Introduction
Colorectal cancer is one of the most prevalent
cancers worldwide, with the third highest global mortality rate
(1–3). The incidence and mortality of
colorectal cancer varies widely by race and ethnicity (4). The second most common cancer sites were
cancers of the colorectal in Europe (1). The World Health Organization estimates
that by 2030, the number of newly diagnosed colorectal cancer cases
will increase by 77% and the number of colorectal cancer deaths
will increase by 80% (2).
Western-style lifestyle-related cancers such as
breast and colorectal cancers are rapidly increasing in Chinese
cities (5). Studies have identified
numerous prognostic markers for colorectal cancer, such as age,
T-stage and N-stage (6). An
important independent predictor is the number of invaded lymph
nodes (LNs) (7–9). The accurate identification of
metastatic LNs (LN+) contributes to pre-operative cancer staging
and influences treatment selection in clinical practice such as
endoscopic resection or surgery, preoperative neoadjuvant
chemotherapy (10).
With advances in CT technology, the value of using
CT to assess the tumor and node stages of patients with colorectal
cancer has been demonstrated (11).
However, the accuracy of assessing LN status remains unreliable and
quite low; with sensitivity, specificity and accuracy of detecting
regional lymph nodes metastases being 71, 41 and 54%, respectively
(11,12). To the best of our knowledge, no
effective imaging criteria for assessing LN+ in colorectal cancer
have currently been identified. At present, LN size is the most
common predictor of LN status in the clinical practice (12). A threshold size of 10 mm is
considered to be strong evidence of LN+ (13–16), and
in a previous systematic review and meta-analysis, the sensitivity,
specificity and odds ratio (OR) for LN+ were 71.0, 67.0 and 4.8%,
respectively (12).
Internal enhancement of CT images is an alternative
method to assess the degree of metastasis to LNs (17). Internal heterogeneity is considered a
marker of LN+ (17). Subtle changes
in internal enhancement features may provide additional valuable
information for diagnosing LNs metastasis.
The aim of the present study was to use
contrast-enhanced CT to improve the identification of LN+ in
patients diagnosed with colorectal cancer.
Materials and methods
Patients
The present retrospective study was performed at the
Departments of Surgery and Radiology of the Second Affiliated
Hospital of Zhejiang University School of Medicine (Hangzhou,
China) and was approved by the Local Ethics committee of the Second
Affiliated Hospital of Zhejiang University School of Medicine. The
requirement for written informed consent from patients was waived
due to the retrospective design of the study.
CT images from 284 patients diagnosed with
colorectal cancer that had undergone radical surgery between
January 2013 and July 2018 were collected. The inclusion criteria
for the present study were as follows: i) Pathological diagnosis of
colorectal cancer; ii) pre-operative CT scan; iii) resection of
colorectal cancer, loco-regional LN-bearing mesentery and 12–15
recruited LNs. Exclusion criteria: i) Patients that had previously
received treatment, including preoperative neoadjuvant radiotherapy
or chemotherapy; ii) presented with metastasis to other organs;
iii) had malignant disease of any abdominal or pelvic organ.
Patient medical records were used to collect additional
information, including age, sex and body mass index.
Image acquisition
For all patients, a multidetector-row helical CT
scan (Somatom Definition AS 40-row; Siemens Healthineers) was
performed, which ranged from the cartilago ensiformis and the anal
verge, with 3 mm axial sections and no intersection gap. Non-ionic
contrast agent (Omnipaque 300 g/l; GE Healthcare Sciences) was
intravenously injected at a rate of 3 ml/sec following a
non-enhanced CT scan. The arterial phase (25 sec delay), portal
venous phase (60 sec delay) and equilibrium phase (100 sec delay)
were obtained. Coronal reconstructions were performed in order to
observe the lesions. The following scan parameters were used:
Voltage, 120 kV; tube current, 160 mA/sec; slice collimation, 0.6
mm; slice thickness, 3 mm; pitch, 1.2; overlap, 50%; field of view,
32 cm.
Pathology
All patients underwent radical surgical resection of
the colorectal carcinoma and the loco-regional LNs and mesentery in
the drainage area of the mass (17).
In the Tumor-Node-Metastasis (6)
staging criteria for colorectal cancer, LNs that can be removed by
radical surgery were defined as loco-regional LNs (18,19). A
light microscope was used at 100 × magnification. Histopathological
analysis of the LNs was performed by two experienced pathologists
(written by a junior pathologist, Shi Dan, attending physician,
Department of Pathology, Shaoxing Second Hospital and reviewed by a
senior pathologist, Liu Qing-Meng, chief physician, Department of
Pathology, Shaoxing Second Hospital) in a blind manner. LNs were
sectioned (thickness, 4–5 µm), fixed in 10% formalin at room
temperature for 24–36 h, embedded in paraffin and stained with
hematoxylin and eosin at room temperature for ~55 min (cat. no.
CG008, Ningbo Tongsheng Biotechnology Co., Ltd). For the LN
metastasis negative (LN-) group, no metastasis was observed in all
12–15 loco-regional LNs, nor in the LNs at the origin of the
inferior mesenteric artery (0/1). For the LN+ group, according to
pathological results, cases with a metastatic LN ratio of ≥0.8 were
included, which meant metastatic LNs/harvested LNs ≥0.8. The area
located ≤5 cm from the distal edge of the tumor, which had a higher
incidence of metastatic LNs (20).
