Introduction
As a result of population aging, the number of
elderly patients with cancer is recently increasing, and of
patients with cancer, ~70% are aged ≥65 years and 36% are aged 75
years in daily clinical practice (1). Elderly patients are frequently
vulnerable and frail compared to younger patients due to
comorbidities and geriatric problems, including physical or
cognitive dysfunctions (2), and
non-elderly patients may also have frailty with malnutrition or low
performance status (PS) due to severe advanced malignancies. In
these situations, conventional cancer treatments are often
intolerable and have a particularly high risk of adverse events
(3).
Immune checkpoint inhibitors (ICIs), especially
those targeting the programmed cell death-1 (PD-1) and programmed
cell death ligand 1 (PD-L1), which have improved outcomes for
various advanced cancers, including non-small cell lung cancer
(NSCLC), malignant melanoma (MM), renal cell carcinoma, urothelial
cancer, head and neck cancer and gastric cancer (4–9), are
generally considered to be more tolerable than conventional
chemotherapy and clinical indications for immunotherapy continue to
increase (4,10). These drugs may provide an opportunity
for treatment of elderly or frail patients who cannot tolerate
toxic chemotherapy or invasive treatment.
The age-related decline in the immune system, so
called ‘immunosenescence’ has been recently reported in preclinical
studies (11,12). T cells, the primary effectors of
antitumor response, undergo significant changes with age. The naïve
CD8+ T cells decline with age in part due to thymic
involution and contraction of lymphopoietic stem cells (13), and decreased expression of CD28 on
the surface of CD8+ T cells leads to decreased immune
activation (14–16). While these numeric and functional
defects in T cells have been characterized gradually, the potential
impact of aging on efficacy and tolerability of immunotherapy in
clinical practice remains unclear. Elderly patients has been
underrepresented in clinical trials; moreover, those who were
included had relatively good PS and function reserve capacities,
which may not reflect the real-world population (17). The real-world clinical practice data
in treating more vulnerable or frail patients with ICIs are
severely insufficient, and identifying the safety and efficacy of
ICIs in this population would be profitable.
Hence, based on a real-world cohort, we designed a
two-part study: i) We evaluated the safety and tolerability of
PD-1/PD-L1 monotherapy in elderly patients with advanced
malignancies. ii) Using indirect markers of frailty including
Eastern Cooperative Oncology Group (ECOG)-PS, Charlson Comorbidity
Index (CCI), and neutrophil-lymphocyte ratio (NLR), we estimated
whether vulnerable and frail patients in the elderly and
non-elderly groups were associated with higher risk of
immune-related adverse events (irAEs) or complications.
Materials and methods
Patient population and data
collection
We performed a retrospective review of electronic
medical records of 197 patients who received nivolumab,
pembrolizumab or atezolizumab as monotherapy for metastatic or
unresectable advanced cancers from September 2014 to December 2018
at Kyoto Prefecture University of Medicine. These patients' data
were continuously followed until the data lock on July 31, 2019.
None of the patients had a history of pretreatment with other ICIs,
such as ipilimumab, which is an anti-cytotoxic T-lymphocyte
associated protein-4 antibody. This study was approved by the
Medical Ethics Review Committee of the Kyoto Prefectural University
of Medicine. Given the retrospective nature of this work, informed
consent was waived for the participants included in the study in
accordance with the standards of the Kyoto Prefectural University
of Medicine Institutional Medical Ethics Review Committee.
All enrolled patients received PD-1/PD-L1 inhibitor
intravenously, according to a schedule of 3 mg/kg or 240 mg every 2
weeks for nivolumab, 2 mg/kg or 200 mg every 3 weeks for
pembrolizumab, and 1,200 mg every 3 weeks for atezolizumab. The
treatment was provided until disease progression or unacceptable
toxicity or complication was noted.
We collected information on patients' age, sex, body
mass index (BMI), tumor type, ECOG-PS (18), laboratory values, prior cancer
treatments and comorbidities to calculate CCI scores at the time of
induction of PD-1/PD-L1 inhibitors (19). NLR, an inflammatory marker that is
reported to be associated with frailty and nutritional status
(20,21), and the prognostic nutritional index
(PNI), which is also a nutritional marker used in various cancer
types, was also calculated using laboratory findings (22–25).
Additionally, we assessed the development, severity and clinical
course of all irAEs, namely, thyroid dysfunction, cutaneous
disorders, interstitial pneumonitis, colitis, adrenal
insufficiency, hepatitis, diabetes, and encephalitis. Hepatitis was
defined as liver dysfunction with compatible pathological findings
from liver biopsy or determined by a hepatologist. The severity of
irAE was graded according to the CTCAE 4.0 criteria.
Furthermore, the duration of PD-1/PD-L1 treatment of
each patient was retrieved from the medical records. This was
defined as the time from the start of PD-1/PD-L1 treatment to the
date of documented disease progression or any events that led to
treatment discontinuation. Evaluation of clinical responses was
based on the laboratory findings and the Response Evaluation
Criteria in Solid Tumors version 1.1. The patients were evaluated
after the first 2–3 cycles and underwent computed tomography or
magnetic resonance imaging every 2–3 months according to the
attending physician.
Assessment
Initially, all 197 patients were divided into two
groups based on their age: Elderly group (age ≥75 years) and
non-elderly group (age <75 years). In the assessment of safety,
development and severity of each irAE, hospitalization and
discontinuation of PD-1/PD-L1 inhibitor treatment due to irAEs,
requirement of corticosteroids or immunosuppressants for irAEs, and
development of unexpected critical complications were evaluated.
Moreover, the proportion of patients who continued PD-1/PD-L1
inhibitor treatment for >6 months was analyzed for verification
of tolerability among the two groups.
Since no formal geriatric assessments were
performed, we used indirect markers to assess frailty. Namely, we
constructed a three-element frailty scoring system, including
ECOG-PS, CCI and NLR. These indirect markers were evaluated at the
time of first administration of PD-1/PD-L1 inhibitors. PS ≥2, CCI
score ≥3 and NLR ≥4 were each given one point and the frailty score
(FS) was calculated by adding the points of individual factors, as
shown in Table I. We defined
patients with FS=0 as having low, FS=1 as intermediate and FS=2 or
3 as high frailty. Based on this score, we respectively divided the
elderly and non-elderly patients into low-, intermediate-, and
high-frailty subgroups and evaluated the safety and tolerability of
PD-1/PD-L1 inhibitor treatment as mentioned above.
 | Table I.Frailty scoring system. |
Table I.
