Introduction
Colorectal cancer (CRC) is one of the most common
neoplasms and constitutes a major cause of cancer-associated
mortality worldwide (1). The
incidence of CRC in 2018 was 6.1% (1). In CRC, ~60% of patients have
unresectable or metastatic disease at the time of diagnosis
(2). The lymph nodes and liver are
common sites of metastasis in metastatic (m)CRC (3).
Advancements in targeted therapies developed against
angiogenesis have improved survival in mCRC as angiogenesis is
known to play a key role in the development of several types of
tumor (4). Hypoxia-inducible
factor-1 α (HIF-1α) and vascular endothelial growth factor (VEGF)
are both notable regulators of angiogenesis that are essential for
tumor growth in CRC (5). HIF-1α
exists in the microenvironment in several tumor entities as the
rapid proliferation of tumor cells outpaces the rate of
angiogenesis (6). Overexpression of
HIF-1α is associated with tumor aggressiveness, invasiveness and
resistance to radiotherapy and chemotherapy in CRC (7). VEGF is the principal pro-angiogenic
growth factor, and its expression is mediated by HIF-1α during
hypoxia (8). VEGF markedly increases
vascular permeability, promotes the formation of new blood vessels
and the induces metastases in CRC (9). Thus, inhibiting HIF-1α and VEGF
expression has demonstrated promise for tumor anti-angiogenesis
therapy in both animal models and patients with cancer (10).
In order for a biomarker to be clinically applied,
the relevance of its status in both the primary tumor and
metastatic sites needs to be understood. Currently, the
understanding of cancer predominantly stems from the comparative
study of normal tissues, paracancerous tissues and cancerous
lesions (11). Only a few
comparative studies on primary and metastatic tumors depend on the
assumption that primary and metastatic sites are pathologically
concordant in clinical practice (12). However, research has determined that
different metastatic sites may have different molecular mechanisms
of metastasis (13). To the best of
our knowledge, the difference in HIF-1α and VEGF expression between
primary tumors and corresponding metastases has not yet been fully
investigated in CRC.
The present study assessed HIF-1α, VEGF and CD34
status via immunohistochemistry (IHC) analysis on samples obtained
from primary tumors and paired metastatic sites of CRC. The primary
aim was to analyze the differential HIF-1α, VEGF and microvascular
density (MVD) status between primary tumors and corresponding
metastatic tissues at the protein level, and provide a potential
predictive mechanism to inform the use of anti-angiogenic agents in
the treatment of mCRC.
Materials and methods
Patient characteristics
The present study was approved by the Ethics and
Scientific Committees of Shandong Cancer Hospital (Shandong,
China), and written informed consent was obtained from all patients
prior to the study start. A total of 46 patients (20 men and 26
women) who underwent surgical resection of both the primary CRC and
the corresponding metastatic sites (lymph nodes and liver)
synchronous between April 2010 and June 2017 in Shandong Cancer
Hospital, and had complete clinical data, were reviewed in this
retrospective analysis. The median age of patients was 62 years
(age range, 40–82 years). The clinical and histopathological
characteristics of the patients are presented in Tables I–III. All patients provided available
tissues, including primary tumors and matched metastases. None of
the cases underwent adjuvant therapy prior to surgery. Tumors were
staged according to the American Joint Committee on Cancer (AJCC)
pathological tumor node metastasis (TNM) classification (14).
 | Table I.Association between HIF-1α and VEGF
expression in primary tumors and the clinicopathological
characteristics of patients with colorectal carcinomas (n=46). |
Table I.
Association between HIF-1α and VEGF
expression in primary tumors and the clinicopathological
characteristics of patients with colorectal carcinomas (n=46).
|
| HIF-1α expression
in primary tumors |
|
| VEGF expression in
primary tumors |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Characteristic | Negative | Positive |
χ2-value | P-value | Negative | Positive |
χ2-value | P-value |
|---|
| Patient, n | 14 | 32 |
|
| 12 | 34 |
|
|
| Sex |
|
| 0.00 | 0.96 |
|
| 0.28 | 0.60 |
| Male
(n=20) | 6 | 14 |
|
| 6 | 14 |
|
|
| Female
(n=26) | 8 | 18 |
|
| 6 | 20 |
|
|
| Age, years |
|
| 0.08 | 0.93 |
|
| 0.02 | 0.90 |
| <60
(n=30) | 9 | 21 |
|
| 8 | 22 |
|
|
| ≥60
(n=16) | 5 | 11 |
|
| 4 | 12 |
|
|
| Sites |
|
| 0.07 | 0.79 |
|
| 2.81 | 0.09 |
| Colon
(n=35) | 11 | 24 |
|
| 7 | 28 |
|
|
| Rectum
(n=11) | 3 | 8 |
|
| 5 | 6 |
|
|
| Tumor
differentiation |
|
| 0.04 | 0.98 |
|
| 0.25 | 0.88 |
| Low
(n=14) | 4 | 10 |
|
| 3 | 11 |
|
|
|
Moderate (n=22) | 7 | 15 |
|
| 6 | 16 |
|
|
| High
(n=10) | 3 | 7 |
|
| 3 | 7 |
|
|
| Depth of
invasion |
|
| 2.19 | 0.14 |
|
| 2.31 | 0.13 |
| pT3
(n=22) | 9 | 13 |
|
| 8 | 14 |
|
|
| pT4
(n=24) | 5 | 19 |
|
| 4 | 20 |
|
|
| Primary tumor size,
cm |
|
| 0.15 | 0.70 |
|
| 1.05 | 0.31 |
| ≤3
(n=25) | 7 | 18 |
|
| 5 | 20 |
|
|
| >3
(n=21) | 7 | 14 |
|
| 7 | 14 |
|
|
 | Table III.Association between HIF-1α VEGF in
liver metastases and the clinicopathological characteristics of
patients with colorectal carcinomas (n=46). |
Table III.
