Introduction
Odontogenic keratocyst (OKC) is a common oral cyst
arising from the odontogenic epithelium, which has the
characteristics of a tumor; however, the name OKC is controversial.
Now in the World Health Organization classification,
non-inflammatory odontogenic cyst head and neck tumors are termed
OKCs (1); however, before 2017, they
were termed keratinous cystic odontogenic tumors (2). An OKC grows aggressively with a high
rate of recurrence, exhibiting the behavior of a malignant tumor;
therefore, due to their locally destructive growth they may require
radical surgery (3). Numerous
pathways representing therapeutic targets may be useful for the
treatment of an OKC. For example, mutations in protein patched
homolog 1, a transmembrane protein in the hedgehog (HH) signaling
pathway, have been demonstrated in OKC, and such mutations increase
HH signaling activity that promotes the proliferation and
neoplastic growth of the tumor (4).
Other signaling pathways, such as epidermal growth factor receptor,
Wnt and AKT signaling, have been reported to be abnormal in OKC
(5).
An increasing number of studies have demonstrated
that macrophages perform a central role in the development of
tumors (6,7). The two subtypes of polarized
macrophages are M1 (classical macrophage) and M2 (alternative
macrophage); these macrophages promote tumor rejection or stimulate
growth, respectively (8).
Tumor-associated macrophages (TAMs) are present in abundance in the
microenvironment of solid tumors, and are involved in tumor
angiogenesis and metastasis (6).
TAMs mostly have an M2-like phenotype (6,9). It has
previously been demonstrated that TAMs are involved in the
development of oral squamous cell carcinomas, as well as functions
such as proliferation, angiogenesis and tumor cell invasion in the
stroma of the lesion (10). The
heterogeneity and plasticity of TAMs in the development of cancer
suggests that the differentiation processes of TAMs may be targets
for immunotherapy (11). Hence,
knowledge of TAMs in tumors is important for their diagnosis and
therapy.
Cyclooxygenase (COX)-2 is an inflammation-inducible
enzyme that is upregulated in numerous types of carcinoma (12,13).
COX-2 is able to promote carcinoma progression and metastasis, and
reduce patient survival (14–16). In
addition, COX-2 is generally absent or at very low levels in cells,
but can be induced by pro-inflammatory and mitogenic stimuli
(17,18). Transforming growth factor (TGF)-β1 is
an important growth factor which performs various roles in
biological growth and development (19). A previous study suggested that TGF-β1
may upregulate the expression of COX-2 in the early stages of human
dental pulp inflammation (19); thus
indicating that TGF-β1-induced COX-2 expression may serve an
important role in human dental disease.
A previous study demonstrated that M2-polarized
macrophages are present in OKCs where they promote tumor
angiogenesis (20). The present
study aimed to explore whether TGF-β1-induced COX-2 affects the
development of OKC and angiogenesis, as well the possible
mechanisms of action.
Materials and methods
Preparation of tissue homogenate
A total of 14 OKC samples were collected by The
Department of Stomatology, Zhejiang Provincial People's Hospital
(Zhejiang, China) between March 2017 to July 2017. Patients with a
history of tumors other than OKC were excluded. The patients
comprised of 8 males and 6 females, with an average age of 32 years
and age range, 21–48 years. This study was approved by the Ethics
Committee of Zhejiang Provincial People's Hospital. All patients
were informed of the purpose of this study and provided written
informed consent.
Tissue homogenate was prepared as previously
described (20). Fresh OKC tissues
were collected and washed thoroughly. The samples were immediately
placed in serum-free RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.). Samples were homogenized in a glass homogenizer,
and were then transferred into tubes and centrifuged at 10,000 × g
for 1 h at room temperature. Each supernatant was collected and
stored at −80°C.
Cell culture
THP-1 cells were purchased from The Kunming Cell
Bank, Chinese Academy of Sciences and human umbilical vein
endothelial cells (HUVECs) were from Shanghai Fuxiang Biotechnology
Co., Ltd. THP-1 cells and HUVECs were cultured in RPMI-1640 and
high-glucose DMEM (Gibco; Thermo Fisher Scientific, Inc.),
respectively. Both were supplemented with 10% FBS (Hyclone; GE
Healthcare life Sciences) and 1% penicillin/streptomycin (Gibco;
Thermo Fisher Scientific, Inc.). Cells were cultured at 37°C in an
atmosphere containing 5% CO2.
Treatment of cells
THP-1 cells were cultured in 6-well plates then
treated with 25 ng/ml phorbol-12-myristate-13-acetate (PMA, M2
macrophages inducer; Sigma-Aldrich; Merck KGaA) at 37°C. After 12
h, the culture medium was removed and OKC tissue supernatant was
added, with or without niflumic acid (NA, COX inhibitor; Selleck
Chemicals). FollowingTHP-1 cell induction to M2 macrophages with
PMA treatment, M2 macrophages were cultured with 1, 5 and 10 ng/ml
concentrations of TGF-β1 with or without NA, 20 ng/ml interleukin
(IL)-4 and 2 ng/ml IL-13, then polarized for 36 h. The cells were
collected for flow cytometric analysis, reverse
transcription-quantitative PCR (RT-qPCR) and western blot analysis.
Collected medium was centrifuged at 500 × g for 20 min at room
temperature and stored at −80°C.
Flow cytometry
After cells were treated, they were collected and
washed in PBS three times. The cells were suspended in loading
buffer (Sangon Biotech Co., Ltd.,) to a density of
2–5×105 cells/ml, then incubated with a phycoerythrin
(PE)-conjugated mouse anti-CD163 monoclonal antibody (1:100; cat.
no. sc-20066; Santa Cruz Biotechnology) in the dark for 30 min at
37°C. After removal of the antibody solution, the cells were washed
with PBS twice and then resuspended in loading buffer. A BD
FACSCanto™ II flow cytometer (BD Biosciences) was used to detect
surface markers of the cells. The results were analyzed by FlowJo
software v.10.0.7 (FlowJo LLC).
