Introduction
Lung cancer is one of the most common malignant
tumors, and its incidence and mortality rank first in China
(1). Non-small cell lung cancer
(NSCLC) is the most common pathological subtype, accounting for
approximately 80% of the total number of lung cancer cases. The
early symptoms of NSCLC are not obvious and the diagnosis is
difficult, leading to the fact that the majority of patients are
already in the middle and late stages of the disease at the time of
diagnosis, thus severely threating the efficacy of surgical
treatment (2,3). The 5-year survival rate of patients
with stage I NSCLC is 80%, while that of patients with advanced
NSCLC is only 15%, and the post-operative recurrence rate is very
high (4). Therefore, it is of utmost
clinical significance to identify specific tumor markers for the
early diagnosis and treatment of NSCLC (5).
MicroRNAs (miRNAs or miRs) are endogenous non-coding
small-molecule RNAs expressed in various types of tissues, and can
be used for the regulation of the proliferation, differentiation
and apoptosis of cells (6). Exosomes
are a type of membrane vesicle (30–150 nm) containing a variety of
RNA and proteins, which can be secreted under both normal and
pathological conditions. Exosomes are one of the main vectors of
miRNA transport and can stably exist in the circulatory system
(7). There are a number of miRNAs
with a dysregulated expression in patients with NSCLC. These miRNAs
can be released into the circulation through exosome release. Serum
exosomal miRNA can become a tumor biomarker of NSCLC (8). miR-23b-3p is one of the newly
discovered and identified miRNAs, which is highly expressed in
colon cancer, liver cancer and gastric cancer (9–11). In
the present study, the expression of miR-23b-3p in serum exosomes
in NSCLC, patients with pneumonia and healthy subjects was compared
and analyzed, and the diagnostic efficacy of miR-23b-3p in serum
exosomes in NSCLC was discussed.
Patients and methods
Patient information
The present study was approved by the Ethics
Committee of the People's Hospital of Yangzhong City. Patients who
participated in this research, signed the informed consent and had
complete clinical data. A total of 80 patients with NSCLC and 60
patients with pneumonia were included in the present study. They
were admitted to the People's Hospital of Yangzhong City from
October, 2017 to October, 2019. During the same period, 30 healthy
subjects in the physical examination center were included as the
controls. The inclusion criteria were as follows: All patients with
NSCLC were confirmed by histopathological examination, and staging
was conducted according to the latest TNM stage 8 version of lung
cancer (12). All patients with
pneumonia were confirmed by clinical examination and did not
receive antibiotics or other relevant treatment prior to specimen
collection. The healthy subjects were those who had a regular
medical examination in the physical examination center of our
hospital for >2 years and did not have tumors in any part of the
body. The exclusion criteria were as follows: Patients with NSCLC
and pneumonia with incomplete clinical data, or healthy subjects
with incomplete physical examination results. A total of 80
patients with NSCLC, 60 patients with pneumonia and 30 healthy
subjects were included. Among the 80 patients with NSCLC, 54 were
male and 26 were female. The mean age was (51.67±4.96) years.
Smoking was found in 47 cases, accounting for 58.75%. There were 41
cases of adenocarcinoma and 39 cases of squamous cell carcinoma. As
regards the clinical stage, 50 patients had stage I–II disease and
30 patients had stage III–IV disease. There were 49 cases of lymph
node metastasis. Among the 60 patients with pneumonia, 36 were
males and 24 were females. The mean age was (51.23±4.42) years.
There were 31 smokers, accounting for 51.67%. Among the 30 healthy
patients, 17 were males and 13 were females. The average age was
(50.14±3.57) years. There were 17 smokers, accounting for 56.67%.
No statistically significant differences were found in sex, age and
smoking status between the patients with NSCLC and those with
pneumonia and the healthy subjects (P>0.05).
Instruments and reagents
The Jem-100cx II transmission electron microscope
was purchased from Hitachi, Ltd., and the nano-particle tracking
analyzer Nanosight NS300 was from Malvern Instruments. The
spectrophotometer was from Bole Life Medicine Products (Shanghai)
Co., Ltd.; the exosome extraction kit was obtained from Applied
Biosystems, Inc.. The 7500 real-time time PCR instrument was from
Applied Biosystems; Thermo Fisher Scientific, Inc.. The reverse
transcription kit and qPCR Kit from also from Applied Biosystems;
Thermo Fisher Scientific, Inc., and the total RNA extraction kit
and (TRIzol) reagent were from Invitrogen; Thermo Fisher
Scientific, Inc.