According to the surgical records, the area of the corresponding
LNs was determined.
Image analysis
The CT images were analyzed by two radiologists
specialized in gastrointestinal imaging (Second Affiliated
Hospital, Zhejiang University School of Medicine and Shaoxing
Second Hospital) and were blinded to the experimental groups. If
the radiologists disagreed, a third radiologist with >30 years
of experience was consulted and provided the final decision. LN
size, margin, morphology and internal characteristics in the
equilibrium phase were recorded.
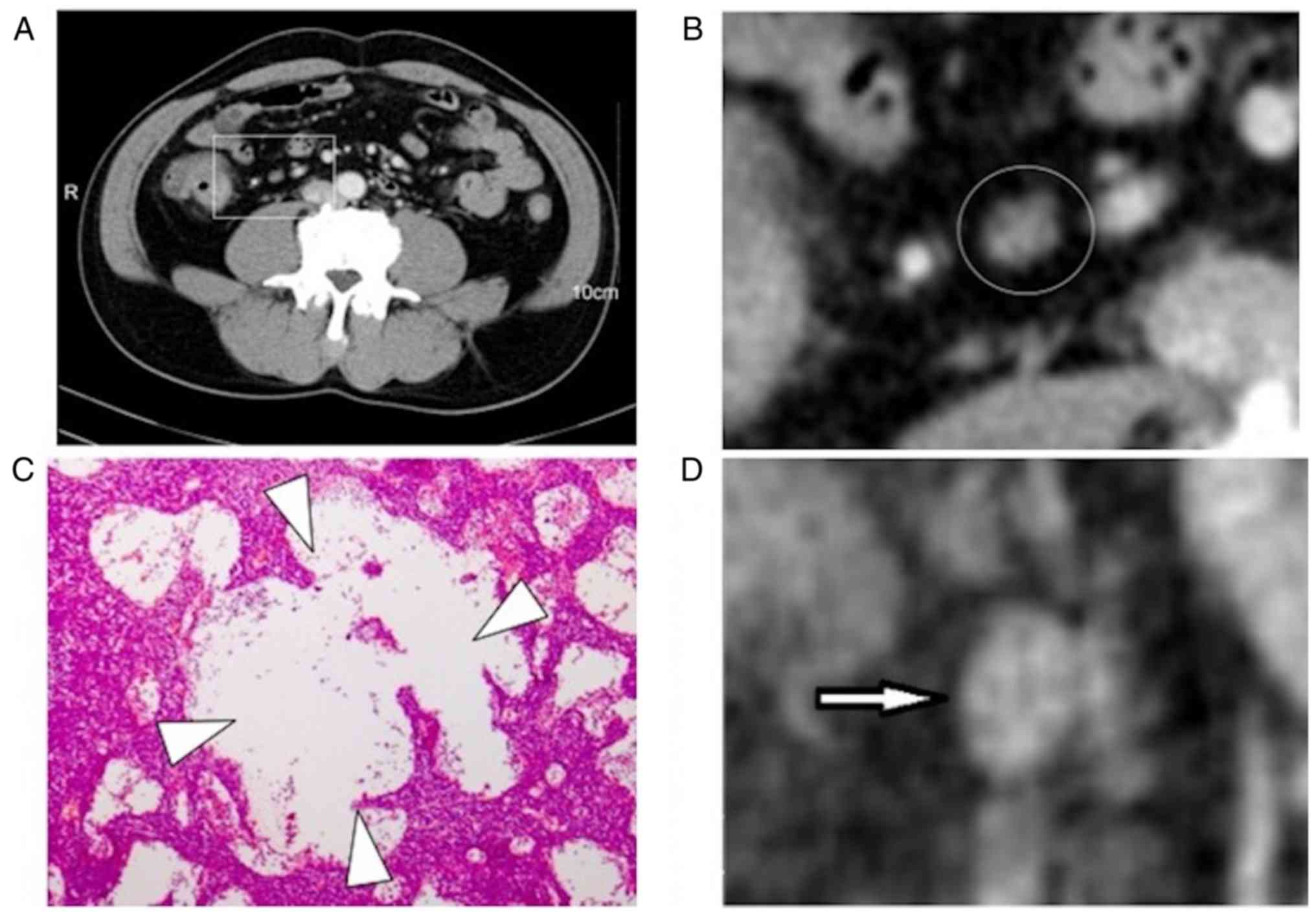
Round-shaped LNs were considered as those with a
short/long axis ratio of ≥0.7 (21).