RT-qPCR
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) in accordance with the manufacturer's protocol. First-strand
cDNA was synthesized according to the manufacturer's instructions
at 42°C for 60 min using a RevertAid first strand cDNA synthesis
kit (Thermo Fisher Scientific, Inc.). cDNA and primers were mixed
with SYBR® Green Master Mix (Starbiolab) and RT-qPCR was
conducted on a Roche LightCycler® 480 system (Roche
Diagnostics) by using the following thermocycling procedure: 10 min
at 95°C, followed by 40 cycles of 20 sec at 95°C, 30 sec at 60°C
and 30 sec at 72°C. The following primers were used: TGF-β, forward
5′-GGGACTATCCACCTGCAAGA-3′, reverse 5-CCTCCTTGGCGTAGTAGTCG-3′;
COX-1, forward 5′-TGTGACTTCCCTTCTAACCCC-3′, reverse
5′-CTCTGTCCTCTCTCTCTGCTG-3′; COX-2, forward
5′-CTTCCTCCTGTGCCTGATGAT-3′, reverse 5′-GCCCTCGCTTATGATCTGTCT-3′;
matrix metalloproteinase (MMP)-9, forward
5′-GTGAAGACGCAGACGGTGGATTC-3′, reverse
5′-GGTACTCACACGCCAGAAGAAGC-3′; and GAPDH, forward
5′-ATGGGGAAGGTGAAGGTCG-3′ and reverse 5′-TAAAAGCAGCCCTGGTGACC-3′.
GAPDH was used as the endogenous reference gene and gene expression
was comparatively quantified using the 2−ΔΔCq method
(21).
Western blotting
Cells were lysed in RIPA buffer (Beyotime Institute
of Biotechnology) containing protease and phosphatase inhibitor
cocktails (Thermo Fisher Scientific, Inc.). Protein concentrations
in samples were quantified using a bicinchoninic acid assay kit
(Beyotime Institute of Biotechnology). Equal quantities of protein
(25 µg) were separated by SDS-PAGE on 10% gels. Proteins were then
transferred onto polyvinylidene difluoride membranes (EMD
Millipore). Membranes were blocked with 5% non-fat milk for 1 h at
room temperature, then incubated with the appropriate primary
antibodies overnight at 4°C. The following primary antibodies were
used to detect proteins: Rabbit polyclonal anti-COX-2 (1:500; cat.
no. 12375-1-AP; Wuhan Sanying Biotechnology), rabbit polyclonal
anti-TGF-β1 (1:500; cat. no. 21898-1-AP; Wuhan Sanying
Biotechnology) and rabbit polyclonal anti-MMP-9 (1:500; cat. no.
27306-1-AP; Wuhan Sanying Biotechnology). Membranes were then
washed with TBST and incubated with a horseradish peroxidase
(HRP)-conjugated goat anti-rabbit IgG antibody or a HRP-conjugated
goat anti-mouse IgG antibody (1:1,000, Beyotime Institute of
Biotechnology), as appropriate, for 1 h at room temperature.
Incubation with a mouse anti-GAPDH monoclonal antibody (1:1,000;
cat. no. ab8245; Abcam) was used as a loading control. Blots were
analyzed and proteins comparatively semi-quantified using a Clarity
MAX™ Western ECL Substrate (Bio-Rad Laboratories, Inc.) in a
ChemiDoc™ XRS+ system (Bio-Rad Laboratories, Inc.).
In vitro tube formation assay and
immunofluorescence staining
A tube formation assay was performed to measure the
effects of OKC supernatant on angiogenesis in vitro. A total
of 100 µl Matrigel (BD Biosciences) was placed into the wells of a
48-well plate (Corning, Inc.) and then chilled at 4°C overnight.
The Matrigel was then polymerized at 37°C for 30 min. M2
macrophages (20,000 cells/well) in combination were added to the
Matrigel-coated wells. After 24 h at 37°C, images of the tubular
structures were captured using a light microscope (magnification,
×100; Leica DM18 microscope, Leica Microsystems, Inc.) equipped
with a Leica MC170 HD digital camera (Leica Microsystems, Inc.) and
were analyzed using ImageJ software v.1.8.0 (National Institutes of
Health).
Tubular structures were fixed in 4% paraformaldehyde
(Sangon Biotech Co., Ltd.) for 30 min at room temperature, then
washed in PBS (Sangon Biotech Co., Ltd.) three times. Slides were
blocked with 10% goat serum (Beyotime Institute of Biotechnology)
for 1 h at room temperature and then stained using a mouse
anti-CD31 monoclonal antibody conjugated with FITC (1:100; cat. no.
sc-376764; Santa Cruz Biotechnology, Inc.) overnight at 4°C in the
dark. The antibodies were removed and the slides were washed in PBS
three times prior to staining the cell nuclei with DAPI for 15 min
at room temperature in the dark. After washing three times in PBS,
the images were captured using a fluorescent microscope and
(magnification, ×100; Leica Microsystems, Inc.) equipped with a
Leica DFC450C digital camera (Leica Microsystems, Inc.).
MTT cell viability assay
A total of 5,000 cells/well were seeded in 96-well
plates and various treatments (PMA, PMA+OKC, PMA+OKC+NA or
PMA+IL4/13) were added, as appropriate. After 48 h at room
temperature of incubation, 100 µl MTT solution (1 mg/ml) was added
to the cells and incubated at 37°C for 4 h. The MTT solution was
aspirated and 150 µl isopropanol was added to solubilize the
formazan crystals formed by cell metabolism, followed by gentle
agitation for 15 min. Cell viability was calculated from relative
absorbance of 595 nm light using a Cytation 3 Multi-Mode plate
reader (BioTek Instruments, Inc.).
Statistical analysis
Each experiment was repeated three times. Data are
presented as the mean ± standard deviation. Data were compared
using a two-tailed Student's t-test (unpaired) or one-way analysis
of variance with a Tukey's multiple comparison test. Data were
presented graphically using GraphPad Prism 6 (GraphPad Software).
P<0.05 was considered to indicate a statistically significant
difference.
