Introduction
Hepatocellular carcinoma (HCC) has developed into
one of the most important medical problems, with high incidence
(6th in terms of new cases) and mortality rates (5-year survival
rate is ~18%) worldwide in recent years (1). With advances in understanding the
pathogenesis of HCC, many treatments have been used to increase
survival in patients with HCC, including surgical treatment,
radiotherapy and chemotherapy (2).
Emerging evidence suggests that many chemotherapeutic drugs,
including cisplatin, sorafenib and paclitaxel (PTX), are used for
the treatment of HCC, however, resistance is regarded as a major
hindrance of these drugs in HCC (3,4). Hence,
much hope is placed in probing novel target to ameliorate
resistance to PTX in HCC.
MicroRNAs (miRNAs/miRs) are a class of small
non-coding RNAs with 20–25 nucleotides, which play essential roles
in the diagnosis and prognosis of HCC via multiple pathways
(5). Moreover, miRNAs have vital
roles in PTX resistance in HCC by regulating different molecular
signaling pathways (6). For
instance, miR-877 was found to regulate PTX sensitivity in HCC by
targeting forkhead box protein M1 (FOXM1) (7). In addition, miR-153 contributes to
resistance of HCC cells to chemotherapeutic agents, such as
sorafenib, etoposide and PTX (8). As
for miR-212-3p, a miRNA plays an important role in cancer
progression by regulating cell proliferation and apoptosis
(9). Furthermore, previous study
suggested that miR-212 is associated with radio-sensitivity in
glioma cells by regulating breast cancer susceptibility gene 1
(BRCA1) (10). Besides, miR-212
could suppress cell proliferation and promote cell apoptosis by
regulating FOXA1 in HCC (11).
Notably, miR-212-3p, a mature form of miR-212, is expressed and may
be used as a potential target for the diagnosis, prognosis and
treatment of HCC (12). However,
there is no direct evidence that miR-212-3p participates in
resistance to PTX in HCC.
Zinc finger E-box binding homeobox 2 (ZEB2) has been
reported as a transcription factor, which exerts an important
impact on the development of the nervous system (13). Moreover, ZEB2 is a key factor of
epithelial-mesenchymal transition (EMT), which is associated with
resistance to cisplatin or PTX in human lung cancer cells (14). Notably, ZEB2 plays essential roles in
HCC progression, through regulating EMT, invasion and metastasis
(15). Intriguingly, bioinformatics
analysis using TargetScan provided putative binding sites of
miR-212-3p and ZEB2. Hence, it was hypothesized that ZEB2 may be
involved in miR-212-3p-mediated PTX resistance in HCC. In the
present study the expression of miR-212-3p, EMT, migration and
invasion were assessed in PTX-resistant HCC cells. Moreover, the
effect of miR-212-3p on PTX resistance, EMT, migration and invasion
were investigated. In addition, the association between miR-212-3p
and ZEB2, and their effect on PTX resistance, was explored in HCC
cells.
Materials and methods
Cell culture and treatment
The human HCC cell lines Huh7 and HCCLM3 cells were
purchased from Japanese Collection of Research (JCRB Cell Bank,
Japan). All cells were maintained in RPMI-1640 culture medium
(Gibco; Thermo Fisher Scientific, Inc.) containing 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.), 1% penicillin and
streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C
with 5% CO2 during the study. The PTX-resistant Huh7
cells were constructed by continuous treatment for 24 h at 37°C
with increasing concentrations of PTX (0–20 nM) and removing any
dead PTX-sensitive cells. Briefly, Huh7 cells were treated with 1
nM PTX for 24 h. Subsequently, the medium was replaced with fresh
PTX-free medium. After cells reached 80% confluence, they were
incubated with gradually increasing concentrations of PTX (2, 5, 10
and 20 nM) for 24 h at 37°C, and PTX-resistant Huh7 cells were
collected (resistant to 20 nM PTX). Subsequently, PTX-resistant
cells (Huh7/PTX) were cultured in RPMI-1640 medium with 10 nM
PTX.
Cell transfection
miR-212-3p mimic (miR-212-3p;
5′-UAACAGUCUCCAGUCACGGCC-3′), mimic negative control (miR-NC; the
non-targeting sequence, 5′-CGAUCGCAUCAGCAUCGAUUGC-3′), miR-212-3p
inhibitor (5′-GGCCGUGACUGGAGACUGUUA-3′), inhibitor negative control
(miR-NC inhibitor; the non-targeting sequence,
5′-CUAACGCAUGCACAGUCGUACG-3′), pcDNA3.1-ZEB2 overexpression vector
(ZEB2), pcDNA3.1 vector (Thermo Fisher Scientific, Inc.) alone as
overexpression vector negative control (vector), small interfering
(si)RNA against ZEB2 (siZEB2; 5′-UGAUAUUGUUUCUCAUUCGGC-3′), and
siRNA negative control (scrambled; 5′-UUCUCCGAACGUGUCACGUTT-3′)
were obtained from Shanghai GenePharma Co., Ltd. Transient
transfection of Huh7 and Huh7/PTX cells with 40 nM oligonucleotides
or vector was performed by using Lipofectamine™ 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
instructions. Cells were collected for further analyses after 48 h
post-transfection.
Cell viability assay
Cell viability was measured by using the 3-(4,
5-dimethylthiazol-2-yl)-2, 5-diphenyl-trtrazolium bromide (MTT)
assay. Briefly, Huh7 and Huh7/PTX cells were seeded into 96-well
plates at the density of 1×104 cells/well and incubated
with varying concentrations of PTX for 24 h. Each sample was
conducted in triplicate. Then cell medium was replaced with 0.5%
MTT (Sigma-Aldrich; Merck KGaA) and incubated for another 4 h at
37°C. After the removal of the supernatant, dimethyl sulfoxide (100
µl/well; Sigma-Aldrich; Merck KGaA) was added to cells to dissolve
the formazan. The absorbance was detected at 490 nm with a
microplate reader (Bio-Rad Laboratories, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from cells was extracted with mirVanaTM
miRNA isolation kit (Thermo Fisher Scientific, Inc.), following the
manufacturer's instructions. Subsequently, the target miRNA was
reverse transcribed into cDNA using the TaqMan microRNA Reverse
Transcription kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.), with the following parameters: 16°C for 30 min, 42°C for 30
min and 85°C for 5 min. q-PCR was conducted using SYBR-Green
(Applied Biosystems; Thermo Fisher Scientific, Inc.), following the
amplification instructions. The following thermocycling conditions
were used: 95°C for 5 min; 40 cycles at 95°C for 10 sec, 60°C for 1
min and a final extension at 72°C for 5 min. All primers were
obtained from Sangon Biotech Co., Ltd.: miR-212-3p forward,
5′-GGTAACAGTCTCCAGTCA-3′; miR-212-3p reverse,
5′-GCAATTGCACTGGATACG-3′; U6 forward,
5′-CTCGCTTCGGCAGCACATATACT-3′; U6 reverse,
5′-CGCTTCACGAATTTGCGTGT-3′. Each sample was conducted in
triplicate. The expression of miR-212-3p was normalized to U6 small
nuclear RNA, using the 2−ΔΔCq method (16).
Cell apoptosis assay
Cell apoptosis was investigated by flow cytometry
using the Annexin V-FITC/PI detection kit (Sigma-Aldrich; Merck
KGaA). Following treatment with PTX for 24 h, Huh7 and Huh7/PTX
were resuspended in binding buffer and then incubated with 10 µl
Annexin V-FITC and PI for 20 min at room temperature in the dark.
