Introduction
In 2018, the incidence rate of colon cancer was
reported as 6.1, and 5.8% mortality rate worldwide (1). Despite recent advances in radical
surgery, adjuvant chemotherapy and target therapy, tumor recurrence
and metastasis remain a major concern, and contribute to impaired
survival in patients with colon cancer (2). The complexity of cancer makes it
difficult to predict the prognosis for colon cancer (3,4).
Therefore, identifying prognostic biomarkers is warranted to
improve the direction of treatment strategies and survival
(5).
Small proline-rich protein 1A (SPRR1A), a member of
the SPRR family, is a cross-linked envelope protein of
keratinocytes (6,7). Previously, it has been described as a
specific marker of differentiation of keratinocytes and squamous
epithelial cells (6,8). Accumulating evidence has revealed the
prognostic importance of the high expression of SPRR1A in various
types of cancer, including diffuse large B-cell lymphomas (9), head and neck squamous cell carcinoma
(10), and breast cancer (11). In addition, high SPRR1A expression
was also reported in a murine preneoplastic intestine and in the
normal intestinal mucosa of patients with colorectal cancer (CRC)
(12). Colonic carcinogenesis is a
multistage process involving environmental and genetic changes
(13). Therefore, the aim of the
present study was to examine whether high expression of SPRR1A may
serve as a promising biomarker for colon cancer prognosis
assessment. To the best of our knowledge, few studies have focused
on this issue (9,11,12).
In order to address this literature gap, the present
study aimed to examine SPRR1A expression in cancerous and adjacent
non-cancerous colon tissues using immunohistochemical staining. The
associations between SPRR1A expression and clinicopathological
parameters of colon cancer were subsequently investigated. The
prognostic value of SPRR1A expression was examined using Cox
regression and bioinformatics analysis.
Materials and methods
Patient tissue samples
Patients with colon cancer who underwent radical
surgery between June 2010 and December 2010 at Fujian Medical
University Union Hospital were identified from the prospectively
maintained database. This study was approved by the Medical Ethics
Committee of Fujian Medical University Union Hospital and all
patients provided written informed consent on admission. Inclusion
criteria included pathologically proven colon adenocarcinoma, and
tumor location in the ascending, transverse, descending or sigmoid
colon. Patient exclusion criteria were as follows: i) Age, <18
years; ii) contraindications for surgery; iii) hereditary CRC
syndromes; iv) multiple primary neoplasms; v) loss of follow-up;
and vi) incomplete clinical records.
A total of 114 patients were included in this study.
Cancerous colon tissues and adjacent non-cancerous tissues were
collected and stored in liquid nitrogen until further analysis. The
clinicopathological features were obtained from medical records
based on the CRC database, including age, sex, body mass index
(BMI), gross type, histological differentiation, pathological TNM
staging (14) and survival outcome.
The variables of colon cancer are presented in Table I. Survival outcomes were obtained
from the post-operative surveillance.
 | Table I.Patient characteristics (n=114). |
Table I.
Patient characteristics (n=114).
| Characteristics | P-value |
|---|
| Mean age ± SD,
years |
62.6±13.4 |
| BMI (mean ± SD,
kg/m2) | 21.9±2.8 |
| Sex, n (%) |
|
| Male | 76
(66.7) |
|
Female | 38
(33.3) |
| Pretreatment CEA
level, n (%) |
|
| <5
ng/ml | 68
(59.6) |
| ≥5
ng/ml | 46
(40.4) |
| Pretreatment CA19-9
level, n (%) |
|
| <37
U/ml | 98
(86.0) |
| ≥37
U/ml | 16
(14.0) |
| Tumor location, n
(%) |
|
| Ascending
colon | 34
(29.8) |
|
Transverse colon | 4
(3.5) |
|
Descending colon | 11 (9.6) |
| Sigmoid
colon | 65
(57.0) |
| Gross type, n
(%) |
|
|
Expanding | 45
(39.5) |
|
Ulcerated | 64
(56.1) |
|
Infiltrating | 5
(4.4) |
| Histopathology, n
(%) |
|
|
Adenocarcinoma | 101 (88.6) |
| Mucinous
or signet ring adenocarcinoma | 13
(11.4) |
| Tumor
differentiation, n (%) |
|
| Grade
1+2 | 87
(76.3) |
| Grade
3+4 | 27
(23.7) |
| T stage, n (%) |
|
| T1 | 7
(6.1) |
| T2 | 9
(7.9) |
| T3 | 61
(53.5) |
| T4 | 37
(32.5) |
| Lymph node invasion,
n (%) |
|
| No | 61
(53.5) |
| Yes | 53
(46.5) |
| Pathological TNM
stage, n (%) |
|
| I | 13
(11.4) |
| II | 44
(38.6) |
| III | 44
(38.6) |
| IV | 13
(11.4) |
| SPRR1A expression, n
(%) |
|
| High | 82
(71.9) |
| Low | 32
(28.1) |
| Mean OS time ± SD,
months |
76.5±32.8 |
Patient follow-up
Post-operative surveillance was conducted four times
in the first 3 years, then twice for the next 2 years and annually
thereafter. Follow-up items included a physical examination, serum
carcinoembryonic antigen (CEA) test, chest X-ray or CT scans,
abdominopelvic MRI or CT scans, and an annual colonoscopy. The last
follow-up time was recorded as the mortality of the patient or the
cutoff date of September 30, 2018.