Results
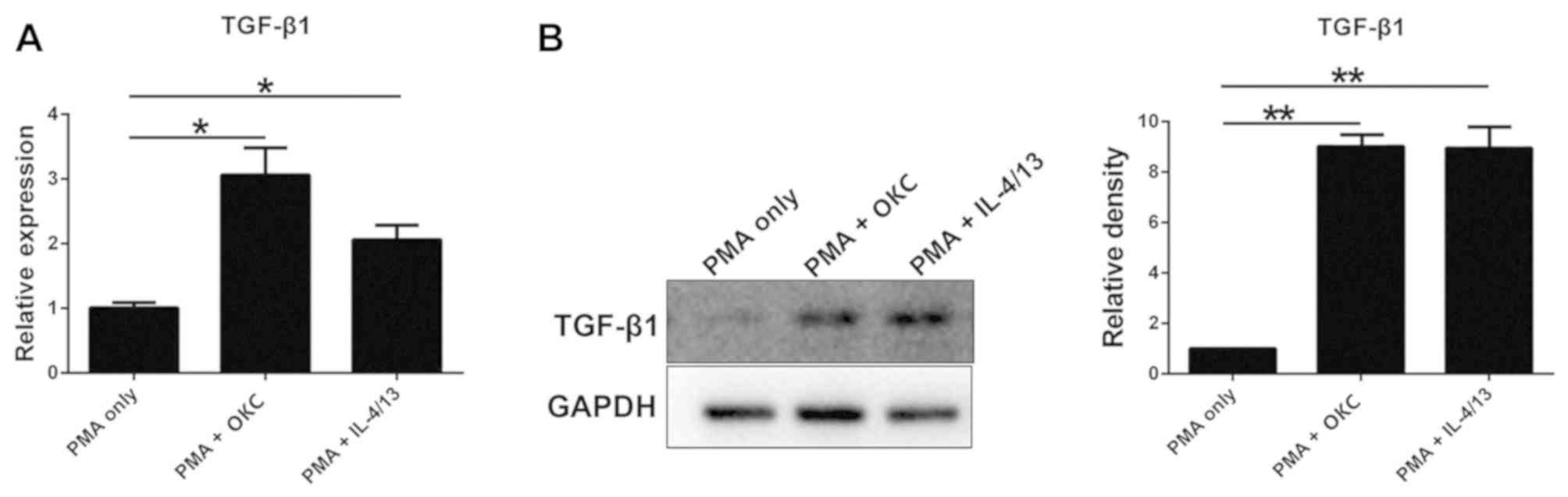
TGF-β1 is upregulated in M2-polarized
macrophages treated with OKC homogenate supernatant
In this study, RT-qPCR and western blotting were
used to find that TGF-1 in M2-polarized macrophages was upregulated
when treated with OKC homogenate supernatant. IL-4/13 promotes
M2-polarization of macrophages; therefore, these two factors were
used to induce M2 macrophages as a positive control. The mRNA and
protein expression levels of TGF-β1 were clearly upregulated in
M2-polarized macrophages induced by treatment with a homogenate
supernatant of OKC tissues compared with the control group (PMA
only) (Fig. 1). These findings
suggested that OKC increased the expression of TGF-β1.
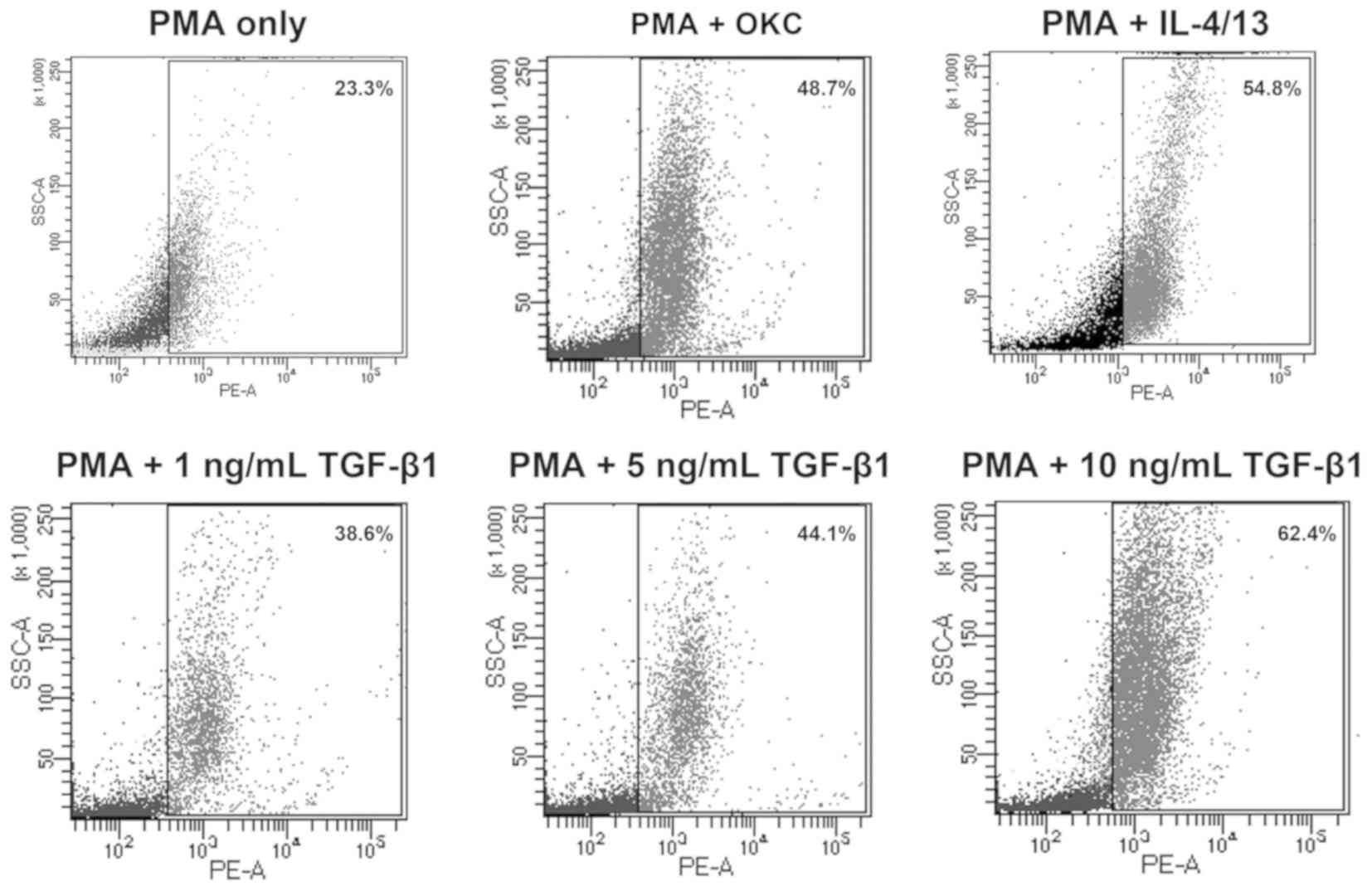
TGF-β1 induces M2-polarization of
macrophages
Multiple markers are differentially expressed on the
surface of different macrophages. For example, CD206, CD163 and
CD301 are M2-specific markers (22).
In the present study, CD163+ was used to characterize
M2-polarization of macrophages using flow cytometry.
The results demonstrated that M2-polarization of
macrophages was induced by treatment with OKC homogenate
supernatant, IL-4/13 and TGF-β1 (Fig.