Association between HIF-1α VEGF in
liver metastases and the clinicopathological characteristics of
patients with colorectal carcinomas (n=46).
|
| HIF-1α expression
in liver metastases |
|
| VEGF expression in
liver metastases |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Characteristic | Negative | Positive |
χ2-value | P-value | Negative | Positive |
χ2-value | P-value |
|---|
| Patient, n | 16 | 30 |
|
| 15 | 31 |
|
|
| Sex |
|
| 0.36 | 0.55 |
|
| 2.56 | 0.11 |
| Male
(n=20) | 6 | 14 |
|
| 4 | 16 |
|
|
| Female
(n=26) | 10 | 16 |
|
| 11 | 15 |
|
|
| Age, years |
|
| 1.04 | 0.31 |
|
| 0.02 | 0.89 |
| <60
(n=30) | 12 | 18 |
|
| 10 | 20 |
|
|
| ≥60
(n=16) | 4 | 12 |
|
| 5 | 11 |
|
|
| Sites |
|
| 1.76 | 0.19 |
|
| 0.19 | 0.67 |
| Colon
(n=35) | 14 | 21 |
|
| 12 | 23 |
|
|
| Rectum
(n=11) | 2 | 9 |
|
| 3 | 8 |
|
|
| Tumor
differentiation |
|
| 2.83 | 0.24 |
|
| 0.10 | 0.95 |
| Low
(n=14) | 6 | 8 |
|
| 5 | 9 |
|
|
|
Moderate (n=22) | 5 | 17 |
|
| 7 | 15 |
|
|
| High
(n=10) | 5 | 5 |
|
| 3 | 7 |
|
|
| Depth of
invasion |
|
| 0.70 | 0.40 |
|
| 0.01 | 0.91 |
| pT3
(n=22) | 9 | 13 |
|
| 7 | 15 |
|
|
| pT4
(n=24) | 7 | 17 |
|
| 8 | 16 |
|
|
| Primary tumor size,
cm |
|
| 0.66 | 0.42 |
|
| 3.23 | 0.07 |
| ≤3
(n=25) | 10 | 15 |
|
| 11 | 14 |
|
|
| >3
(n=21) | 6 | 15 |
|
| 4 | 17 |
|
|
IHC
The formalin-fixed paraffin-embedded tissues of
primary tumors (46 specimens), matched lymph node metastases (46
specimens) and liver metastases (46 specimens) were collected to
detect HIF-1α, VEGF and CD34 expression at the protein level.
Briefly, specimens had been fixed in 10% formalin and embedded in
paraffin, and were subsequently sectioned into 4-µm sections. IHC
staining was performed as previously described (15). Briefly, deparaffinized sections were
pretreated with 0.4% pepsin for 60 min at 37°C, and endogenous
peroxidase activity was blocked by treatment with 0.2%
H2O2 for 3 h. The antibodies against CD34
(cat. no. Kit-0004) and the MaxVisionTM IHC kit (cat. no. Kit-5030)
that immunostained HIF-1α and VEGF were all purchased from Fuzhou
Maixin Biotech Co., Ltd. Tissue sections were incubated with
primary antibodies against HIF-1α (1:50) and VEGF (1:100) at 4°C
for 12 h, and CD34 was ready to use. The
streptavidin-peroxidase-biotin method was performed according to
the manufacturer's protocol (16).
Following the primary incubation, membranes were incubated with
horseradish peroxidase-labeled secondary antibody (Supervision™
Universal Detection Reagent; cat. no. D-3004; Shanghai Changdao
Biotech Co., Ltd.) The slides were subsequently stained with
3,3′-diaminobenzidine for 5 min at room temperature prior to
counterstaining with haematoxylin. PBS was used as a negative
control. Sections were examined by using the image analyzer of a
light microscope (Olympus BX43; Olympus Corporation).
IHC assessment
Prior to pairing, both the primary tumors and
metastases tissues in each patient were assessed by two independent
pathologists at the pathology department of Shandong Cancer
Hospital in a blinded manner. Staining was evaluated as reported by
Qiu and Zhou (10), with a
semi-quantitative analysis incorporating both the proportion of
positively stained cells and the staining intensity. The
immunoreactions for HIF-1α were divided into four groups as
follows: 0, <1% of tumor cells exhibiting nuclear
immunostaining; 1, 1–10% of tumor cells; 2, 11–50% of tumor cells
and 3, >50% of tumor cells exhibiting nuclear immunostaining.
For VEGF, the evaluation was as follows: 0, <10% of tumor cells
exhibiting cytoplasmic immunostaining; 1, 11–25% of tumor cells; 2,
26–50% of tumor cells and 3, >50% of tumor cells exhibiting
cytoplasmic immunostaining. The intensity of HIF-1α and VEGF
staining was also determined semi-quantitatively on a scale of 0–3
as follows: 0, negative; 1, weakly positive; 2, moderately
positive; and 3, strongly positive. The final staining score was
determined by combining the percentage scores and staining
intensities, as follows: 0 (negative), + (1–4), ++
(5–8)
and +++ (9). Final staining scores
of 0 or + were classified as negative expression, while final
staining scores of ++ and +++ were classified as positive
expression. Microvessel density was visualized using
immunohistochemical detection of CD34 antigen and subsequently
quantified using Image Capture software (Panasonic Corporation;
version 3.7) (17,18). Briefly, tumor sections were observed
under a low power light microscope (magnification, ×40) in three
areas with the greatest degree of vascularization (hot spots of
vascularization). These three hot spots were then examined at high
magnification (×200) and the mean MVD for each specimen was
calculated. Stained endothelial cells or endothelial cell clusters
clearly separated from adjacent microvessels by tumor cells and/or
stroma elements were considered a single countable microvessel.