Frailty scoring system.
| Marker | Value | Score |
|---|
| Performance
Status | 0-1 | 0 |
|
| ≥2 | 1 |
| Charlson
Comorbidity Index | 0-2 | 0 |
|
| ≥3 | 1 |
|
Neutrophil-Lymphocyte Ratio | <4 | 0 |
|
| ≥4 | 1 |
| Total | Low | 0 |
|
| Intermediate | 1 |
|
| High | 2, 3 |
Statistical analysis
Continuous variables were presented as median with
range according to their distribution. Student's t-test and
Mann-Whitney U test were used to compare continuous variables
between the elderly and non-elderly groups. The Chi-square test or
Fisher's exact test was used to compare categorical variables. A
Bonferroni adjustment was used for multiple comparisons. All
statistical tests were two-sided, and a P-value <0.05 indicated
statistical significance. All statistical analyses were performed
using JMP® 13 (SAS Institute Inc.).
Results
Baseline characteristics
This study group of 197 patients consisted of 77
patients with NSCLC, 32 with MM, 29 with head and neck cancer, 27
with renal cell carcinoma, 23 with gastric cancer, and 9 with
urothelial cancer. Of these patients, 146 received nivolumab, 42
received pembrolizumab and 9 received atezolizumab. At the time of
analysis, the median follow-up duration was 43 weeks (range 2–254
weeks). There were 58 patients (29.4%) aged ≥75 years and 139
patients (70.6%) aged <75 years, which constituted the elderly
and non-elderly groups, respectively. The baseline clinical
characteristics of the patients in the two groups are shown in
Table II. The average age in the
non-elderly and elderly groups was 62.5 and 79.4 years
(P<0.001), respectively. There were no significant differences
in sex, BMI, follow-up duration, type of PD-1/PD-L1 inhibitor
administered, prior therapy lines, PS, NLR and PNI. A certain
deviation in tumor type was observed, and the elderly group showed
fewer prior therapy lines and higher CCI scores, although these did
not reach statistical significance. In addition, 2 out of 7
patients with preexisting autoimmune disease were in use of
corticosteroids or immunosuppressants at baseline; namely, a 70
years old man with dermatomyositis used corticosteroids and a 79
years old woman with rheumatoid arthritis used corticosteroids and
immunosuppressants.
 | Table II.Baseline characteristics of
non-elderly (n=139) and elderly (n=58) patients. |
Table II.
Baseline characteristics of
non-elderly (n=139) and elderly (n=58) patients.
|
Characteristics | Non-elderly (age
<75 years) | Elderly (age ≥75
years) | P-value |
|---|
| Age, years | 62.5 | 79.4 | <0.01 |
| Sex, n
(male/female) | 93/46 | 39/19 | 0.96 |
| PS, n |
|
| 0.72 |
| 0 | 78 | 32 |
|
| 1 | 45 | 17 |
|
| ≥2 | 16 | 9 |
|
| Median follow-up
period, weeks (range) | 40 (2–254) | 52 (8–170) | 0.12 |
| Tumor type, n |
|
| 0.08 |
|
NSCLC | 56 | 21 |
|
| MM | 18 | 14 |
|
| Head
and neck | 25 | 4 |
|
|
RCC | 20 | 7 |
|
|
Gastric | 16 | 7 |
|
|
Urothelial | 4 | 5 |
|
| Treatment, n |
|
| 0.84 |
|
Nivolumab | 106 | 40 |
|
|
Pembrolizumab | 26 | 16 |
|
|
Atezolizumab | 7 | 2 |
|
| Prior therapy
lines, n (≤1/≥2) |
68/71 | 36/22 | 0.09 |
| CCI, n |
|
| 0.09 |
| 0 | 74 | 25 |
|
| 1,
2 | 55 | 23 |
|
| ≥3 | 10 | 10 |
|
| BMI | 21.2 | 20.9 | 0.58 |
| NLR | 4.1 | 3.8 | 0.50 |
| PNI | 43.2 | 43.6 | 0.71 |
| Preexisting
autoimmune disease, n | 4 | 3 | 0.43 |
Profiles of irAEs in the elderly and
non-elderly groups
The profiles of irAEs in the elderly and non-elderly
groups are shown in Table III.
Moreover, 52 patients (37.4%) in the non-elderly group and 28
patients (48.3%) in the elderly group developed any irAEs of any
grade, which showed a slight tendency of more irAEs in elderly
patients, although the difference was not statistically
significant. Moreover, the analysis of irAEs showed no significant
difference in the development of cutaneous disorders, thyroid
dysfunction, interstitial pneumonitis, adrenal insufficiency,
hepatitis, and diabetes. The severity of irAEs and clinical
outcomes related to irAEs are shown in Table IV. Grade 3–5 irAEs developed equally
in the non-elderly and elderly groups [12 patients (8.6%) in the
non-elderly group and 5 (8.6%) in the elderly group], although one
elderly patient with renal cell carcinoma developed grade 5
hepatitis (fulminant hepatitis), which showed resistance to pulse
steroid therapy and died after the 4th cycle of nivolumab. Although
the following data did not show statistical significance,
hospitalization due to irAEs and use of corticosteroids or
immunosuppressants for irAEs were slightly higher in the elderly
patients, and PD-1/PD-L1 inhibitor treatment was more easily
discontinued in the elderly group than in the non-elderly group [9
patients (15.5%) vs. 13 patients (9.4%) (P=0.21)]. Furthermore,
unexpected critical complications developed more frequently in the
elderly patients (P=0.03), including each patient with acute
exacerbation of chronic heart failure, cerebral hemorrhage, femoral
neck fracture due to fall and sudden unexplained death.
 | Table III.Development of irAEs among the
non-elderly (n=139) and elderly (n=58) groups. |
Table III.