Immunohistochemistry (IHC)
Immunohistochemical staining was performed using the
streptavidin-biotin complex method (15), in order to determine SPRR1A protein
expression. For immunohistochemical studies, surgical specimens
were harvested and fixed in 10% formalin overnight at room
temperature, prior to being embedded in paraffin at room
temperature. Paraffin-embedded tissue samples were cut into
4-μm-thick sections. In brief, the slides were blocked with 5%
normal goat serum (Fuzhou Maixin Biotech Co., Ltd.) for 20 min at
room temperature. Tissue sections were incubated with primary
antibody directed against SPRR1A (1:200; cat. no. bs-11162R; BIOSS)
overnight at 4°C. After 24 h, membranes were incubated with
horseradish peroxidase-labeled secondary antibody (1:500; cat. no.
SP KIT-C1; Fuzhou Maixin Biotech Co., Ltd.) for 1 h at room
temperature. The slides were subsequently stained with
3,3′-diaminobenzidine for 30 min at room temperature and observed
under an optical light microscope (magnification, ×100). PBS was
used as a negative control.
Tissue sections were observed and blindly scored by
two independent pathologists from the Department of Pathology,
Fujian Medical University Union Hospital (Fuzhou, China). The
percentage of positive cells and the color were determined based on
the intensity score (16). The
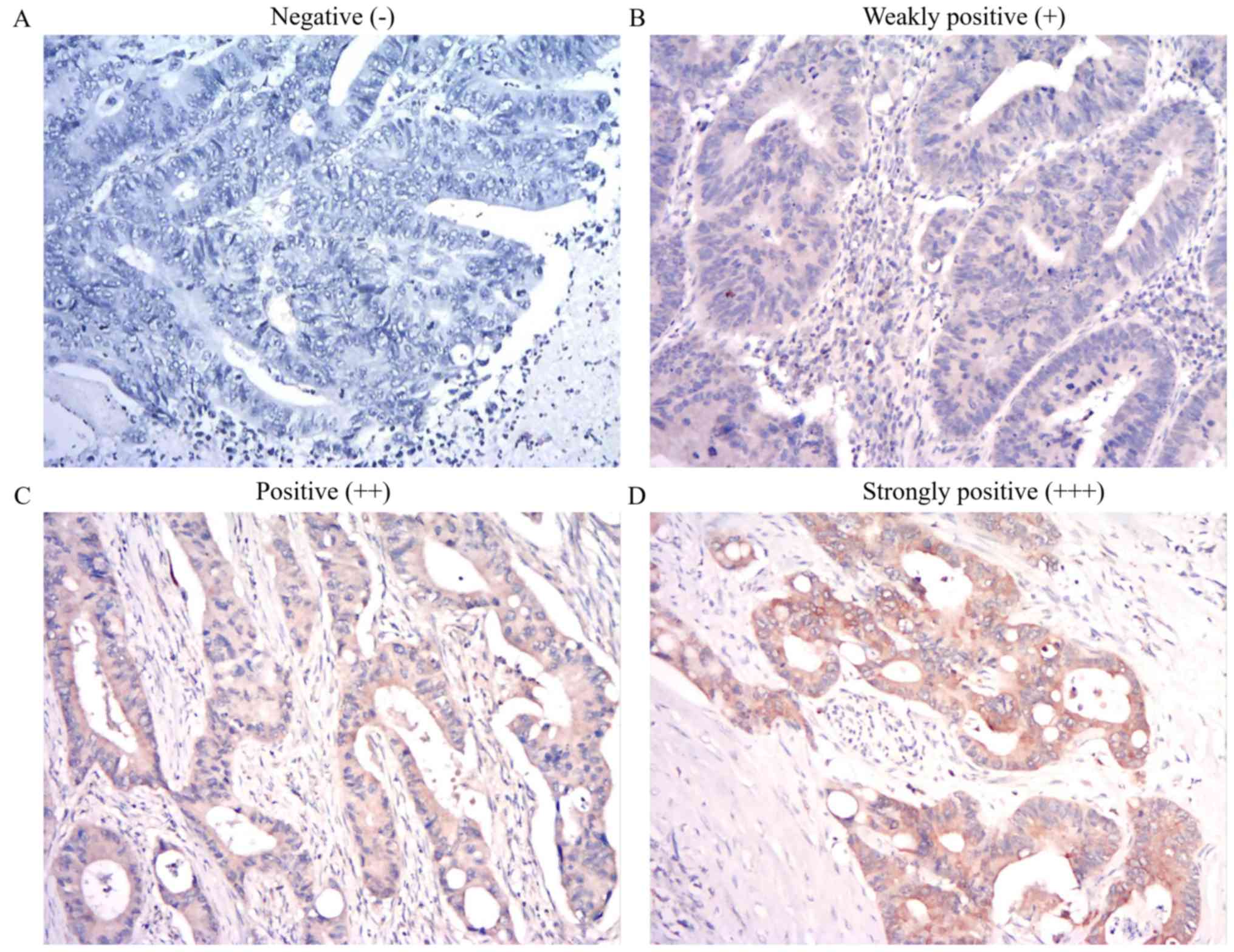
staining intensity was scored as follows: 0, no staining; 1, light
yellow; 2, brown and 3, deep brown. The percentage of positive
cells was scored as follows: 0, <5%; 1 (5-≤25%); 2 (>25–50%);
3, (51–75%) and 4 (>75%). The final score was obtained by
multiplying these two scores: 0, negative (−); 1–4, weakly positive
(+); 5–8, positive (++) and 9–12, strongly positive (+++) (16). SPRR1A expression was divided into two
groups as follows: High SPRR1A expression, final score 5–12; and
low SPRR1A expression, final score 0–4.
