Introduction
Esophageal cancer was the eighth most common cancer
worldwide, and the sixth most common cause of cancer-associated
death in 2012 (1). Adenocarcinoma of
the cervical esophagus is uncommon, and squamous cell carcinoma is
usually observed (2). Cervical
esophageal squamous cell carcinoma (CESCC) has been reported to
represent ~5% of esophageal cancer cases (3–5), and
tobacco and alcohol consumption are risk factors for CESCC, the
same as for thoracic esophageal cancer (6,7).
Surgical resection or chemoradiotherapy (CRT) are
widely accepted as initial treatments, but a standard therapy has
not yet been established for patients with CESCC (8–13). CRT
is often selected for patients with unresectable tumors or those
who are not candidates for surgery based on patient selection and
general condition (9,14). Some patients who undergo surgery for
CESCC require total pharyngo-laryngectomy, which is associated with
speech impairment and compromises a quality of life (12).
The cervical esophagus is defined as the upper part
of the esophagus between the cricopharyngeal muscle and the
thoracic inlet, and is ~18 cm from the incisor teeth (15). CESCC is surrounded by various
structures, such as the hypopharynx, larynx, trachea and thyroid
gland (16–19). The anatomical complexity of the
cervical esophagus makes surgery more dangerous (16–19). The
surgical procedure for CESCC is subtotal esophagectomy often with
pharyngo-laryngectomy, depending on tumor progression and the
superior extent of the tumor (20,21). It
is well known that laryngeal preservation is more difficult if a
tumor extends to the oral side (20,21).
Marmuse et al (22) reported
that a 2 cm surgical margin was needed for laryngeal preservation
for CESCC. However, few studies have investigated the association
between the upward extension of the tumor and laryngeal
preservation or prognosis in CESCC.
In previous studies, the 5-year survival rates of
CESCC were reported to be 18–35% (8,23,24).
These results are similar to those for thoracic esophageal cancer
(3–5). Yamada et al (25) reported that performance status and
tumor length >6 cm were prognostic risk factors for CESCC.
However, there are few reports investigating the risk factors for
CESCC. Therefore, the aim of the present study was to measure the
distance between the inferior border of the cricoid cartilage and
upper edge of the tumor using positron emission tomography and
computed tomography (PET-CT), and to evaluate the association
between this distance and clinicopathological factors.
Materials and methods
Patients
Between January 2000 and December 2016, 73 patients
with CESCC underwent treatments at the University Hospital of Kyoto
Prefectural University of Medicine. Of these, four cases were
excluded from the study because they were clinically diagnosed with
distant metastasis or had a history of esophagectomy. The median
age of patients was 66 years (range, 60–72 years), and 53 patients
were males and 16 were females. Preoperative age, sex, body mass
index (BMI) and American Society of Anesthesiologists' Physical
Status (ASA-PS) were recorded (26).
The present study was conducted in accordance with the principles
of the Declaration of Helsinki, and written informed consent was
obtained from all patients. The study was approved by the Research
Ethics Committee of the Kyoto Prefectural University of Medicine
(approval no. ERB-C-1414-1).
Surgery
A total of 48 out of 69 patients underwent surgery.
In 20 patients with invasion of the pharynx or trachea and upper
thoracic esophagus, pharyngo-laryngo-total esophagectomy with neck
and mediastinal lymph node dissection and reconstruction with a
gastric tube was performed. In seven patients with invasion of the
pharynx or trachea, pharyngo-laryngo-cervical esophagectomy with
neck and upper mediastinal lymph node dissection and reconstruction
with free jejunal transfer was performed. Subtotal esophagectomy
with neck and mediastinal lymph node dissection and reconstruction
with a gastric tube or ileocolonic reconstruction was performed in
20 cases in which it was possible to preserve the larynx and the
tumor extended to upper thoracic esophagus. One patient underwent
cervical esophagectomy with laryngeal preservation with neck and
upper mediastinal lymph node dissection and reconstruction with
free jejunal transfer.
The absence of cancer cells in the proximal margin
was confirmed pathologically, but if the patients wanted to
preserve the larynx, there were some cases where a
laryngeal-preserving procedure was later performed with residual
cancer and CRT. The definitions of degrees of resection are defined
in R0 (complete resection), R1 (incomplete resection, with
microscopic residual disease) and R2 (incomplete resection, with
gross residual disease). A positive surgical margin was classified
as R1/R2 (27).
Distance from the cricoid cartilage to
the upper edge of the tumor
Makino et al (28), reported a method to evaluate the
upward extension of a tumor. As the cricoid cartilage is at the
same height as the esophageal entrance, this method can measure the
distance from the esophagus entrance. According to this method, the
distance between the inferior border of the cricoid cartilage and
upper edge of the tumor was measured using a sagittal PET-CT. At
the University Hospital of Kyoto Prefectural University of
Medicine, values of standard fluorodeoxyglucose uptake (SUV) were
measured by placing volumetric regions of interest over PET/CT
images, and SUV ≥2.5 was considered to indicate a malignant lesion.
Positive and negative values indicate oral and anal directions,
respectively. The distance from the cricoid cartilage was measured
using PET-CT when patients were diagnosed with CESCC.