Development of irAEs among the
non-elderly (n=139) and elderly (n=58) groups.
| irAEs | Non-elderly (age
<75 years), n (%) | Elderly (age ≥75
years), n (%) | P-value |
|---|
| Total of irAEs | 52 (37.4) | 28 (48.3) | 0.16 |
| Thyroid
dysfunction | 18 (13.1) | 12 (20.7) | 0.18 |
| Cutaneous
disorders | 25 (18.0) | 12 (20.7) | 0.66 |
| Interstitial
pneumonitis | 10 (7.2) | 5 (8.6) | 0.73 |
| Colitis | 6 (4.3) | 2 (3.5) | 0.78 |
| Adrenal
insufficiency | 5 (3.6) | 2 (3.5) | 0.96 |
| Hepatits | 3 (2.1) | 1 (1.7) | 0.84 |
| Diabetes | 2 (1.4) | 1 (1.7) | 0.70 |
 | Table IV.Severity of irAEs and clinical
outcomes related to irAEs in the non-elderly (n=139) and elderly
(n=58) patients. |
Table IV.
Severity of irAEs and clinical
outcomes related to irAEs in the non-elderly (n=139) and elderly
(n=58) patients.
| Variables | Non-elderly (age
<75 years), n (%) | Elderly (age ≥75
years), n (%) | P-value |
|---|
| Total of irAEs | 52 (37.4) | 28 (48.3) | 0.16 |
| Grade 3–5
irAEs | 12 (8.6) | 5 (8.6) | 0.99 |
|
| Gr3: N=9, Gr4:
N=3 | Gr3: N=2, Gr4: N=2,
Gr5: N=1 |
|
| Hospitalization due
to irAE | 12 (8.6%) | 7 (12.1%) | 0.46 |
| Use of
corticosteroids or immunosuppressants for irAE | 17 (12.2) | 8 (13.8) | 0.76 |
| Treatment
discontinuation due to irAE | 13 (9.4) | 9 (15.5) | 0.21 |
| Unexpected critical
complications | 1
(0.7)a | 4
(6.9)b | 0.03 |
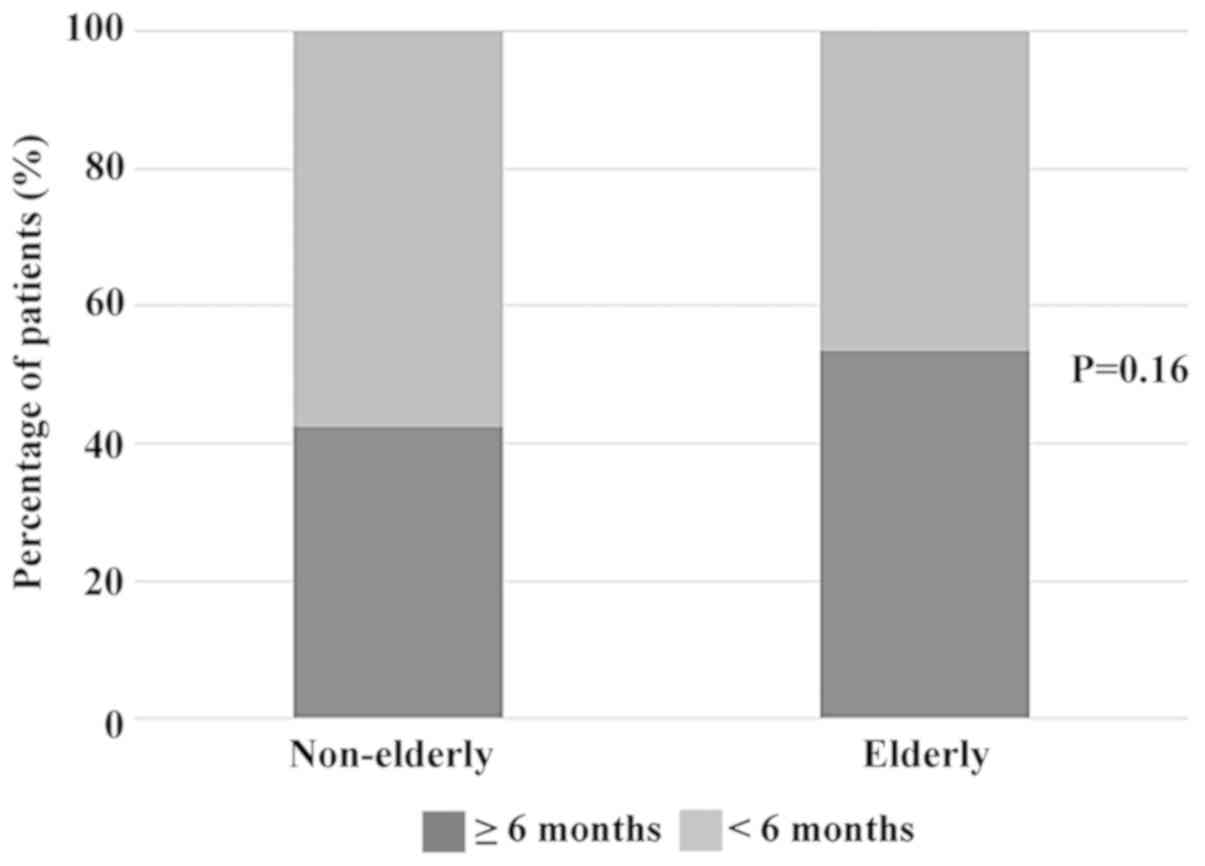
When we analyzed the treatment duration among the
two groups, 59 patients (42.5%) in the non-elderly group and 31
patients (53.5%) in the elderly group were able to continue
PD-1/PD-L1 inhibitor treatment for >6 months (Fig. 1). In addition, patients who had
longer treatment duration showed tendency to develop more irAEs in
the two groups, especially in the non-elderly patients [29 patients
(49.2%) with treatment period ≥6 months and 23 patients (28.8%)]
with treatment period <6 months, P=0.01, data not shown).
According to these results, PD-1/PD-L1 inhibitors seemed to have
comparable tolerability in elderly patients.