Bioinformatic analysis of SPRR1A
Meta-analysis of SPRR1A expression in colon cancer
tissues was performed using the Oncomine database (https://www.oncomine.org). The prognostic impact of
SPRR1A on CRC was evaluated using the R2: Genomics Analysis and
Visualization Platform (17).
Statistical analysis
Statistical analyses were conducted using SPSS
(version 24.0; IBM, Corp.). Data were presented as the mean ±
standard deviation (SD), as appropriate. Clinicopathological
characteristics between groups were compared using the
χ2, Student's t-test and paired Student's t-test, when
appropriate. Survival outcomes were evaluated with the Kaplan-Meier
method and compared via the log-rank test. Univariate and
multivariate analysis were conducted by using a Cox regression
model to evaluate the prognostic value of SPRR1A expression.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Baseline characteristics
A total of 114 patients with colon cancer (76 men
and 38 women) were included in the present study. The mean age was
62.6 years (range, 49.2–76.1 years). Baseline clinicopathological
characteristics are presented in Table
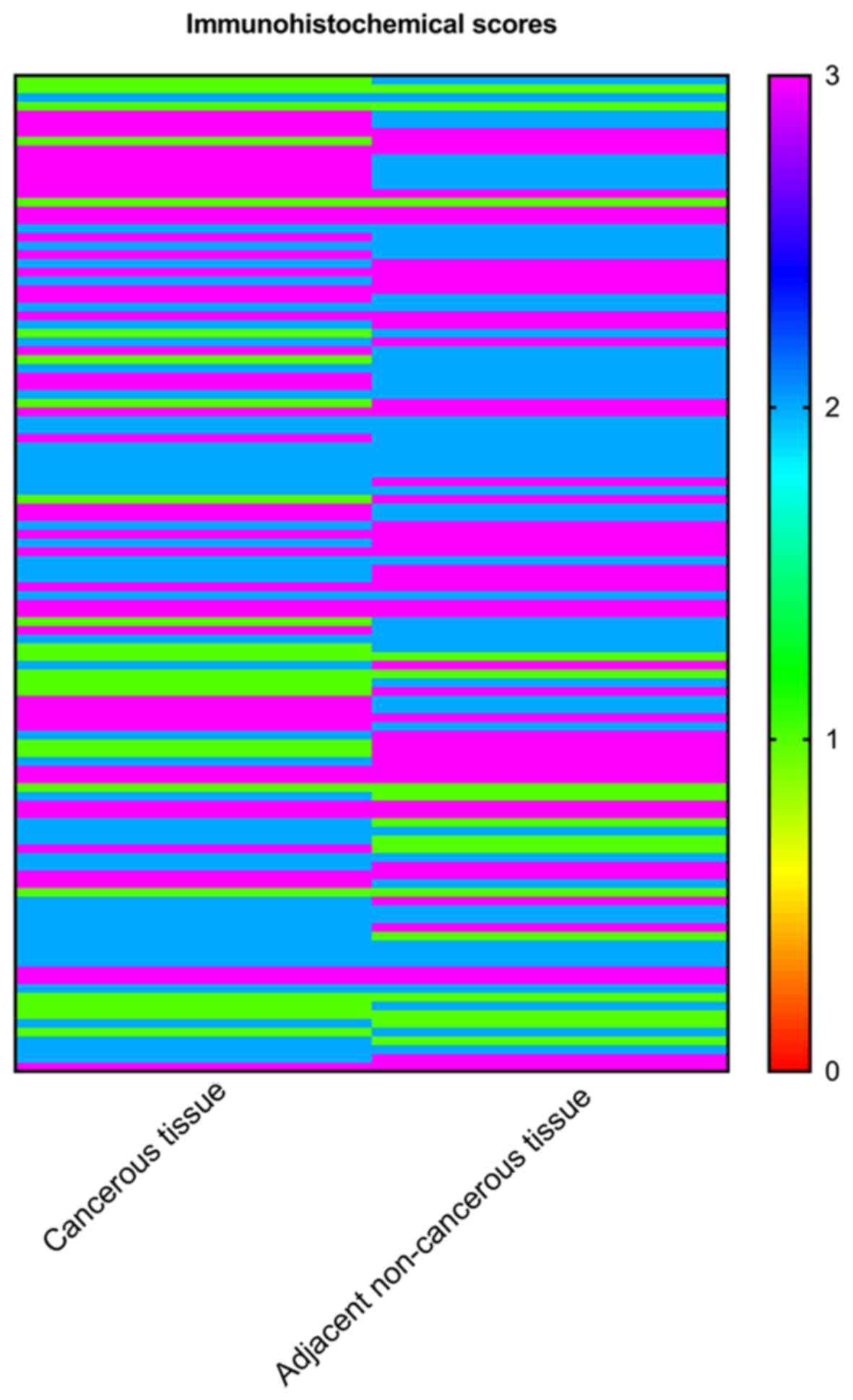
I. SPRR1A expression significantly increased in colon cancer
tissues compared with adjacent non-cancerous tissues via paired
sample t-test analysis (P<0.001; Fig.
