig.
3B). Furthermore, flow cytometry detection revealed that
miR-1291 mimics induces cell cycle arrest in G0/G1 phase while
miR-1291 inhibitors promote cell cycle transition from G0/G1 phase
to S and G2/M phase. These data indicate that miR-1291 inhibits
cell proliferation and induces cell cycle arrest of PCa cells.
MED1 is a direct target of miR-1291 in
PCa
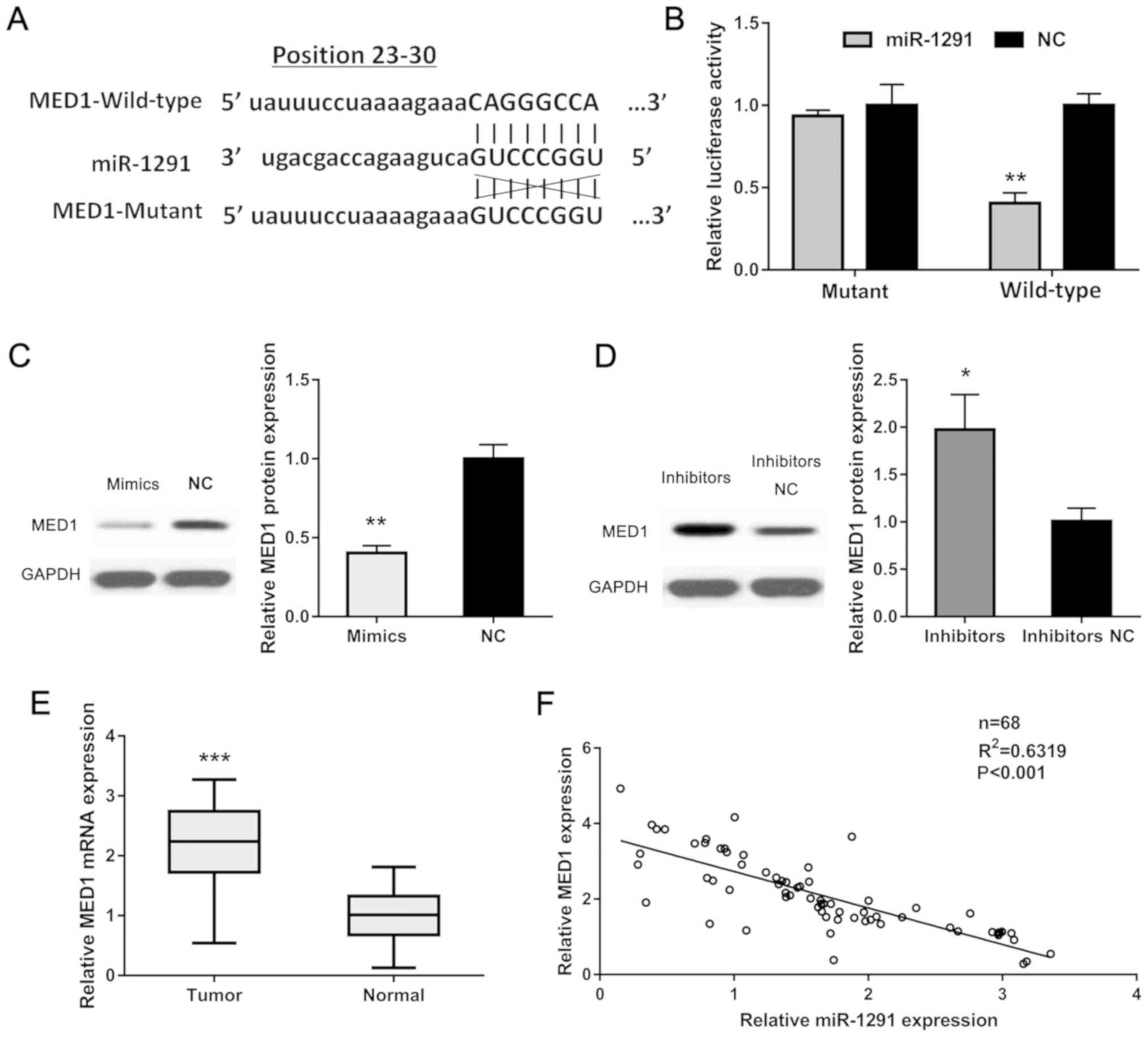
To explain the underlying mechanism of miR-1291 in
PCa, due to miRNAs and whether they play their roles via binding to
the 3′-UTR of their target genes, we searched several databases
including miRWalk (http://mirwalk.umm.uni-heidelberg.de/), TargetScan
(http://www.targetscan.org), PicTar
(https://pictar.mdc-berlin.de/) and
miRanda (http://www.microrna.org/). After
cross-checking, we found MED1 as a potential target for miR-1291.