Frailty scores
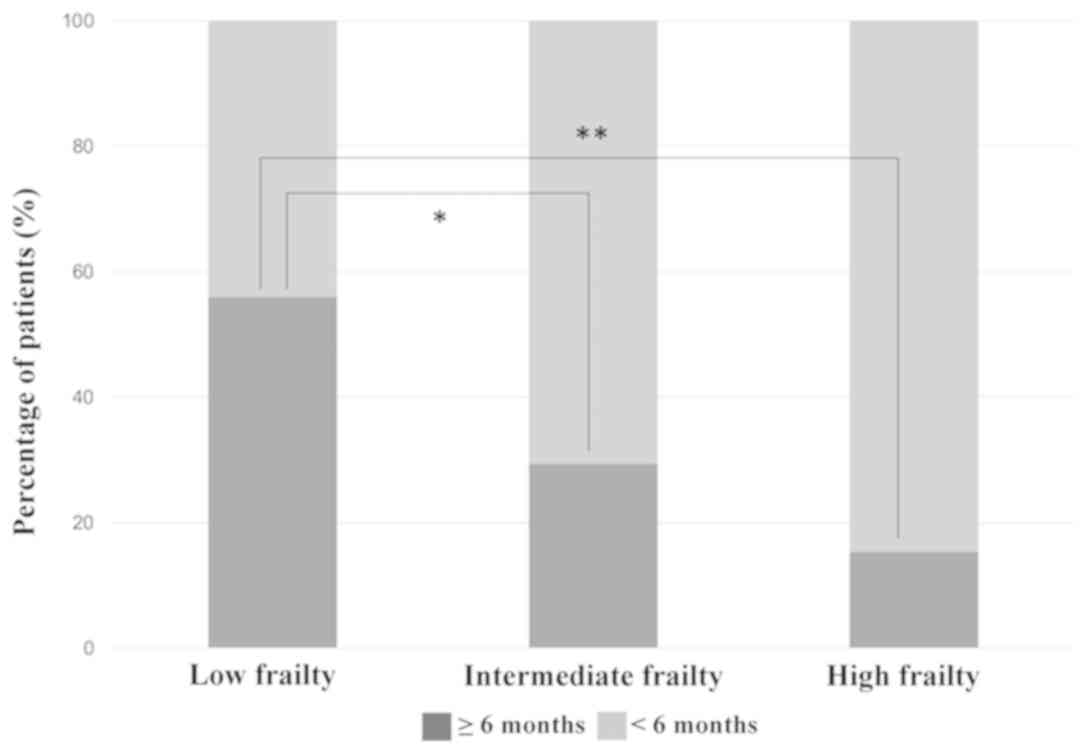
In the non-elderly group, 75 patients (54.0%) with
FS=0, 51 patients (36.7%) with FS=1 and 13 patients (9.3%) with
FS=2 or 3 were defined as patients with low, intermediate and high
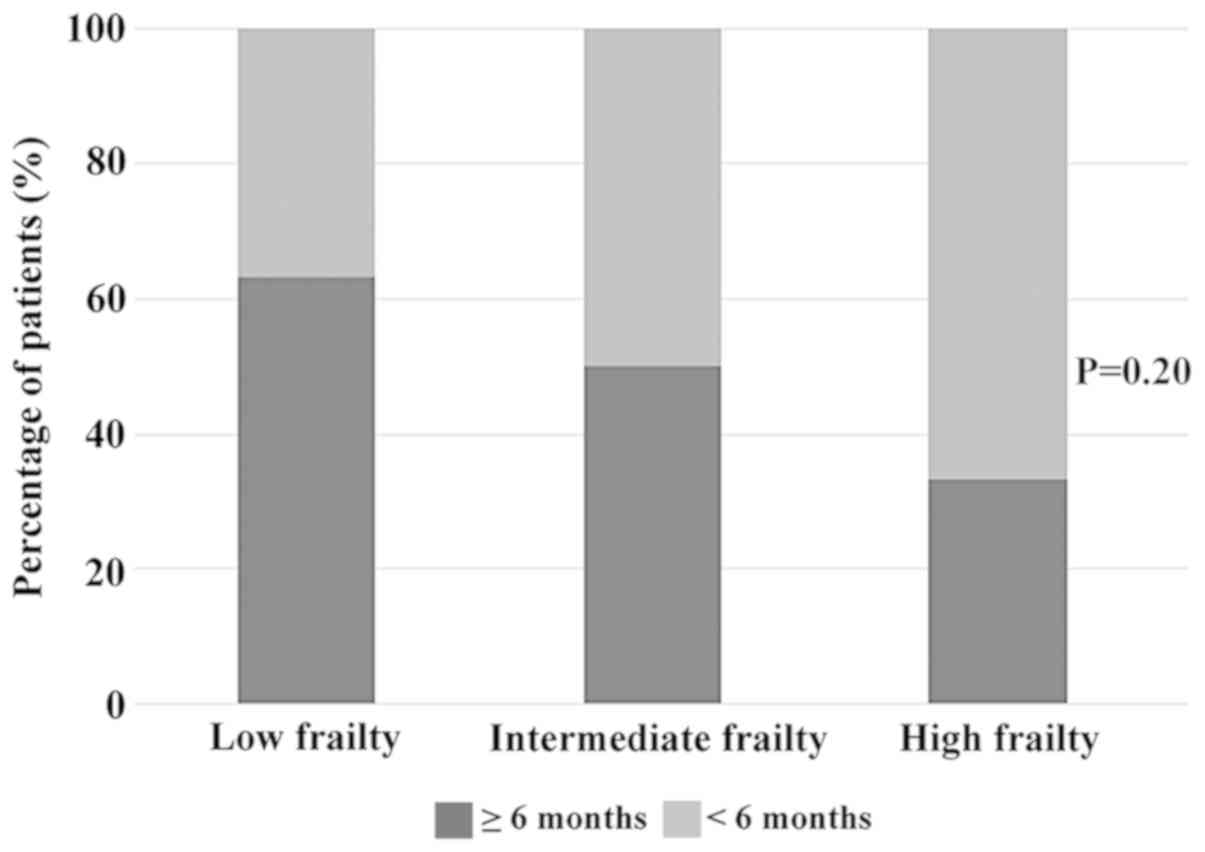
frailty, respectively. In the elderly group, 30 patients (51.7%)
with FS=0, 16 patients (27.6%) with FS=1, and 12 patients (20.7%)
with FS=2 or 3 were defined as patients with low, intermediate and
high frailty, respectively. The proportion of patients with high
frailty was considerably greater in the elderly group and close to
significance (P=0.08).
Development of irAEs and clinical
outcomes in patients with low, intermediate, and high frailty
The development of irAEs and unexpected critical
complications in the three frailty subgroups of the non-elderly and
elderly patients are demonstrated in Tables V and VI. In the non-elderly patients, 35
patients (46.7%) with low frailty, 15 patients (29.4%) with
intermediate frailty and 2 patients (15.4%) with high frailty
developed any irAEs of any grade, which showed a tendency of lower
development of irAEs in patients with high frailty (P=0.09). No
significant difference was shown in the development of severe irAEs
among the three groups. One patient with low frailty had
exacerbation of the preexisting dermatomyositis 17 days after
initiation of atezolizumab.
 | Table V.Development of irAEs and unexpected
critical complications among the three subgroups of frailty (age
<75 years). |
Table V.