2). In addition, the proportion of M2-polarization increased
with TGF-β1 in a concentration-dependent manner. These results
indicated that TGF-β1 induced M2-polarization of macrophage-like
cells in vitro.
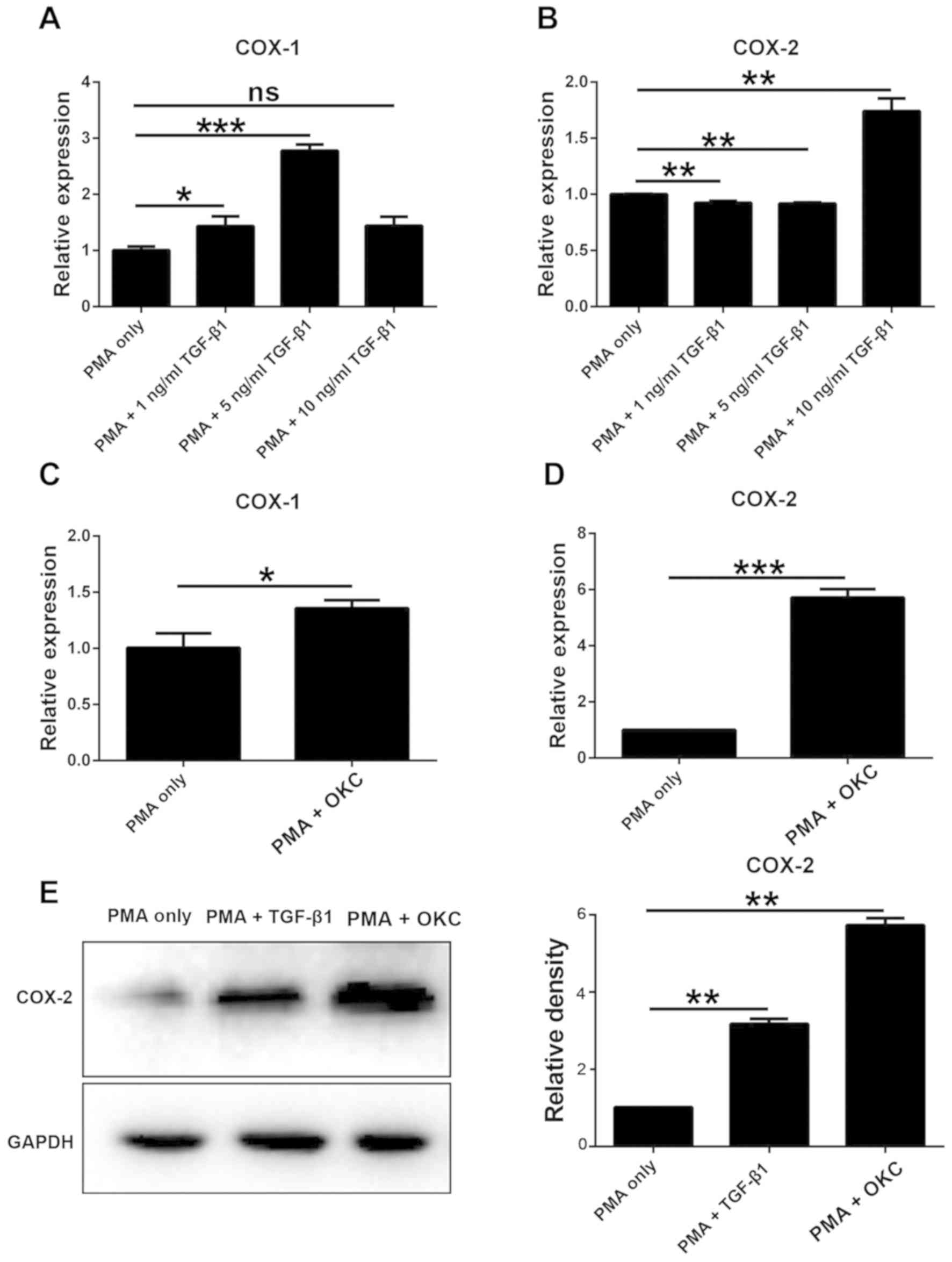
COX-2 is upregulated in TGF-β1- and
OKC homogenate supernatant-induced M2-polarized macrophages
COX exists as two isoforms: COX-1 and COX-2. COX-1
is universally expressed in high concentrations, whereas COX-2 is
expressed in low concentrations but can be induced by multiple
stimuli (19,23). In the present study, the expression
levels of the two genes were quantified in response to different
treatments.
TGF-β1 induced a significant increase in COX-1 mRNA
expression levels, except for the highest concentration (Fig. 3A), compared with the levels in the
PMA-only group. Conversely, the mRNA expression levels of COX-2
were significantly upregulated in a concentration-dependent manner
by TGF-β1 (Fig. 3B). In addition,
following treatment with OKC homogenate supernatant, the mRNA and
protein expression levels of COX-1 and COX-2 were significantly
upregulated compared with those in the PMA-only group (Fig. 3C and D). In subsequent experiments,
10 ng/ml TGF-β1 was selected and western blot analysis was
conducted to evaluate the protein expression levels of COX-2.
Treatment with TGF-β1 or OKC homogenate supernatant significantly
increased the protein expression levels of COX-2 compared with
those in the PMA-only group (Fig.
3E). These results suggested that COX-2 can be induced by both
OKC and TGF-β1, in particular in high doses.
