Introduction
The cancer stroma, which is generally thought to be
derived from non-neoplastic cells represented by cancer-associated
fibroblasts, plays an important role in various tumour behaviours,
such as invasion, metastasis, and chemotherapeutic response
(1–3).
Carcinoma-associated fibroblasts promote cancer progression
(4), angiogenesis (5), and metastasis (6). In several cancers, interactions between
cancer cells and the associated stroma lead to drug resistance
(7). Recent studies have suggested
that some carcinoma cells and stromal cells may share an origin,
based on gene landscapes shared by both cell types in certain
cancers, including breast (8), colon
(9), bladder (10), and ovarian cancers (11,12).
However, the origin and role of cancer stroma have been
controversial yet.
Epithelial ovarian cancer (EOC) is the leading cause
of death arising from gynecological malignancies (13). EOCs, including serous carcinoma,
endometrioid carcinoma (EMC), clear cell carcinoma (CCC), and
mucinous carcinoma, have specific clinical and genetic features.
EMC and CCC are histogenetically associated with endometriosis and
are often characterized by ARID1A (30 and 40–60%,
respectively), PIK3CA (40 and 51%, respectively),
KRAS (33 and 20%, respectively), and PTEN (17 and
13%, respectively) (14–17). But the frequency of TP53
mutation in EMC and CCC are considerably lower (7 and 13%,
respectively), compared to the four genes (14–17). The
stroma of EOCs sometimes consists of a specialized ovarian stroma
with luteinisation and/or hyperthecosis with endocrine function,
called a ‘functioning stroma’ (18).
The relationship between functioning stroma and the response to
chemotherapy or prognosis remains to be clarified. In addition, the
histogenetic mechanism of functioning stroma is poorly defined.
Functioning stroma is observed not only in mucinous carcinoma but
also in EMC and CCC (19). However,
serous carcinoma characterized by TP53 mutation rarely has a
functioning stroma (19). Therefore,
this study aimed to evaluate the localization of gene abnormalities
commonly detected in EMC and CCC in carcinoma cells and functioning
stromal cells separately. We believe that some of functioning
stroma may share an origin with carcinoma cells.
Materials and methods
Patients and samples
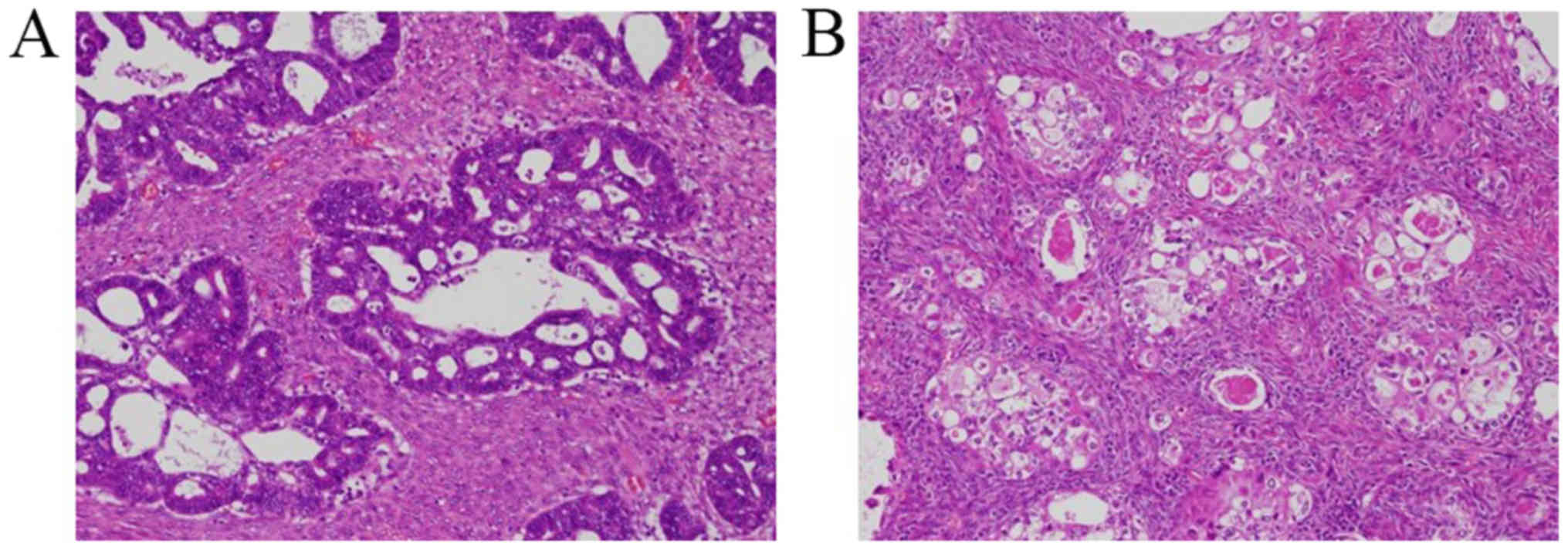
Subjects eligible for this study had histologically
confirmed ovarian EMC or CCC with functioning stroma (Fig. 1). Patient and clinicopathological
data, including age, menopause, International Federation of
Obstetrics and Gynecology (FIGO) stage, histological subtype,
histological grade, surgery (optimal, residual tumour <1 cm;
suboptimal, residual tumour ≥1 cm), serum oestrogen level, serum
follicle-stimulating hormone (FSH) level, recurrence, and death,
were reviewed. Serum levels of oestrogen (Eclusys E2 IV; Roche
Diagnostics, Tokyo, Japan) and FSH (FSH II; Roche Diagnostics) were
analysed by enzyme immunoassays. All patients had a follow-up
period of at least three years. The study was approved by the
Institutional Review Board of the Saitama Medical University
International Medical Center (Saitama, Japan), and written informed
consent was obtained from all patients.