Development of irAEs and unexpected
critical complications among the three subgroups of frailty (age
<75 years).
| Variables | Low frailty (FS=0)
(n=75), n (%) | Intermediate
frailty (FS=1) (n=51), n (%) | High frailty (FS=2,
3) (n=13), n (%) | P-value |
|---|
| Total of irAEs | 35 (46.7) | 15 (29.4) | 2 (15.4) | 0.09 |
| Grade 3–5
irAEs | 5 (6.7) | 5 (9.8) | 2 (15.4) | 0.55 |
| Unexpected critical
complications | Worsening of
preexisting dermatomyositis (n=1) | None | None |
|
 | Table VI.Development of irAEs and unexpected
critical complications among the three subgroups of frailty (age
≥75 years). |
Table VI.
Development of irAEs and unexpected
critical complications among the three subgroups of frailty (age
≥75 years).
| Variables | Low frailty (FS=0)
(n=30), n (%) | Intermediate
frailty (FS=1) (n=16), n (%) | High frailty (FS=2,
3) (n=12), n (%) | P-value |
|---|
| Total of irAEs | 17 (56.7) | 7 (43.8) | 4
(33.3) | 0.36 |
| Grade 3–5
irAEs | 2 (6.7) | 2 (12.5) | 1 (8.3) | 0.80 |
| Unexpected critical
complications | Cerebral hemorrhage
(n=1), femoral neck fracture (n=1) | None | Exacerbation of
chornic heart failure (n=1), sudden unexplained death (n=1) |
|
In the elderly patients, 17 patients (56.7%) with
low frailty, 7 patients (43.8%) with intermediate frailty, and 4
patients (33.3%) with high frailty developed any irAEs of any grade
(P=0.36). There was also no significant difference in the
development of grade 3–5 irAEs among the three subgroups. However,
it should be noted that two patients with high frailty developed
critical complications, including a case of sudden death.
Tables VII and
VIII show the clinical outcomes
of all patients (12 patients in the non-elderly group, 5 in the
elderly group) who developed grade 3–5 irAEs. All non-elderly
patients recovered from severe irAEs, including one patient with
low and one patient with intermediate frailty restarting the same
PD-1/PD-L1 inhibitor. In contrast, one elderly patient with high
frailty died of fulminant hepatitis as mentioned above, and no
other patient resumed ICI treatment afterward.
 | Table VII.Clinical features of the non-elderly
patients with severe irAEs. |
Table VII.
Clinical features of the non-elderly
patients with severe irAEs.
| Case | Age, years | Sex | Tumor type | Treatment | Frailty | irAE | Grade | Remission of
irAE | Restart of
PD-1/PD-L1 treatment |
|---|
| 1 | 60 | M | RCC | Nivolumab | Low | Diabetes | 4 (diabetic
ketoacidosis) | Yes | Yes |
| 2 | 67 | F | NSCLC | Nivolumab | Low | Diabetes | 4 (diabetic
ketoacidosis) | Yes | No |
| 3 | 71 | M | Urothelial | Pembrolizumab | Low | Colitis | 3 | Yes | No |
| 4 | 68 | M | MM | Nivolumab | Low | Aderenal
insufficienecy | 3 | Yes | No |
| 5 | 70 | F | NSCLC | Nivolumab | Low | Interstitial
pneumonitis | 3 | Yes | No |
| 6 | 74 | F | NSCLC | Nivolumab | Intermediate | Hepatitis | 4 | Yes | No |
| 7 | 73 | M | NSCLC | Pembrolizumab | Intermediate | interstitial
pneumonitis | 3 | Yes | No |
| 8 | 71 | M | NSCLC | Atezolizumab | Intermediate | Encephatitis | 3 | Yes | No |
| 9 | 63 | M | NSCLC | Nivolumab | Intermediate | Interstitial
pneumonitis | 3 | Yes | No |
| 10 | 60 | M | Head and neck | Nivolumab | Intermediate | Hepatitis | 3 | Yes | Yes |
| 11 | 44 | M | Gastric | Nivolumab | High | Interstitial
pneumonitis | 3 | Yes | No |
| 12 | 73 | M | NSCLC | Pembrolizumab | High | Aderenal
insufficinecy | 3 | Yes | No |
 | Table VIII.Clinical features of the elderly
patients with severe irAEs. |
Table VIII.
Clinical features of the elderly
patients with severe irAEs.
| Case | Age, years | Sex | Tumor type | Treatment | Frailty | irAE | Grade | Remission of
irAE | Restart of
PD-1/PD-L1 treatment |
|---|
| 1 | 78 | M | NSCLC | Pembrolizumab | Low | Aderenal
insufficienecy | 3 | Yes | No |
| 2 | 75 | M | RCC | Nivolumab | Low | Aderenal
insufficienecy | 3 | Yes | No |
| 3 | 84 | F | MM | Nivolumab | Intermediate | Cutaneous
disorder | 4 (Stevens-Johnson
syndrome) | Yes | No |
| 4 | 77 | F | MM | Nivolumab | Intermediate | Diabetes | 4 (diabetic
ketoacidosis) | Yes | No |
| 5 | 80 | F | RCC | Nivolumab | High | Hepatitis | 5 (fulminant
hepatits) | No (died) | No |
When we focused on the treatment duration of
PD-1/PD-L1 inhibitor among the three frailty subgroups, patients
with low frailty showed a tendency to continue treatment for >6
months in both non-elderly and elderly population (Figs. 2 and 3). Nevertheless, 33.3% of patients with
high frailty aged ≥75 years were able to continue PD-1/PD-L1
inhibitor treatment for >6 months, suggesting a certain level of
efficacy and tolerability of these drugs in this population.
Discussion
In this study, we described the safety and
tolerability of PD-1 inhibitors in elderly and frail patients with
advanced malignancies. To the best of our knowledge, this is the
first study to evaluate the correlation between indirect frailty
markers and clinical outcomes in patients with advanced cancer
treated by PD-1/PD-L1 inhibitors.