Microvessels were counted in three hot spots, and MVD was divided
into low and high groups, according to a median MVD value from all
samples from the present study.
Statistical analysis
Statistical analyses were performed using SPSS
software (version 17.0; SPSS, Inc.). Data were expressed as the
means ± standard deviation. The associations between HIF-1α and
VEGF expression, and the clinicopathological characteristics of
patients with CRC were assessed using the χ2 test.
Pearson's correlation test and McNemar test were used to compare
HIF-1α and VEGF staining between primary tumors and associated
metastatic sites (perfect correlation, 1.0). P<0.05 was
considered to indicate a statistically significant difference and
all statistical tests were two-sided.
Results
Association between HIF-1α, VEGF and
CD34 expression in primary and metastatic sites of CRC and
clinicopathological characteristics
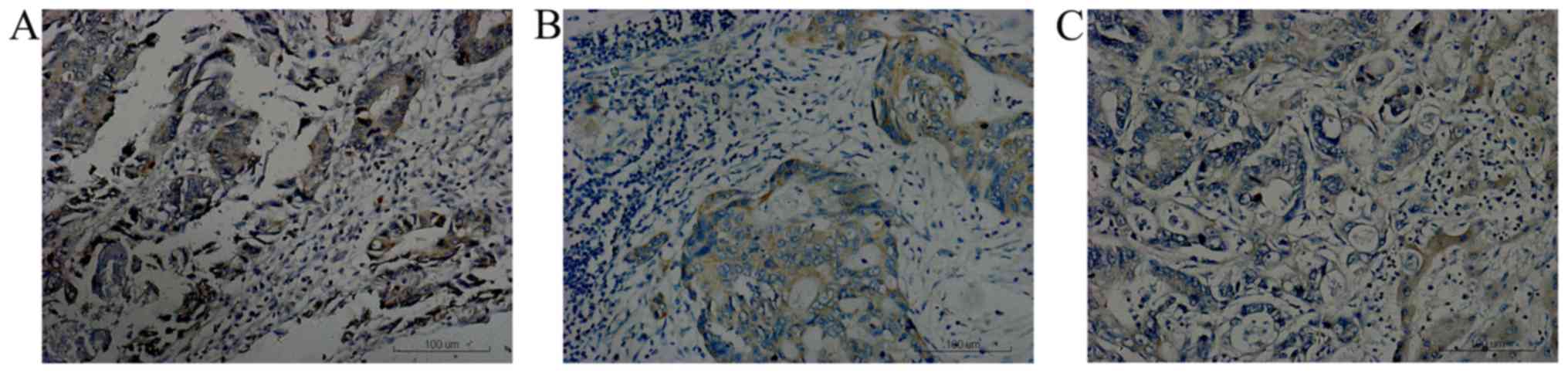
Positive HIF-1α staining was predominantly located
in the nucleus or cytoplasm with brown or brown yellow granules
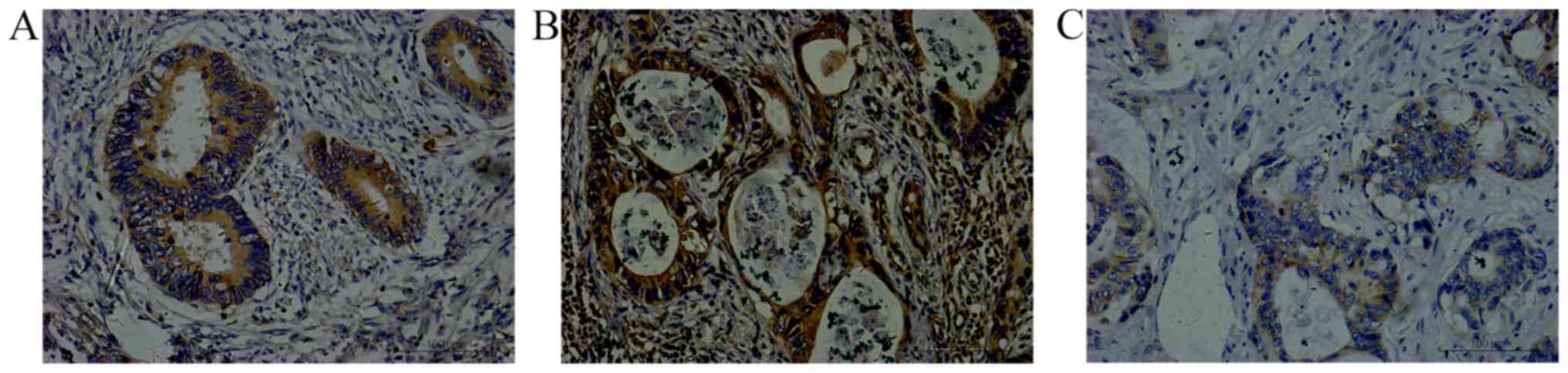
(Fig. 1), while positive VEGF
staining was indicated by the presence of brownish-yellow granules
in the cytoplasm or cell membrane (Fig.