1). Based on the IHC results of SPRR1, 32 patients with a final
score of 0–4 were classified in the low SPRR1A expression group
(Fig. 2A and B), and 82 patients
with a final score of 5–12 were classified in the high SPRR1A
expression group (Fig. 2C and
D).
Association of SPRR1A expression with
clinicopathological characteristics of patients with colon
cancer
The present study subsequently evaluated the
association of SPRR1A expression with the clinicopathological
features patients with colon cancer (Table II). High SPRR1A expression was
significantly associated with lymph node invasion (P=0.042);
however, no significant associations were demonstrated between
SPRR1A expression and other clinicopathological features, including
age, sex, BMI, pretreatment CEA and carbohydrate antigen 19-9
(CA19-9) levels, tumor location, gross type of tumor, tumor
histopathology, tumor differentiation and pathological T stage (all
P>0.05). The incidence of distant metastasis was 15.9% in the
high SPRR1A expression group and 3.1% in the low SPRR1A expression
group; however, no significant differences were observed between
the two groups.
 | Table II.Association between SPRR1A expression
and clinicopathological characteristics in patients with colon
cancer. |
Table II.
Association between SPRR1A expression
and clinicopathological characteristics in patients with colon
cancer.
|
| SPRR1A
expression |
|
|---|
|
|
|
|
|---|
| Characteristics | Low (n=32) | High (n=82) | P-value |
|---|
| Mean age ± SD,
years |
64.0±12.4 |
62.1±13.8 | 0.685 |
| Mean BMI ± SD,
kg/m2 | 21.5±2.8 | 22.1±2.8 | 0.683 |
| Pretreatment CEA, n
(%) |
|
| 0.525 |
| <5
ng/ml | 21 (65.6) | 47 (57.3) |
|
| ≥5
ng/ml | 11 (34.4) | 35 (42.7) |
|
| Pretreatment
CA19-9, n (%) |
|
| 0.760 |
| <37
ng/ml | 27 (84.4) | 71 (86.6) |
|
| ≥37
ng/ml | 5
(15.6) | 11 (13.4) |
|
| Pathological T
stage, n (%) |
|
| 0.337 |
| T1 | 4
(12.5) | 3 (6.1) |
|
| T2 | 3 (9.4) | 6 (7.9) |
|
| T3 | 17 (53.1) | 50 (61.0) |
|
| T4 | 8
(25.0) | 23 (28.0) |
|
| Lymph node
invasion, n (%) |
|