The positive cells (Annexin V-FITC+/PI−/+)
were examined using a flow cytometer (BD Biosciences) and BD
FACStation software (version 6.1; BD Biosciences)
Western blotting
Total protein was extracted using RIPA lysis buffer
(Thermo Fisher Scientific, Inc.) containing 1% protease inhibitor
and quantified by bicinchoninic acid protein assay kit
(Sigma-Aldrich; Merck KGaA), according to the manufacturer's
instructions. Denatured proteins (20 µg/lane) were loaded onto 6 or
10% SDS-PAGE gels and then transferred to polyvinylidene difluoride
membranes (Millipore). Membranes were subsequently blocked in 5%
non-fat milk for 1 h at room temperature. Membranes were then
incubated with primary antibodies against p-glycoprotein (P-gp)
(1:1,000; cat. no. ab235954), glutathione S-transferase π (GST-π)
(1:2,000; cat. no. ab153949), E-cadherin (1:500; cat. no. ab15148),
cytokeratin 18 (1:10,000; cat. no. ab133263), N-cadherin (1:1,000;
cat. no. ab18203), vimentin (1:1,000; cat. no. ab92547), ZEB2
(1:500, cat. no. ab138222) or β-actin (1:5,000; cat. no. ab227387)
(all from Abcam) overnight at 4°C. β-actin was regarded as a
housekeeping protein in this study. After three washes in
Tris-buffer saline, containing 0.1% Tween-20 (TBST), membranes were
incubated in horseradish peroxidase-conjugated secondary antibodies
(1:10,000; cat. no. ab6721; Abcam) for 2 h at room temperature. The
protein bands were visualized using enhanced chemiluminescence
(ECL) chromogenic substrate (GE Healthcare) and analyzed using the
Image Lab software (version 2.1; Bio-Rad Laboratories. Inc.).
Transwell assay
Cell migration and invasion were investigated by
transwell assay. For cell invasion assay, chambers were pre-coated
with Matrigel (BD Biosciences). Huh7 and Huh7/PTX cells
(2×104 cells/well) were suspended in serum-free
RPMI-1640 medium and then incubated in the upper chambers (Costar;
Corning, Inc.) at 37°C with 5% CO2 for 12 h. Invasive
cells on the basal side of the membrane were fixed with 4%
paraformaldehyde for 20 min at room temperature, stained with 0.1%
crystal violet (Sigma-Aldrich; Merck KGaA) for 10 min at room
temperature, and subsequently counted under a light microscope
(magnification, ×100) (Olympus Corporation). Migrated cells were
investigated by using transwell chambers, following a similar
approach, without Matrigel.
Luciferase assays
The putative binding sites of miR-212-3p and ZEB2
were predicted by TargetScan Human Release 7.1 (www.targetscan.org/vert_71/). The wild-type or
mutant sequence of 3′ untranslated regions (3′-UTR) of ZEB2 were
amplified by PCR and cloned into the pGL3 luciferase reporter
vector (Promega Corporation), in order to generate the ZEB2-Wt or
ZEB2-Mut, respectively. Wt or Mut luciferase reporter vector was
co-transfected with miR-212-3p mimic, miR-NC, miR-212-3p inhibitor
or miR-NC inhibitor in Huh7 and Huh7/PTX cells, using
Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocols. Subsequently, cells were
lysed and used for the luciferase activity analysis by using the
Dual-Luciferase Assay kit (Promega Corporation) after 48 h of
transfection. Firefly luciferase activity was normalized to Renilla
luciferase activity.
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments. The differences were investigated
by Student's t-test or one-way analysis of variance followed by
Tukey's post hoc test using the GraphPad Prism 7 software (GraphPad
Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-212-3p expression is downregulated
in PTX-resistant HCC cells
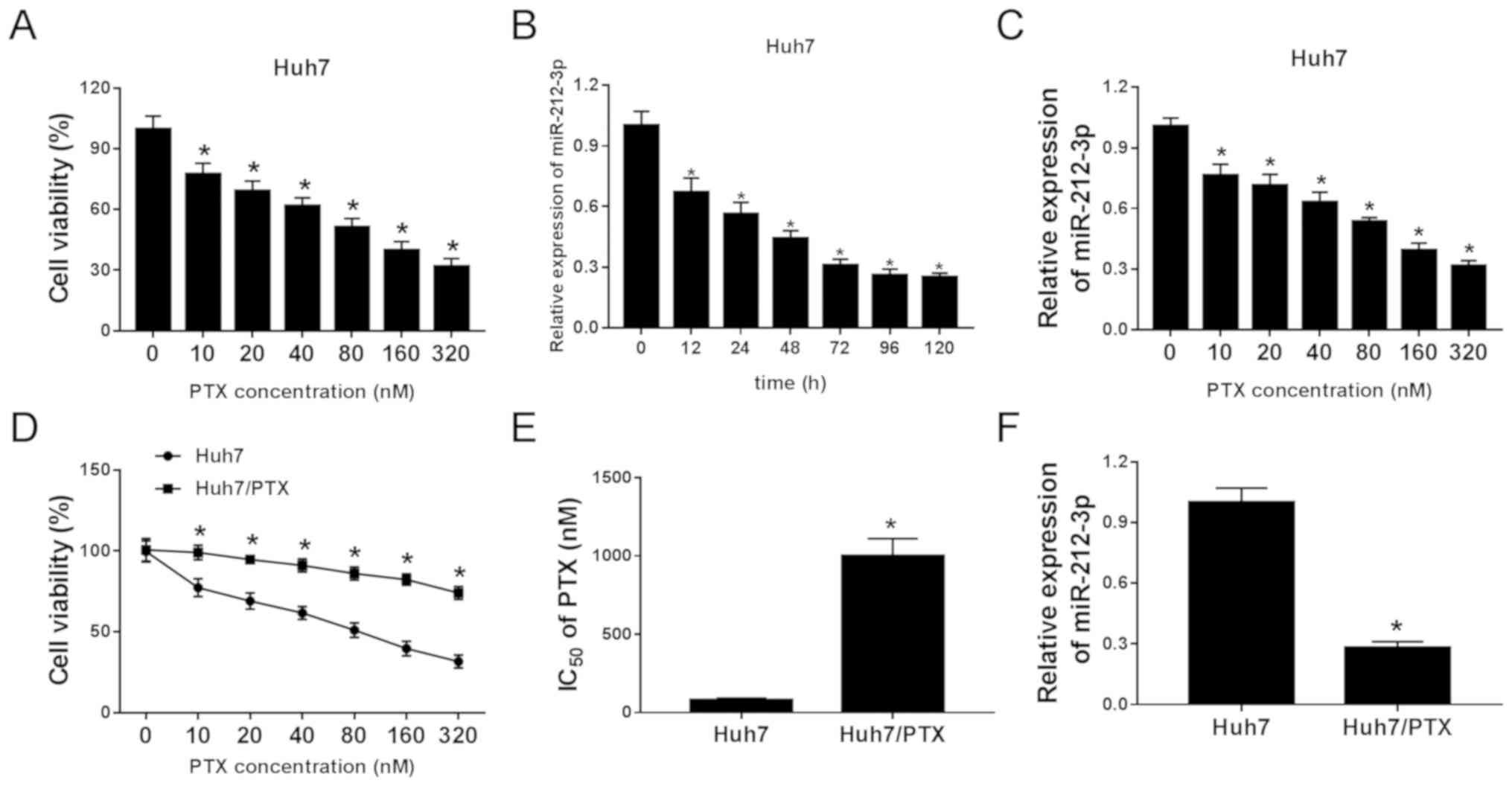
Following treatment with different concentrations of
PTX for 24 h, the viability of Huh7 cells was significantly
decreased in a concentration-dependent manner (Fig. 1A). Moreover, the data from the
RTq-PCR assay revealed that the expression of miR-212-3p was
progressively reduced in Huh7 cells after treatment with PTX at
various time points or exposure to different doses at 24 h
(Fig. 1B and C). In order to
investigate the potential role of miR-212-3p in HCC resistance,
PTX-resistant Huh7/PTX cells were constructed. MTT analysis showed
that Huh7/PTX cells displayed higher cell viability compared with
Huh7 cells in response to different concentrations of PTX (Fig. 1D). Meanwhile, the IC50 at
24 h of PTX was ~80 nM in Huh7 cells and 1,000 nM in Huh7/PTX cells
(Fig. 1E). Furthermore,
downregulation of miR-212-3p was demonstrated in Huh7/PTX cells
compared with Huh7 cells (Fig. 1F).