Laser microdissection and DNA
extraction
Formalin-fixed, paraffin-embedded sections (10 µm)
prepared from tumour tissue specimens were affixed to 2-µm-thick
LCM Film glass slides (Membrane Slides PEN Membrane 2; Leica,
Wetzlar, Germany) and stained with 0.05% toluidine blue solution
(pH 2.5; Wako, Osaka, Japan). Stained sections were microdissected
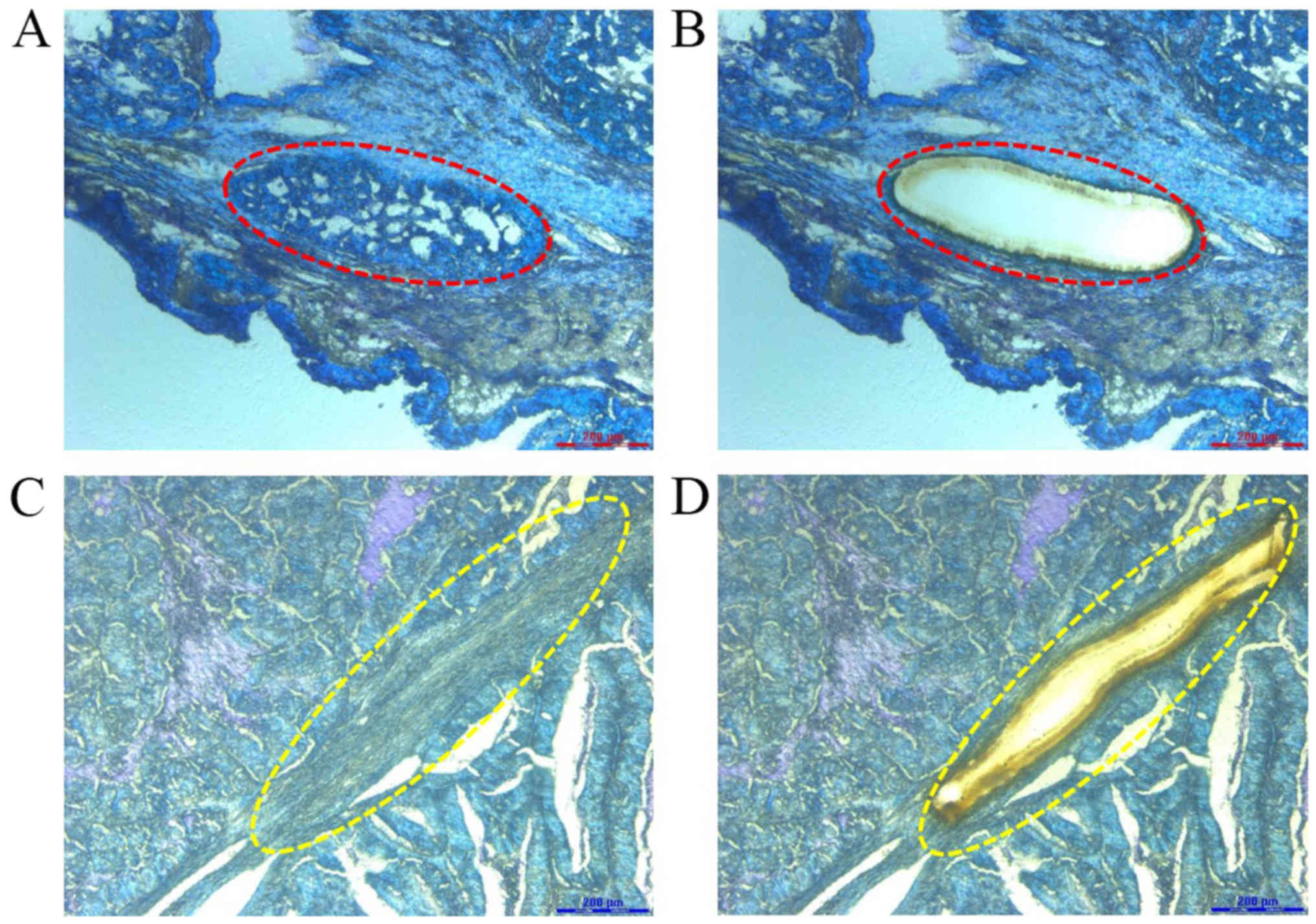
using a Leica LMD7000 laser microdissection microscope. Carcinoma
cells and adjacent functional stromal cells were visualized under
the microscope and were selectively detached by activation of the
laser (Fig. 2). DNA was extracted
using the Maxwell RSC DNA FFPE kit (Promega, Madison, WI, USA)
according to the manufacturer's instructions.