Using dual-luciferase assay, we employed pLG3 promoter vector
containing wild-type and mutant 3′-UTR of MED1 to verify our
hypothesis (Fig. 4A). The luciferase
activity of the wild-type group showed a markedly decrease while
the mutant group showed no difference compared to each relative
control group (Fig. 4B). We also
detected the MED1 protein level in established cells by western
blot analysis and found that MED1 expression was lower in miR-1291
upregulated DU-145 cells while higher in miR-1291 downregulated
LNCaP cells compared to the relative negative control group
(Fig. 4C and D). Furthermore, the
mRNA level of MED1 in 98 paired PCa tissues was measured and it was
found to be significantly increased than that in the adjacent
normal tissues (Fig. 4E). Moreover,
we analyzed the relationship of miR-1291 and MED1 in PCa tissues
and verified an obvious negative correlation between them
(R2=0.6319, P<0.001). Our results suggest that MED1
as a direct target for miR-1291 in PCa.
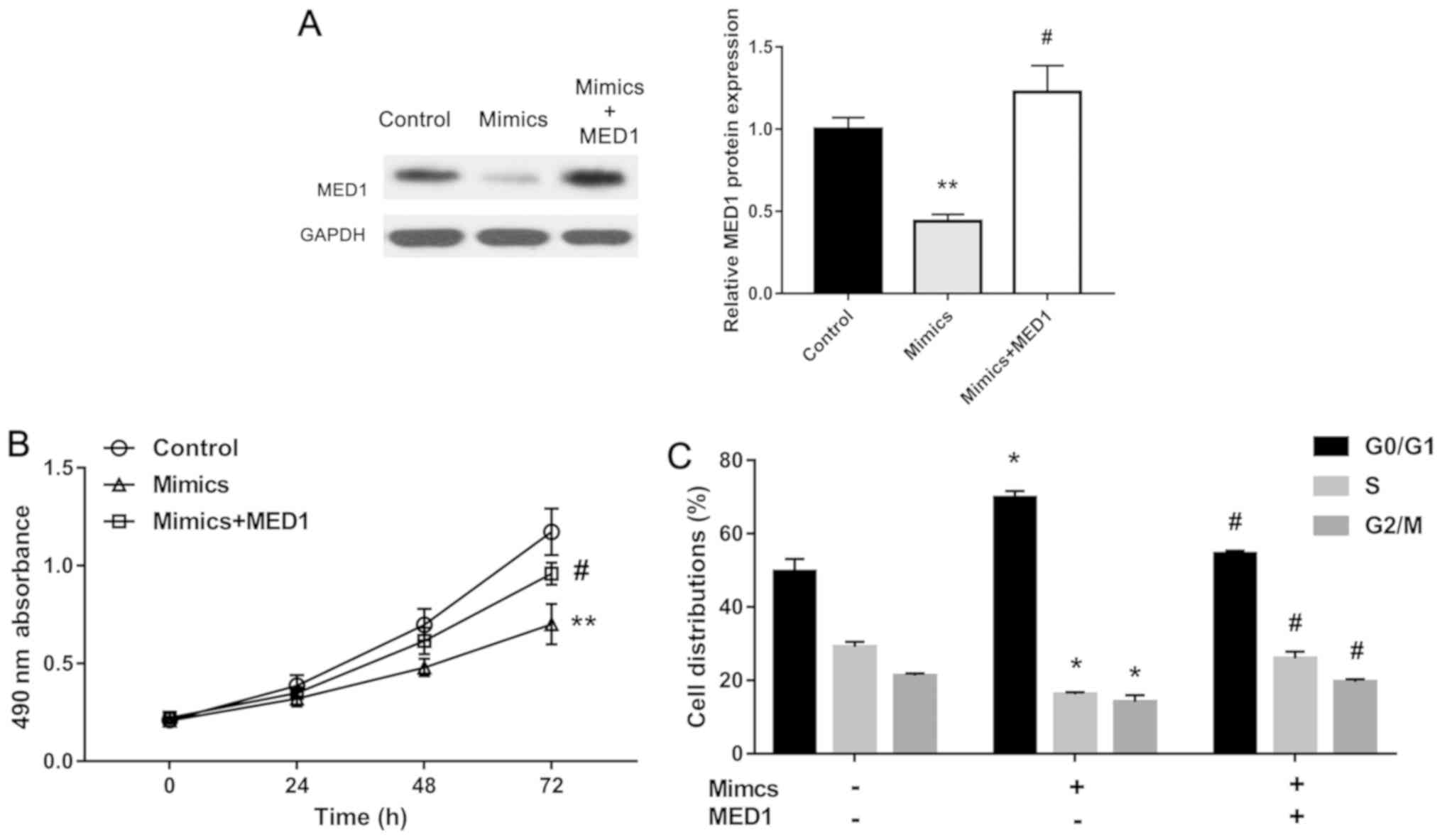
Overexpression of MED1 restored the
effect of miR-1291 upregulation
To further identify our assumption, we overexpressed