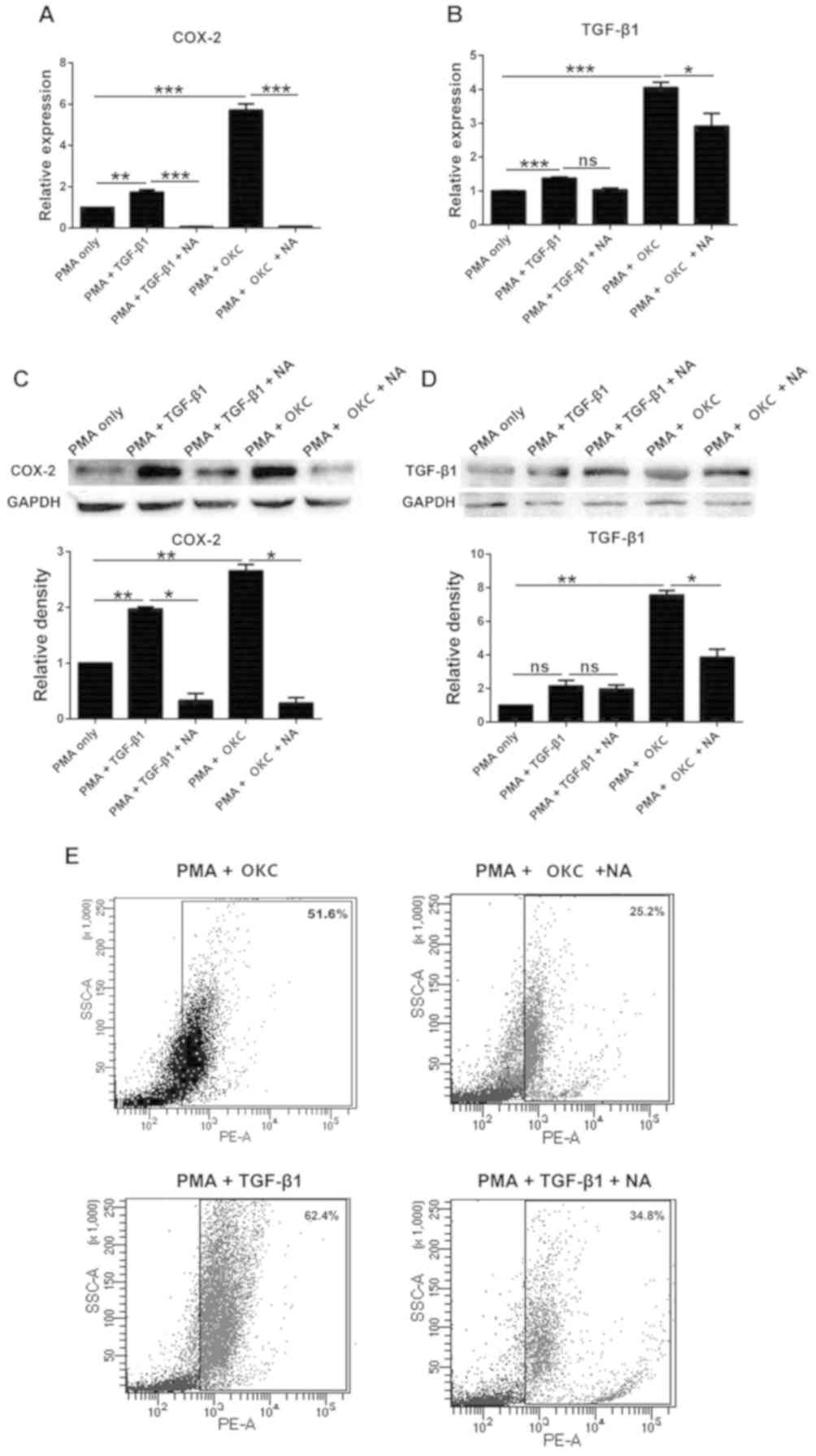
Inhibition of COX-2 influences
M2-polarization of macrophages induced by OKC
NA is an inhibitor of COX-2 (24), and can inhibit cancer cell
proliferation and migration (25).
After treatment with TGF-β1 combined with NA, or OKC homogenate
supernatant combined with NA, the protein expression levels of
COX-2 were significantly downregulated compared with those in the
PMA-only group (Fig. 4C), indicating
that NA effectively inhibited COX-2 in cells. The mRNA expression
levels of TGF-β1 were significantly upregulated following treatment
with TGF-β1, but the protein expression levels were not
significantly different compared with those in the PMA-only group
(Fig. 4B and D). Following treatment
with NA, TGF-β1 was downregulated compared with the PMA-only group
(Fig. 4D), suggesting that TGF-β1
was affected when COX-2 was inhibited.
Flow cytometry was then used to analyze whether
M2-polatrized macrophages were affected by NA. These results
demonstrated that NA reduced the M2-polarization of macrophages by
OKC homogenate supernatant and TGF-β1 (from 51.6 to 25.2 and 62.4
to 34.8%, respectively) (Fig.