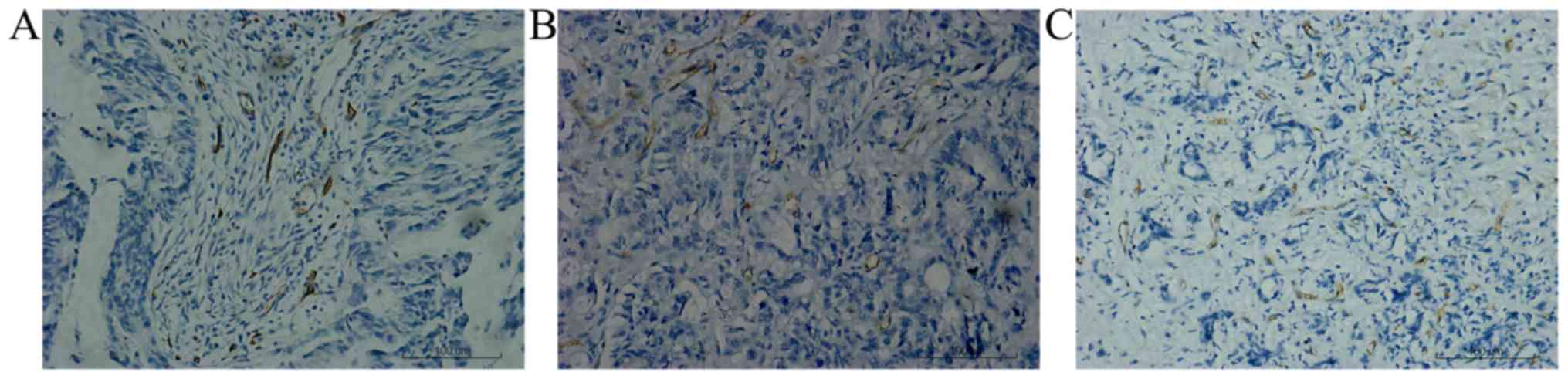
2) and CD34 staining was observed in the vascular endothelium
as brown sediments (Fig. 3). HIF-1α
was detectable (++ to +++) in 70.0% of the primary tumors, 60.9% of
the metastatic lymph nodes and 65.2% of the liver metastases. VEGF
was detectable (++ to +++) in 73.9% of the primary tumors, 58.7% of
the metastatic lymph nodes and 67.4% of the liver metastases. The
mean microvessel density was 16.2 (standard deviation=9.3), with a
range of 4–37. For the high group of MVD, it accounted for 78.3% of
the primary samples, 32.6% of the metastatic lymph nodes and 41.3%
of the liver metastases. Although HIF-1α and VEGF expression levels
were frequently positive in primary tumors compared with
corresponding metastases, no significant difference was observed.
However, MVD scores demonstrated a significant difference between
primary CRC and matched metastases (all P<0.05). Univariate
analysis demonstrated that no significant associations were
observed between HIF-1α and VEGF expression, and
clinicopathological characteristics in primary lesions and
metastatic sites (all P>0.05; Tables
I–III).
Association in individual tumor marker
expression between primary tumors and corresponding metastases
The association between HIF-1α and VEGF status in
primary tumors and paired metastatic lesions, and histological
sections demonstrated consistency (Table IV). A total of 38 patients (83%)
with HIF-1α and 35 patients (76%) with VEGF exhibited concordance
in expression between primary tumors and matched lymph node
metastases. The correlation coefficient of HIF-1α and VEGF were
(K=0.62; P<0.001) and (K=0.48; P=0.001), respectively.
Conversely, a total of 34 patients (74%) with HIF-1α and 39
patients (85%) with VEGF exhibited concordance with regards to
expression levels in primary tumors and liver metastases. The
correlation coefficient of HIF-1α and VEGF were (K=0.41; P=0.005)
and (K=0.64; P<0.001), respectively.
 | Table IV.HIF-1α and VEGF expression between
primary tumors and corresponding metastases. |
Table IV.
HIF-1α and VEGF expression between
primary tumors and corresponding metastases.
| A, HIF-1α
expression between primary tumors and corresponding lymph node
metastases (n=46) |
|---|
|
|---|
|
| Regional lymph node
metastasis |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Primary tumor | Negative, n | Positive, n | Concordance,
n/total n (%) | Discordance,
n/total n (%) | McNemar
P-value |
|---|
| Negative, n | 12 | 2 | 38/46 (83) | 8/46 (17) | 0.29 |
| Positive, n | 6 | 26 |
|
|
|
|
| B, HIF-1α
expression between primary tumors and synchronous liver metastasis
(n=46) |
|
|
| Synchronous
liver metastasis |
|
|
|
|
|
|
|
|
|
| Primary
tumor | Negative,
n | Positive,
n | Concordance,
n/total n (%) | Discordance,
n/total n (%) | McNemar
P-value |
|
| Negative, n | 9 | 7 | 34/46 (74) | 12/46 (26) | 0.29 |
| Positive, n | 7 | 25 |
|
|
|
|
| C, VEGF
expression between primary tumors and corresponding lymph node
metastases (n=46) |
|
|
| Regional lymph
node metastasis |
|
|
|
|
|
|
|
|
|
| Primary
tumor | Negative,
n | Positive,
n | Concordance,
n/total n (%) | Discordance,
n/total n (%) | McNemar
P-value |
|
| Negative, n | 10 | 2 | 35/46 (76) | 11/46 (24) | 0.45 |
| Positive, n | 9 | 25 |
|
|
|
|
| D, VEGF
expression between primary tumors and synchronous liver metastasis
(n=46) |
|
|
| Synchronous
liver metastasis |
|
|
|
|
|
|
|
|
|
| Primary
tumor | Negative,
n | Positive,
n | Concordance,
n/total n (%) | Discordance,
n/total n (%) | McNemar
P-value |
|
| Negative, n | 10 | 5 | 39/46 (85) | 7/46 (15) | 0.29 |
| Positive, n | 2 | 29 |
|
|
|
Minor differences between primary tumors and
metastatic lesions were still presented. Regarding HIF-1α
expression, eight cases demonstrated a discordance in expression
between primary tumors and lymph node metastases (P=0.29; Table IV), while 12 cases indicated
discordance between primary tumors and liver metastases (P=0.29;
Table IV). Among these, HIF-1α
immunostaining were negative expression in the primary tumor and
positive expression in the metastatic lesions in seven cases, while
opposing results were exhibited in the remaining 13 cases.