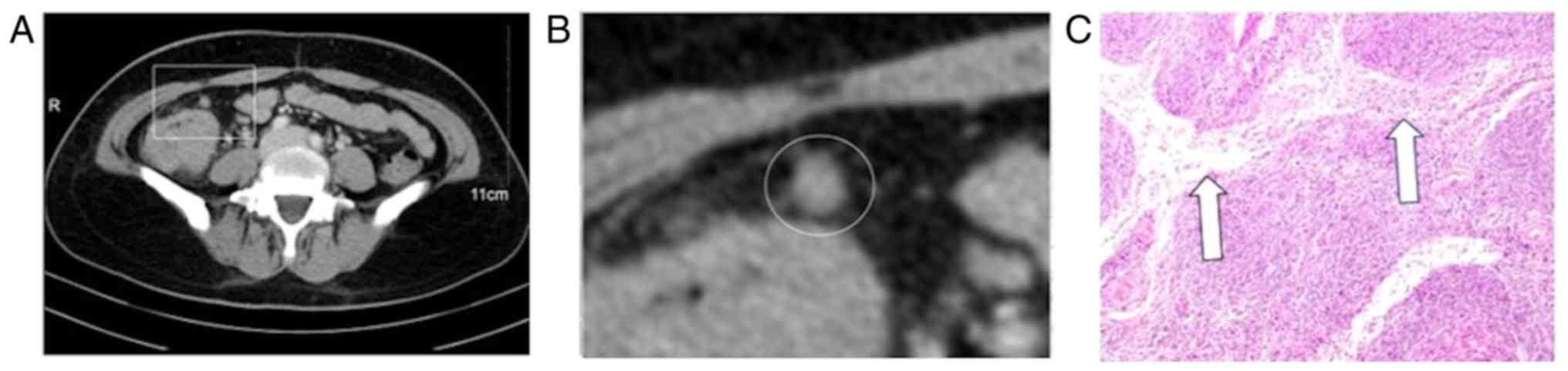
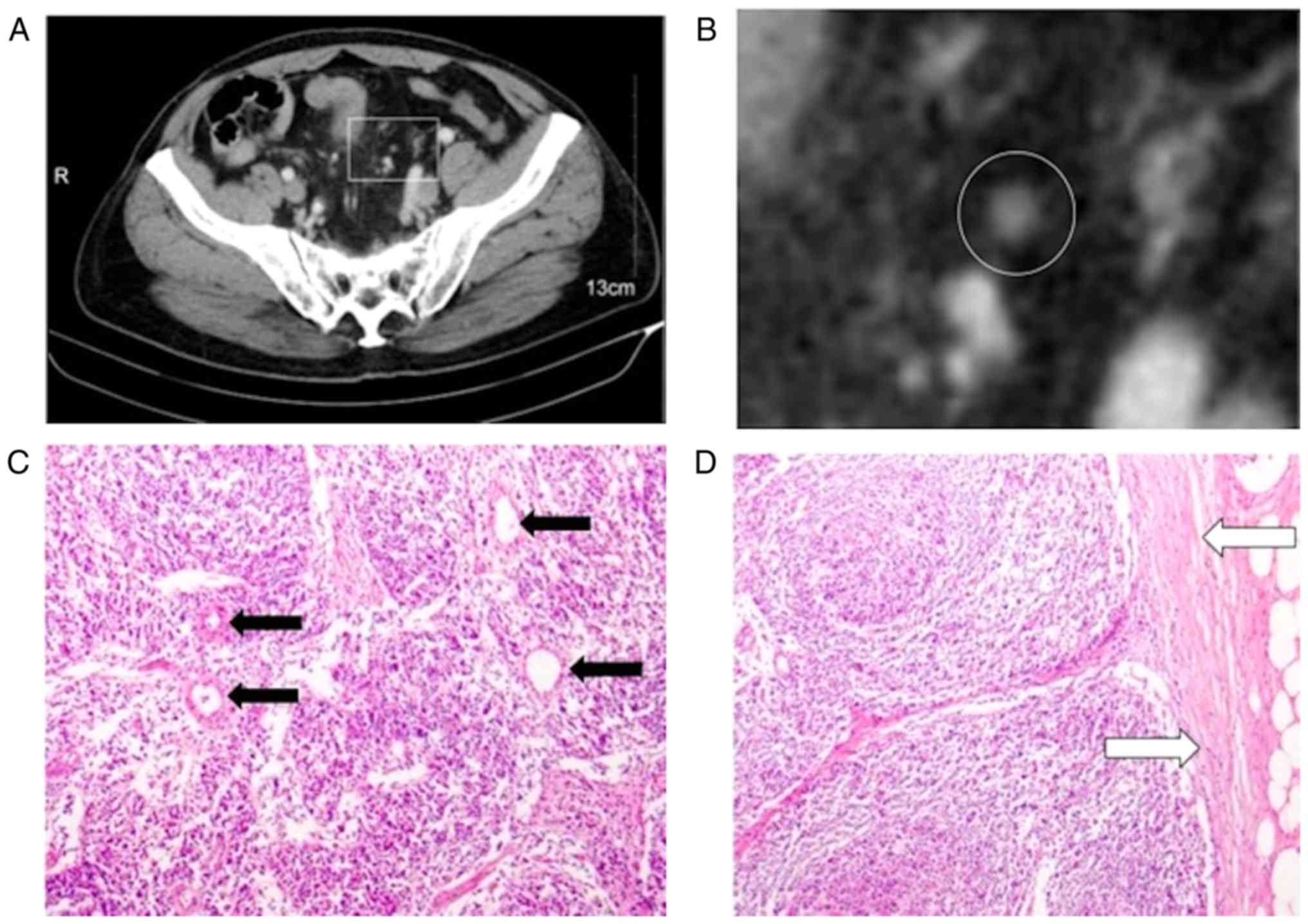
In magnified images, detailed internal enhancement characteristics
were classified into the following 6 types: Homogeneous, spotted
(Fig. 1), striped (Fig. 2), core (Fig. 3), rim and heterogeneous. The spotted
characteristic manifested as the appearance of small low intensity
circles, which were similar in size (≤3 mm), with clear boundaries.