Furthermore, similar events were also observed in HCCLM3 cells
(Fig. S1A-D). Taken together,
dysregulated miR-212-3p may be associated with PTX resistance in
HCC cells.
miR-212-3p inhibits PTX resistance in
HCC cells
To explore the function of miR-212-3p on PTX
resistance in HCC cells, Huh7 cells were transfected with miR-NC
inhibitor or miR-212-3p inhibitor and Huh7/PTX cells were
transfected with miR-212-3p mimic or miR-NC, respectively. The
transfection efficiency was confirmed (Fig. S2A and B). Following treatment with
different concentrations of PTX, MTT assay demonstrated that the
knockdown miR-212-3p reversed the inhibitory effect of PTX on the
viability of Huh7 cells and led to increased IC50 of PTX
from 85 to 624 nM (Fig. 2A and B).
However, the overexpression of miR-212-3p exacerbated PTX-mediated
inhibition of cell viability in Huh7/PTX cells and resulted in
reduced IC50 of PTX from 1,086 to 365 nM (Fig. 2C and D). In addition, the protein
levels of P-gp and GST-π were significantly increased in Huh7 cells
by miR-212-3p knockdown and decreased in Huh7/PTX cells following
miR-212-3p overexpression (Fig. 2E and
F). Moreover, cell apoptosis was measured in Huh7 and Huh7/PTX
cells, following treatment with 80 or 1,000 nM PTX for 24 h. The
results demonstrated that treatment with 80 nM PTX induced Huh7
cells apoptosis, whereas miR-212-3p depletion protected cells from
PTX-induced apoptosis (Fig. 2G).
Furthermore, the introduction of miR-212-3p aggravated PTX-induced
cells apoptosis in Huh7/PTX cells (Fig.
2H). Overall, these findings suggest that miR-212-3p attenuates
PTX resistance in HCC cells.
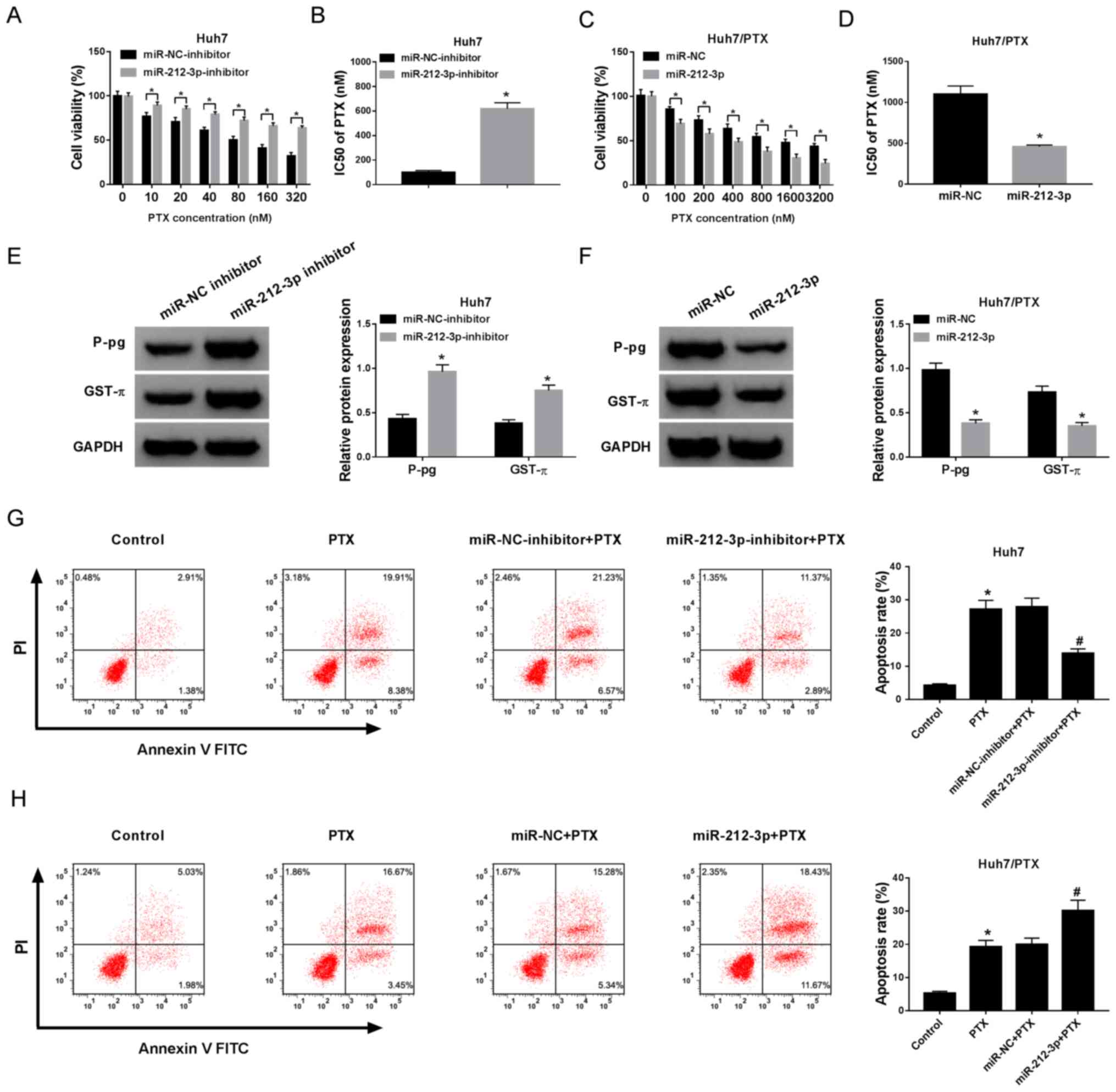 | Figure 2.Addition of miR-212-3p inhibited PTX
resistance in HCC cells. (A) The effect of miR-212-3p inhibition on
cell viability was investigated in Huh7 cells treated with
different concentrations of PTX for 24 h. (B) The IC50
of PTX in Huh7 cells transfected with miR-212-3p inhibitor or
miR-NC inhibitor. (C) The effect of miR-212-3p overexpression on
cell viability was detected in Huh7/PTX cells treated with
different concentrations of PTX for 24 h. (D) The IC50
of PTX in Huh7/PTX cells transfected with miR-212-3p or miR-NC. (E
and F) The protein expression levels of P-gp and GST-π were
detected in the cells. (G) Cell apoptosis was evaluated in Huh7
cells treated with PTX and miR-212-3p inhibitor by flow cytometry.