4E).
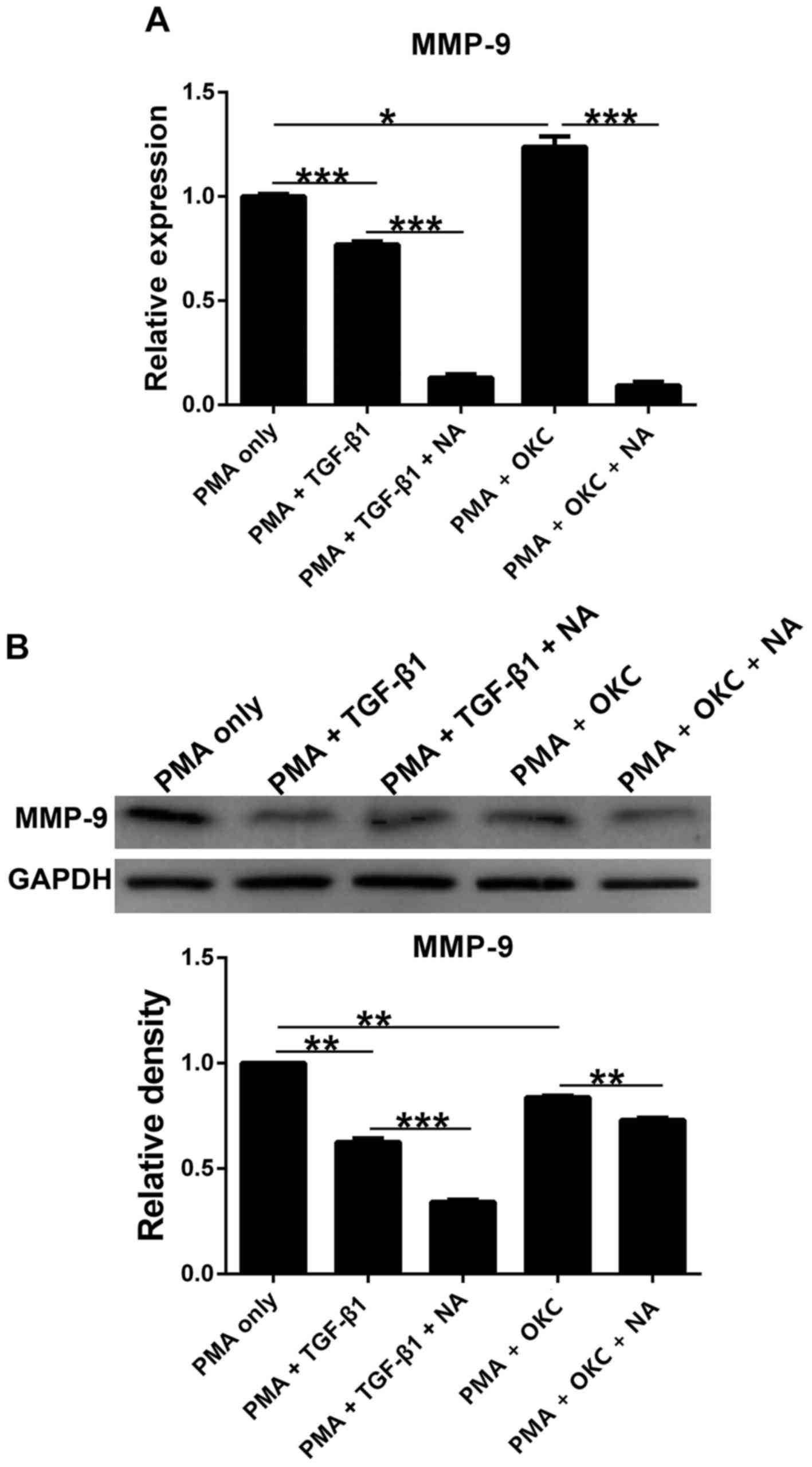
MMP-9 levels in cells treated with
TGF-β1 and OKC homogenate supernatant with or without NA
Regulation of the ECM by TGF-β1 is altered in a
number of chronic diseases (26) and
MMP serves a significant role in ECM degradation; therefore, MMP-9
expression levels were measured to determine whether the ECM was
affected as a result of different treatments. The results
demonstrated that compared with in the PMA-only group, the
expression levels of MMP-9 were significantly downregulated in all
groups except for the mRNA levels after OKC homogenate supernatant
treatment (Fig. 5). In addition,
MMP-9 expression levels were markedly downregulated following
treatment with NA. These results indicated that MMP-9 expression
may be altered following treatment with TGF-β1 and OKC, especially
when COX-2 is inhibited.
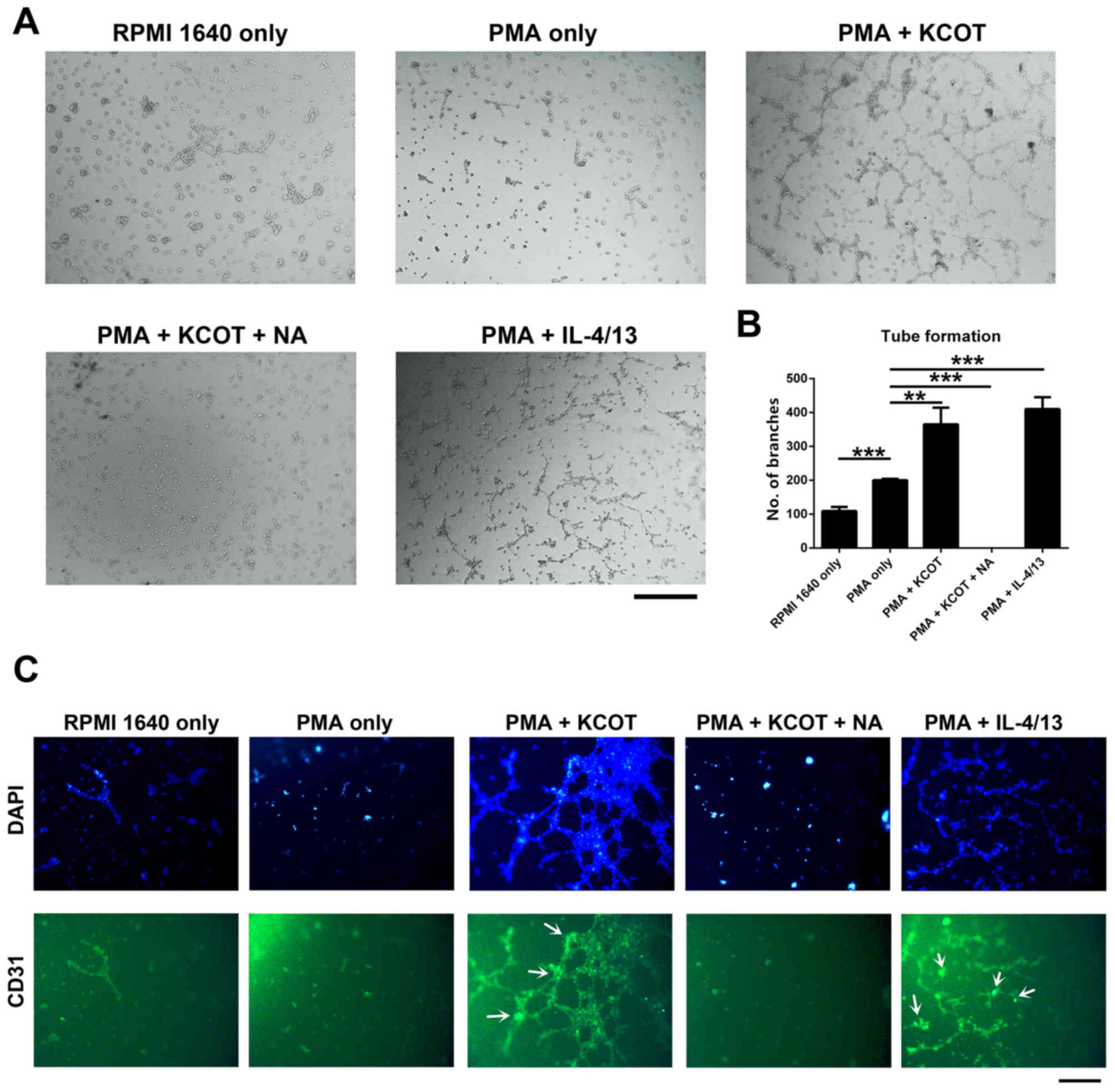
OKC promotes angiogenesis, as
determined by measuring microvessel density (MVD)
A previous study has indicated that angiogenesis is
observed in OKC tissue (20);
therefore, the ability of OKC to induce angiogenesis was examined.