Regarding VEGF expression, 11 cases demonstrated discordance in
pairs of primary tumors and matched lymph node metastases (P=0.45;
Table IV), while seven cases
indicated discordant between primary tumors and matched liver
metastases (P=0.29; Table IV).
Among these, four cases exhibited positive expression in metastasis
but negative expression in primary tumors, while opposing results
were exhibited in the remaining 14 cases.
Discussion
Advanced colorectal cancer continues to present a
major health problem worldwide. Similar to other types of malignant
tumor, CRC is a systemic disease. Eliminating metastatic lesions
allows for successful comprehensive surgical treatment. Hypoxia and
angiogenesis are a common phenomenon in solid tumors, which play
key roles in cancer progression (19). HIF-1α and VEGF are the most potent
angiogenic proteins, which promote malignant transformation,
angiogenesis and metastatic dissemination (20). Currently, patients with mCRC
resistant to chemotherapy benefit from anti-angiogenesis-targeted
therapies, such as bevacizumab, which has been widely applied in
clinical practice (21). Unlike
epidermal growth factor receptor (EGFR), angiogenesis inhibitors do
not have specific target populations and present no clear
indications for clinical application in CRC. The benefit of
angiogenesis inhibitors is that the unselected patient population
are modest (22). However, whether
primary CRC and their associated metastases have similar levels of
angiogenesis has not yet been determined. This gap in the
literature is primarily attributable to the incorrect assumption
that primary CRC and metastatic lesions are pathologically
consistent (12). Thus, the
comparative analysis of HIF-1α, VEGF and MVD differences between
primary and metastatic tumors may improve understanding of the
changes of metastases, promote research and application of novel
targeted drugs and provide information to predict whether
anti-angiogenesis targeted drugs may benefit patients with
mCRC.
According to published data, HIF-1α and VEGF
upregulation in CRC at the protein level ranges from 55–65 and
44–64%, respectively, while less expression is observed in normal
tissues (23,24), and relatively little is known about
the role of hypoxia and angiogenesis in metastases. In the present
study, hypoxia and angiogenesis in lymph node and liver metastases
tissues of 46 patients with mCRC were investigated and compared
with the primary tumor. HIF-1α, VEGF and CD34 were positively
expressed at both sites, indicating that they were closely
associated with the occurrence and development of mCRC (25). Further research and analysis
demonstrated no significant difference in the expression levels of
HIF-1α and VEGF across different ages, sex, tumor sizes and degrees
of histological differentiation. The current results are consistent
with previous findings of the major of studies published to date
(26,27). A previous study reported that HIF-1α
and VEGF expression are frequently associated with depth of
invasion (28). Although the
positive rate of HIF-1α and VEGF in patients with pT4 was slightly
higher than that of patients with pT3 in both primary tumors and
metastases in the present study, no significant difference was
observed. These results are also inconsistent with findings
reported in the literature and may be associated with all patients
with distant metastasis in stage IV of the present study.
In order to fully utilize individualized treatment
in molecular targeted therapies, an immunohistochemical evaluation
of target molecule expression at the primary tumor site, as well as
at metastatic sites is required to provide value for therapeutic
decisions and decrease high costs (29). However, regarding the expression of
angiogenesis markers in metastatic sites of CRC, only a few cases
have been reported in the literature, and the research results are
inconsistent. For example, Shim et al (30) demonstrated that HIF-1α expression is
higher in metastatic lymph nodes compared with primary lesions in
breast cancer. However, Fraga et al (31) reported that there is no difference in
HIF-1α protein expression between primary lesions and metastatic
sites in the upper aerodigestive tract of patients with cancer. Due
to the fact that metastases are not easily accessible from the same
patients in clinical work, several aspects of the molecular
mechanism of CRC metastasis are yet to be elucidated. The results
of the present study demonstrated that HIF-1α and VEGF expression
levels in corresponding metastases were lower than in primary
tumors; however, only the difference in MVD value was statistically
significant. This indicates that compared with metastases, there
may be less oxygen in primary tumors, and hypoxia may provide the
stimulus that upregulates HIF-1α and VEGF transcription and
malignancy (27). In most types of
human malignant tumor, the vasculature is a direct result of
angiogenesis. MVD is one marker of tumor angiogenesis (32). The present study used CD34 to detect
MVD in primary colorectal tumors and matched metastases, and it was
revealed that the metastases were poorly vascularised compared with
the primary tumors. This was not consistent with the trends seen in
HIF-1α and VEGF expression. In CRC, the primary tumor suppresses
the vascularization of distant metastases, and the vascular density
of the primary tumor is higher than its metastases (33). Furthermore, a variety of internal
environmental factors may affect angiogenesis in metastatic
lesions. For example, in lymph node metastases, which may have a
good blood supply, the growth of metastatic lesions in the lymph
nodes is not dependent on angiogenesis (34). Additionally, anti-tumor immunity in
lymph nodes may be associated with the inhibition of
neovascularization, thereby explaining the low MVD (35,36). In
clinical studies, although patients with CRC received neoadjuvant
chemotherapy and bevacizumab, lymph node metastases were still
observed at the time of surgery and pathological evaluation,
suggesting a lack of response of lymph node metastasis to
anti-angiogenic therapy (37). The
replacement pattern is one of three growth patterns of liver
metastases of CRC, in which tumor cells simply replace hepatocytes,
and where the liver architecture and sinusoidal blood vessels
provide an angiogenic prosperous environment for metastatic tumor
growth (38,39). Additionally, blood vessels
surrounding liver metastasis are heterogeneous, and the relatively
high levels of oxygen in the highly vascular liver that cause MVD
are less of an angiogenic driving force (40,41). MVD
is different in primary CRC and metastatic lesions, suggesting that
close attention should be paid to the changes of molecular
indicators in the course of disease progression, which may inform
the treatment strategy of patients. Thus, the blood vessels of
metastases may respond in a different way to anti-angiogenic
therapy and partially explain the unsatisfactory therapeutic effect
of anti-angiogenic and/or anti-vascular treatments, such as
bevacizumab, supporting the low MVD measured in previous studies of
metastases (41,42). Nevertheless, HIF-1α and VEGF were
highly expressed in corresponding metastases in the present study,
supporting the notion of targeted therapies, such as
anti-HIF-1α/anti-VEGF monoclonal antibodies, which may be involved
in the metastasis of CRC and may serve as a novel target to treat
mCRC (43).