Collection of blood samples
Fasting venous blood was collected from all patients
and the healthy controls. Serum was obtained following
centrifugation and stored at −80°C for analysis. At the same time,
2 ml blood samples were collected from each subject and sent to the
nuclear medicine department for carcinoembryonic antigen (CEA) and
Cyfra21-1 detection (the normal upper limit of CEA and Cyfra21-1 as
indicated in the kit instructions was 5 and 7 µg/l,
respectively).
Extraction and identification of serum
exosomes
A total of 400 µl serum samples were gradually
melted on an ice surface. The exosome extraction kit from Applied
Biosystems, Inc. was used and the serum exosomes were extracted
strictly according to the instructions provided with the kit. The
exosomal samples were placed under an electron microscope to
observe the morphology of the particles. The exosomal samples were
diluted 5,000-, 10,000- or 20,000-fold to yield concentrations
between 108 and 109/ml. The NanoSight NS300
instrument (Malvern Panalytical) was used for detection, the
threshold was set to 5, and all detection parameters were kept
unaltered during the detection process.
Detection of miR-23b-3p in
exosomes
The same amount (100 µl) of exosomes was used for
each sample. Total RNA was extracted from the exosomal samples
using TRIzol reagent and purified by a silica gel adsorption column
(Life Technologies; Thermo Fisher Scientificc, Inc.). cDNA was
obtained by reverse transcription. The reaction conditions were as
follows: 16°C for 15 min, 42°C for 60 min, 85°C for 5 min, and 4°C
for 5 min. miR-23b-3p expression in exosomes was detected
quantitatively by fluorescent dye participation method [SYBR-Green,
Tiangen Biotech (Beijing) Co., Ltd.]. The primer sequences were as
follows: miR-23b-3p forward, 5′-TCACGGTCCAGTTTTCCCAG-3′ and
reverse, 5′-GGAAGGAGGCAAATCCAGCT-3′; miRNA-39 forward,
5′-TGGAAAGGACGAAACACCGT-3′ and reverse, 5′-ATTTGCGTGTCATCCTTGCG-3.
The expression level of miR-23b-3p in exosomes was calculated using
the 2−ΔΔCq method (13)
and miRNA-39 was used as the external reference gene.
Statistical analysis
SPSS 18.0 statistical software was used to analyze
the data. The measurement data are expressed as the means ±
standard deviation (SD). One-way variance analysis followed by the
LSD test was used for comparisons between multiple groups. The
counting data were expressed by case number and rate (%), and the
Chi-squared (χ2) test was used for comparisons between
groups. The diagnostic efficacy of serum exosomal miR-23b-3p, serum
CEA and Cyfra21-1 in NSCLC was evaluated by receiver operating
characteristic (ROC) curve analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Identification of serum exosomes
The structure and morphology of the serum exosomes
extracted from the samples of the 3 groups of study subjects were
relatively similar (data not shown). Under an electron microscope,
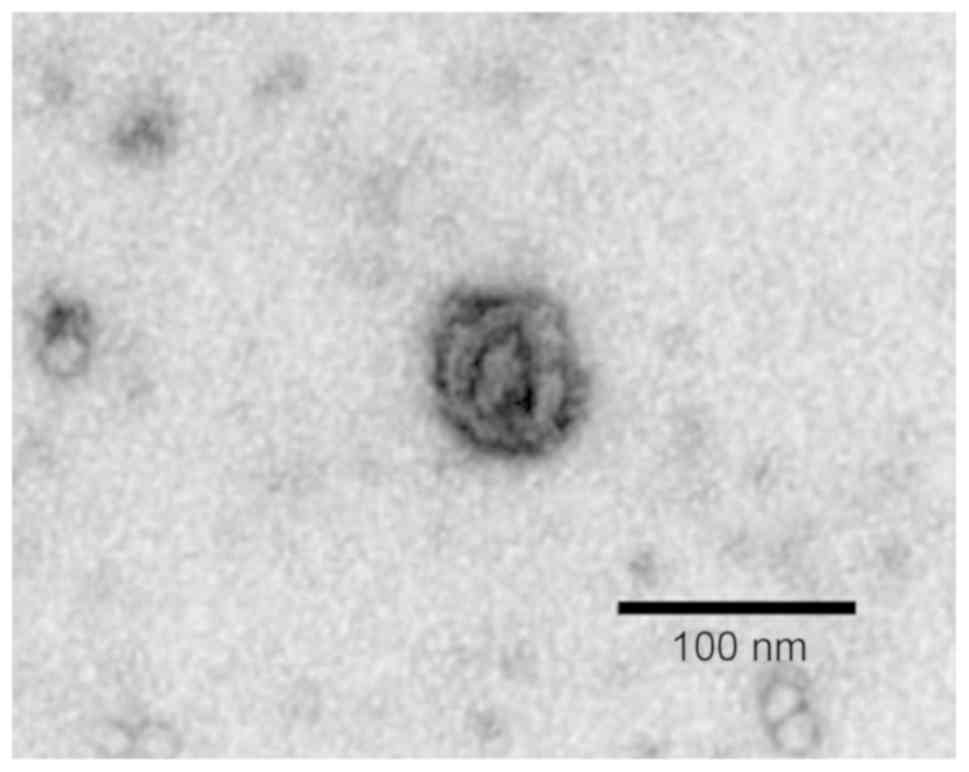
the serum exosome sample from a patient with NSCLC exhibited a
lipid bilayer membrane with cystic round particles with a diameter
of approximately 100 nm (Fig. 1).