Amplification and sequence analysis of
ARID1A, PIK3CA, KRAS, and PTEN
We analysed ARID1A (exons 18 and 20),
PIK3CA (exons 9 and 20), KRAS (exons 2 and 3), and
PTEN (exons 5–8) sequences in DNA extracted from the whole
tumours of 14 patients. For cases with mutations, carcinoma cells
and functioning stromal cells were analysed separately to clarify
the histological localization of the mutations. Target sequences
were PCR-amplified using Accuprime Taq DNA Polymerase (Invitrogen,
Carlsbad, CA, USA) on a 9800 Fast Thermal Cycler (Applied
Biosystems, Foster City, CA, USA). Primer sequences are shown in
Table I. The thermal cycles were as
follows: 95°C for 10 min, followed by 40 cycles of 94°C for 30 sec,
60°C for 30 sec, and 68°C for 60 sec. Products were electrophoresed
on a 2% agarose gel. Purified products were subjected to direct
sequencing on an ABI PRISM 3100 (Applied Biosystems) using the ABI
PRISM Big Dye Terminator Ver3.1 Cycle Sequencing kit according to
the manufacturer's instructions. Sequencing was conducted twice to
confirm reproducibility of the results.
 | Table I.Sequence information for primers used
to amplify ARID1A, PIK3CA, KRAS and PTEN. |
Table I.
Sequence information for primers used
to amplify ARID1A, PIK3CA, KRAS and PTEN.
| A, ARID1A |
|---|
|
|---|
| Gene | Primer (5′-3′) |
|---|
| Exon 18 |
|
| S |
TGGCATCTGTGGGCTTTATGT |
| AS |
CCATACTGGTTGTATACATCTTGCT |
| S |
GGAGATGTACAGCGTGCCATA |
| AS |
TTGTGGTGGCATGTTTTGCTG |
| S |
CAGAACCAATTTCCATTCCAGT |
| AS |
GTGTGCAGCATTTCATCTGTTC |
| S |
GGGGCGTAATGACATGACCTAT |
| AS |
AATGTGATTCTGCATGCTTGGTG |
| S |
CAAGGCCCCCTCCATCTAAC |
| AS |
TGCTAGGAGAGGTGCGGTTC |
| S |
ATGCAGAATCACATTCCTCAGGTAT |
| AS |
GGCAGATTAGGCAACCGAATG |
| Exon 20 |
|
| S |
GGGGAGGTCTCTCAAGTCAAT |
| AS |
ATGGGAGCTGGACTAGACAC |
| S |
GGACAGAGAACGCTACTGGAT |
| AS |
AATGGATCATTCTTCTGTACGATCT |
| S |
GAGGAGAAGCTGATCAGTAAGTTTG |
| AS |
CTGCTGTTGTCACATGCTTCC |
| S |
CTGAGCATATCCAGACCCACTTC |
| AS |
GCCTCTGAACTCTTAGCTCCATC |
| S |
CAGCCACTATGGATGACATGTT |
| AS |
GGTGTTTGGACATCTCAAAGTCA |
| S |
GCGTCTGTGTGTCCAATACCA |
| AS |
CACTCCACTTTGTTGCAGCTC |
| S |
CAGGCACCACTAACTTATGAAAAGG |
| AS |
GAGTTTGCTGAGGGTTTCCAAGA |
| S |
TCCTTTCCCCGCAGAGACT |
| AS |
CCAGGAGGTTGCCGATACTG |
| S |
GTGCCATTGCAGTGCAGAA |
| AS |
GAGATGTCCAACAGCCGTGAT |
| S |
TGGACGAGAACCACTCAGAGTTTAC |
| AS |
AGGGCAACAGTCAGTTTCTAAGTTC |
|
| B,
PIK3CA |
|
| Gene | Primer
(5′-3′) |
|
| Exon 9 |
|
| S |
TGTAAAACGACGGCCAGTGGGAAA