The striped characteristic was defined as regular arrangements of
linear belts of low enhancement. The core characteristic appeared
as a central bright spot. Heterogeneous characteristics were
defined as LNs with spots ≥3 mm in size and with irregular
boundaries. The rim characteristic appeared as a central low
intensity and high intensity rim peripherally.
Statistical analysis
Data analysis was performed using SPSS software
(version 23.0; IBM Corp.). Descriptive statistics (Table I, median and range, percentage;
Table II/III, percentage) were
calculated for the LN characteristics, including the sensitivity,
specificity, positive predictive value (PPV), negative predictive
value (NPV) and ORs. The following formula was used to analyze
data: Diagnostic accuracy = (true positive + true
negative)/totality. The χ2 test (Tables II and III) was performed to ascertain
significant predictors of LN+. For χ2 test the group
lacking the characteristic A (reference to the characteristics
involved in the present study) was used as the baseline to
calculate the risk of characteristic A. In Table III, using first columns as criteria
for predicting LNs status, LNs were classified into the two groups
to form a crosstab, and finally the χ2 test was used to
calculate the P-value. P<0.05 was considered to indicate a
statistically significant difference.
 | Table I.Patient characteristics including CT
scan variables of 284 patients and 794 lymph nodes. |
Table I.
Patient characteristics including CT
scan variables of 284 patients and 794 lymph nodes.
| Variable | Value |
|---|
| Age, years, median
(range) | 61.71 (17–91) |
| BMI (median,
range) | 22.76
(15.67–38.50) |
| Size, mm, median
(range) | 7.95
(4.60–30.00) |
| Sex, male/female, n
(%) | 132 (46.5)/152
(53.5) |
| Lymph node status,
positive/negative (%) | 217 (27.3)/577
(72.7) |
| Tumor T stage, n
(%) |
|
| T1 | 10 (3.5) |
| T2 | 25 (8.8) |
| T3 | 148 (52.1) |
| T4 | 101 (35.6) |
| Tumor N stage, n
(%) |
|
| N0 | 217 (76.4) |
| N2 | 67
(23.6) |
| Tumor localization,
n (%) |
|
|
Ascending colon | 113 (39.8) |
|
Transverse colon | 19 (6.7) |
|
Descending colon | 22 (7.7) |
| Sigmoid
colon | 51
(18.0) |
|
Rectum | 79
(27.8) |
| Shape, n (%) |
|
|
Round | 355 (44.7) |
|
Kidney-bean | 176 (22.2) |
|
Oblong | 148 (18.6) |
|
Lobulated | 42 (5.3) |
|
Irregular | 73 (9.2) |
| Inner enhancement,
n (%) |
|
|
Homogeneity | 30 (3.8) |
|
Spotted | 311 (39.2) |
|
Stripe | 267 (33.6) |
|
Core | 26 (3.3) |
|
Rim | 91
(11.5) |
|
Heterogeneity | 69 (8.7) |
 | Table II.χ2 test to detect
metastatic lymph nodes of different characteristics. |
Table II.
χ2 test to detect
metastatic lymph nodes of different characteristics.
|
| Risk |
|---|
|
|
|
|---|
|
|
| 95% confidence
interval |
|
|---|
|
|
|
|
|
|---|
| Variables | Odds ratio | Lower | Upper | P-value |
|---|
| Margin | 1.59 | 1.12 | 2.24 | 0.009 |
| Size, ≥10 mm | 3.77 | 2.69 | 5.28 | <0.001 |
| Size, ≥8 mm | 3.46 | 2.47 | 4.85 | <0.001 |
| Shape |
|
|
|
|
|
Round | 1.14 | 0.83 | 1.55 | 0.425 |
|
Kidney-bean | 0.65 | 0.43 | 0.97 | 0.033 |
|
Oblong | 0.56 | 0.36 | 0.87 | 0.009 |
|
Lobulated | 2.21 | 1.19 | 4.12 | 0.020 |
|
Irregular | 2.41 | 1.47 | 3.93 | <0.001 |
| Shape
criteria | 1.96 | 1.4 | 2.7 | <0.001 |
| Inner
enhancement |
|
|
|
|
|
Homogeneity | 0.52 | 0.2 | 1.38 | 0.260 |
|
Spotted | 0.43 | 0.3 | 0.6 | <0.001 |
|
Striped | 0.59 | 0.41 | 0.83 | 0.002 |
|
Core | 0.1 | 0.01 | 0.76 | 0.012 |
|
Rim | 9.06 | 5.56 | 14.78 | <0.001 |
|
Heterogeneity | 3.28 | 1.99 | 5.41 | <0.001 |
 | Table III.Sensitivity, specificity, PPV, NPV,
diagnostic accuracy and distributions of the different
computed-tomography characteristics of lymph nodes. |
Table III.