Whether HIF-1α and VEGF expression between primary
and metastatic colorectal cancer is consistent has rarely been
reported in the literature. Shimomura et al (19) reported that HIF-1α expression levels
in liver metastases is significantly associated with that in the
corresponding primary tumor. Furthermore, Nakamoto et al
(44) analysed VEGF tissue samples
from pairs of primary tumors and corresponding metastatic liver
tumors and reported that the primary and associated metastatic
liver demonstrated concordant immunoreactivity for VEGF in CRC.
Kobayashi et al (45) also
reported that VEGF expression in hepatic metastatic tumors is
positively associated with its expression level in primary tumors
in CRC. The results of the present study demonstrated that in 46
pairs of primary tumors and matched metastases, 38 patients (83%)
for HIF-1α and 35 cases (76%) for VEGF demonstrated concordance in
lymph node metastases, and 34 patients (74%) for HIF-1α and 39
cases (85%) for VEGF indicated consistency in liver metastases.
This suggests that patients with high angiogenesis activity in
primary cancer also exhibit a high degree of angiogenesis in the
corresponding metastatic sites. Concordance may indicate that
primary tumors and corresponding metastases have the same genomic
status and that cancer cells remain notably stable in metastatic
tumors (13). Thus, the expression
profiles of HIF-1α and VEGF during the metastatic process were
mainly unchanged. The data suggest that detection of HIF-1α and
VEGF expression in either a primary tumor or metastases may be
reliable indicator used to inform treatment decisions with HIF-1α
and/or VEGF inhibitors (19).
Targeted therapy for HIF-1α and VEGF in metastases may achieve good
therapeutic effects; however, further investigations with more
cases are needed to confirm these findings.
Minor differences were observed between primary
tumors and metastatic lesions in the present study. The molecular
mechanism by which metastasis occurs is hypothesized to exist in a
small population of cells in the primary tumor, and these changes
result in the emergence of metastases (12). This hypothesis would predict a degree
of difference in protein expression between primary tumors and
metastases. Additionally, the expression of biomarkers is prone to
be influenced by several clinicopathological and local
microenvironments of the liver and lymph node (46). Thus, a certain degree of difference
in protein expression may exist between primary tumors and
corresponding metastases. In the present study, when matched tissue
sets were compared on an individual basis, eight cases demonstrated
discordant HIF-1α expression, and 11 cases exhibited discordant
VEGF expression, in 46 pairs of primary tumors and paired
metastatic lymph nodes. A discordant rate of 26 and 15% were
observed for HIF-1α and VEGF expression between primary tumors and
liver metastases, respectively, with no statistical difference.
Thus, major concordance and minor differences are observed between
primary CRCs and corresponding metastases, suggesting that the
inhibitors of HIF-1α and VEGF are effective in mCRC.
In conclusion, HIF-1α and VEGF were overexpressed in
both primary and matched metastatic tissues of CRC. HIF-1α and VEGF
expression status of the primary tumor were concordant with
corresponding metastases, suggesting that the markers are stable in
the process of metastasis and provide a reliable basis for
predicting the angiogenic activity of metastases by analysing the
primary tumor. Based on the present data, the results represent
important implications for understanding the biology of metastasis
in mCRC, providing evidence for further use of inhibitors of HIF-1α
and VEGF. However, the local microenvironment of metastases may
affect the angiogenesis of tumor cells. Differences in angiogenesis
of different metastatic sites may have different therapeutic
consequences when treatment with anti-angiogenic therapy is
considered, thus further research is required.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Development Plan of Jinan (grant nos. 201805091 and
201401253) and the Postdoctoral Science Foundation of China (grant
no. 2017M612317).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YS conceived and designed the present study. LY, JL
and DM performed the experiments. DL participated in
immunohistochemistry and performed the statistical analysis. LY and
DL reviewed and edited the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics and
Scientific Committees of Shandong Cancer Hospital (Shandong, China)
and performed in accordance with The Declaration of Helsinki.
Written informed consent was obtained from all patients prior to
the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Song X, Zhao Z, Barber B, Gregory C,
Schutt D and Gao S: Characterizing medical care by disease phase in
metastatic colorectal cancer. Am J Manag Care. 17 (Suppl
5):SP20–SP25. 2011.PubMed/NCBI
|
|
3
|
Devesa H, Pereira L, Gonçalves A, Brito T,
Almeida T, Torres R and Midoes A: Axillary lymph node metastasis of
colon cancer-case report and literature review. Case Rep Clin Med.
12:669–673. 2014. View Article : Google Scholar
|
|
4
|
Kirstein MM, Lange A, Prenzler A, Manns
MP, Kubicka S and Vogel A: Targeted therapies in metastatic
colorectal cancer: A systematic review and assessment of currently
available data. Oncologist. 19:1156–1168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim DH, Sung B, Kang YJ, Hwang SY, Kim MJ,
Yoon JH, Im E and Kim ND: Sulforaphane inhibits hypoxia-induced
HIF-1α and VEGF expression and migration of human colon cancer
cells. Int J Oncol. 47:2226–2232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ajith TA: Current insights and future
perspectives of hypoxia-inducible factor targeted therapy in
cancer. J Basic Clin Physiol Pharmacol. 30:11–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Semenza GL: HIF-1 mediates metabolic
responses to intratumoral hypoxia and oncogenic mutations. J Clin
Invest. 123:3664–3671. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nieves BJ, D'Amore PA and Bryan BA: The
function of vascular endothelial growth factor. Biofactors.