MED1 in miR-1291 mimics-treated DU-145 cells. The MED1 protein
level was obviously restored by pcDNA-MED1 (Fig. 5A). Using MTT assay, we found that the
inhibition effect of miR-1291 on cell proliferation was restored by
overexpression of MED1 (Fig. 5B).
Also, upregulation of MED1 reversed the effect of miR-1291 on cell
cycle (Fig. 5C). These results
demonstrate that miR-1291 functions as a tumor suppressor in PCa
via repressing MED1 expression.
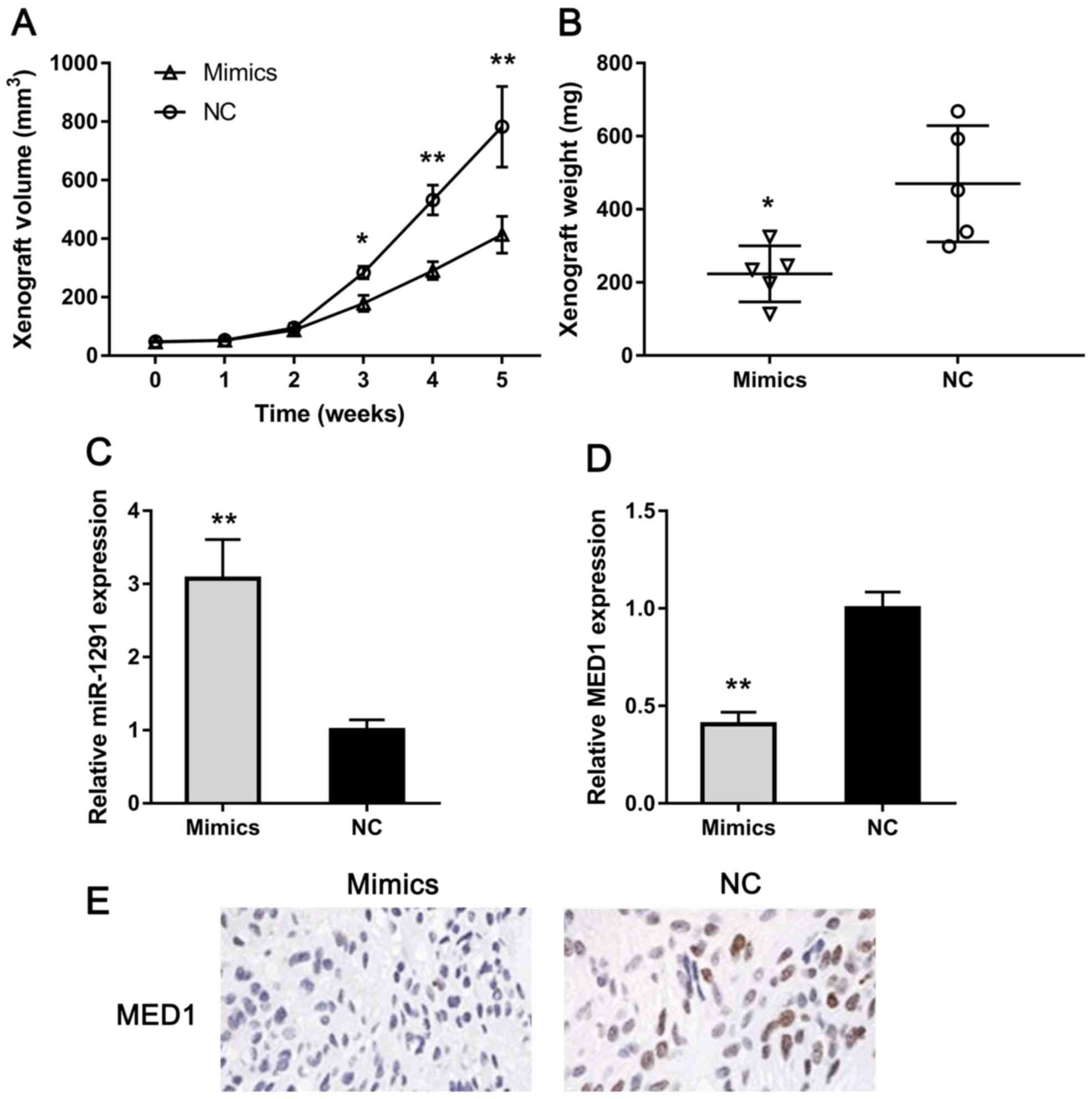
miR-1291 inhibits tumor growth in
vivo
To explore the influence of miR-1291 in PCa in
vivo, we constructed a xenograft model using nude mice. As
clearly shown in Fig. 6A, tumors grew
significantly slower in miR-1291 mimics-treated group than the NC
group. Also, we measured the weight of xenografts and found that
the weight of miR-1291-upregulated group was obviously lower than
that of the control group (Fig. 6B).
Furthermore, the expression of miR-1291 and MED1 was detected using
RT-qPCR. The MED1 expression was remarkably lower in miR-1291
mimics group compared with the NC group (Fig. 6C and D). Also, we detected MED1
protein expression in xenografts using IHC and MED1 level decreased
in the miR-1291 upregulation group (Fig.
6E). These results indicate that miR-1291 could inhibit PCa
cell growth in vivo via downregulating MED1.
Discussion
With the increase of the incidence of PCa, exploring
the pathogenesis and progression of PCa has become an increasingly
important research topic (3). In the
present study we identified for the first time, up to our
knowledge, that miR-1291 is significantly downregulated in PCa
tissues and cells compared to normal tissues and cell lines. This
result predicts that miR-1291 may serve as a new target in the
pathogenesis and development of PCa.
As important factors regulating the progression of
tumors, miRNAs have been reported to participate in the
tumorigenesis and metastasis of PCa (21,22). In
the present study, we overexpressed and knocked down miR-1291
levels in PCa cell lines using miR-1291 mimics and inhibitors, and
performed several functional experiments to confirm the role of
miR-1291 in PCa. Upregulation of miR-1291 significantly inhibited
PCa cell proliferation and cell cycle transition while
downregulation of miR-1291 promoted cell growth. These assays
indicate that miR-1291 could function as a tumor suppressor in PCa.