AATATGACAAAGAAAGC |
| AS |
CAGGAAACAGCTATGACCTGAGAT
CAGCCAAATTCAGTT |
| Exon 20 |
|
| S |
TGTAAAACGACGGCCAGTCTCAATG
ATGCTTGGCTCTG |
| AS |
CAGGAAACAGCTATGACTGGAATCC
AGAGTGAGCTTTC |
|
| C,
KRAS |
|
| Gene | Primer
(5′-3′) |
|
| Exon 2 |
|
| S |
TAACCTTATGTGTGACATGTTC |
| AS |
ATGCATATTAAAACAAGATTTACC |
| Exon 3 |
|
| S |
CTCCCTTCTCAGGATTCCTA |
| AS |
AGTCCTCATGTACTGGTCCC |
|
| D,
PTEN |
|
| Gene | Primer
(5′-3′) |
|
| Exon 5 |
|
| S |
ACCTGTTAAGTTTGTATGCAAC |
| AS |
TCCAGGAAGAGGAAAGGAAA |
| Exon 6 |
|
| S |
CATAGCAATTTAGTGAAATAACT |
| AS |
GATATGGTTAAGAAAACTGTTC |
| Exon 7 |
|
| S |
TGACAGTTTGACAGTTAAAGG |
| AS |
GGATATTTCTCCCAATGAAAG |
| Exon 8 |
|
| S |
ACACATCACATACATACAAGTC |
| AS |
GTGCAGATAATGACAAGGAATA |
Results
Patient characteristics
The median age of the 14 patients was 67 years
(range, 52 to 85 years); 13 patients were postmenopausal, and one
was in perimenopause. Serum oestrogen levels ranged from 10 to 129
ng/ml, with a median of 51 ng/ml, before surgery, but were reduced
to less than 10 ng/ml in all available postoperative patients.
Serum FSH levels ranged from 6 to 89 mIU/ml, with a median of 32
mIU/ml, preoperatively, but increased after surgery, ranging from
62 to 96 mIU/ml and with a median of 82 mIU/ml. Ten patients had
FIGO stage I cancer, two patients had stage II, and two patients
had stage IV. Five patients with EMC had grade 1 cancer, and two
patients with EMC had grade 2 cancer. Two patients with FIGO stage
IV had suboptimal surgeries, and the others had optimal surgeries.
Among CCC patients, four patients experienced recurrence, and three
of them died of the disease. In EMC, there was no recurrence or
death.
Localization of gene mutations
As shown in Table II,
one patient had an ARID1A mutation (7%), two patients had
PIK3CA mutations (14%), three patients had KRAS
mutations (21%), and one patient had a PTEN mutation (7%).
In EMC, three patients had KRAS mutations (43%), one patient
had a PIK3CA mutation (14%), and none of the patients had
ARID1A or PTEN mutations (0%). In CCC, one patient
had both PIK3CA and PTEN mutations (14%), one patient
had an ARID1A mutation (14%), and none of the patients had
KRAS mutations (0%).
 | Table II.Demographic and clinicopathological
data for 14 patients, and gene mutations in whole tumours in the 14
cases. |
Table II.
Demographic and clinicopathological
data for 14 patients, and gene mutations in whole tumours in the 14
cases.