| 0.042 |
| No | 22 (68.8) | 39 (47.6) |
|
|
Yes | 10 (31.3) | 43 (52.4) |
|
| Distant metastasis,
n (%) |
|
| 0.108 |
| No | 31 (96.9) | 69 (84.1) |
|
|
Yes | 1 (3.1) | 13 (15.9) |
|
| Tumor location, n
(%) |
|
| 0.444 |
|
Ascending colon | 13 (40.6) | 21 (25.7) |
|
|
Transverse colon | 1 (3.1) | 3 (3.6) |
|
|
Descending colon | 2 (6.3) | 9
(10.9) |
|
| Sigmoid
colon | 16 (50.0) | 49 (59.8) |
|
| Tumor
differentiation, n (%) |
|
| 0.836 |
| Grade
1+2 | 24 (75.0) | 63 (76.8) |
|
| Grade
3+4 | 8
(25.0) | 19 (23.2) |
|
High SPRR1A expression is associated
with poor survival in colon cancer
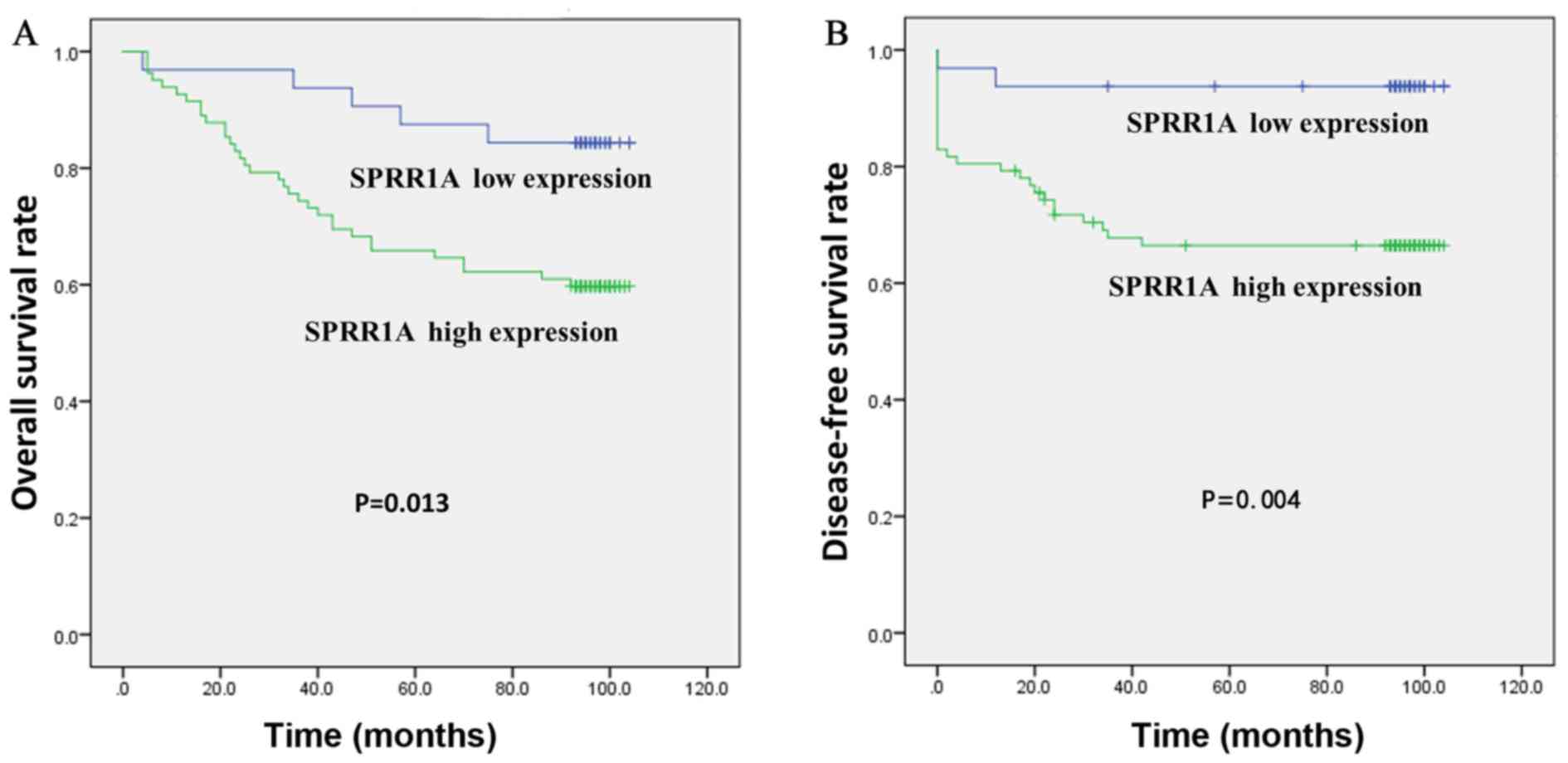
The Kaplan-Meier curve demonstrated that high SPRR1A
expression was significantly associated with a worse overall
survival (OS) (P=0.013; Fig. 3A) and
disease-free survival (DFS) (P=0.0.004, Fig. 3B) rates. The 5-year OS rates were
significantly lower in patients with high SPRR1A expression levels
than that of patients with low SPRR1A expression levels (64.6% vs.
87.5%; Fig. 3A). The 5-year DFS rate
was significantly lower in patients with high SPRR1A expression
levels than that of patients with low SPRR1A expression levels
(66.4% vs. 93.8%; Fig. 3B).
High SPRR1A expression is a prognostic
indicator in patients with colon cancer
Univariate analysis identified 10 potential risk
factors associated with OS, including pathological T stage [hazard
ratio (HR), 2.669; P<0.001], pathological N stage (HR, 4.323;
P<0.001) and high SPRR1A expression (HR, 3.083; P=0.019). In
addition, it was indicated that age, sex, BMI, histology,
differentiation, pretreatment CEA and CA19-9 levels were not
significantly associated with OS. Cox regression analysis
demonstrated that pathological T stage (HR, 2.099; P=0.012),
pathological N stage (HR, 2.832; P=0.007) and high SPRR1A
expression (HR, 2.606; P=0.047) remained independent prognostic
factors for OS, as presented in Table
III.
 | Table III.Univariate and multivariate analyses
of risk factors for overall survival of patients with colon
cancer. |
Table III.