Furthermore, we verified MED1 as a direct target for miR-1291 in
PCa via western blot analysis and luciferase assay. To our best
knowledge, this is the first study to validate the relationship
between miR-1291 and MED1 in PCa.
MED1 is a subunit of MED family which is a
multiprotein complex and regulates eukaryotic mRNA synthesis
(23). MED1 has been identified to
play a role in the progression of several tumors. It acts as an
antitumor gene in lung cancer and melanoma via inhibiting
metastasis and invasion of cells (23,24). In
PCa, MED1 functions as a target of miR-205 and upregulation of MED1
has been associated with a poor prognosis of PCa (25). Furthermore, loss of MED1 obviously
reduces proliferation of PCa cells (26,27). In
this study, we found that MED1 is inhibited by miR-1291
overexpression and its low expression causes the decrease of PCa
cell growth, in consistency with the previously reported
conclusions. Furthermore, we restored MED1 expression in miR-1291
overexpressed DU-145 cells and the ability of cell proliferation
was restored. Taken together, we validated that miR-1291 inhibited
PCa cell proliferation via repressing MED1.
We further constructed a xenograft model to verify
the function of miR-1291 in PCa in vivo. With miR-1291 mimic
treatment, tumors grew obviously slower than the control group and
showed higher miR-1291 expression but lower MED1 level. Clearly,
the protein level of MED1 in xenograft was repressed by miR-1291.
In combination with the results of previous in vitro
experiments, we have reason to believe that miR-1291 could inhibit
PCa tumorigenesis and progression through MED1. Although regulation
of miR-1291 in PCa is likely a more complex network-like system,
this study explains to a certain extent the role and mechanism of
miR-1291 in PCa.
In conclusion, this study demonstrated that miR-1291
participates in the regulation of PCa progression and regulates
cell proliferation by downregulating MED1 expression in
vitro and in vivo. These findings might suggest miR-1291
as a novel target for PCa biological diagnosis and therapy.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (no. 81602217), the Science and
Technology Planning Project of Zhejiang Province (no. 2015C33096),
and the Medical Scientific Research Foundation of Zhejiang Province
(no. 2015117161).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
QC and WZ designed the study and performed the
experiments. AZ, LR and JC acquired the data. KL and ZW analyzed
the data. QC and WZ prepared the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Tongde Hospital of Zhejiang Province (Hangzhou, China). Signed
informed consents were obtained from the patients or the
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blumenthal-Barby JS, Lee D and Volk RJ:
Toward ethically responsible choice architecture in prostate cancer
treatment decision-making. CA Cancer J Clin. 65:257–260. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chan JM, Stampfer MJ and Giovannucci EL:
What causes prostate cancer? A brief summary of the epidemiology.
Semin Cancer Biol. 8:263–273. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Levesque C and Nelson PS: Cellular
constituents of the prostate stroma: Key contributors to prostate
cancer progression and therapy resistance. Cold Spring Harb
Perspect Med. 8(pii): a0305102018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Garisto JD and Klotz L: Active
surveillance for prostate cancer: How to do it right. Oncology
(Williston Park). 31:333–340, 345. 2017.PubMed/NCBI
|
|
6
|
Shepard DR and Raghavan D: Innovations in
the systemic therapy of prostate cancer. Nat Rev Clin Oncol.
7:13–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ameres SL and Zamore PD: Diversifying
microRNA sequence and function. Nat Rev Mol Cell Biol. 14:475–488.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berindan-Neagoe I, Monroig PC, Pasculli B
and Calin GA: MicroRNAome genome: A treasure for cancer diagnosis
and therapy. CA Cancer J Clin. 64:311–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carthew RW and Sontheimer EJ: Origins and
mechanisms of miRNAs and siRNAs. Cell. 136:642–655. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bartels CL and Tsongalis GJ: MicroRNAs:
Novel biomarkers for human cancer. Clin Chem. 55:623–631. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cai C, Chen QB, Han ZD, Zhang YQ, He HC,
Chen JH, Chen YR, Yang SB, Wu YD, Zeng YR, et al: miR-195 inhibits
tumor progression by targeting RPS6KB1 in human prostate cancer.