The present study performed a tube formation assay using HUVECs
in vitro. The results demonstrated that M2-polarization of
macrophage-like cells induced by OKC enhanced angiogenesis compared
with in the PMA-only group (Fig. 6A and
B). After NA was added to the PMA + OKC group to observe the
effects of NA on angiogenesis caused by M2-polarized
macrophage-like cells, the effect was inhibited (Fig. 6A and B). In addition, analysis of
HUVEC viability, as measured by the MTT assay, demonstrated that
M2-polarized macrophage-like cells induced by OKC homogenate
supernatant promoted HUVEC viability, whereas this was inhibited by
NA treatment (Fig. S1). These data
indicated that inhibition of COX-2 prevents the angiogenesis caused
by OKC-induced M2-polarized macrophage-like cells.
MVD can be used as a useful marker for evaluation of
angiogenesis in tumors. In this assay, CD31 represents the MVD
marker (27). Immunofluorescence
staining was performed to measure MVD in capillary-like structures.
The results demonstrated that CD31 was highly expressed in the PMA
+ OKC and PMA + IL-4/13 groups, but not expressed in the PMA + OKC
+ NA group (Fig. 6C). These results
suggested that OKC may increase angiogenesis and enhance tumor cell
invasion. Additionally, the effects of OKC on tumor development may
be dependent on the regulation of COX-2.
Discussion
OKC is a common oral disease arising from the
odontogenic epithelium, which has characteristics similar to those
of malignant tumors such as aggressive growth and high recurrence
(28). Tumor development always
requires angiogenesis and the expression of numerous cytokines; OKC
also has this characteristic (29–31). The
aim of the present study was to elucidate the regulation of OKC,
its mechanism of action and disease progression.
In this study, THP-1 cells were used as a model of
macrophages (32–34). However, THP-1 cells are derived from
the peripheral blood of a patient with acute monocytic leukemia.
Therefore, the results of the present study will be further
verified using normal tissue-derived monocytes collected from
patients in future studies. The existence of M2-polarized
macrophages in OKC was first reported by Zhong et al
(20), who also reported that TGF-β1
was highly expressed in OKC homogenate supernatant; therefore, to
investigate the effects of TGF-β1 on OKC, RT-qPCR and western
blotting were conducted to confirm its upregulation in the present
study. At present, studies have reported that the rise of TGF-β1 is
often associated with tumor development (35,36),
thus the OKC cancerous characteristics may be related to TGF-β1.
Accordingly, the mechanism by which TGF-β1 increases and how to
inhibit this pathway will be explored in future studies. The
present study also explored the relationships between TGF-β1 and
M2-polarized macrophages. Flow cytometry measured the percentage of
M2-polarized macrophages induced by different concentrations of
TGF-β1. The results demonstrated that TGF-β1 induced
M2-polarization of macrophages in a concentration-dependent manner.
Since a number of studies have demonstrated that COX-2 can be
induced by TGF-β1 (19,37), the present study aimed to determine
whether the TGF-β1/COX-2 pathway also had a role in OKC. The
results of the present study indicated that COX-2 was upregulated
by TGF-β1 in a concentration-dependent manner and that a high
expression was observed in OKC-induced M2-polarization of
macrophages. Unfortunately, the specific molecular mechanism was
not determined in this experiment, and this will be studied further
in future experiments. Taken together, these results indicated that
the TGF-β1/COX-2 pathway may have a role in OKC.
The present study also inhibited COX-2 expression
through the addition of NA and observed that the number of
M2-polarized macrophages decreased when treated with NA. In
addition, the expression of MMP-9, a protease with an active role
in ECM remodeling, was downregulated in OKC-induced M2-polarized
macrophages. These results suggested that the TGF-β1/COX-2 pathway
not only affects macrophage polarization, but also the ECM of cells
in OKC progression.
The present study also performed a tube formation
assay with immunofluorescence staining. The results of the present
study indicated that OKC induced capillary-like structures and
markers of MVD were demonstrated in these structures; however, when
COX-2 was inhibited by NA, these structures were destroyed. These
results suggested that OKC enhanced angiogenesis and tumor
development, and that this effect that may be dependent on
COX-2.
In summary, the present study demonstrated that
TGF-β1/COX-2 may serve an important role in OKC-induced
M2-polarization of macrophages and angiogenesis, suggesting that
the TGF-β1/COX-2 pathway may regulate OKC progression.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by The Zhejiang Provincial
Medicine Science and Technology Plan (grant no. 2017205515) and The
National Science Foundation of China (grant no. 81602706).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
GC and SW designed the research study. JG and LW
performed the experiments. YD, QW and QBW analyzed the data. JX
performed the flow cytometry experiments. GC wrote the manuscript.
All authors have read and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wright JM and Vered M: Update from the 4th
edition of the World Health Organization classification of head and
neck tumours: Odontogenic and maxillofacial bone tumors. Head Neck
Pathol. 11:68–77. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thompson L: World Health Organization
classification of tumours: Pathology and genetics of head and neck
tumours. Ear Nose Throat J. 85:742006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharif FN, Oliver R, Sweet C and Sharif
MO: Interventions for the treatment of keratocystic odontogenic
tumours. Cochrane Database Syst Rev. 2015:CD0084642015.
|
|
4
|
Pan S and Li TJ: PTCH1 mutations in
odontogenic keratocysts: Are they related to epithelial cell
proliferation? Oral Oncol. 45:861–865. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amm HM and MacDougall M: Molecular
signaling in benign odontogenic neoplasia pathogenesis. Curr Oral
Health Rep. 3:82–92. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qian BZ and Pollard JW: Macrophage
diversity enhances tumor progression and metastasis. Cell.
141:39–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ruffell B, Affara NI and Coussens LM:
Differential macrophage programming in the tumor microenvironment.
Trends Immunol. 33:119–126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gordon S and Martinez FO: Alternative
activation of macrophages: Mechanism and functions. Immunity.
32:593–604. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chanmee T, Ontong P, Konno K and Itano N:
Tumor-associated macrophages as major players in the tumor
microenvironment. Cancers (Basel). 6:1670–1690. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Petruzzi MN, Cherubini K, Salum FG and de
Figueiredo MA: Role of tumour-associated macrophages in oral
squamous cells carcinoma progression: An update on current
knowledge. Diagn Pathol. 12:322017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fujimura T, Kambayashi Y, Fujisawa Y,
Hidaka T and Aiba S: Tumor-associated macrophages: Therapeutic
targets for skin cancer. Front Oncol. 8:32018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li ZL, Ye SB, OuYang LY, Zhang H, Chen YS,
He J, Chen QY, Qian CN, Zhang XS, Cui J, et al: COX-2 promotes
metastasis in nasopharyngeal carcinoma by mediating interactions
between cancer cells and myeloid-derived suppressor cells.