Univariate and multivariate analyses
of risk factors for overall survival of patients with colon
cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (years) | 1.021
(0.994–1.048) | 0.136 |
|
|
| Sex (female vs.
male) | 1.818
(0.860–3.842) | 0.117 |
|
|
| BMI | 1.064
(0.955–1.187) | 0.261 |
|
|
| Pretreatment CEA
level (<5 vs. ≥ 5 ng/ml) | 0.611
(0.323–1.155) | 0.129 |
|
|
| Pretreatment CA19-9
level (<37 vs. ≥37 U/ml) | 0.876
(0.366–2.096) | 0.766 |
|
|
| Pathological T
stage | 2.669
(1.551–4.594) | <0.001 | 2.099
(1.175–3.749) | 0.012 |
| Pathological N
stage | 4.323
(2.093–8.928) | <0.001 | 2.832
(1.335–6.008) | 0.007 |
| Histopathology
(adenocarcinoma vs. mucinous adenocarcinoma) | 1.745
(0.784–3.886) | 0.173 |
|
|
| Tumor
differentiation (grade 1+2 vs. 3+4) | 0.755
(0.367–1.554) | 0.445 |
|
|
| SPRR1A expression
(high vs. low) | 3.083
(1.203–7.903) | 0.019 | 2.606
(1.012–6.708) | 0.047 |
Validation of SPRR1A in Oncomine and
R2
SPRR1A expression was verified, along with its
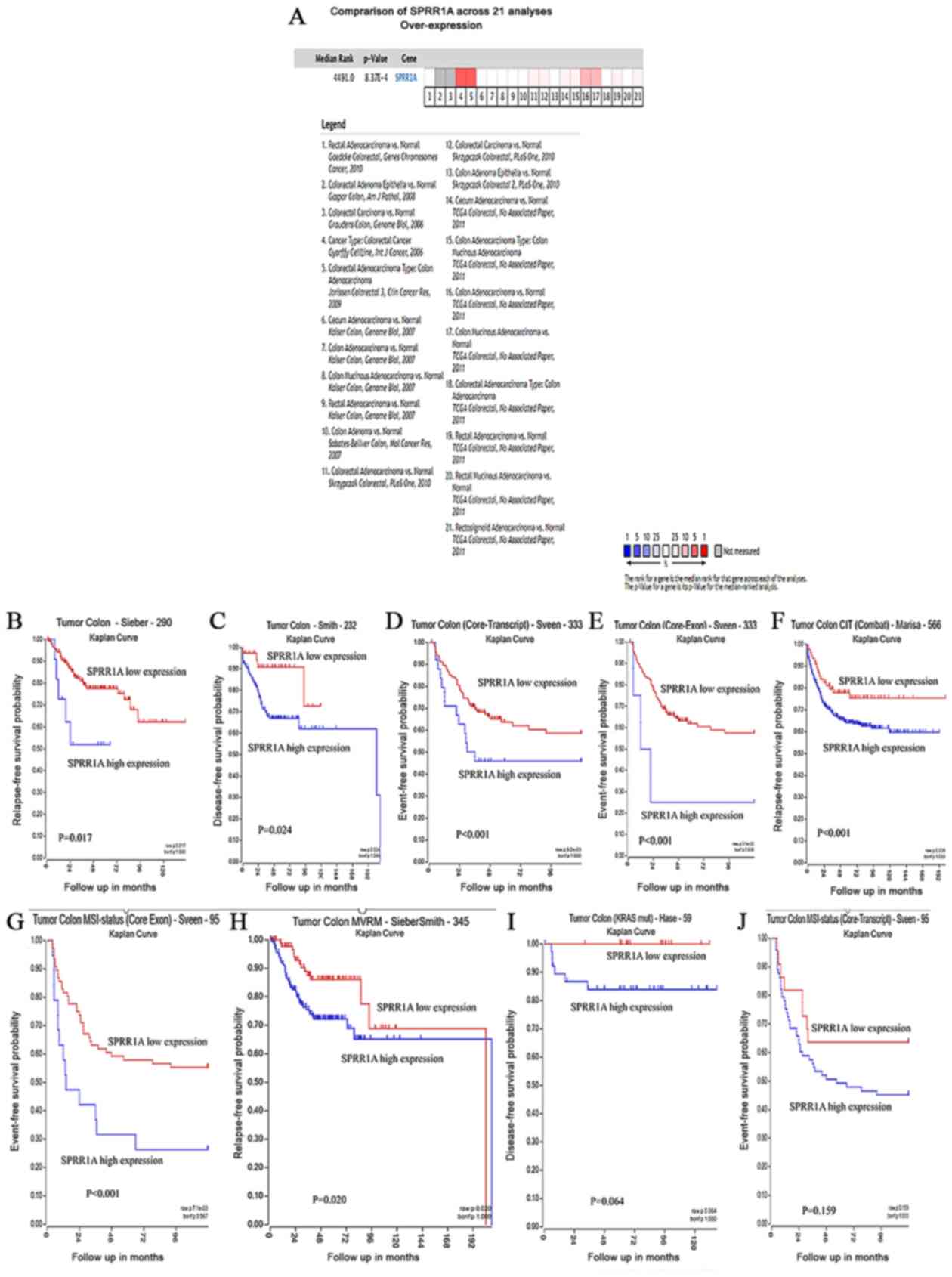
prognostic value via bioinformatics analysis. Meta-analysis of 21
GEO-sourced datasets from the Oncomine database was performed and
the results revealed that SPRR1A mRNA levels were significantly
increased in CRC tissues (P<0.001; Fig. 4A). The R2 platform was used to
generate Kaplan-Meier OS curves using the following datasets:
‘Tumor Colon-Sieber-290-MAS5.0-u133p2’, ‘Tumor
Colon-Smith-232-MAS5.0-u133p2’, ‘Tumor Colon
(Core-Exon)-Sveen-333-rma_sketch-huex10p’, ‘Tumor Colon
(Core-Transcript)-Sveen-333-rma_sketch-huex10t’, ‘Tumor Colon CIT
(Combat)-Marisa-566-rma-u133p2’, ‘Tumor Colon MSI-status (Core
Exon)-Sveen-95-rma_sketch-huex10p’ and ‘Tumor Colon
MVRM-SieberSmith-345-fRMA(bc)-u133p2’ (17). High SPRR1A expression was associated
with significantly worse rates of event- and relapse-free survival
rate (all P<0.05; Fig. 4B-H). In
the ‘Tumor Colon (KRAS mut)-Hase-59-MAS5.0-u133p2’ (P=0.064;
Fig. 4I) and ‘Tumor Colon MSI-status
(Core-Transcript)-Sveen-95-rma_sketch-huex10t’ (P=0.259; Fig. 4J) data sets, high SPRR1A expression
was associated with a worse OS; however, no statistically
significant differences were observed.
Discussion
Few studies have focused on the association between
SPRR1A expression and the prognosis of colon cancer (9,11,12).
Through use of immunohistochemical analysis, the present study
demonstrated that SPRR1A expression is increased in colon cancer
tissues compared with adjacent noncancerous tissues, which was
significantly associated with lymph node involvement. In addition,
high SPRR1A expression was an independent predictor for OS. Using
bioinformatics analysis, the association between higher SPRR1A