(H) The effect of miR-212-3p on PTX-induced apoptosis was analyzed
in Huh7/PTX cells. *P<0.05; #P<0.05: A, B and E,
miR-212-3p inhibitor group vs. miR-NC inhibitor group; C, D and F,
miR-212-3p group vs. miR-NC group; G and H, PTX group vs. control
group, miR-212-3p inhibitor group + PTX vs. miR-NC inhibitor + PTX
group, and miR-212-3p group + PTX vs. miR-NC + PTX group. PTX,
paclitaxel; miR, microRNA; NC, negative control; IC50,
half maximal inhibitory concentration; P-gp, p-glycoprotein; GST-π,
glutathione S-transferase π. |
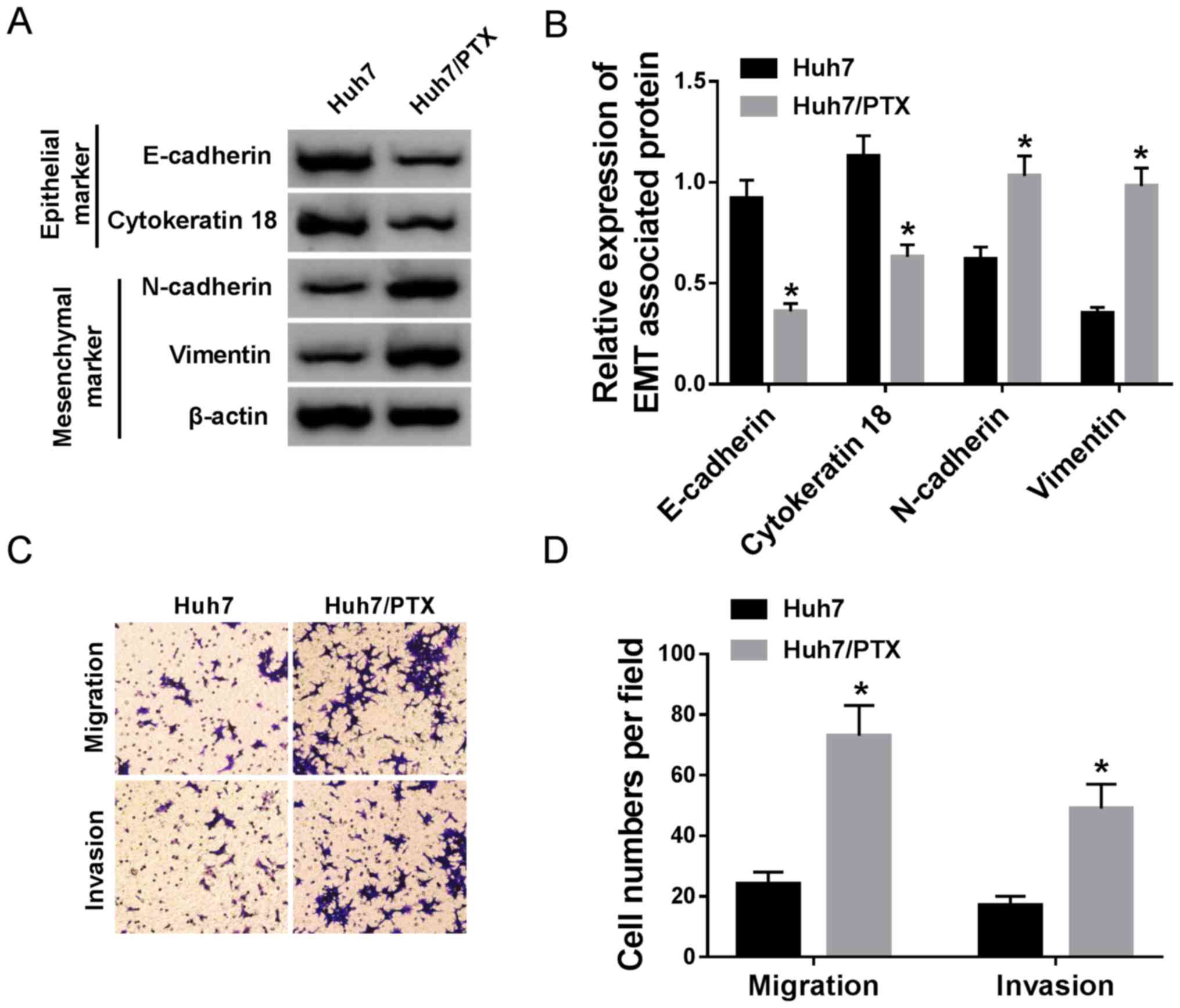
EMT, migration and invasion are
associated with PTX resistance in HCC cells
Since EMT, migration and invasion may play essential
roles in chemoresistance, these processes were assessed in Huh7 and
Huh7/PTX cells. The protein levels of EMT markers were investigated
by western blotting. The results demonstrated decreased E-cadherin
and cytokeratin 18, whilst increased N-cadherin and vimentin, in
Huh7/PTX cells compared with Huh7 cells, suggesting that PTX
resistance promoted EMT (Fig. 3A and
B). Furthermore, cell migration and invasion were detected in
HCC cells. The transwell assays demonstrated increased cell
migration and invasion abilities in Huh7/PTX cells compared with
PTX-sensitive cells (Fig. 3C and D).
Overall, these results suggest that EMT, migration and invasion are
positively associated with PTX resistance in HCC cells.
miR-212-3p controls PTX resistance by
regulating EMT, migration and invasion in HCC cells
Based on the aforementioned results, the effect of
miR-212-3p on EMT, migration and invasion was investigated in Huh7
and Huh7/PTX cells. Western blotting assay showed that miR-212-3p
deficiency promoted EMT in Huh7 cells, demonstrated by decreased
E-cadherin, cytokeratin 18 and increased of N-cadherin, vimentin
expression levels (Fig. 4A and B).
However, the overexpression of miR-212-3p resulted in the opposite
effects on EMT in Huh7/PTX cells (Fig.
4A and B). Moreover, inhibition of miR-212-3p resulted in
increased migration and invasion abilities of Huh7 cells, whereas
its addition blocked cell migration and invasion in (Fig. 4C and D). These results indicate that
miR-212-3p regulates PTX resistance by suppressing EMT, migration
and invasion in HCC cells.