|
|
|
|
|
|
|
|
|
| Oestrogen,
ng/ml | FSH, mIU/ml |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Case | Age, years | Age at menopause,
years | Histology | FIGO stage | FIGO grade | Surgery | Recurrence | Death | Pre | Post | Pre | Post | ARID1A | PIK3CA | KRAS | PTEN |
|---|
| 1 | 79 | 53 | EMC | 1C | 1 | Opt | No | No | 50 | <10 | 23 | 62 | WT | WT | Q12V | WT |
| 2 | 80 | 50 | EMC | 2B | 2 | Opt | No | No | 50 | <10 | 28 | 84 | WT | WT | WT | WT |
| 3 | 63 | 48 | EMC | 1C | 1 | Opt | No | No | 103 | <10 | 16 | 85 | WT | WT | E31K, Q61L | WT |
| 4 | 74 | 50 | EMC | 4A | 1 | Sub | No | No | 36 | NA | 35 | NA | WT | H1047R | E31K | WT |
| 5 | 70 | 50 | EMC | 1C | 1 | Opt | No | No | 36 | <10 | 39 | 89 | WT | WT | WT | WT |
| 6 | 65 | 52 | EMC | 1A | 2 | Opt | No | No | 48 | NA | NA | NA | WT | WT | WT | WT |
| 7 | 85 | 47 | EMC | 1C | 1 | Opt | No | No | 129 | NA | NA | NA | WT | WT | WT | WT |
| 8 | 68 | 50 | CCC | 1A | NA | Opt | No | No | 31 | NA | 29 | 96 | WT | WT | WT | WT |
| 9 | 64 | 52 | CCC | 2A | NA | Opt | Yes | Yes | 29 | NA | NA | NA | WT | H1047R | WT | C105fs*8 |
| 10 | 62 | 51 | CCC | 1C | NA | Opt | Yes | No | 88 | <10 | 6 | 76 | WT | WT | WT | WT |
| 11 | 63 | 53 | CCC | 1C | NA | Opt | Yes | Yes | 21 | NA | 55 | NA | L2155L | WT | WT | WT |
| 12 | 62 | 52 | CCC | 1A | NA | Opt | No | No | 10 | NA | 79 | NA | WT | WT | WT | WT |
| 13 | 52 | 51 | CCC | 1C | NA | Opt | No | No | NA | NA | NA | NA | WT | WT | WT | WT |
| 14 | 52 | Peri | CCC | 4B | NA | Sub | Yes | Yes | 34 | NA | 12 | NA | WT | WT | WT | WT |
In total, eight mutations were detected in whole
tumours of five patients: ARID1A (L2155L), PIK3CA
(H1047R), KRAS (Q12V, E31K, Q61L), and PTEN
(C105fs*8) (Table III). Seven of
the eight mutations were detected in only carcinoma cells; thus,
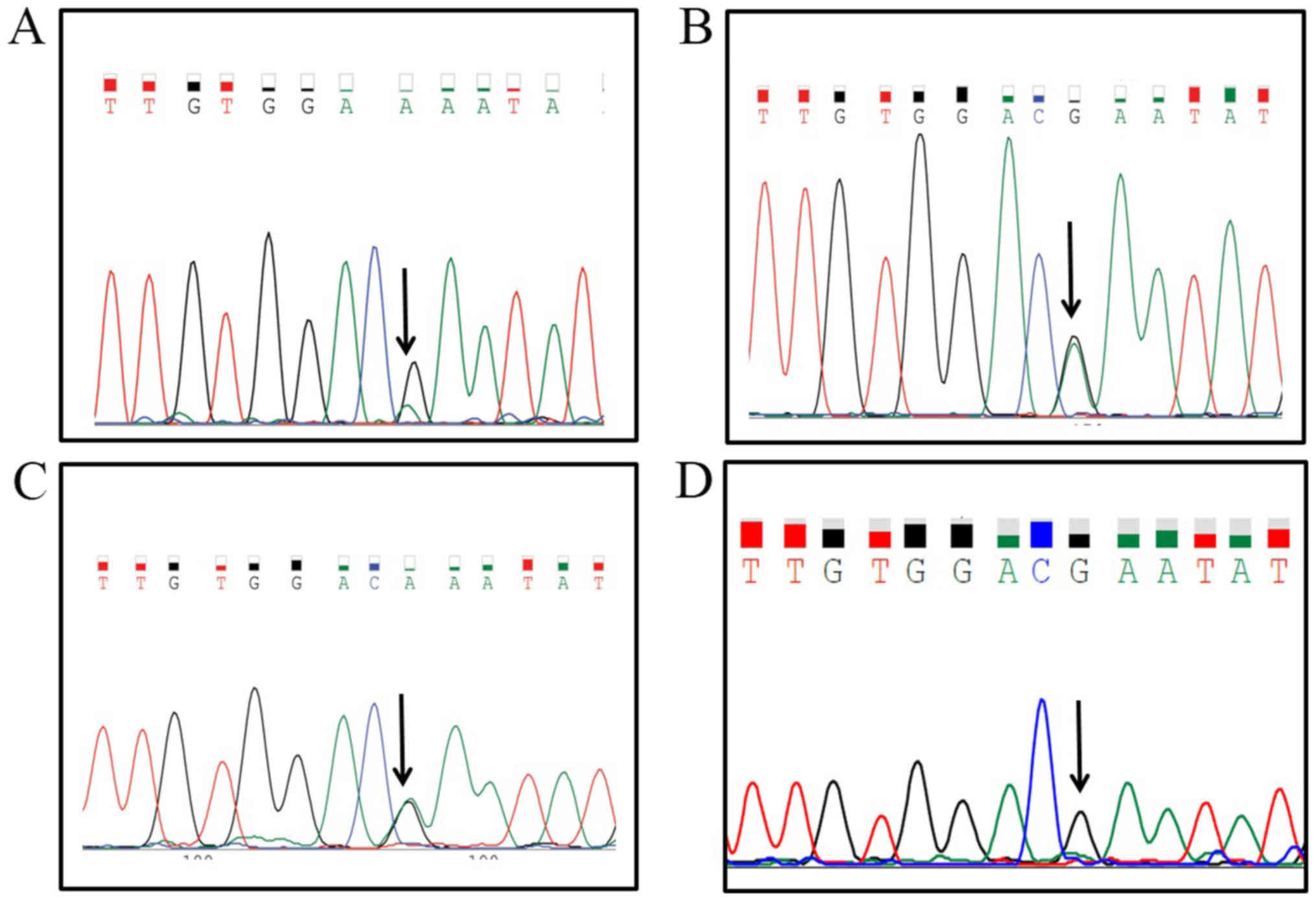
only one patient (case 4) had a KRAS (E31K) mutation in both
carcinoma and functioning stromal cells. The KRAS mutation
in this case (Fig. 3) was repeatedly
confirmed. In non-functioning stroma, composed of non-specific
fibroblasts, no KRAS mutation was detected in this patient.
Moreover, germline analysis of this patient revealed no KRAS
mutation.
 | Table III.Localization of ARID1A, PIK3CA,
KRAS and PTEN mutations. |
Table III.
Localization of ARID1A, PIK3CA,
KRAS and PTEN mutations.
|
|
| ARID1A | PIK3CA | KRAS | PTEN |
|---|
|
|
|
|
|
|
|
|---|
| Case | Histology | Whole | CA | FS | Whole | CA | FS | Whole | CA | FS | Whole | CA | FS |
|---|
| 1 | EMC | WT | WT | WT | WT | WT | WT | Q12V | Q12V | WT | WT | WT | WT |