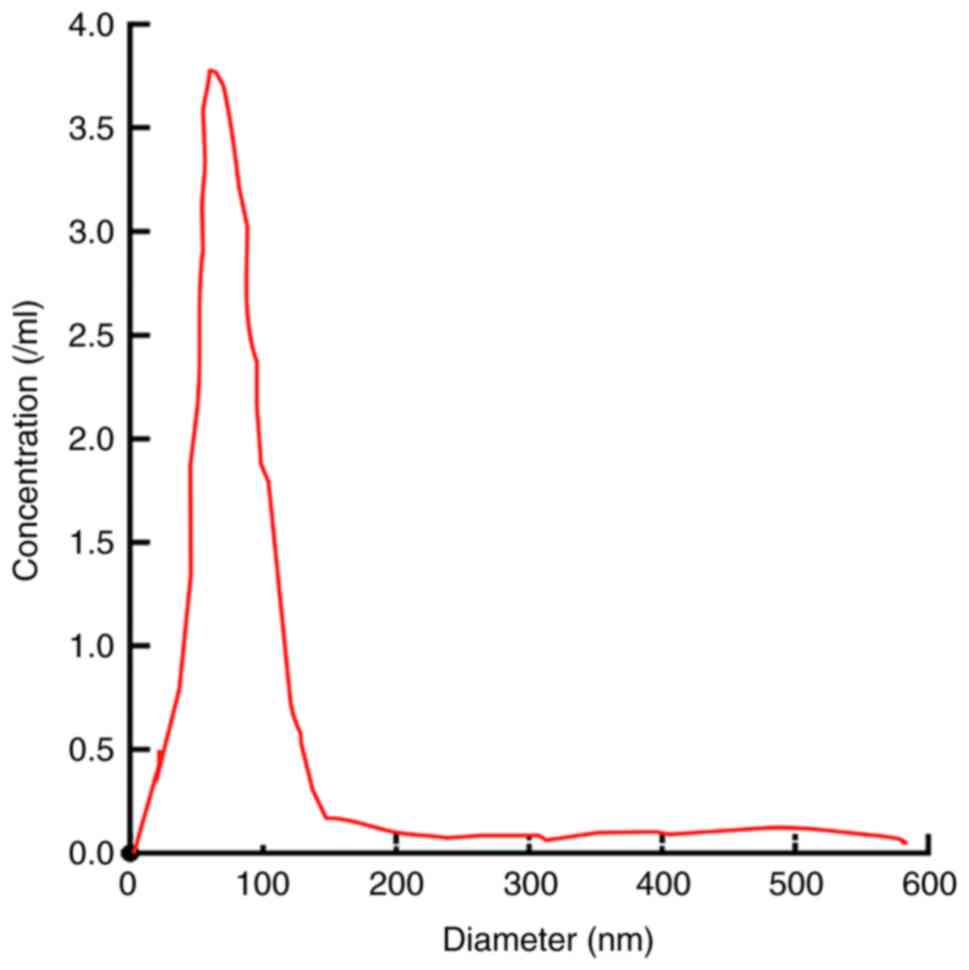
NanoSight revealed that the serum exosome particles were uniformly
dispersed, and the diameter of the exosome body was approximately
50–180 nm, the distribution curve exhibited a single peak normal
distribution and the peak value was approximately 104 nm (Fig. 2).