Sensitivity, specificity, PPV, NPV,
diagnostic accuracy and distributions of the different
computed-tomography characteristics of lymph nodes.
| Variable | Sensitivity, % | Specificity, % | PPV, % | NPV, % | Diagnostic
accuracy, % | OR | P-value |
|---|
| Margin | 31.30 | 77.60 | 34.50 | 75.00 | 65.00 | 1.59 | 0.009 |
| Size ≥10 mm | 47.00 | 80.90 | 48.10 | 80.20 | 71.70 | 3.77 | <0.001 |
| Size ≥8 mm | 71.40 | 58.10 | 39.00 | 84.40 | 56.70 | 3.46 | <0.001 |
| Shape criteria | 70.40 | 45.10 | 32.60 | 80.20 | 52.00 | 1.96 | <0.001 |
| Internal
enhancement criteria | 46.50 | 89.90 | 63.50 | 81.70 | 78.10 | 7.79 | <0.001 |
| Size ≥10 mm +
internal enhancement | 32.30 | 96.40 | 76.90 | 79.00 | 79.10 | 12.01 | <0.001 |
| Size ≥8 mm +
internal enhancement | 38.70 | 93.40 | 68.90 | 80.20 | 78.50 | 8.71 | <0.001 |
Results
Patients and histopathology
A total of 284 patients diagnosed with colorectal
cancer (confirmed by histopathological analysis) that had undergone
radical surgical resection were analyzed in the present study and
794 LNs were obtained. Among these patients, 132 were male and 152
were female (median age, 61.71 years; range, 17–91 years; median
body mass index, 22.76; range, 15.67–38.50). Out of the 794 LNs
obtained, 217 were LN+ and 577 were LN-, with a median size of 7.95
mm (range, 4.60–30.00 mm). The tumor location, and T and N stages
of tumors are presented in Table I.
No significant association between body mass index, age, sex and
the size of LNs was observed (P=0.492, P=0.950 and P=0.555,
respectively; data not shown).
Internal enhancement and morphology of
LNs
According to the ORs (Table II), kidney bean and oblong shapes
were most likely to be LN-, while rounded, lobulated and irregular
shapes were most likely to predict LN+, with sensitivity,
specificity, PPVs and NPVs of 70.50% (153/217), 45.10% (260/577),
32.60% (153/470) and 80.20% (260/324), respectively (OR, 1.96; 95%
CI, 1.40–2.70; Table III).
According to the ORs (Table II), homogeneous, spotted, striped
and core internal enhancement characteristics were indicators of
LN-, while rim and heterogeneous characteristics indicated LN+,
with sensitivity, specificity, PPVs and NPVs of 46.50% (101/217),
89.90% (519/577), 63.50% (101/159) and 81.70% (519/635),
respectively (OR, 7.79; 95% CI, 5.33–11.40; Table III). Statistical analysis of the
results demonstrated that a rounded shape (P=0.425) and internal
homogeneity (P=0.26) on their own were not significantly different
between LN- and LN+.
Evaluation of LN size
As presented in Table
III, LNs ≥10 mm in size demonstrated sensitivity, specificity,
PPV and NPVs of 47.00% (102/217), 80.90% (467/577), 48.10%
(102/212) and 80.20% (467/582), respectively [OR, 3.77; 95%
confidence interval (CI), 2.69–5.28). LNs ≥8 mm in size
demonstrated sensitivity, specificity, PPVs and NPVs of 71.40%
(155/217), 58.10% (335/577), 39.00% (155/397) and 84.40% (335/397),
respectively (OR, 3.46; 95% CI, 2.47–4.85). A significant
association was observed between the size of LNs and tumor stage
(P=0.001).
Combining size and internal
enhancement characteristics
By combining LN size and internal enhancement
characteristics, the metastatic status of LNs was subsequently
estimated in the present study. The results revealed that LNs
<10 mm or ≥10 mm in size with benign internal enhancement
characteristics predicted LN-. These features demonstrated
sensitivity, specificity, PPVs, NPVs and a diagnostic accuracy of
32.30% (70/217), 96.40% (556/577), 76.90% (70/91), 79.00% (556/704)
and 79.10% (628/794), respectively (Table III). In addition, LNs <8 or ≥8
mm in size with benign internal enhancement features predicted LN-,
with sensitivity, specificity, PPVs, NPVs and a diagnostic accuracy
of 38.70% (84/217), 93.40% (539/577), 68.90% (84/122), 80.2%
(539/672) and 78.5% (623/794), respectively (Table III). For LNs ≥10 mm in size
(n=212), using the internal enhancement criteria would prevent
42.0% (n=89) of LNs from being wrongly diagnosed as LN+ and neglect
9.9% (n=21) of metastatic LNs. For LNs ≥8 mm in size (n=397), using
the internal enhancement criteria would prevent 51.4% (n=204) of
LNs from being wrongly diagnosed as LN+ and neglect 9.6% (n=38) of
metastatic LNs.