| 3 | EMC | WT | WT | WT | WT | WT | WT | E31K, Q61L | E31K, Q61L | WT | WT | WT | WT |
| 4 | EMC | WT | WT | WT | H1047R | H1047R | WT | E31K | E31K | E31K | WT | WT | WT |
| 9 | CCC | WT | WT | WT | H1047R | H1047R | WT | WT | WT | WT | C105fs*8 | C105fs*8 | WT |
| 11 | CCC | L2155L | L2155L | WT | WT | WT | WT | WT | WT | WT | WT | WT | WT |
Trends in prognosis, hormone levels,
and gene mutations
Five patients with high serum oestrogen levels (≥50
ng/ml) survived, whereas three patients (38%) with low serum
oestrogen levels (<50 ng/ml) died of the disease. In the four
patients with recurrence, one patient (case 10) with a high serum
oestrogen level (88 ng/ml) and low FSH level (6 mIU/ml) experienced
disease-free survival after chemotherapy, but the other three
patients died of the disease.
Discussion
In EOC, the cancer stroma is classified as either
non-specific fibroblastic type or functioning stroma, the latter of
which frequently occurs in postmenopausal EOCs. However,
functioning stroma had not yet been thoroughly characterized in
terms of histogenesis, response to chemotherapy, and prognosis. A
close association between mucinous tumours and functioning stroma
has been reported (19), and Katoh
et al (20) found that
functioning stroma is more common in EMCs resembling sex
cord-stromal tumours. In the seven EMC and seven CCC patients
recruited in the present study, functioning stroma was identified
histologically and endocrinologically. Five EOCs in four patients
carried mutations in ARID1A, PIK3CA, KRAS, and PTEN
in carcinoma cells, but not in functioning stromal cells. However,
one case of ovarian EMC (case 4) harboured an identical KRAS
mutation (E31K) in both carcinoma and functioning stromal cells,
whereas no mutation was detected in the tissue surrounding the
tumour. Akahane et al (11)
reported a case of ovarian EMC with carcinoma and stromal cells
harbouring the same mutation in TP53 (R2489) as indicated by
direct sequence analysis. Tuhkanen et al (12) reported 39 similar genetic alterations
in carcinoma and stromal cells in 11 EOC tumours based on multiplex
ligation-dependent probe amplification. These findings suggest that
the cancer stroma may contain cells of epithelial origin that are
generated by epithelial-mesenchymal transition and that this
transformed tumour stroma may have an effect on epithelial-stromal
cell interactions and tumorigenesis (12). Therefore, it may be possible to
elucidate the mechanisms of tumour initiation by evaluating the
association between carcinoma cells and functioning stromal
cells.