Expression of miR-23b-3p in serum
exosomes
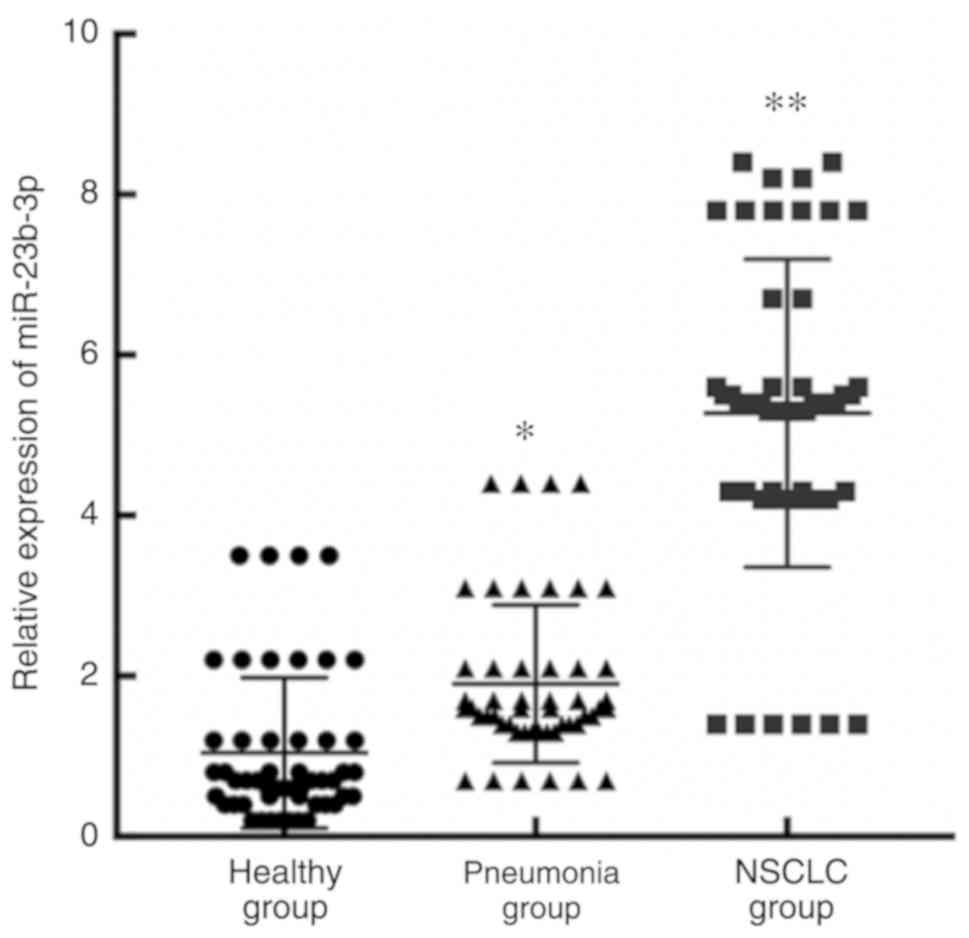
A significant difference was observed in the
expression of miR-23b-3p in serum exosomes from the patients with
NSCLC, the patients with pneumonia and the healthy subjects
(F=16.202, P<0.01). The expression level of serum exosomal
miR-23b-3p in patients with NSCLC was significantly higher than
that in patients with pneumonia (t=10.332, P<0.001) and healthy
subjects (t=12.810, P<0.001; Fig.
3).
Association between serum exosomal
miR-23b-3p and clinicopathological parameters of patients with lung
cancer
The 80 patients with NSCLC were divided into the
high expression group and low expression group according to the
relative expression level of miR-23b in exosomes (median relative
expression level of mir-23b-3p in exosomes was 3.98). There were 40
cases with a high exosomal miR-23b-3p expression and 40 cases with
a low exosomal miR-23b-3p expression. There was a significant
association between serum exosomal miR-23b-3p and tumor size, depth
of invasion, liver metastasis and TNM stage (Table I).
 | Table I.Association between serum exosomal
miR-23b-3p expression and clinicopathological parameters of
patients with NSCLC. |
Table I.
Association between serum exosomal
miR-23b-3p expression and clinicopathological parameters of
patients with NSCLC.