expression and impaired prognosis was further confirmed.
Previous studies have demonstrated that SPRR1A plays
a crucial role in the pathogenesis of various types of cancer, such
as diffuse large B-cell lymphomas (9), head and neck squamous cell carcinoma
(10) and breast (12) cancer. Leclerc et al (12) reported high expression of SPRR1A in a
murine preneoplastic intestine and in the normal intestinal mucosa
of patients with CRC. In addition, only a limited number of mice
with high expression of SPRR1A protein eventually develop tumors
(12). The present study showed that
SPRR1A expression was significantly higher in cancerous tissues.
However, the underlying mechanism remains unclear. Given that
colonic carcinogenesis is a multistage process involving
environmental and genetic changes (13), one of the possible explanations may
be that the normal intestinal mucosa in patients with colon cancer
has sufficient time for compensatory expression changes. Therefore,
the present study investigated whether the high expression of
SPRR1A serves as an oncogene in colon cancer progression. To the
best of our knowledge, this study provided novel insight
demonstrating that the high expression of SPRR1A is associated with
lymph node metastasis, suggesting the clinical importance of SPRR1A
in colon cancer.
The prognostic significance of SPRR1A has been
demonstrated in different types of cancer including, diffuse large
B-cell lymphomas, epithelial-like head and neck carcinoma and
progesterone receptor-positive breast cancer (9–11).
However, the clinical impact of SPRR1A as a prognostic biomarker
for colon cancer remains unclear. In the present study, the
prognostic significance of SPRR1A expression was further examined
in colon cancer. It was revealed that high SPRR1A expression was
associated with significantly worse OS and DFS rates. After
adjusting for confounding factors, it was demonstrated that the
expression of SPRR1A was an independent predictor for OS. In
accordance with previous findings (10,11),
these results revealed the prognostic implication of SPRR1A in
colon cancer. A tendency was indicated between high SPRR1A
expression and the incidence of distant metastasis (high expression
vs. low expression, 15.9% vs. 3%); however, no significant
differences were indicated. In addition, this study was not able to
identify high SPRR1A expression as an independent predictor for
tumor recurrence. The small patient sample size of this study
cohort could contribute to the lack of statistical difference.
Given the complex mechanism of tumor recurrence and metastasis,
large-scale studies are required to investigate the underlying
mechanism.