Discussion
Metastasis to regional LNs is an independent risk
factor for the prognosis of colorectal cancer (22). According to the American Joint
Committee on Cancer (AJCC; 8th Edition) (6), node stage represents the number of
positive LNs (22,23). However, the criteria for predicting
LN+ based on CT images varies (17,24). The
most common criteria for LN+ is the presence of regional LNs >10
mm in size and/or clusters of ≥3 LNs (10,25–28). In
addition, LNs ≥8 mm in size also predicts LN+ (29). Rodriguez-Bigas et al (30) reported that LN size did not affect
whether the tumor became metastatic, and the majority of metastatic
LNs were <5 mm in size. Using CT scan images, de Vries et
al (12) demonstrated that the
diagnostic accuracy of using LNs in patients diagnosed with colon
cancer was only 54%. At present, the major disadvantage of using CT
scan images is the poor efficiency in differentiating malignant and
benign LNs (12). Relying on LN size
to predict LN+ may be problematic, as the size of LN- may be
falsely diagnosed as LN+, due to inflammation (21). Despite this, larger LNs are more
likely to be LN+, with increasing specificity, but decreasing
sensitivity (11). Consistent with
these results, the present study demonstrated that LNs ≥8 mm in
size exhibited sensitivity and specificity values of 71.40 and
58.10%, respectively, while LNs ≥10 mm in size demonstrated
sensitivity and specificity values of 47.00 and 80.90%,
respectively. However, regardless of size (8 or 10 mm), false
positive rates are high, which is the major disadvantage of using
these criteria.
Internal enhancement characteristics of LNs may be
helpful in estimating LNs status (17). Previous studies have indicated that
heterogeneity and rim enhancement features on CT images may be
characteristics of LN+ (21). This
may be explained by the invasion of tumor cells into the sub
capsular sinus via afferent lymphatic vessels (31), leading to infiltration and damage of
lymphoid tissue, which is then replaced by tumor cells (32). A lack of blood supply and subsequent
central necrosis may then occur in the medulla (32). In the present study, spotted
enhancement features in pathological sections revealed several
dilated subcapsular sinuses, which may coincide with the low
enhancement and small circle area (Fig.
1). The low enhancement and striped area of the striped
characteristic may correspond to the interlinked capsular sinus
(Fig. 2). Compared with the size
criteria, internal enhancement criteria demonstrated improved PPV
and diagnostic accuracy, while other parameters remained stable.
Among the internal enhancement characteristics, core enhancement
demonstrated excellent efficiency in estimating LN-. The spotted
and striped characteristics were also able to differentiate between
LN+ and LN-. These results may lead to changes in cancer staging
according to the AJCC criteria (6),
particularly for patients diagnosed with T3-4 stage. During
pre-operative assessment of these patients, LN+ is a criterion for
upgrading the lesion from stage II to stage III (22).
The present study identified internal enhancement
characteristics and classified them into several groups. A previous
study suggested that internal heterogeneity may be a feature of LN+
(17). The present study
demonstrated that internal enhancement features of LNs varied upon
magnification of the images, even though they may appear similar in
normal unmagnified CT images. Therefore, to the best of our
knowledge, the present study is the first to classify the detailed
internal enhancement features of LN CT images to differentiate
between LN- and LN+. The results revealed that detailed internal
enhancement characteristics were superior to LN size when assessing
LN status.
The present study attempted to combine internal
enhancement characteristics with LN size to increase the diagnostic
accuracy, in order to resolve problems with using LN size alone as
an objective criterion. For LNs ≥10 mm in size (n=212), using the
internal enhancement criteria would prevent 42.0% (n=89) of LNs
from being wrongly diagnosed as LN+; however, it would neglect 9.9%
(n=21) of metastatic LNs. For LNs ≥8 mm in size (n=397), using the
internal enhancement criteria would prevent 51.4% (n=204) of LNs
from being wrongly diagnosed as LN+ and neglect 9.6% (n=38) of
metastatic LNs. Therefore, the present study suggests that
combining LN size with internal enhancement criteria may decrease
false positive results and decrease false negative results.
LN morphology is an additional variable used to
assess the metastatic status of LNs (17,21). LN-
are generally kidney bean-shaped (33) and oblong (21,34,35),
whereas LN+ are irregularly-shaped and lobulated (17). McMahon et al (21) revealed that a short-to-long axis
ratio of >0.7 was able to effectively differentiate LN- from
LN+. However, according to the results of the present study, the
rounded shape of LNs was not observed to be a significant predictor
of LN+. The underlying reasons for these inconsistencies are
currently unclear and require further investigation.
Previous studies have assessed the value of MRI and
positron emission tomography in predicting the metastatic status of
LNs (10,36,37).
Doyon et al (38) and
Gagliardi et al (39)
demonstrated that a threshold value of >5 mm could be used to
identify LN+. Margin characteristics and the signal intensity of
LNs were also significant variables for the assessment of LN
metastatic status in patients with colon cancer (24). However, the use of MRI to assess LN
status may lead to overdiagnosis (36), and therefore may be an unreliable
tool (40,41). The performance of positron emission
tomography combined with fluorine-18 fluorodeoxyglucose for
predicting LN+ may also be insufficient to assess LN status
(42,43).
The present study has several limitations. First, it
was a retrospective study involving the analysis of loco-regional
LNs from patients with colorectal cancer, thereby introducing bias
when interpreting the results. Secondly, the imaging and
histopathological analysis of LNs in the present study were not
matched one by one. However, for the LN metastasis negative (LN-)
group, no metastasis was observed in all 12–15 loco-regional LNs,
nor in the LNs at the origin of the inferior mesenteric artery
(0/1). For the LN+ group, cases with a metastatic LN ratio of ≥0.8
were included. Third, LNs with a diameter of ≤4.5 mm were difficult
to identify from the internal enhancement of CT images, as the
diameters of harvested LNs ranged from 4.60–30.00 mm. Finally, the
number of LNs analyzed was insufficient, and a larger sample size
is required in order to confirm the results.