Oncoimmunology. 4:e10447122015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu B, Qu L and Yan S: Cyclooxygenase-2
promotes tumor growth and suppresses tumor immunity. Cancer Cell
Int. 15:1062015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmitz KJ, Lang H, Wohlschlaeger J, Reis
H, Sotiropoulos GC, Schmid KW and Baba HA: Elevated expression of
cyclooxygenase-2 is a negative prognostic factor for overall
survival in intrahepatic cholangiocarcinoma. Virchows Arch.
450:135–141. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Solanki R, Agrawal N, Ansari M, Jain S and
Jindal A: COX-2 expression in breast carcinoma with correlation to
clinicopathological parameters. Asian Pac J Cancer Prev.
19:1971–1975. 2018.PubMed/NCBI
|
|
16
|
Pang LY, Hurst EA and Argyle DJ:
Cyclooxygenase-2: A role in cancer stem cell survival and
repopulation of cancer cells during therapy. Stem Cells Int.
2016:20487312016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Clarkin CE, Garonna E, Pitsillides AA and
Wheeler-Jones CP: Heterotypic contact reveals a COX-2-mediated
suppression of osteoblast differentiation by endothelial cells: A
negative modulatory role for prostanoids in VEGF-mediated cell:
Cell communication? Exp Cell Res. 314:3152–3161. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Caporarello N, Lupo G, Olivieri M,
Cristaldi M, Cambria MT, Salmeri M and Anfuso CD: Classical VEGF,
Notch and Ang signalling in cancer angiogenesis, alternative
approaches and future directions (Review). Mol Med Rep.
16:4393–4402. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin PS, Cheng RH, Chang MC, Lee JJ, Chang
HH, Huang WL, Yeung SY, Chang YC and Jeng JH: TGF-β1 stimulates
cyclooxygenase-2 expression and PGE2 production of human
dental pulp cells: Role of ALK5/Smad2 and MEK/ERK signal
transduction pathways. J Formos Med Assoc. 116:748–754. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhong WQ, Chen G, Zhang W, Xiong XP, Zhao
Y, Liu B and Zhao YF: M2-polarized macrophages in keratocystic
odontogenic tumor: Relation to tumor angiogenesis. Sci Rep.
5:155862015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang YH, He M, Wang Y and Liao AH:
Modulators of the balance between M1 and M2 macrophages during
pregnancy. Front Immunol. 8:1202017.PubMed/NCBI
|
|
23
|
Yi Y, Cheng JC, Klausen C and Leung PCK:
TGF-β1 inhibits human trophoblast cell invasion by upregulating
cyclooxygenase-2. Placenta. 68:44–51. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cao C, Gao R, Zhang M, Amelio AL, Fallahi
M, Chen Z, Gu Y, Hu C, Welsh EA, Engel BE, et al: Role of
LKB1-CRTC1 on glycosylated COX-2 and response to COX-2 inhibition
in lung cancer. J Natl Cancer Inst. 107:3582014.PubMed/NCBI
|
|
25
|
Luo S, Huang G, Wang Z, Wan Z, Chen H,
Liao D, Chen C, Li H, Li B, Chen L, et al: Niflumic acid exhibits
anti-tumor activity in nasopharyngeal carcinoma cells through
affecting the expression of ERK1/2 and the activity of MMP2 and
MMP9. Int J Clin Exp Pathol. 8:9990–10001. 2015.PubMed/NCBI
|
|
26
|
Xi PP, Xu YY, Chen XL, Fan YP and Wu JH:
Role of the prostaglandin E2 receptor agonists in TGF-β1-induced
mesangial cell damage. Biosci Rep. 36:e003832016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miyata Y and Sakai H: Reconsideration of
the clinical and histopathological significance of angiogenesis in
prostate cancer: Usefulness and limitations of microvessel density
measurement. Int J Urol. 22:806–815. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Godhi SS and Kukreja P: Keratocystic
odontogenic tumor: A review. J Maxillofac Oral Surg. 8:127–131.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kouhsoltani M, Moradzadeh Khiavi M, Jamali
G and Farnia S: Immunohistochemical assessment of mast cells and
small blood vessels in dentigerous cyst, odontogenic keratocyst,
and periapical cyst. Adv Pharm Bull. 5 (Suppl 1):S637–S641. 2015.
View Article : Google Scholar
|
|
30
|
Fatemeh M, Sepideh A, Sara BS and Nazanin
M: P53 protein expression in dental follicle, dentigerous cyst,
odontogenic keratocyst, and inflammatory subtypes of cysts: An
immunohistochemical study. Oman Med J. 32:227–232. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sadri D, Farhadi S and Nourmohamadi P:
Angiogenesis in odontogenic keratocyst and dentigerous cyst:
Evaluation of JunB and VEGF expression. Dent Res J (Isfahan).
16:327–332. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Diaz-Jimenez D, Petrillo MG, Busada JT,
Hermoso MA and Cidlowski JA: Glucocorticoids mobilize macrophages
by transcriptionally up-regulating the exopeptidase DPP4. J Biol
Chem. 295:3213–3227. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bellmunt AM, Lopez-Puerto L, Lorente J and
Closa D: Involvement of extracellular vesicles in the
macrophage-tumor cell communication in head and neck squamous cell
carcinoma. PLoS One. 14:e02247102019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kletting S, Barthold S, Repnik U,
Griffiths G, Loretz B, Schneider-Daum N, de Souza Carvalho-Wodarz C
and Lehr CM: Co-culture of human alveolar epithelial (hAELVi) and
macrophage (THP-1) cell lines. ALTEX. 35:211–222. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang X, Zhang P, Shao M, Zang X, Zhang J,
Mao F, Qian H and Xu W: SALL4 activates TGF-β/SMAD signaling
pathway to induce EMT and promote gastric cancer metastasis. Cancer
Manag Res. 10:4459–4470. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Soni UK, Chadchan SB, Kumar V, Ubba V,
Khan MTA, Vinod BSV, Konwar R, Bora HK, Rath SK, Sharma S and Jha
RK: A high level of TGF-B1 promotes endometriosis development via
cell migration, adhesiveness, colonization, and invasiveness†. Biol
Reprod. 100:917–938. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Abdallah MS, Kennedy CRJ, Stephan JS,
Khalil PA, Mroueh M, Eid AA and Faour WH: Transforming growth
factor-β1 and phosphatases modulate COX-2 protein expression and
TAU phosphorylation in cultured immortalized podocytes. Inflamm
Res. 67:191–201. 2018. View Article : Google Scholar : PubMed/NCBI
|