Clin Cancer Res. 21:4922–4934. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Josson S, Gururajan M, Hu P, Shao C, Chu
GY, Zhau HE, Liu C, Lao K, Lu CL, Lu YT, et al: miR-409-3p/-5p
promotes tumorigenesis, epithelial-to-mesenchymal transition, and
bone metastasis of human prostate cancer. Clin Cancer Res.
20:4636–4646. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu C, Kelnar K, Liu B, Chen X,
Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, et
al: The microRNA miR-34a inhibits prostate cancer stem cells and
metastasis by directly repressing CD44. Nat Med. 17:211–215. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rajendiran S, Parwani AV, Hare RJ,
Dasgupta S, Roby RK and Vishwanatha JK: MicroRNA-940 suppresses
prostate cancer migration and invasion by regulating MIEN1. Mol
Cancer. 13:2502014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo H, Guo W, Wang F, You Y, Wang J, Chen
X, Wang J, Wang Y, Du Y, Chen X, et al: miR-1291 targets mucin 1
inhibiting cell proliferation and invasion to promote cell
apoptosis in esophageal squamous cell carcinoma. Oncol Rep.
34:2665–2673. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tu MJ, Pan YZ, Qiu JX, Kim EJ and Yu AM:
MicroRNA-1291 targets the FOXA2-AGR2 pathway to suppress pancreatic
cancer cell proliferation and tumorigenesis. Oncotarget.
7:45547–45561. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamasaki T, Seki N, Yoshino H, Itesako T,
Yamada Y, Tatarano S, Hidaka H, Yonezawa T, Nakagawa M and Enokida
H: Tumor-suppressive microRNA-1291 directly regulates glucose
transporter 1 in renal cell carcinoma. Cancer Sci. 104:1411–1419.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Soufi-Zomorrod M, Hajifathali A, Kouhkan
F, Mehdizadeh M, Rad SM and Soleimani M: MicroRNAs modulating
angiogenesis: miR-129-1 and miR-133 act as angio-miR in HUVECs.
Tumour Biol. 37:9527–9534. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Turkbey B, Brown AM, Sankineni S, Wood BJ,
Pinto PA and Choyke PL: Multiparametric prostate magnetic resonance
imaging in the evaluation of prostate cancer. CA Cancer J Clin.
66:326–336. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fendler A, Stephan C, Yousef GM and Jung
K: MicroRNAs as regulators of signal transduction in urological
tumors. Clin Chem. 57:954–968. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gade P, Singh AK, Roy SK, Reddy SP and
Kalvakolanu DV: Down-regulation of the transcriptional mediator
subunit Med1 contributes to the loss of expression of
metastasis-associated dapk1 in human cancers and cancer cells. Int
J Cancer. 125:1566–1574. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang Y, Leonard M, Zhang Y, Zhao D,
Mahmoud C, Khan S, Wang J, Lower EE and Zhang X: HER2-driven breast
tumorigenesis relies upon interactions of the estrogen receptor
with coactivator MED1. Cancer Res. 78:422–435. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hulf T, Sibbritt T, Wiklund ED, Patterson
K, Song JZ, Stirzaker C, Qu W, Nair S, Horvath LG, Armstrong NJ, et
al: Epigenetic-induced repression of microRNA-205 is associated
with MED1 activation and a poorer prognosis in localized prostate
cancer. Oncogene. 32:2891–2899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jin F, Irshad S, Yu W, Belakavadi M,
Chekmareva M, Ittmann MM, Abate-Shen C and Fondell JD: ERK and AKT
signaling drive MED1 overexpression in prostate cancer in
association with elevated proliferation and tumorigenicity. Mol
Cancer Res. 11:736–747. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Z, Zhang C, Wu D, Chen H, Rorick A,
Zhang X and Wang Q: Phospho-MED1-enhanced UBE2C locus looping
drives castration-resistant prostate cancer growth. EMBO J.
30:2405–2419. 2011. View Article : Google Scholar : PubMed/NCBI
|