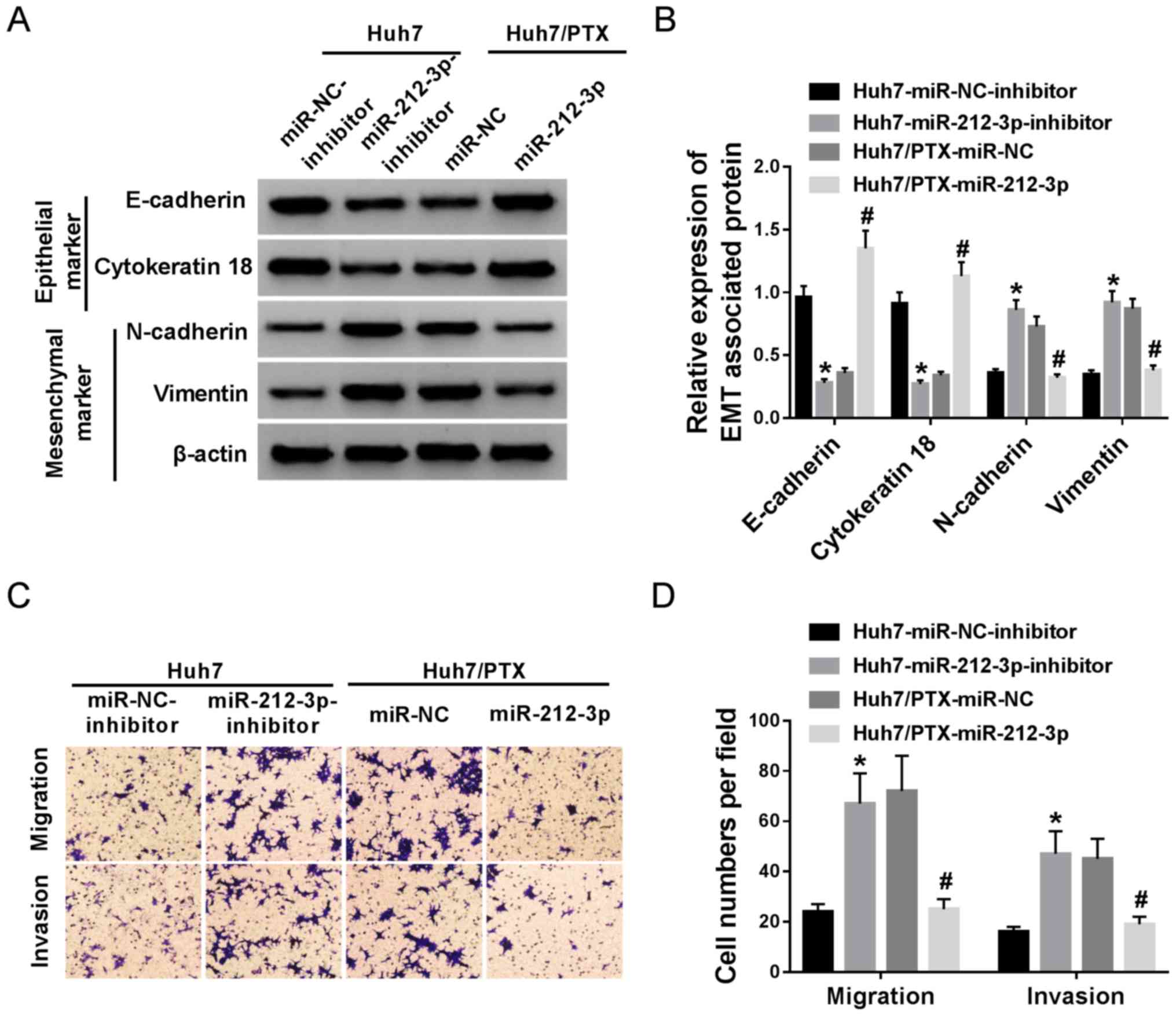 | Figure 4.miR-212-3p overexpression suppresses
EMT, migration and invasion in PTX-resistant hepatocellular cells.
(A and B) The effect of miR-212-3p on E-cadherin, cytokeratin 18,
N-cadherin and vimentin protein expression levels was investigated
in Huh7 and Huh7/PTX cells, following transfection with miR-212-3p
inhibitor or miR-212-3p. (C and D) Cell migration and invasion were
measured in miR-212-3p inhibitor-transfected Huh7 and
miR-212-3p-transfected Huh7/PTX cells. *P<0.05, miR-212-3p
inhibitor group vs. miR-NC inhibitor group; #P<0.05,
miR-212-3p group vs. miR-NC group. PTX, paclitaxel; miR, microRNA;
NC, negative control; EMT, epithelial-mesenchymal transition. |
ZEB2 is a target of miR-212-3p
Given that functional miRNAs exert their effects by
regulating target genes, the targets of miR-212-3p were predicted
by TargetScan Human Release 7.1 online. Potential binding sites of
miR-212-3p were predicted in ZEB2, suggesting that ZEB2 might be a
target of miR-212-3p (Fig. 5A).
Hence, the prediction was next validated by luciferase activity
assay. Results showed that the knockdown of miR-212-3p resulted in
enhanced luciferase activity in ZEB2-Wt-transfected Huh7 cells,
while little effect was observed with ZEB2-Mut cells (Fig. 5B). Moreover, a notable decrease in
luciferase activity was demonstrated in Huh7/PTX cells
co-transfected with miR-212-3p and ZEB2-Wt, whereas the effect was
lost in the ZEB2-Mut group (Fig.
5C). Besides, ZEB2 protein expression was significantly
enhanced in Huh7/PTX compared with Huh7 cells (Fig. 5D and E). In addition, the effect of
miR-212-3p on ZEB2 expression was also investigated in Huh7 and
Huh7/PTX cells. As shown in Fig. 5F and
G, miR-212-3p abrogation induced ZEB2 expression in Huh7 cells,
while miR-212-3p overexpression blocked ZEB2 abundance in Huh7/PTX
cells. These findings suggest ZEB2 as a direct target of miR-212-3p
in HCC cells.
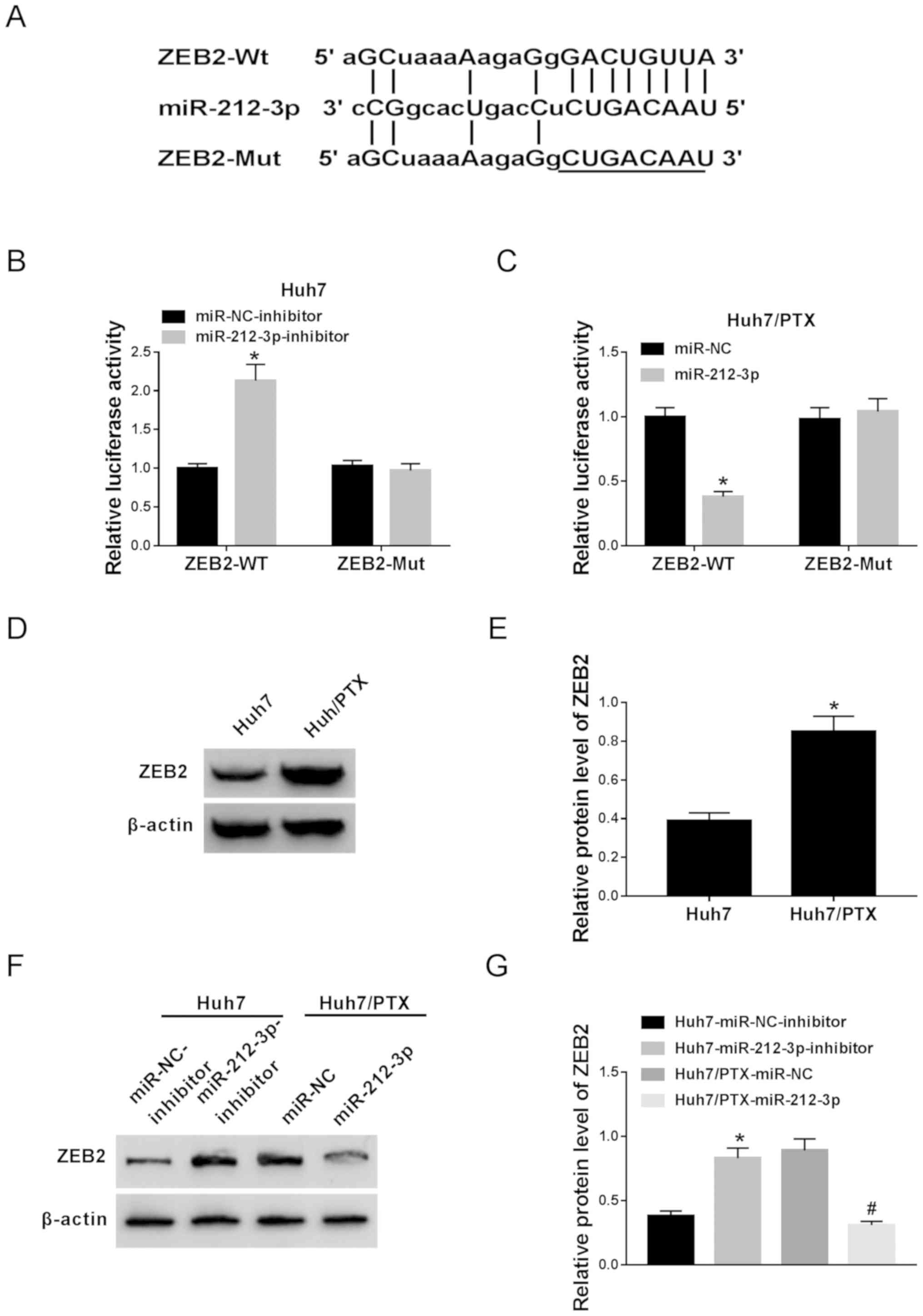 | Figure 5.ZEB2 is a direct target of miR-212-3p.