KRAS mutations are typically detected in
20–30% of EMC cases (14,21); however, in the present study, EMC with
functioning stroma had a higher frequency (43%) of KRAS
mutations. KRAS mutation is more common in
endometriosis-associated EMC than in non-endometriosis-associated
EMC (21). These findings suggest
that EMC with functioning stroma may be associated with
endometriosis. Mutations in ARID1A, which are distributed
evenly across the gene, are detected in 46% of ovarian CCC and 30%
of EMC cases (16). In the present
study, there was only one case of CCC (7%) with ARID1A
mutation. However, we immunohistochemically confirmed the deletion
of ARID1A in eight of the 14 patients (57%) (data not
shown). This discrepancy may be explained by the fact that only
exons 18 and 20 were sequenced in the present study. EOC patients
with functioning stroma exhibited elevated serum oestrogen levels
and reduced serum FSH levels. Following surgery, the levels of both
hormones were restored to normal postmenopausal levels. Patients
with high oestrogen levels (≥50 ng/ml) experienced no death from
the disease, as demonstrated by one patient with EMC (case 10) who
had a long period of disease-free survival after chemotherapy for
recurrence; however, patients with low oestrogen levels (<50
ng/ml) exhibited poor outcomes. Thus, the functioning stroma,
characterized by morphological and endocrinological
differentiation, may be related to the biological behaviours of
cancer.
Our study had several limitations. The sample size
was small and the range of genetic mutations analysed was narrow.
In addition, the detection of identical mutations in both carcinoma
and functioning stromal cells was not replicated, and differences
between EMC with and without functioning stroma were not assessed.
Therefore, this research should be considered a preliminary study.
Confirmation of our findings in a larger population, including
patients with and without functioning stroma, is warranted.
In conclusion, we are the first to report a case of
EMC in which the same KRAS mutation was observed in both
carcinoma cells and functioning stromal cells. This suggests that
some regions of the tumour and stroma may have a common origin.
Further studies are needed to clarify the molecular association of
functioning stroma with carcinoma cells in a larger population.
Acknowledgements
Not applicable.
Funding
The present study was supported by Grants-in-Aid
from the Ministry of Education, Science, Sports and Culture of
Japan (grant nos. 15K08355 and 18K06997), a Sekiguchi Memorial
Award (grant no. 18-C-1-02) and Saitama Medical University.
Availability of data and materials
All datasets generated in this study are available
from the corresponding author upon reasonable request.
Authors' contributions
MN took part in the study conception and design, as
well as the acquisition, analysis and interpretation of data. MiY
took part in the interpretation of data and drafting of the
manuscript. KK and TK took part in the acquisition and analysis of
data. MS and KI took part in the conception and design of the
study, and revised the manuscript for important intellectual
content. HK participated in the analysis of data and critically
revised the manuscript for important intellectual content. MaY took
part in the conception and design of the study, critically revised
the manuscript for important intellectual content, and supervised
the study.
Ethics approval and consent to
participate
The study protocol was approved by the institutional
review board of Saitama Medical University International Medical
Center (Saitama, Japan), and written informed consent was obtained
from all patients.
Patient consent for publication
The present study obtained written informed consent
for publication from the all patients.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EOC
|
epithelial ovarian cancer
|
|
EMC
|
endometrioid carcinoma
|
|
CCC
|
clear cell carcinoma
|
|
FIGO
|
The International Federation of
Obstetrics and Gynecology
|
|
FSH
|
follicle-stimulating hormone
|
References
|
1
|
Bhowmick NA and Moses HL: Tumor-stroma
interactions. Curr Opin Genet Dev. 15:97–101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim JB, Stein R and O'Hare MJ:
Tumour-stromal interactions in breast cancer: The role of stroma in
tumourigenesis. Tumour Biol. 26:173–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tlsty TD and Coussens LM: Tumor stroma and
regulation of cancer development. Annu Rev Pathol. 1:119–150. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Olumi AF, Grossfeld GD, Hayward SW,
Carroll PR, Tlsty TD and Cunha GR: Carcinoma-associated fibroblasts
direct tumor progression of initiated human prostatic epithelium.
Cancer Res. 59:5002–5011. 1999.PubMed/NCBI
|
|
5
|
Nagasaki T, Hara M, Nakanishi H, Takahashi
H, Sato M and Takeyama H: Interleukin-6 released by colon
cancer-associated fibroblasts is critical for tumour angiogenesis:
Anti-interleukin-6 receptor antibody suppressed angiogenesis and
inhibited tumour-stroma interaction. Br J Cancer. 110:469–478.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu J, Chen S, Wang W, Ning BF, Chen F,
Shen W, Ding J, Chen W, Xie WF and Zhang X: Cancer-associated
fibroblasts promote hepatocellular carcinoma metastasis through
chemokine-activated hedgehog and TGF-β pathways. Cancer Lett.