| Variable | miR-23b-3p low
expression group | miR-23b-3p high
expression group | P-value |
|---|
| Sex |
|
| 0.400 |
| Male | 28 | 26 |
|
|
Female | 12 | 14 |
|
| Size of tumors
(cm) |
|
| 0.019 |
|
<5 | 22 | 20 |
|
| ≥5 | 18 | 20 |
|
| Differentiation
degree |
|
| 0.161 |
|
High/moderate | 25 | 26 |
|
| Poor | 15 | 14 |
|
| Depth of
invasion |
|
| 0.029 |
| pT1 | 10 | 11 |
|
| ≥pT2 | 30 | 29 |
|
| Lymphatic
metastasis |
|
| 0.136 |
| No | 15 | 16 |
|
| Yes | 25 | 24 |
|
| Liver metastasis |
|
| 0.032 |
| No | 32 | 37 |
|
| Yes | 8 | 3 |
|
| Peritoneal
carcinomatosis |
|
| 0.248 |
| No | 31 | 30 |
|
| Yes | 9 | 10 |
|
| TNM staging |
|
| 0.034 |
| I | 13 | 12 |
|
| II | 12 | 13 |
|
|
III | 8 | 7 |
|
| IV | 7 | 8 |
|
Diagnostic efficacy of miR-23b-3p in
serum exosomes in NSCLC
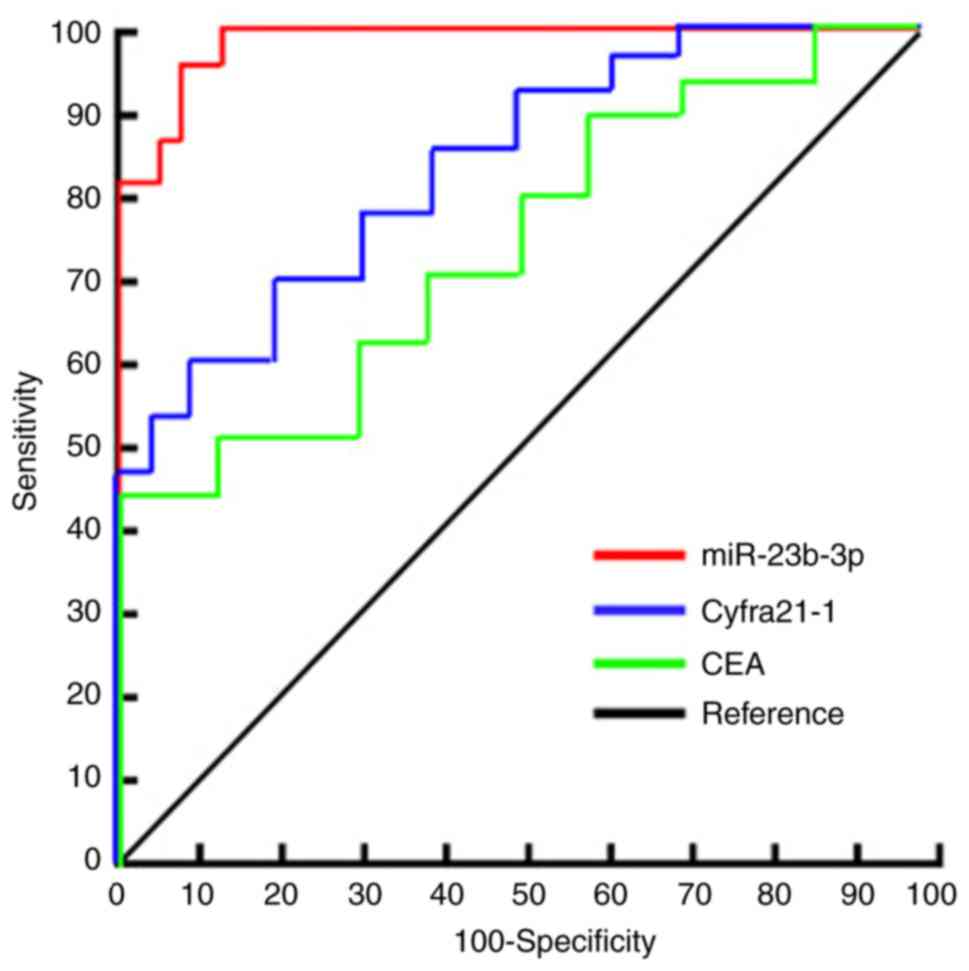
ROC curve analysis was used to evaluate the
diagnostic efficacy of serum exosomal miR-23b-3p in NSCLC. The area
under the curve of serum exosomal miR-23b-3p was 0.915 (95% CI:
0.84-0.92). The optimal critical value of miR-23b-3p relative
expression was 3.46, the diagnostic sensitivity was 87.4%, and the
specificity was 93.8%. The area under the ROC curve (AUC) for serum
CEA, a traditional tumor marker, was 0.645 (95% CI, 0.641-0.772) in
the diagnosis of NSCLC. The critical value of CEA relative
expression was 7.46, the diagnostic sensitivity was 67.4% and the
specificity was 66.8%. The AUC of serum Cyfra21-1 for the diagnosis
of NSCLC was 0.745 (95% CI, 0.701-0.812), the critical value of
Cyfra21-1 relative expression was 8.56, and the diagnostic
sensitivity and specificity were 70.4 and 71.8%, respectively
(Fig. 4).