In conclusion, the present study identified novel
internal enhancement characteristics, including the spotted,
striped and core enhancement features on magnified CT images that
may facilitate the identification of LN- in patients with
colorectal cancer.
Acknowledgements
The authors would like to thank Dr Dan Shi
(attending physician, Department of Pathology, Shaoxing Second
Hospital, Shaoxing, China) for reviewing and supervising the
histopathological analysis of the lymph nodes.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SM and YL analyzed and interpreted the patient data
regarding colorectal cancers. SM, YL and HC wrote the manuscript.
HC made substantial contributions to analysis and interpretation of
data. QL performed the histological examination of the lesions. JC
and YP contributed to acquisition of data for the work. RY
conceived the concept and designed the study. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the local Ethics committee
of the Second Affiliated Hospital of Zhejiang University School of
Medicine (Hangzhou, China). The requirement for written informed
consent from patients was waived due to the retrospective design of
the study.
Patient consent for publication
The patient(s) referred to in this study provided
consent for the publication of their information.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
LN
|
lymph node
|
|
PPV
|
positive predictive value
|
|
NPV
|
negative predictive value
|
|
OR
|
odds ratio
|
|
CI
|
confidence interval
|
References
|
1
|
Ferlay J, Colombet M, Soerjomataram I,
Dyba T, Randi G, Bettio M, Gavin A, Visser O and Bray F: Cancer
incidence and mortality patterns in Europe: Estimates for 40
countries and 25 major cancers in 2018. Eur J Cancer. 103:356–387.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Binefa G, Rodriguez-Moranta F, Teule A and
Medina-Hayas M: Colorectal cancer: From prevention to personalized
medicine. World J Gastroenterol. 20:6786–6808. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pan R, Zhu M, Yu C, Lv J, Guo Y, Bian Z,
Yang L, Chen Y, Hu Z, Chen Z, et al: Cancer incidence and
mortality: A cohort study in China, 2008–2013. Int J Cancer.
141:1315–1323. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weiser MR: AJCC 8th Edition: Colorectal
cancer. Ann Surg Oncol. 25:1454–1455. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ceelen W, Van Nieuwenhove Y and Pattyn P:
Prognostic value of the lymph node ratio in stage III colorectal
cancer: A systematic review. Ann Surg Oncol. 17:2847–2855. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosenberg R, Friederichs J, Schuster T,
Gertler R, Maak M, Becker K, Grebner A, Ulm K, Höfler H, Nekarda H
and Siewert JR: Prognosis of patients with colorectal cancer is
associated with lymph node ratio: A single-center analysis of 3,026
patients over a 25-year time period. Ann Surg. 248:968–978. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim YS, Kim JH, Yoon SM, Choi EK, Ahn SD,
Lee SW, Kim JC, Yu CS, Kim HC, Kim TW and Chang HM: lymph node
ratio as a prognostic factor in patients with stage III rectal
cancer treated with total mesorectal excision followed by
chemoradiotherapy. Int J Radiat Oncol Biol Phys. 74:796–802. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choi J, Oh SN, Yeo DM, Kang WK, Jung CK,
Kim SW and Park MY: Computed tomography and magnetic resonance
imaging evaluation of lymph node metastasis in early colorectal
cancer. World J Gastroenterol. 21:556–562. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dighe S, Purkayastha S, Swift I, Tekkis
PP, Darzi A, A'Hern R and Brown G: Diagnostic precision of CT in
local staging of colon cancers: A meta-analysis. Clin Radiol.
65:708–719. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Vries FE, da Costa DW, van der Mooren
K, van Dorp TA and Vrouenraets BC: The value of pre-operative
computed tomography scanning for the assessment of lymph node
status in patients with colon cancer. Eur J Surg Oncol.
40:1777–1781. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leufkens AM, van den Bosch MA, van Leeuwen
MS and Siersema PD: Diagnostic accuracy of computed tomography for
colon cancer staging: A systematic review. Scand J Gastroenterol.
46:887–894. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burton S, Brown G, Bees N, Norman A,
Biedrzycki O, Arnaout A, Abulafi AM and Swift RI: Accuracy of CT
prediction of poor prognostic features in colonic cancer. Br J
Radiol. 81:10–19. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gazelle GS, Gaa J, Saini S and Shellito P:
Staging of colon carcinoma using water enema CT. J Comput Assist
Tomogr. 19:87–91. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Acunas B, Rozanes I, Acunas G, Celik L,
Sayi I and Gokmen E: Preoperative CT staging of colon carcinoma
(excluding the recto-sigmoid region). Eur J Radiol. 11:150–153.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rollven E, Abraham-Nordling M, Holm T and
Blomqvist L: Assessment and diagnostic accuracy of lymph node
status to predict stage III colon cancer using computed tomography.
Cancer Imaging. 17:32017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The Eighth Edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamaoka Y, Kinugasa Y, Shiomi A, Yamaguchi
T, Kagawa H, Yamakawa Y, Furutani A and Manabe S: The distribution
of lymph node metastases and their size in colon cancer.