(A) The potential binding sites of miR-212-3p and ZEB2 were
predicted by TargetScan Human Release 7.1. (B) The luciferase
activity was investigated in Huh7 cells co-transfected with
miR-212-3p inhibitor or miR-NC inhibitor and ZEB2-WT or ZEB2-Mut.
(C) The luciferase activity was evaluated in Huh7/PTX cells
co-transfected with miR-212-3p or miR-NC and ZEB2-WT or ZEB2-Mut.
(D and E) The protein expression level of ZEB2 was detected in Huh7
and Huh7/PTX cells by western blotting. (F and G) The effect of
miR-212-3p on ZEB2 expression level was investigated in miR-212-3p
inhibitor-transfected Huh7 and miR-212-3p-transfected Huh7/PTX
cells. *P<0.05: B and G, miR-212-3p inhibitor group vs. miR-NC
inhibitor group; C, miR-212-3p group vs. miR-NC group; E, Huh7/PTX
group vs. Huh7 group. #P<0.05: G, miR-212-3p group
vs. miR-NC group. PTX, paclitaxel; miR, microRNA; NC, negative
control; ZEB, zinc finger E-box binding homeobox 2; WT, wild type;
Mut, mutant. |
ZEB2 is involved in
miR-212-3p-mediated PTX resistance in HCC cells
In order to assess whether ZEB2 was required for
miR-212-3p-mediated PTX resistance, Huh7 cells were co-transfected
with miR-212-3p inhibitor and siZEB2 or scrambled siRNA and
Huh7/PTX cells were co-transfected with miR-212-3p and ZEB2 or
vector. The transfection efficacy was confirmed (Fig. S3A-D). Cell viability and apoptosis
were detected in transfected cells, following treatment with PTX
for 24 h. MTT assay showed that the silencing of ZEB2 decreased
cell viability and IC50 of PTX in Huh7 cells, whereas
ZEB2 restoration protected the cell viability and increased
IC50 of PTX in Huh7/PTX cells (Fig. 6A-D). Moreover, data from flow
cytometry reflected that the absence of ZEB2 induced cell apoptosis
in miR-212-3p-deficient Huh7 cells, while the introduction of ZEB2
weakened miR-212-3p-induced apoptosis in Huh7/PTX cells (Fig. 6E and F). In addition, a significant
loss of ZEB2 protein expression was demonstrated in
siZEB2-transfected Huh7 cells and a notable increase in ZEB2
expression was displayed in Huh7/PTX cells transfected with ZEB2
overexpression vector, compared with their corresponding control,
respectively (Fig. 6G). Furthermore,
ZEB2 interference overturned the effect of miR-212-3p inhibition on
EMT, revealed by elevated E-cadherin, cytokeratin 18 and reduced
N-cadherin and vimentin (Fig. 6G)
expression levels. However, ZEB2 overexpression indicated opposite
trends in miR-212-3p-transfected Huh7/PTX cells (Fig. 6G). Besides, ZEB2 abrogation inhibited
cell migration and invasion in Huh7 cells transfected with
miR-212-3p inhibitor and siZEB2 compared with cells transfected
with miR-212-3p inhibitor and scrambled (Fig. 6H). Conversely, ZEB2 restoration
promoted cell migration and invasion in miR-212-3p-transfected
Huh7/PTX cells (Fig. 6I).
Collectively, these results indicate that miR-212-3p mediates PTX
resistance by targeting ZEB2 in HCC cells.
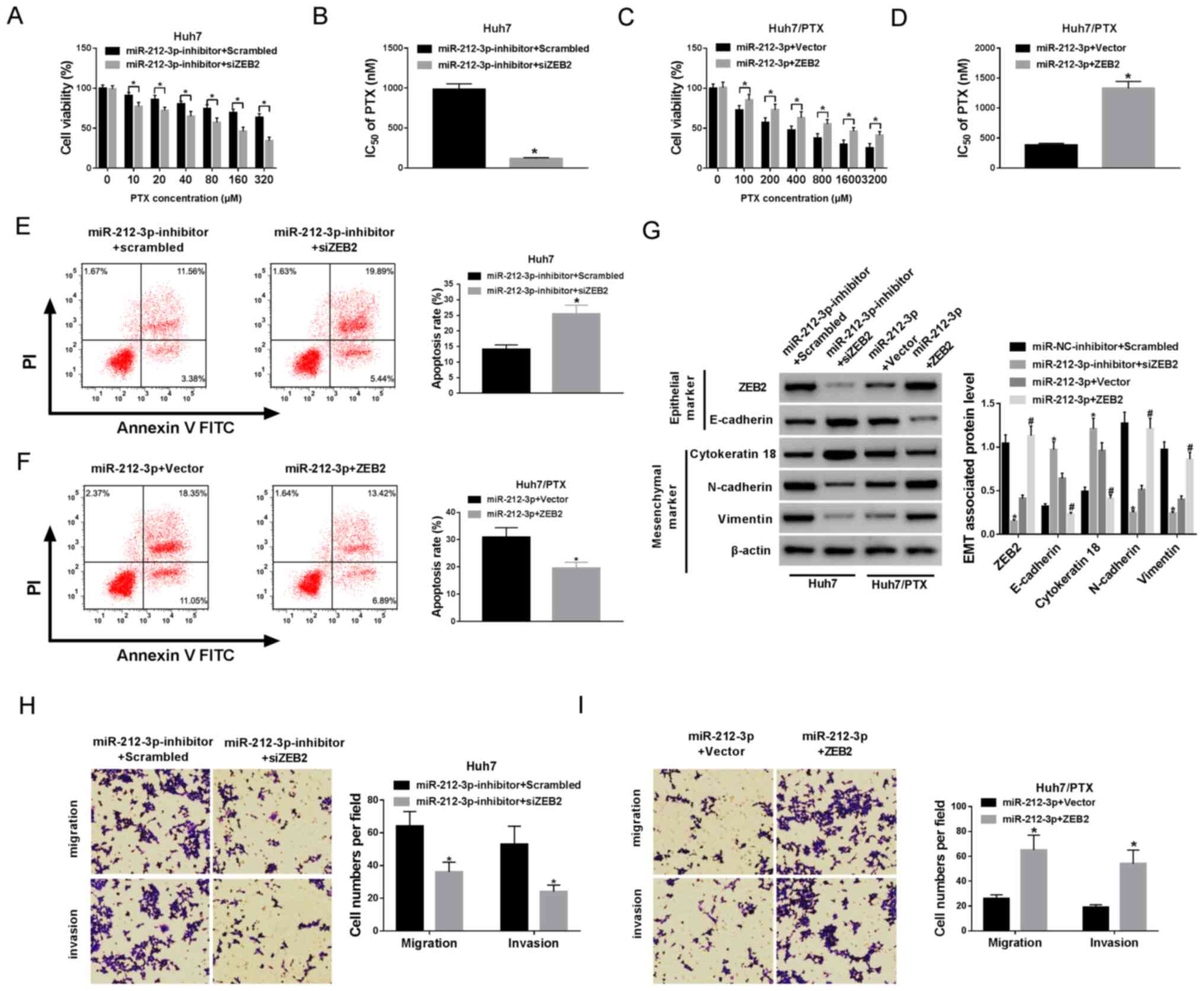 | Figure 6.ZEB2 is required for
miR-212-3p-mediated PTX resistance. Huh7 cells were co-transfected
with miR-212-3p inhibitor and scrambled or siZEB2. Huh7/PTX cells
were co-transfected with miR-212-3p and vector or ZEB2. (A-D) Cell
viability and IC50 of PTX were measured in transfected
Huh7 and Huh7/PTX cells after treatment with different
concentrations of PTX for 24 h. (E and F) Cell apoptosis was
detected in transfected Huh7 and Huh7/PTX cells after treatment
with the corresponding concentration (80 nM for Huh7 cells and
1,000 nM for Huh7/PTX cells) of PTX for 24 h. (G) The protein
levels of ZEB2, E-cadherin, cytokeratin 18, N-cadherin and vimentin
were examined in transfected Huh7 and Huh7/PTX cells. (H and I)
Cell migration and invasion were analyzed in transfected Huh7 and
Huh7/PTX cells, respectively. *P<0.05: A, B, E, G and H,
miR-212-3p inhibitor + siZEB2 group vs. miR-212-3p inhibitor +
scrambled group; C, D, F and I, miR-212-3p + ZEB2 group vs.
miR-212-3p + vector group. #P<0.05: G, miR-212-3p +
ZEB2 group vs. miR-212-3p + vector group. PTX, paclitaxel; miR,
microRNA; ZEB, zinc finger E-box binding homeobox 2;
IC50, half maximal inhibitory concentration. |
Discussion
The efficacy of chemotherapy is unsatisfactory,
owing to chemoresistance in HCC (3).
PTX, as an important chemotherapeutic agent, has been widely used