Discussion
NSCLC is the most common malignant tumor in China,
which severely threatens the health of residents and places a heavy
burden on families and society. The search for non-invasive serum
markers for patients with NSCLC is of utmost importance for the
early diagnosis and follow-up evaluation of patients with lung
cancer (14). Surgery combined with
simultaneous radiotherapy and chemotherapy can improve the clinical
cure rate of early-stage NSCLC; however, there is still a lack of
effective treatment for patients with advanced disease, whose
survival rate is <15%. No significant improvements have been
made in this field for a number of years (15). Thus, it is of utmost clinical
significance to explore early NSCLC diagnostic markers.
miRNAs, short chain non-coding RNAs, play a key role
in the occurrence and progression of cancer, and have gradually
become molecular markers for cancer diagnosis and prognosis
(16,17). In recent years, a large number of
studies have indicated that the expression of miRNAs in tumor
tissues or cells differs from that in normal tissues, and this
differential expression is closely related to tumorigenesis and
progression. miRNAs are potential molecular markers of tumors and
can play an important role in the early diagnosis and prognosis of
tumors (18,19). It has been found that a variety of
miRNAs, such as miR-17, miR-190b, miR-19a, miR-19b, miR-26b and
miR-375, are related to the occurrence and progression of NSCLC,
and have certain diagnostic value for NSCLC (20,21). A
recent study (22) also demonstrated
that serum miRNA is expected to be a diagnostic marker of
NSCLC.
Although serum miRNAs are valuable for the diagnosis
of clinical NSCLC, there are certain shortcomings: The composition
of serum is complex and the source of miRNAs is unknown, which
affects the specificity of the detection results. miRNAs in serum
are also affected by a number of enzymes, resulting in poor
stability (23). Recently, it has
been found that there is a certain difference in the expression
profiles between serum miRNAs and serum exosomes. Compared with
serum miRNAs, serum exosomes are less disturbed and have better
stability (24). miRNAs exist stably
in serum exosome bodies and are less affected by various enzymes in
serum. The samples can be obtained in a non-invasive manner, which
renders serum exosomal miRNAs a non-invasive and reliable tumor
biomarkers (25).
In the present study, exosomes in serum were
directly extracted using a serum exosome extraction kit.
Examination by a transmission electron microscope and a NasoSight
instrument revealed that there was a large number of high-abundance
exosome particles in the samples of the 3 groups. At present, there
are 4 main methods reported in the literature for the extraction
and purification of exosomes: Ultracentrifugation, immunomagnetic
bead method, polyethylene glycol precipitation method and the kit
method (26). In the present study,
the kit from Applied Biosystems, Inc. was used to extract and
confirm the high purity and abundance of serum exosomes, which
provided the basis for subsequent research (26). In this study, it was found that the
purified serum samples contained a large number of exosome
particles. The expression of miR-23b-3p in serum exosomes of
patients with NSCLC was significantly higher than that of patients
with pneumonia and healthy controls. ROC curve analysis was used to
evaluate the diagnostic efficacy of miR-23b-3p in NSCLC. The
results suggested that serum exosomal miR-23b-3p was more effective
in the diagnosis of NSCLC. CEA and Cyfra21-1 are common traditional
biomarkers in the diagnosis of NSCLC and for monitoring tumor
recurrence (27). In the present
study, it was found that the AUC of miR-23b-3p was greater than
that of CEA and Cyfra21-1, and the sensitivity and specificity of
miR-23b-3p were greater than those of CEA and Cyfra21-1. In
comparison, miR-23b-3p in serum exosomes was superior to the
traditional tumor markers, CEA and Cyfra21-1, in the diagnosis of
NSCLC.