35:332–337. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iwasaki K, Yabushita H, Ueno T and
Wakatsuki A: Role of hypoxia-inducible factor-1α, carbonic
anhydrase-IX, glucose transporter-1 and vascular endothelial growth
factor associated with lymph node metastasis and recurrence in
patients with locally advanced cervical cancer. Oncol Lett.
10:1970–1978. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brahimi-Horn MC, Chiche J and Pouyssegur
J: Hypoxia and cancer. J Mol Med (Berl). 85:1301–1307. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cohen SA, Yu M, Baker K, Redman M, Wu C,
Heinzerling TJ, Wirtz RM, Charalambous E, Pentheroudakis G, Kotoula
V, et al: The CpG island methylator phenotype is concordant between
primary colorectal carcinoma and matched distant metastases. Clin
Epigenetics. 9:462017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luo KJ, Hu Y, Wen J and Fu JH: CyclinD1,
p53, E-cadherin, and VEGF discordant expression in paired regional
metastatic lymph nodes of esophageal squamous cell carcinoma: A
tissue array analysis. J Surg Oncol. 104:236–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Knosel T, Schluns K, Dietel M and Petersen
I: Chromosomal alterations in lung metastases of colorectal
carcinomas: Associations with tissue specific tumor dissemination.
Clin Exp Metastasis. 22:533–538. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu Y, Jin M, Xu H, Shimin Z, He S, Wang L
and Zhang Y: Clinicopathologic significance of HIF-1α, CXCR4, and
VEGF expression in colon cancer. Clin Dev Immunol. 2010(pii):
5375312010.PubMed/NCBI
|
|
15
|
Qiu Y and Zhou H: Expression of HIF-1alpha
and VEGF in human laryngeal carcinoma and its relationship with
angiogenes. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi.
28:389–393. 2014.(In Chinese). PubMed/NCBI
|
|
16
|
Matsuo Y, Ding Q, Desaki R, Maemura K,
Mataki Y, Shinchi H, Natsugoe S and Takao S: Hypoxia inducible
factor-1 alpha plays a pivotal role in hepatic metastasis of
pancreatic cancer: An immunohistochemical study. J Hepatobiliary
Pancreat Sci. 21:105–112. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schlingemann RO, Rietveld FJ, de Waal RM,
Bradley NJ, Skene AI, Davies AJ, Greaves MF, Denekamp J and Ruiter
DJ: Leukocyte antigen CD34 is expressed by a subset of cultured
endothelial cells and on endothelial abluminal microprocesses in
the tumor stroma. Lab Invest. 62:690–696. 1990.PubMed/NCBI
|
|
18
|
Hlatky L, Hahnfeldt P and Folkman J:
Clinical application of antiangiogenic therapy: microvessel
density, what it does and doesn't tell us. J Natl Cancer Inst.
94:883–893. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shimomura M, Hinoi T, Kuroda S, Adachi T,
Kawaguchi Y, Sasada T, Takakura Y, Egi H, Okajima M, Tashiro H, et
al: Overexpression of hypoxia inducible factor-1 alpha is an
independent risk factor for recurrence after curative resection of
colorectal liver metastases. Ann Surg Oncol. 20 (Suppl
3):S527–S536. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jensen NF, Smith DH, Nygard SB, Romer MU,
Nielsen KV and Brunner N: Predictive biomarkers with potential of
converting conventional chemotherapy to targeted therapy in
patients with metastatic colorectal cancer. Scand J Gastroenterol.
47:340–355. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ulivi P, Marisi G and Passardi A:
Relationship between hypoxia and response to antiangiogenic therapy
in metastatic colorectal cancer. Oncotarget. 7:46678–46691. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao D, Hou M, Guan YS, Jiang M, Yang Y and
Gou HF: Expression of HIF-1alpha and VEGF in colorectal cancer:
Association with clinical outcomes and prognostic implications. BMC
cancer. 9:4322009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Simiantonaki N, Taxeidis M, Jayasinghe C,
Kurzik-Dumke U and Kirkpatrick CJ: Hypoxia-inducible factor 1 alpha
expression increases during colorectal carcinogenesis and tumor
progression. BMC Cancer. 8:3202008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Berk V, Deniz K, Bozkurt O, Ozaslan E,
Karaca H, Inanc M, Duran AO and Ozkan M: Predictive significance of
VEGF and HIF-1α expression in patients with metastatic colorectal
cancer receiving chemotherapy combinations with bevacizumab. Asian
Pac J Cancer Prev. 16:6149–6154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kurokawa T, Miyamoto M, Kato K, Cho Y,
Kawarada Y, Hida Y, Shinohara T, Itoh T, Okushiba S, Kondo S and
Katoh H: Overexpression of hypoxia-inducible-factor 1alpha
(HIF-1alpha) in oesophageal squamous cell carcinoma correlates with
lymph node metastasis and pathologic stage. Br J Cancer.