Furthermore, the association between SPRR1A
expression and survival in colon cancer was evaluated using
bioinformatics analysis. The results demonstrated significantly
higher SPRR1A mRNA levels in CRC tissues, and high SPRR1A
expression was associated with a worse survival time. Collectively,
these findings were consistent with the aforementioned results.
In summary, SPRR1A expression was upregulated in
colon cancer tissues and high SPRR1A expression was associated with
lymph node metastasis and impaired survival in patients with colon
cancer. The results suggest that SPRR1A may act as a potential
biomarker for the prognosis of colon cancer.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant no. 81472777), the Foundation of
Science and Technology Innovation Project in Fujian Province (grant
no. 2016Y9026), the Training Plan of Middle-Aged and Science
Foundation of the Fujian Province (grant no. 2017J01296) and the
National Clinical Key Specialty Construction Project (General
Surgery) of China (grant no. 2012-649).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YD, XZ, YYZ, XRL, and ZBX designed the study,
performed the experiments, interpreted the data and drafted the
initial manuscript. XZ and MFX observed and blindly scored IHC. JP,
CWY, PC and MXL provided the tissue specimens and interpreted the
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was ethically approved by the
Institutional Review Board of Fujian Medical University Union
Hospital. Written informed consent was obtained from all patients
prior to the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mahar AL, Compton C, Halabi S, Hess KR,
Weiser MR and Groome PA: Personalizing prognosis in colorectal
cancer: A systematic review of the quality and nature of clinical
prognostic tools for survival outcomes. J Surg Oncol. 116:969–982.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bardhan K and Liu K: Epigenetics and
colorectal cancer pathogenesis. Cancers (Basel). 5:676–713. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mouradov D, Domingo E, Gibbs P, Jorissen
RN, Li S, Soo P, Lipton L, Desai J, Danielsen HE, Oukrif D, et al:
Survival in stage II/III colorectal cancer is independently
predicted by chromosomal and microsatellite instability, but not by
specific driver mutations. Am J Gastroenterol. 108:1785–1793. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Das V, Kalita J and Pal M: Predictive and
prognostic biomarkers in colorectal cancer: A systematic review of
recent advances and challenges. Biomed Pharmacother. 87:8–19. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fujimoto W, Nakanishi G, Arata J and
Jetten AM: Differential expression of human cornifin alpha and beta
in squamous differentiating epithelial tissues and several skin
lesions. J Invest Dermatol. 108:200–204. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gibbs S, Fijneman R, Wiegant J, van Kessel
AG, van De Putte P and Backendorf C: Molecular characterization and
evolution of the SPRR family of keratinocyte differentiation
markers encoding small proline-rich proteins. Genomics. 16:630–637.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Heller-Milev M, Huber M, Panizzon R and
Hohl D: Expression of small proline rich proteins in neoplastic and
inflammatory skin diseases. Br J Dermatol. 143:733–740. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang H, Gao J, Zhao Z, Li M and Liu C:
Clinical implications of SPRR1A expression in diffuse large B-cell
lymphomas: A prospective, observational study. BMC Cancer.
14:3332014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pavón MA, Arroyo-Solera I, León X,
Téllez-Gabriel M, Virós D, Gallardo A, Céspedes MV, Casanova I,
Lopez-Pousa A, Barnadas A, et al: The combined use of EFS, GPX2,
and SPRR1A expression could distinguish favorable from poor
clinical outcome among epithelial-like head and neck carcinoma
subtypes. Head Neck. 41:1830–1845. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen G, Li G, Luo M, Wei X, Wang D, Zhang
H, Zhao X, Chen B and Liu C: Clinical significance of SPRR1A
expression in progesterone receptor-positive breast cancer. Tumour
Biol. 36:2601–2605. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leclerc D, Lévesque N, Cao Y, Deng L, Wu
Q, Powell J, Sapienza C and Rozen R: Genes with aberrant expression
in murine preneoplastic intestine show epigenetic and expression
changes in normal mucosa of colon cancer patients. Cancer Prev Res
(Phila). 6:1171–1181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hammoud SS, Cairns BR and Jones DA:
Epigenetic regulation of colon cancer and intestinal stem cells.
Curr Opin Cell Biol. 25:177–183. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Edge S: American Joint Committee on
Cancer: AJCC cancer staging manual. 7th. New York: Springer;
2010
|
|
15
|
Qiu J, Liu P, Shi C and Han B: Low-grade
myofibroblastic sarcomas of the maxilla. Oncol Lett. 9:619–625.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Xu Z, Sun Y, Chi P and Lu X:
Knockdown of KLK11 reverses oxaliplatin resistance by inhibiting
proliferation and activating apoptosis via suppressing the PI3K/AKT
signal pathway in colorectal cancer cell. Onco Targets Ther.
11:809–821. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koster J, Molenaar J and Versteeg R:
Abstract B1-05: R2: Accessible web-based genomics analysis and
visualization platform for biomedical researchers. Cancer Res.
757:B1–B5. 2015.
|