The results of the present study demonstrated that
there was a statistically significant association between serum
exosomal miR-23b-3p and tumor size, depth of invasion, liver
metastasis and TNM stage in patients with NSCLC, suggesting that
exosomal miR-23b-3p was closely related to the clinical progress of
NSCLC. It has been reported (8–10) that
miR-23b-3p plays an important role in the progression of colon
cancer, liver cancer and gastric cancer, which is consistent with
the results of the present study. It has been found that miR-23b-3p
is an important miRNA that plays the role of a proto-oncogene. The
results of whole gene sequencing of cancer and normal tissues in
patients with NSCLC have demonstrated that the expression in NSCLC
tissues seems to be significantly higher than that in healthy
cancer tissues (28). A high
expression of miR-23b-3p was considered to be a molecular marker of
a poor prognosis in a single sample of 114 NSCLC patients (28). In the present study, the expression
of miR-23b-3p in the serum of patients with NSCLC was not
investigated. A recent study reported that a high expression of
miR-23b-3p in serum exosomes was a marker of a poor prognosis of
patients with pancreatic cancer (29). Although its involvement in the
development and progression of pancreatic cancer has not yet been
elucidated, it may promote the progress of pancreatic cancer by
regulating annexin A2 expression (30). The above-mentioned results indicate
that miR-23b-3p may regulate the expression of different related
target genes in different tumor types, and may generally play a
role similar to that of a proto-oncogene. The results of the
present study initially confirmed that serum exosomal miRNA was
closely related to the occurrence and progression of NSCLC. Since
the detection of serum exosomal miRNA can be obtained in a
non-invasive manner, it may become a non-invasive diagnostic marker
of NSCLC.
In conclusion, the present study demonstrates that
miR-23b-3p is highly expressed in serum exosomes of patients with
NSCLC and has a high diagnostic efficacy for NSCLC; thus, it may
become a novel diagnostic marker for NSCLC. However, further
studies are required with an increased number of samples in order
to further explore the asociation between serum exosomal miRNA and
NSCLC, so as to provide reference for the early clinical diagnosis
and treatment of NSCLC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW and HX conceived and designed the study. ZZ and
JG were responsible for the collection and analysis of the
experimental data. MZ interpreted the data and drafted the
manuscript. ZM performed the detection of miR-23b-3p in exosomes
and revised the manuscript critically for important intellectual
content. JW wrote the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the People's Hospital of Yangzhong City. Patients who
participated in this research, signed the informed consent and had
complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang ML, Peng P, Wu CX, Gong YM, Zhang
SW, Chen WQ and Bao PP: Report of breast cancer incidence and
mortality in China registry regions, 2008–2012. Zhonghua Zhong Liu
Za Zhi. 41:315–320. 2019.(In Chinese). PubMed/NCBI
|
|
2
|
He Y, Li D, Shan B, Liang D, Shi J, Chen W
and He J: Incidence and mortality of esophagus cancer in China,
2008–2012. Chin J Cancer Res. 31:426–434. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Caballero Vázquez A, García Flores P,
Romero Ortiz A, Del Moral RG and Alcázar-Navarrete B: Changes in
non-small cell lung cancer diagnosis, molecular testing and
prognosis 2011–2016. J Thorac Dis. 10:5468–5475. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fernandez FG, Kosinski AS, Furnary AP,
Onaitis M, Kim S, Habib RH, Tong BC, Cowper P, Boffa D, Jacobs JP,
et al: Differential effects of operative complications on survival
after surgery for primary lung cancer. J Thorac Cardiovasc Surg.
155:1254–1264.e1. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mishra S, Yadav T and Rani V: Exploring
miRNA based approaches in cancer diagnostics and therapeutics. Crit
Rev Oncol Hematol. 98:12–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen R, Xu X, Qian Z, Zhang C, Niu Y, Wang
Z, Sun J, Zhang X and Yu Y: The biological functions and clinical
applications of exosomes in lung cancer. Cell Mol Life Sci.
76:4613–4633. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nigita G, Distefano R, Veneziano D, Romano
G, Rahman M, Wang K, Pass H, Croce CM, Acunzo M and Nana-Sinkam P:
Tissue and exosomal miRNA editing in non-small cell lung cancer.
Sci Rep. 8:102222018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grisard E, Coan M, Cesaratto L, Rigo I,
Zandonà L, Paulitti A, Andreuzzi E, Rampioni Vinciguerra GL,
Poletto E, Del Ben F, et al: Sleeping beauty genetic screen
identifies miR-23b::BTBD7 gene interaction as crucial for
colorectal cancer metastasis. EBioMedicine. 46:79–93. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang T, He X, Chen A, Tan K and Du X:
LncRNA HOTAIR contributes to the malignancy of hepatocellular
carcinoma by enhancing epithelial-mesenchymal transition via
sponging miR-23b-3p from ZEB1. Gene. 670:114–122. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xian X, Tang L, Wu C and Huang L:
miR-23b-3p and miR-130a-5p affect cell growth, migration and
invasion by targeting CB1R via the Wnt/β-catenin signaling pathway
in gastric carcinoma. Onco Targets Ther. 11:7503–7512. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hattori A, Takamochi K, Oh S and Suzuki K:
New revisions and current issues in the eighth edition of the TNM
classification for non-small cell lung cancer. Jpn J Clin Oncol.