89:1042–1047. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maxwell PH, Dachs GU, Gleadle JM, Nicholls
LG, Harris AL, Stratford IJ, Hankinson O, Pugh CW and Ratcliffe PJ:
Hypoxia-inducible factor-1 modulates gene expression in solid
tumors and influences both angiogenesis and tumor growth. Proc Natl
Acad Sci USA. 94:8104–8109. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ioannou M, Paraskeva E, Baxevanidou K,
Simos G, Papamichali R, Papacharalambous C, Samara M and Koukoulis
G: HIF-1α in colorectal carcinoma: Review of the literature. J
BUON. 20:680–689. 2015.PubMed/NCBI
|
|
29
|
Bar J, Herbst RS and Onn A: Targeted drug
delivery strategies to treat lung metastasis. Expert Opin Drug
Deliv. 6:1003–1016. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shim H, Lau SK, Devi S, Yoon Y, Cho HT and
Liang Z: Lower expression of CXCR4 in lymph node metastases than in
primary breast cancers: Potential regulation by ligand-dependent
degradation and HIF-1alpha. Biochem Biophys Res Commun.
346:252–258. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fraga CA, de Oliveira MV, de Oliveira ES,
Barros LO, Santos FB, Gomez RS, De-Paula AM and Guimarães AL: A
high HIF-1α expression genotype is associated with poor prognosis
of upper aerodigestive tract carcinoma patients. Oral Oncol.
48:130–135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jilaveanu LB, Puligandla M, Weiss SA, Wang
XV, Zito C, Flaherty KT, Boeke M, Neumeister V, Camp RL, Adeniran
A, Pins M, et al: Tumor microvessel density as a prognostic marker
in high-risk renal cell carcinoma patients treated on ECOG-ACRIN
E2805. Clin Cancer Res. 24:217–223. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Peeters CF, Westphal JR, de Waal RM,
Ruiter DJ, Wobbes T and Ruers TJ: Vascular density in colorectal
liver metastases increases after removal of the primary tumor in
human cancer patients. Int J Cancer. 112:554–559. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guo M, Mu Y, Yu D, Li J, Chen F, Wei B, Bi
S, Yu J and Liang F: Comparison of the expression of TGF-β1,
E-cadherin, N-cadherin, TP53, RB1CC1 and HIF-1α in oral squamous
cell carcinoma and lymph node metastases of humans and mice. Oncol
Lett. 15:1639–1645. 2018.PubMed/NCBI
|
|
35
|
Jeong HS, Jones D, Liao S, Wattson DA, Cui
CH, Duda DG, Willett CG, Jain RK and Padera TP: Investigation of
the lack of angiogenesis in the formation of lymph node metastases.
J Natl Cancer Inst. 107(pii): djv1552015.PubMed/NCBI
|
|
36
|
Roberts N, Kloos B, Cassella M,
Podgrabinska S, Persaud K, Wu Y, Pytowski B and Skobe M: Inhibition
of VEGFR-3 activation with the antagonistic antibody more potently
suppresses lymph node and distant metastases than inactivation of
VEGFR-2. Cancer Res. 66:2650–2657. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Willett CG, Duda DG, Ancukiewicz M, Shah
M, Czito BG, Bentley R, Poleski M, Fujita H, Lauwers GY, Carroll M,
et al: A safety and survival analysis of neoadjuvant bevacizumab
with standard chemoradiation in a phase I/II study compared with
standard chemoradiation in locally advanced rectal cancer.
Oncologist. 15:845–851. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stessels F, Van den Eynden G, Van der
Auwera I, Salgado R, V n den Heuvel E, Harris AL, Jackson DG,
Colpaert CG, van Marck EA, Dirix LY and Vermeulen PB: Breast
adenocarcinoma liver metastases, in contrast to colorectal cancer
liver metastases, display a non-angiogenic growth pattern that
preserves the stroma and lacks hypoxia. Br J Cancer. 90:1429–1436.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
van der Wal GE, Gouw AS, Kamps JA, Moorlag
HE, Bulthuis ML, Molema G and de Jong KP: Angiogenesis in
synchronous and metachronous colorectal liver metastases: The liver
as a permissive soil. Ann Surg. 255:86–94. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rajaganeshan R, Prasad R, Guillou PJ,
Scott N, Poston G and Jayne DG: Expression patterns of hypoxic
markers at the invasive margin of colorectal cancers and liver
metastases. Eur J Surg Oncol. 35:1286–1294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Raluca BA, Cimpean AM, Cioca A, Cretu O,
Mederle O, Ciolofan A, Gaje P and Raica M: Endothelial cell
proliferation and vascular endothelial growth factor expression in
primary colorectal cancer and corresponding liver metastases. Asian
Pac J Cancer Prev. 6:4549–4553. 2015. View Article : Google Scholar
|
|
42
|
Sandhu J, Lavingia V and Fakih M: Systemic
treatment for metastatic colorectal cancer in the era of precision
medicine. J Surg Oncol. 119:564–582. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim YW, Ko YT, Kim NK, Chung HC, Min BS,
Lee KY, Park JP and Kim H: A comparative study of protein
expression in primary colorectal cancer and synchronous hepatic
metastases: The significance of matrix metalloproteinase-1
expression as a predictor of liver metastasis. Scand J
Gastroenterol. 45:217–225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nakamoto RH, Uetake H, Iida S, Kolev YV,
Soumaoro LT, Takagi Y, Yasuno M and Sugihara K: Correlations
between cyclooxygenase-2 expression and angiogenic factors in
primary tumors and liver metastases in colorectal cancer. Jpn J
Clin Oncol. 37:679–685. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kobayashi H, Sugihara K, Uetake H, Higuchi
T, Yasuno M, Enomoto M, Kuramochi H, Lenz HJ, Danenberg KD and
Danenberg PV: Messenger RNA expression of vascular endothelial
growth factor and its receptors in primary colorectal cancer and
corresponding liver metastasis. Ann Surg Oncol. 15:1232–1238. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Klein CA: Parallel progression of primary
tumors and metastases. Nat Rev Cancer. 9:302–312. 2009. View Article : Google Scholar : PubMed/NCBI
|