379:49–59. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Straussman R, Morikawa T, Shee K,
Barzily-Rokni M, Qian ZR, Du J, Davis A, Mongare MM, Gould J,
Frederick DT, et al: Tumour micro-environment elicits innate
resistance to RAF inhibitors through HGF secretion. Nature.
487:500–504. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kurose K, Gilley K, Matsumoto S, Watson
PH, Zhou XP and Eng C: Frequent somatic mutations in PTEN and TP53
are mutually exclusive in the stroma of breast carcinomas. Nat
Genet. 32:355–357. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wernert N, Locherbach C, Wellmann A,
Behrens P and Hügel A: Presence of genetic alterations in
microdissected stroma of human colon and breast cancers. Anticancer
Res. 21:2259–2264. 2001.PubMed/NCBI
|
|
10
|
Paterson RF, Ulbright TM, MacLennan GT,
Zhang S, Pan CX, Sweeney CJ, Moore CR, Foster RS, Koch MO, Eble JN
and Cheng L: Molecular genetic alterations in the
laser-capture-microdissected stroma adjacent to bladder carcinoma.
Cancer. 98:1830–1836. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akahane T, Hirasawa A, Tsuda H, Kataoka F,
Nishimura S, Tanaka H, Tominaga E, Nomura H, Chiyoda T, Iguchi Y,
et al: The origin of stroma surrounding epithelial ovarian cancer
cells. Int J Gynecol Pathol. 32:26–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tuhkanen H, Anttila M, Kosma VM, Heinonen
S, Juhola M, Helisalmi S, Kataja V and Mannermaa A: Frequent gene
dosage alterations in stromal cells of epithelial ovarian
carcinomas. Int J Cancer. 119:1345–1353. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Permuth-Wey J and Sellers TA: Epidemiology
of ovarian cancer. Methods Mol Biol. 472:413–437. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McConechy MK, Ding J, Senz J, Yang W,
Melnyk N, Tone AA, Prentice LM, Wiegand KC, McAlpine JN, Shah SP,
et al: Ovarian and endometrial endometrioid carcinomas have
distinct CTNNB1 and PTEN mutation profiles. Mod Pathol. 27:128–134.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murakami R, Matsumura N, Brown JB, Higasa
K, Tsutsumi T, Kamada M, Abou-Taleb H, Hosoe Y, Kitamura S,
Yamaguchi K, et al: Exome sequencing landscape analysis in ovarian
clear cell carcinoma shed light on key chromosomal regions and
mutation gene networks. Am J Pathol. 187:2246–2258. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wiegand KC, Shah SP, Al-Agha OM, Zhao Y,
Tse K, Zeng T, Senz J, McConechy MK, Anglesio MS, Kalloger SE, et
al: ARID1A mutations in endometriosis-associated ovarian
carcinomas. N Engl J Med. 363:1532–1543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim SI, Lee JW, Lee M, Kim HS, Chung HH,
Kim JW, Park NH, Song YS and Seo JS: Genomic landscape of ovarian
clear cell carcinoma via whole exome sequencing. Gynecol Oncol.
148:375–382. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scully RE YR and Clement PB: Tumors with
functioning stroma. Tumors of the ovary, maldeveloped gonads,
fallopian tube and broad ligament. In: Atlas of Tumor Pathology
Armed Forces Institute of Pathology; Washington, DC: pp. pp373–378.
1998
|
|
19
|
Kato N, Hayasaka T, Takeda J, Osakabe M
and Kurachi H: Ovarian tumors with functioning stroma: A
clinicopathologic study with special reference to serum estrogen
level, stromal morphology and aromatase expression. Int J Gynecol
Pathol. 32:556–561. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Katoh T, Yasuda M, Hasegawa K, Kozawa E,
Maniwa J and Sasano H: Estrogen-producing endometrioid
adenocarcinoma resembling sex cord-stromal tumor of the ovary: A
review of four postmenopausal cases. Diagn Pathol. 7:1642012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stewart CJ, Leung Y, Walsh MD, Walters RJ,
Young JP and Buchanan DD: KRAS mutations in ovarian low-grade
endometrioid adenocarcinoma: Association with concurrent
endometriosis. Hum Pathol. 43:1177–1183. 2012. View Article : Google Scholar : PubMed/NCBI
|