49:3–11. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cao M and Chen W: Epidemiology of lung
cancer in China. Thorac Cancer. 10:3–7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen W, Sun K, Zheng R, Zeng H, Zhang S,
Xia C, Yang Z, Li H, Zou X and He J: Cancer incidence and mortality
in China, 2014. Chin J Cancer Res. 30:1–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie Y, Zhang Y, Du L, Jiang X, Yan S, Duan
W, Li J, Zhan Y, Wang L, Zhang S, et al: Circulating long noncoding
RNA act as potential novel biomarkers for diagnosis and prognosis
of non-small cell lung cancer. Mol Oncol. 12:648–658. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Y, Yin Z, Fan J, Zhang S and Yang W:
The roles of exosomal miRNAs and lncRNAs in lung diseases. Signal
Transduct Target Ther. 4:472019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rupaimoole R, Calin GA, Lopez-Berestein G
and Sood AK: miRNA deregulation in cancer cells and the tumor
microenvironment. Cancer Discov. 6:235–246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gallach S, Jantus-Lewintre E,
Calabuig-Fariñas S, Montaner D, Alonso S, Sirera R, Blasco A, Usó
M, Guijarro R, Martorell M and Camps C: MicroRNA profiling
associated with non-small cell lung cancer: Next generation
sequencing detection, experimental validation, and prognostic
value. Oncotarget. 8:56143–56157. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu S, Kong H, Hou Y, Ge D, Huang W, Ou J,
Yang D, Zhang L, Wu G, Song Y, et al: Two plasma microRNA panels
for diagnosis and subtype discrimination of lung cancer. Lung
Cancer. 123:44–51. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zaporozhchenko IA, Morozkin ES,
Ponomaryova AA, Rykova EY, Cherdyntseva NV, Zheravin AA,
Pashkovskaya OA, Pokushalov EA, Vlassov VV and Laktionov PP:
Profiling of 179 miRNA expression in blood plasma of lung cancer
patients and cancer-free individuals. Sci Rep. 8:63482018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang P, Yang D, Zhang H, Wei X, Ma T,
Cheng Z, Hong Q, Hu J, Zhuo H, Song Y, et al: Early detection of
lung cancer in serum by a panel of microRNA biomarkers. Clin Lung
Cancer. 16:313–319.e1. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen X, Xu Y, Liao X, Liao R, Zhang L, Niu
K, Li T, Li D, Chen Z, Duan Y and Sun J: Plasma miRNAs in
predicting radiosensitivity in non-small cell lung cancer. Tumour
Biol. 37:11927–11936. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Munagala R, Aqil F and Gupta RC: Exosomal
miRNAs as biomarkers of recurrent lung cancer. Tumour Biol.
37:10703–10714. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Li S, Li L, Li M, Guo C, Yao J
and Mi S: Exosome and exosomal microRNA: Trafficking, sorting, and
function. Genomics Proteomics Bioinformatics. 13:17–24. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tang YT, Huang YY, Zheng L, Qin SH, Xu XP,
An TX, Xu Y, Wu YS, Hu XM, Ping BH and Wang Q: Comparison of
isolation methods of exosomes and exosomal RNA from cell culture
medium and serum. Int J Mol Med. 40:834–844. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang ZF, Wang M and Xu JL: Thymidine
kinase 1 combined with CEA, Cyfra21-1 and NSE improved its
diagnostic value for lung cancer. Life Sci. 194:1–6. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Begum S, Hayashi M, Ogawa T, Jabboure FJ,
Brait M, Izumchenko E, Tabak S, Ahrendt SA, Westra WH, Koch W, et
al: An integrated genome-wide approach to discover deregulated
microRNAs in non-small cell lung cancer: Clinical significance of
miR-23b-3p deregulation. Sci Rep. 5:132362015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen D, Wu X, Xia M, Wu F, Ding J, Jiao Y,
Zhan Q and An F: Upregulated exosomic miR-23b-3p plays regulatory
roles in the progression of pancreatic cancer. Oncol Rep.
38:2182–2188. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wei DM, Dang YW, Feng ZB, Liang L, Zhang
L, Tang RX, Chen ZM, Yu Q, Wei YC, Luo DZ and Chen G: Biological
effect and mechanism of the miR-23b-3p/ANXA2 axis in pancreatic
ductal adenocarcinoma. Cell Physiol Biochem. 50:823–840. 2018.
View Article : Google Scholar : PubMed/NCBI
|