Our data showed that elderly patients developed
slightly more irAEs of any grade than non-elderly patients,
although this was not statistically significant. In detail, no
significant difference was found in the development of cutaneous
disorders, thyroid dysfunction, interstitial pneumonitis, adrenal
insufficiency, hepatitis, and diabetes. Moreover, the development
of severe irAEs was similar between the non-elderly and elderly
patients. However, hospitalization or treatment discontinuation due
to irAEs and use of corticosteroids or immunosuppressants were more
frequent in the elderly group. Additionally, unexpected critical
complications developed more often in this group, including a case
of acute exacerbation of chronic heart failure, cerebral hemorrhage
and sudden death.
When we assessed frailty using indirect markers such
as PS, CCI and NLR, the incidence of severe irAEs was not
associated with the frailty levels in both non-elderly and elderly
groups. In non-elderly patients, all recovered with proper
treatment and each case with low/intermediate frailty were able to
restart the same PD-1 inhibitor. On the contrary, none of the
patients were able to restart PD-1 treatment, and grade 5 hepatitis
and fatal complications were observed in the high-frailty subgroup.
This supports the idea that assessment of frailty is especially
important in elderly patients.
In previous clinical trials, no major increase in
the incidence of irAEs was noted in this population (26–28).
However, only relatively fit patients were enrolled and patients
with reduced functional reserve or age-related comorbidities were
excluded in these trials and therefore not representative of the
‘real-world’ population. In limited observational studies based on
more general population of patients, contradictory results are
reported. Sattar et al (29)
and Leroy et al (30) showed
a trend of higher incidence of irAEs in elderly patients. Muchnik
et al (31) reported that a
larger proportion of elderly patients were hospitalized and
required corticosteroids and treatment discontinuation. Although
not statistically significant, our data also showed a trend to
increased irAEs of any grade with more frequent hospitalization,
need of immune-modulating medication and treatment discontinuation
in elderly patients. Meanwhile, our results also showed that the
proportion of patients who were able to continue PD-1/PD-L1
inhibitor treatment for >6 months was slightly larger in the
elderly patients. This suggests that PD-1/PD-L1 inhibitors may have
comparable tolerability and provide similar levels of benefit to
elderly patients. These ambivalent findings were found
retrospectively with small sample size, although it implies further
investigation of the application of ICIs in elderly patients.
Patients with advanced malignancies have an
increased risk of frailty due to cancer cachexia or tumor-related
disabilities, and more than half of elderly patients are vulnerable
or frail (32). Selecting frail
patients using geriatric assessment can help personalize treatment
decisions, which leads to less treatment-related adverse events and
mortality (3). However, the best
geriatric assessment tool to evaluate frailty has still not reached
consensus and consequently not widely used in daily clinical
practice (2). ICI treatment is
generally considered to be more tolerable than chemotherapy,
although there are still concerns about the greater impact of irAEs
in frail patients than in fit patients due to comorbidities and
reduced functional reserve. From the abovementioned background,
only a few studies have described the correlation between frailty
and safety of immunotherapy. Welaya et al (33) did not find any relationships between
impairment in geriatric assessment domains and complications but
showed that patients with impairments of instrumental activities of
daily living had shorter treatment duration. Archibald et al
(34) used indirect frailty markers
reporting that frail elderly patients had similar response rates
and treatment toxicity compared to fit patients. We also used
indirect markers such as ECOG-PS, CCI and NLR, which have been
reported to correlate with frailty (20,35,36), and
created an original scoring system showing marginally difference
between elderly and non-elderly patients. Indeed, this system is
not sufficient, although provided a certain indication of frailty
in cancer patients. Further evaluation of frailty markers,
classification systems and geriatric assessment tools are urgently
warranted in this field.
In this study, we did not find a correlation between
the development of grade 3–5 irAEs and frailty levels but warned
that fatal irAEs and complications may develop more frequently in
frail elderly patients. This may be a result of reduced functional
reserve or interaction of adverse events and comorbidities.
Although some recent studies have reported the safety and efficacy
of retreatment with ICIs after irAEs, contradictory reports exist
and no consensus has been reached yet (37–39).
Therefore, restarting ICIs after irAEs may be too challenging for
these patients. Regardless of age, the treatment duration was
shorter in frail patients. Perhaps, this was because frailty was
defined based on PS and NLR, which can be related to disease
activity (40,41), and patients may have had more rapidly
progressive cancer, in which PD-1 inhibitors are less effective
(42). Besides, the incidence of
irAEs of any grade was low in frail patients, especially in the
younger group. Although, we speculate that this was influenced by
the shorter treatment duration. It is noteworthy that 33.3% of
elderly patients with high frailty were able to continue PD-1/PD-L1
inhibitor treatment for >6 months. We consider this data
valuable because these patients may have no indication of
conventional chemotherapy, and PD-1/PD-L1 inhibitors may provide an
opportunity to prolong life expectancy with safety and mild
toxicity. This data may become more meaningful when PD-1/PD-L1
inhibitor treatment indication has expanded more broadly, and we
hope this result serves as a basis for future research.
There are several limitations in this study. First,
this was a single-center retrospective study. Therefore, the
analyzed data were limited by its retrospective nature, and the
patient sample size was relatively small. However, the collected
data were purely based on daily clinical practice in our hospital
and we did not include patients on clinical trials because these
patients could have closer monitoring of adverse events; thus, we
believe that our cohort was representative of the general
population. Second, we used indirect markers for the evaluation of
frailty instead of formal geriatric assessment, because it was not
performed in our study cohort. Indeed, geriatric assessment will
help us clearly identify vulnerable and frail patients.
Nevertheless, our three-element frailty scoring system showed more
frail patients in the elderly patients indicating that this method
was reasonable.
Therefore, based on data of the ‘real-world’
population, we demonstrated that mild and severe irAEs developed
similarly in non-elderly and elderly patients treated with
PD-1/PD-L1 inhibitors. In elderly patients, frailty evaluation
seemed to be essential in the safe management of PD-1/PD-L1
inhibitor treatment. Simultaneously, we also note that a certain
proportion of frail elderly patients can receive great benefit from
these drugs with close monitoring. Therefore, further prospective
studies are required to evaluate the safety of ICI treatment in
frail patients using geriatric assessment and identify predictive
biomarkers for optimizing the application of antitumor
immunotherapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TS, TI and YI were responsible for the design of the
study, selection and analysis and interpretation of the data. They
also have revised critically the manuscript for important
intellectual content. TI, JU, YT, SK, JA, AA, HT, TK, HK, FH, MI,
SH, OU, TT and KT were responsible for the acquisition and clinical
interpretation of the data. All authors had full access to all of
the data in the study and had final responsibility for the decision
to submit for publication. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was designed under the
responsibility of Kyoto Prefectural University of Medicine, in
conjunction with the steering committee (approval no. ERB-C-867-1).