for the treatment of HCC (17). In
the present study, Huh7 cells were treated with PTX in order to
establish Huh7/PTX cells. MTT analysis showed that PTX treatment
suppressed cell viability of Huh7 and Huh7/PTX cells, and Huh7/PTX
cells had higher viability and IC50 of PTX, suggesting
the anti-HCC role of PTX and successful establishment of
PTX-resistant HCC cells. The present study was the first to
demonstrate that miR-212-3p inhibited PTX resistance by targeting
ZEB2 in HCC cells.
A previous study suggested that miR-212-3p was
downregulated in HCC (12), while
the effect of miR-212-3p on PTX resistance was unclear. In the
present study, miR-212-3p expression was decreased in Huh7 cells,
following treatment with PTX, and Huh7/PTX cells displayed lower
expression level of miR-212-3p, suggesting that miR-212-3p may play
an important role in PTX resistance in HCC cells. Moreover,
following treatment with PTX, analyses by MTT, western blotting and
flow cytometry revealed that miR-212-3p decreased PTX resistance in
HCC cells, indicating miR-212-3p as a promising target for
improving the efficacy of chemotherapy in HCC.
Several mechanisms, such as EMT, inflammation,
autophagy and oxidative stress, play key roles in regulating drug
resistance via varying pathways (18). EMT has been reported to be involved
in the advancement of HCC and is associated with the development of
drug resistance in advanced HCC (19). The expression levels of EMT markers
showed that EMT was induced in Huh7/PTX cells, suggesting that EMT
was positively associated with PTX resistance. This is consistent
with a previous study that also indicated the association between
EMT and PTX resistance by regulating the expression levels of
E-cadherin, cytokeratin 18, N-cadherin and vimentin (20). Moreover, it was found that miR-212-3p
inhibited PTX resistance by regulating EMT. Furthermore, current
evidence indicates that EMT is associated with cell migration and
invasion in HCC (21). Furthermore,
the acquisition of PTX resistance was suggested to be associated
with metastatic properties (22,23). In
the present study, the contribution of migration and invasion to
PTX resistance was demonstrated, and the potential role of
miR-212-3p in suppressing drug resistance by regulating migration
and invasion.
miRNAs function by mediating the expression of
target genes in different conditions. For example, miR-212-3p was
reported to inhibit cell proliferation and induce apoptosis by
regulating FOXA1 expression in osteosarcoma (9). Moreover, miR-212-3p mediated cell
proliferation, migration, cell cycle and EMT by targeting
Ras-associated binding-GTPase 1a (Rab1a) in intrahepatic
cholangiocarcinoma, in response to hypoxia treatment (24). Besides, miR-212-3p may inhibit
proliferation and increase apoptosis via suppressing sex
determining region Y-box 5 (SOX5) in rheumatoid arthritis (25). Apart from these, serum and
glucocorticoid-inducible kinase 3 (SGK3) was also a target of
miR-212-3p, which regulated cell proliferation in glioblastoma
(26). ZEB2, an EMT-associated gene,
induced EMT to regulate cell migration and invasion in HCC by
targeting miR-145 (15). In
addition, miR-139-5p also inhibited EMT, migration and invasion by
mediating ZEB2 in HCC (27).
Promisingly, it was found that miR-212-3p could also target ZEB2 in
HCC cells, which was identified by luciferase activity analysis.
Western blotting showed increased protein expression level of ZEB2
in PTX-resistant HCC cells compared with PTX-sensitive cells,
suggesting that ZEB2 may contribute towards PTX resistance in HCC
cells, which is similar to findings of other studies, which
demonstrated positive association between ZEB2 and EMT, and
supported chemoresistance in lung cancer (14,28).
Furthermore, rescue experiments demonstrated that ZEB2 interference
or restoration reversed miR-212-3p knockdown or
overexpression-mediated regulation of PTX resistance. These data
reflected that miR-212-3p counteracted PTX resistance by targeting
ZEB2 in HCC cells. In the present study, the potential mechanism
underlying the regulation of PTX resistance by miR-212-3p was only
investigated in vitro. Hence, a preclinical study with an
animal model, as well as clinical experiments are expected in
future.