Langenbecks Arch Surg. 402:1213–1221. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McMahon CJ, Rofsky NM and Pedrosa I:
Lymphatic metastases from pelvic tumors: Anatomic classification,
characterization, and staging. Radiology. 254:31–46. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jin M and Frankel WL: Lymph node
metastasis in colorectal cancer. Surg Oncol Clin N Am. 27:401–412.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hari DM, Leung AM, Lee JH, Sim MS, Vuong
B, Chiu CG and Bilchik AJ: AJCC cancer staging manual 7th edition
criteria for colon cancer: Do the complex modifications improve
prognostic assessment? J Am Coll Surg. 217:181–190. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brown G, Richards CJ, Bourne MW, Newcombe
RG, Radcliffe AG, Dallimore NS and Williams GT: Morphologic
predictors of lymph node status in rectal cancer with use of
high-spatial-resolution MR imaging with histopathologic comparison.
Radiology. 227:371–377. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harvey CJ, Amin Z, Hare CM, Gillams AR,
Novelli MR, Boulos PB and Lees WR: Helical CT pneumocolon to assess
colonic tumors: Radiologic-pathologic correlation. AJR Am J
Roentgenol. 170:1439–1443. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Smith NJ, Bees N, Barbachano Y, Norman AR,
Swift RI and Brown G: Preoperative computed tomography staging of
nonmetastatic colon cancer predicts outcome: implications for
clinical trials. Br J Cancer. 96:1030–1036. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hundt W, Braunschweig R and Reiser M:
Evaluation of spiral CT in staging of colon and rectum carcinoma.
Eur Radiol. 9:78–84. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nerad E, Lahaye MJ, Maas M, Nelemans P,
Bakers FC, Beets GL and Beets-Tan RG: Diagnostic accuracy of CT for
local staging of colon cancer: A systematic review and
meta-analysis. AJR Am J Roentgenol. 207:984–995. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chi YK, Zhang XP, Li J and Sun YS: To be
or not to be: significance of lymph nodes on pretreatment CT in
predicting survival of rectal cancer patients. Eur J Radiol.
77:473–477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rodriguez-Bigas MA, Maamoun S, Weber TK,
Penetrante RB, Blumenson LE and Petrelli NJ: Clinical significance
of colorectal cancer: Metastases in lymph nodes <5 mm in size.
Ann Surg Oncol. 3:124–130. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Servais EL, Colovos C, Bograd AJ, White J,
Sadelain M and Adusumilli PS: Animal models and molecular imaging
tools to investigate lymph node metastases. J Mol Med (Berl).
89:753–769. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li L, Mori S, Sakamoto M, Takahashi S and
Kodama T: Mouse model of lymph node metastasis via afferent
lymphatic vessels for development of imaging modalities. PloS One.
8:e557972013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bontumasi N, Jacobson JA, Caoili E,
Brandon C, Kim SM and Jamadar D: Inguinal lymph nodes: Size,
number, and other characteristics in asymptomatic patients by CT.
Surg Radiol Anat. 36:1051–1055. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Willard-Mack CL: Normal structure,
function, and histology of lymph nodes. Toxicol Pathol. 34:409–424.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van der Valk P and Meijer CJ: The
histology of reactive lymph nodes. Am J Surg Pathol. 11:866–882.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ogawa M, Ichiba N, Watanabe M and Yanaga
K: The usefulness of diffusion MRI in detection of lymph node
metastases of colorectal cancer. Anticancer Res. 36:815–819.
2016.PubMed/NCBI
|
|
37
|
Kaur H, Choi H, You YN, Rauch GM, Jensen
CT, Hou P, Chang GJ, Skibber JM and Ernst RD: MR imaging for
preoperative evaluation of primary rectal cancer: Practical
considerations. Radiographics. 32:389–409. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Doyon F, Attenberger UI, Dinter DJ,
Schoenberg SO, Post S and Kienle P: Clinical relevance of
morphologic MRI criteria for the assessment of lymph nodes in
patients with rectal cancer. Int J Colorectal Dis. 30:1541–1546.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gagliardi G, Bayar S, Smith R and Salem
RR: Preoperative staging of rectal cancer using magnetic resonance
imaging with external phase-arrayed coils. Arch Surg. 137:447–451.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nerad E, Lambregts DM, Kersten EL, Maas M,
Bakers FC, van den Bosch HC, Grabsch HI, Beets-Tan RG and Lahaye
MJ: MRI for Local Staging of Colon Cancer: Can MRI Become the
Optimal Staging Modality for Patients With Colon Cancer? Dis Colon
Rectum. 60:385–392. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hunter C, Blake H, Jeyadevan N, Abulafi M,
Swift I, Toomey P and Brown G: Local staging and assessment of
colon cancer with 1.5-T magnetic resonance imaging. Br J Radiol.
89:201602572016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bae SU, Won KS, Song BI, Jeong WK, Baek SK
and Kim HW: Accuracy of F-18 FDG PET/CT with optimal cut-offs of
maximum standardized uptake value according to size for diagnosis
of regional lymph node metastasis in patients with rectal cancer.
Cancer Imaging. 18:322018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Abdel-Nabi H, Doerr RJ, Lamonica DM,
Cronin VR, Galantowicz PJ, Carbone GM and Spaulding MB: Staging of
primary colorectal carcinomas with fluorine-18 fluorodeoxyglucose
whole-body PET: correlation with histopathologic and CT findings.
Radiology. 206:755–760. 1998. View Article : Google Scholar : PubMed/NCBI
|