Given the retrospective nature of this work, the requirement for
informed consent was waived for the individual participants
included in the study in accordance with the standards of the Kyoto
Prefectural University of Medicine Institutional Medical Ethics
Review Committee.
Patient consent for publication
Not applicable.
Competing interests
YI has received research grants and lecture fees
from Ono Pharmaceutical Co., Ltd., Bristol-Myers Squibb, MSD and
research grants from Chugai Pharmaceutical Co., Ltd. TI and TT
received lecture fees from Ono Pharmaceutical Co., Ltd. and Chugai
Pharmaceutical Co., Ltd. TT has also received research grants from
Chugai Pharmaceutical Co., Ltd. KT and OU have received research
grants from Ono Pharmaceutical Co., Ltd. and Chugai Pharmaceutical
Co., Ltd. KT has also received lecture fees from Ono Pharmaceutical
Co., Ltd., Bristol-Myers Squibb, MSD and Chugai Pharmaceutical Co.,
Ltd. The authors declare that all these conflicts of interest are
not connected with the issue of this paper. The other authors have
no conflicts of interest to declare.
Glossary
Abbreviations
Abbreviations:
|
ICI
|
immune checkpoint inhibitors
|
|
PD-1
|
programmed cell death protein-1
|
|
PD-L1
|
programmed cell death ligand 1
|
|
NSCLC
|
non- small cell lung cancer
|
|
MM
|
malignant melanoma
|
|
irAEs
|
immune-related adverse events
|
|
CTLA-4
|
cytotoxic T-lymphocyte associated
protein-4
|
|
PS
|
performance status
|
|
CCI
|
Charlson Comorbidity Index
|
|
NLR
|
neutrophil-lymphocyte ratio
|
References
|
1
|
National Cancer Institute, . Cancer Stat
Facts: Cancer of Any Site. Available from:
https://seer.cancer.gov/statfacts/html/all.html. December
16–2019
|
|
2
|
Wildiers H, Heeren P, Puts M, Topinkova E,
Janssen-Heijnen ML, Extermann M, Falandry C, Artz A, Brain E,
Colloca G, et al: International Society of Geriatric Oncology
consensus on geriatric assessment in older patients with cancer. J
Clin Oncol. 32:2595–2603. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hurria A, Togawa K, Mohile SG, Owusu C,
Klepin HD, Gross CP, Lichtman SM, Gajra A, Bhatia S, Katheria V, et
al: Predicting chemotherapy toxicity in older adults with cancer: A
prospective multicenter study. J Clin Oncol. 29:3457–3465. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Robert C, Long GV, Brady B, Dutriaux C,
Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C,
Kalinka-Warzocha E, et al: Nivolumab in previously untreated
melanoma without BRAF mutation. N Engl J Med. 372:320–330. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al CheckMate 025 Investigators, : Nivolumab versus
everolimus in advanced renal-cell carcinoma. N Engl J Med.
373:1803–1813. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferris RL, Blumenschein G Jr, Fayette J,
Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE,
Even C, et al: Nivolumab for recurrent squamous-cell carcinoma of
the head and neck. N Engl J Med. 375:1856–1867. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ansell SM, Lesokhin AM, Borrello I,
Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry
D, Freeman GJ, et al: PD-1 blockade with nivolumab in relapsed or
refractory Hodgkin's lymphoma. N Engl J Med. 372:311–319. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang Y-K, Boku N, Satoh T, Ryu M-H, Chao
Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, et al: Nivolumab in
patients with advanced gastric or gastro-oesophageal junction
cancer refractory to, or intolerant of, at least two previous
chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet.
390:2461–2471. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Garon EB, Rizvi NA, Hui R, Leighl N,
Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L,
et al KEYNOTE-001 Investigators, : Pembrolizumab for the treatment
of non-small-cell lung cancer. N Engl J Med. 372:2018–2028. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Elias R, Morales J, Rehman Y and Khurshid
H: Immune checkpoint inhibitors in older adults. Curr Oncol Rep.
18:472016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Elias R, Karantanos T, Sira E and
Hartshorn KL: Immunotherapy comes of age: Immune aging &
checkpoint inhibitors. J Geriatr Oncol. 8:229–235. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tomihara K, Curiel TJ and Zhang B:
Optimization of immunotherapy in elderly cancer patients. Crit Rev
Oncog. 18:573–583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Czesnikiewicz-Guzik M, Lee WW, Cui D,
Hiruma Y, Lamar DL, Yang ZZ, Ouslander JG, Weyand CM and Goronzy
JJ: T cell subset-specific susceptibility to aging. Clin Immunol.
127:107–118. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weng NP, Akbar AN and Goronzy J: CD28(−) T
cells: Their role in the age-associated decline of immune function.
Trends Immunol. 30:306–312. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Filaci G, Fravega M, Negrini S, Procopio
F, Fenoglio D, Rizzi M, Brenci S, Contini P, Olive D, Ghio M, et
al: Nonantigen specific CD8+ T suppressor lymphocytes
originate from CD8+CD28− T cells and inhibit
both T-cell proliferation and CTL function. Hum Immunol.
65:142–156. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Loh KP, Wong ML and Maggiore R: From
clinical trials to real-world practice: Immune checkpoint
inhibitors in older adults. J Geriatr Oncol. 10:384–388. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Charlson ME, Pompei P, Ales KL and
MacKenzie CR: A new method of classifying prognostic comorbidity in
longitudinal studies: Development and validation. J Chronic Dis.
40:373–383. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nishijima TF, Deal AM, Williams GR,
Guerard EJ, Nyrop KA and Muss HB: Frailty and inflammatory markers
in older adults with cancer. Aging (Albany NY). 9:650–664. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sato Y, Gonda K, Harada M, Tanisaka Y,
Arai S, Mashimo Y, Iwano H, Sato H, Ryozawa S, Takahashi T, et al:
Increased neutrophil-to-lymphocyte ratio is a novel marker for
nutrition, inflammation and chemotherapy outcome in patients with
locally advanced and metastatic esophageal squamous cell carcinoma.