In conclusion, low expression of miR-212-3p was
demonstrated in PTX-resistant HCC cells, and overexpression of
miR-212-3p inhibited PTX resistance through regulating EMT,
migration and invasion by targeting ZEB2 in HCC cells. Thus, the
present study provides a novel regulator for studying the efficacy
of chemotherapy in HCC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
JY conceived the present study. RC and YL
contributed to manuscript preparation and data analyses, with
constructive discussions. All authors performed the experiments. JY
performed the data analyses and drafted the initial manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Craig AJ, von Felden J, Garcia-Lezana T,
Sarcognato S and Villanueva A: Tumour evolution in hepatocellular
carcinoma. Nat Rev Gastroenterol Hepatol. 17:139–152. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forner A, Reig M and Bruix J:
Hepatocellular carcinoma. Lancet. 391:1301–1314. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lohitesh K, Chowdhury R and Mukherjee S:
Resistance a major hindrance to chemotherapy in hepatocellular
carcinoma: An insight. Cancer Cell Int. 18:442018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meena AS, Sharma A, Kumari R, Mohammad N,
Singh SV and Bhat MK: Inherent and acquired resistance to
paclitaxel in hepatocellular carcinoma: Molecular events involved.
PLoS One. 8:e615242013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vasuri F, Visani M, Acquaviva G, Brand T,
Fiorentino M, Pession A, Tallini G, D'Errico A and de Biase D: Role
of microRNAs in the main molecular pathways of hepatocellular
carcinoma. World J Gastroenterol. 24:2647–2660. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yan H, Wang S, Yu H, Zhu J and Chen C:
Molecular pathways and functional analysis of miRNA expression
associated with paclitaxel-induced apoptosis in hepatocellular
carcinoma cells. Pharmacology. 92:167–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang X, Qin J and Lu S: Up-regulation of
miR-877 induced by paclitaxel inhibits hepatocellular carcinoma
cell proliferation though targeting FOXM1. Int J Clin Exp Pathol.
8:1515–1524. 2015.PubMed/NCBI
|
|
8
|
Chen Y, Feng F, Gao X, Wang C, Sun H,
Zhang C, Zeng Z, Lu Y, An L, Qu J, et al: MiRNA153 Reduces Effects
of Chemotherapeutic Agents or Small Molecular Kinase Inhibitor in
HCC Cells. Curr Cancer Drug Targets. 15:176–187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie C, Chen B, Wu B, Guo J and Cao Y:
LncRNA TUG1 promotes cell proliferation and suppresses apoptosis in
osteosarcoma by regulating miR-212-3p/FOXA1 axis. Biomed
Pharmacother. 97:1645–1653. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He X and Fan S: hsa-miR-212 modulates the
radiosensitivity of glioma cells by targeting BRCA1. Oncol Rep.
39:977–984. 2018.PubMed/NCBI
|
|
11
|
Tu H, Wei G, Cai Q, Chen X, Sun Z, Cheng
C, Zhang L, Feng Y, Zhou H, Zhou B, et al: MicroRNA-212 inhibits
hepatocellular carcinoma cell proliferation and induces apoptosis
by targeting FOXA1. Onco Targets Ther. 8:2227–2235. 2015.PubMed/NCBI
|
|
12
|
Shen S, Lin Y, Yuan X, Shen L, Chen J,
Chen L, Qin L and Shen B: Biomarker MicroRNAs for Diagnosis,
Prognosis and Treatment of Hepatocellular Carcinoma: A Functional
Survey and Comparison. Sci Rep. 6:383112016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hegarty SV, Sullivan AM and O'Keeffe GW:
Zeb2: A multifunctional regulator of nervous system development.
Prog Neurobiol. 132:81–95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han ML, Zhao YF, Tan CH, Xiong YJ, Wang
WJ, Wu F, Fei Y, Wang L and Liang ZQ: Cathepsin L
upregulation-induced EMT phenotype is associated with the
acquisition of cisplatin or paclitaxel resistance in A549 cells.
Acta Pharmacol Sin. 37:1606–1622. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li C, Lu L, Feng B, Zhang K, Han S, Hou D,
Chen L, Chu X and Wang R: The lincRNA-ROR/miR-145 axis promotes
invasion and metastasis in hepatocellular carcinoma via induction
of epithelial-mesenchymal transition by targeting ZEB2. Sci Rep.
7:46372017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu D, Wu S, Hu C, Chen Z, Wang H, Fan F,
Qin Y, Wang C, Sun H, Leng X, et al: Folate-targeted polymersomes
loaded with both paclitaxel and doxorubicin for the combination
chemotherapy of hepatocellular carcinoma. Acta Biomater.
58:399–412. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen J, Jin R, Zhao J, Liu J, Ying H, Yan
H, Zhou S, Liang Y, Huang D, Liang X, et al: Potential molecular,
cellular and microenvironmental mechanism of sorafenib resistance
in hepatocellular carcinoma. Cancer Lett. 367:1–11. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mir N, Jayachandran A, Dhungel B, Shrestha
R and Steel JC: Epithelial-to-Mesenchymal Transition: A Mediator of
Sorafenib Resistance in Advanced Hepatocellular Carcinoma. Curr
Cancer Drug Targets. 17:698–706. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao YF, Han ML, Xiong YJ, Wang L, Fei Y,
Shen X, Zhu Y and Liang ZQ: A miRNA-200c/cathepsin L feedback loop
determines paclitaxel resistance in human lung cancer A549 cells in
vitro through regulating epithelial-mesenchymal transition. Acta
Pharmacol Sin. 39:1034–1047. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Wang W, Wang Y, Huang X, Zhang Z,
Chen B, Xie W, Li S, Shen S and Peng B: NEK2 promotes
hepatocellular carcinoma migration and invasion through modulation
of the epithelial-mesenchymal transition. Oncol Rep. 39:1023–1033.
2018.PubMed/NCBI
|
|
22
|
Kim JJ, Yin B, Christudass CS, Terada N,
Rajagopalan K, Fabry B, Lee DY, Shiraishi T, Getzenberg RH, Veltri
RW, et al: Acquisition of paclitaxel resistance is associated with
a more aggressive and invasive phenotype in prostate cancer. J Cell
Biochem. 114:1286–1293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li H, Zhang P, Sun X, Sun Y, Shi C, Liu H
and Liu X: MicroRNA-181a regulates epithelial-mesenchymal
transition by targeting PTEN in drug-resistant lung adenocarcinoma
cells. Int J Oncol. 47:1379–1392. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hou P, Kang Y and Luo J: Hypoxia-mediated
miR-212-3p downregulation enhances progression of intrahepatic
cholangiocarcinoma through upregulation of Rab1a. Cancer Biol Ther.
19:984–993. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Y, Zhang XL, Li XF, Tang YC and Zhao
X: miR-212-3p reduced proliferation, and promoted apoptosis of
fibroblast-like synoviocytes via down-regulating SOX5 in rheumatoid
arthritis. Eur Rev Med Pharmacol Sci. 22:461–471. 2018.PubMed/NCBI
|
|
26
|
Liu H, Li C, Shen C, Yin F, Wang K, Liu Y,
Zheng B, Zhang W, Hou X, Chen X, et al: MiR-212-3p inhibits
glioblastoma cell proliferation by targeting SGK3. J Neurooncol.
122:431–439. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qiu G, Lin Y, Zhang H and Wu D: miR-139-5p
inhibits epithelial-mesenchymal transition, migration and invasion
of hepatocellular carcinoma cells by targeting ZEB1 and ZEB2.
Biochem Biophys Res Commun. 463:315–321. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Duan X, Fu Z, Gao L, Zhou J, Deng X, Luo
X, Fang W and Luo R: Direct interaction between miR-203 and ZEB2
suppresses epithelial-mesenchymal transition signaling and reduces
lung adenocarcinoma chemoresistance. Acta Biochim Biophys Sin
(Shanghai). 48:1042–1049. 2016. View Article : Google Scholar : PubMed/NCBI
|