Biomed Rep. 7:79–84. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mirili C, Yılmaz A, Demirkan S, Bilici M
and Basol Tekin S: Clinical significance of prognostic nutritional
index (PNI) in malignant melanoma. Int J Clin Oncol. 24:1301–1310.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang W, Ye B, Liang W and Ren Y:
Preoperative prognostic nutritional index is a powerful predictor
of prognosis in patients with stage III ovarian cancer. Sci Rep.
7:95482017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li D, Yuan X, Liu J, Li C and Li W:
Prognostic value of prognostic nutritional index in lung cancer: A
meta-analysis. J Thorac Dis. 10:5298–5307. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Onodera T, Goseki N and Kosaki G:
Prognostic nutritional index in gastrointestinal surgery of
malnourished cancer patients. Nihon Geka Gakkai Zasshi.
85:1001–1005. 1984.(In Japanese). PubMed/NCBI
|
|
26
|
Singh H, Kim G, Maher VE, Beaver JA,
Pai-Scherf LH and Balasubramaniam S: FDA subset analysis of the
safety of nivolumab in elderly patients with advanced cancers. J
Clin Oncol. 34 (Suppl 15):100102016. View Article : Google Scholar
|
|
27
|
Nosaki K, Saka H, Hosomi Y, Baas P, de
Castro G Jr, Reck M, Wu YL, Brahmer JR, Felip E, Sawada T, et al:
Safety and efficacy of pembrolizumab monotherapy in elderly
patients with PD-L1-positive advanced non-small-cell lung cancer:
Pooled analysis from the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042
studies. Lung Cancer. 135:188–195. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Spigel D, Schwartzberg L, Waterhouse D,
Chandler J, Hussein M, Jotte R, Stepanski E, Mccleod M, Page R, Sen
R, et al: Is nivolumab safe and effective in elderly and PS2
patients with non-small cell lung cancer (NSCLC)? Results of
CheckMate 153. J Thorac Oncol. 12:S1287–S1288. 2017. View Article : Google Scholar
|
|
29
|
Sattar J, Kartolo A, Hopman WM, Lakoff JM
and Baetz T: The efficacy and toxicity of immune checkpoint
inhibitors in a real-world older patient population. J Geriatr
Oncol. 10:411–414. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Leroy V, Gerard E, Dutriaux C, Prey S, Gey
A, Mertens C, Beylot-Barry M and Pham-Ledard A: Adverse events need
for hospitalization and systemic immunosuppression in very elderly
patients (over 80 years) treated with ipilimumab for metastatic
melanoma. Cancer Immunol Immunother. 68:545–551. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Muchnik E, Loh KP, Strawderman M, Magnuson
A, Mohile SG, Estrah V and Maggiore RJ: Immune checkpoint
inhibitors in real-world treatment of older adults with non-small
cell lung cancer. J Am Geriatr Soc. 67:905–912. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Handforth C, Clegg A, Young C, Simpkins S,
Seymour MT, Selby PJ and Young J: The prevalence and outcomes of
frailty in older cancer patients: A systematic review. Ann Oncol.
26:1091–1101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Welaya K, Loh KP, Messing S, Szuba E,
Magnuson A, Mohile SG and Maggiore RJ: Geriatric assessment and
treatment outcomes in older adults with cancer receiving immune
checkpoint inhibitors. J Geriatr Oncol. 11:523–528. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Archibald WJ, Victor AI, Strawderman MS
and Maggiore RJ: Immune checkpoint inhibitors in older adults with
melanoma or cutaneous malignancies: The Wilmot Cancer Institute
experience. J Geriatr Oncol. 11:496–502. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gleason LJ, Benton EA, Alvarez-Nebreda ML,
Weaver MJ, Harris MB and Javedan H: FRAIL questionnaire screening
tool and short-term outcomes in geriatric fracture patients. J Am
Med Dir Assoc. 18:1082–1086. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Facon T, Dimopoulos MA, Meuleman N, Belch
A, Mohty M, Chen WM, Kim K, Zamagni E, Rodriguez-Otero P, Renwick
W, et al: A simplified frailty scale predicts outcomes in
transplant-ineligible patients with newly diagnosed multiple
myeloma treated in the FIRST (MM-020) trial. Leukemia. 34:224–233.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Santini FC, Rizvi H, Plodkowski AJ, Ni A,
Lacouture ME, Gambarin-Gelwan M, Wilkins O, Panora E, Halpenny DF,
Long NM, et al: Safety and efficacy of re-treating with
immunotherapy after immune-related adverse events in patients with
NSCLC. Cancer Immunol Res. 6:1093–1099. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Simonaggio A, Michot JM, Voisin AL, Le
Pavec J, Collins M, Lallart A, Cengizalp G, Vozy A, Laparra A,
Varga A, et al: Evaluation of readministration of immune checkpoint
inhibitors after immune-related adverse events in patients with
cancer. JAMA Oncol. 5:13102019. View Article : Google Scholar
|
|
39
|
Dolladille C, Ederhy S, Sassier M, Cautela
J, Thuny F, Cohen AA, Fedrizzi S, Chrétien B, Da-Silva A, Plane AF,
et al: Immune checkpoint inhibitor rechallenge after immune-related
adverse events in patients with cancer. JAMA Oncol. 6:8652020.
View Article : Google Scholar
|
|
40
|
Bowen RC, Little NAB, Harmer JR, Ma J,
Mirabelli LG, Roller KD, Breivik AM, Signor E, Miller AB and Khong
HT: Neutrophil-to-lymphocyte ratio as prognostic indicator in
gastrointestinal cancers: A systematic review and meta-analysis.
Oncotarget. 8:32171–32189. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Templeton AJ, McNamara MG, Šeruga B,
Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G,
Knox JJ, Tran B, et al: Prognostic role of neutrophil-to-lymphocyte
ratio in solid tumors: A systematic review and meta-analysis. J
Natl Cancer Inst. 106:dju1242014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kato K, Masuishi T, Fushiki K, Nakano S,
Kawakami T, Kawamoto Y, Narita Y, Tsushima T, Nakatsumi H, Kadowaki
S, et al: Impact of tumor growth rate during preceding treatment on
tumor response to nivolumab or irinotecan in advanced gastric
cancer. J Clin Oncol. 37 (Suppl 4):842019. View Article : Google Scholar : PubMed/NCBI
|