Neoadjuvant chemotherapy (NAC)
NAC was administered to patients with cStage II or
III, according to the JCOG9907 study (29), which was diagnosed based on the
Japanese Classification of Esophageal Cancer (27). Two cycles of standard 5-Fluorouracil
(FU) and cisplatin (one cycle was three weeks long, including 800
mg/body/day 5-FU on days 1–5 as a 24-h continuous infusions plus 80
mg/body/day cisplatin on day 1 as a 1-h drip infusion) were used
between 2007 and 2013. Since 2011, two cycles of DCF as NAC for
patients with cStageIII and good general condition (based on age,
ASA-PS and medical history) were administered. Some patients
received two cycles of 5-FU, cisplatin and docetaxel therapy as NAC
(one cycle was three weeks long, including 750 mg/body/day 5-FU on
days 1–5 as a 24-h continuous infusions, plus 70 mg/body/day
cisplatin and 70 mg/body/day docetaxel on day 1 as a 1-h drip
infusion) (30). These patients
underwent upper endoscopy and CT after NAC, and the resectability
of their tumors was re-evaluated.
Neoadjuvant chemoradiotherapy
(NACRT)
NACRT combined with two cycles of low-dose 5-FU and
cisplatin therapy (one cycle was one week long, including 250–500
mg body/day 5-FU on days 1–5 as a 24-h continuous infusion plus 10
mg body/day, cisplatin on days 1–5 as a 1-h drip infusion) with 40
Gy radiotherapy was administered to patients with an invasion depth
of clinical T3 or to the adjacent structures (T4) (27) between 2000 and 2007. If a tumor was
resectable based on upper-endoscopy and CT scans performed after
NACRT, resection surgery was performed.
Definitive chemoradiotherapy
(dCRT)
dCRT was administered to clinical T4 cases or
patients who refused curative surgery. Low-dose or standard 5-FU
and cisplatin therapy were used as combined chemotherapy (31). Salvage surgery after dCRT was
performed on recurrent cases after complete response (CR) or non-CR
that were resectable. Patients who underwent surgery as the initial
treatment were classified as the surgery group and patients who
underwent dCRT as the initial treatment were classified as the dCRT
group. Patients who underwent NACRT were included in the surgery
group, and salvage surgery cases were included in the dCRT
group.
Follow-up
Blood tests (using the tumor markers squamous cell
carcinoma and carcinoembryonic antigens), gastrointestinal
endoscopy and PET-CT scanning of the neck, chest and abdomen were
performed approximately every 6 months after the initiation of
treatment until death or loss to follow-up. Overall survival times
were calculated from the time of diagnosis until either death, loss
during follow-up or the end of the study. Follow-up was performed
in the clinic and the average length of follow-up was 3.03
years.
Statistical analysis
Data in our computerized database were examined in
the present retrospective study. Additional data were obtained by
reviewing medical records. All analyses were performed using JMP
software version 12 (SAS Institute Inc.). Comparisons between
categorical variables were performed between groups using a
χ2 or Fisher's exact test. Mann-Whitney U test was used
for comparisons between continuous variables. The diagnostic
accuracy was determined based on the area under the receiver
operating characteristic (ROC) curve. The optimal cut-off value for
laryngeal preservation was defined as −5 mm using Youden's index.
The patients were divided into two groups according to the presence
or absence of laryngeal preservation, and their clinicopathological
features were compared. In survival analysis, comparisons between
two groups were analyzed using the log-rank test. Multivariate
analysis was performed using Cox's proportional hazards model.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
Table I shows the
clinicopathological features of the two groups according to initial
treatment. There were more patients who were clinical T4 in the
dCRT group compared with in the surgery group. No significant
differences were observed in terms of age, sex, BMI, ASA-PS or
clinical N and stage between the two groups.
 | Table I.Characteristics of 69 patients with
cervical esophageal squamous cell carcinoma at initial
treatment. |
Table I.
Characteristics of 69 patients with
cervical esophageal squamous cell carcinoma at initial
treatment.
| Variable | Total, n | Surgery, n | dCRT, n | P-value |
|---|
| Age, years |
|
|
| 0.145 |
|
≥65 | 40 | 25 | 15 |
|
|
<65 | 29 | 13 | 16 |
|
| Sex |
|
|
| 0.574 |
|
Male | 53 | 28 | 25 |
|
|
Female | 16 | 10 | 6 |
|
| BMI |
|
|
| 0.458 |
|
≥20 | 30 | 15 | 15 |
|
|
<20 | 39 | 23 | 16 |
|
| ASA-PS |
|
|
| 0.757 |
|
1/2 | 57 | 32 | 25 |
|
|
3/4 | 12 | 6 | 6 |
|
| cT |
|
|
| 0.168 |
| T1 | 9 | 6 | 3 |
|
|
T2/T3 | 35 | 22 | 13 |
|
| T4 | 25 | 10 | 15 |
|
| Tumor length,
mm |
|
|
| 0.726 |
|
≥40 | 35 | 20 | 15 |
|
|
<40 | 34 | 18 | 16 |
|
| cN |
|
|
| 0.589 |
| N0 | 20 | 10 | 10 |
|
|
N1-N3 | 49 | 28 | 21 |
|
| cStage |
|
|
| 0.468 |
|
0/I | 7 | 4 | 3 |
|
|
II/III | 43 | 26 | 17 |
|
| IV | 19 | 8 | 11 |
|
Distance from the cricoid
cartilage
The association between laryngeal preservation and
tumor extension was investigated. The distance between the inferior
border of the cricoid cartilage and upper edge of the tumor was
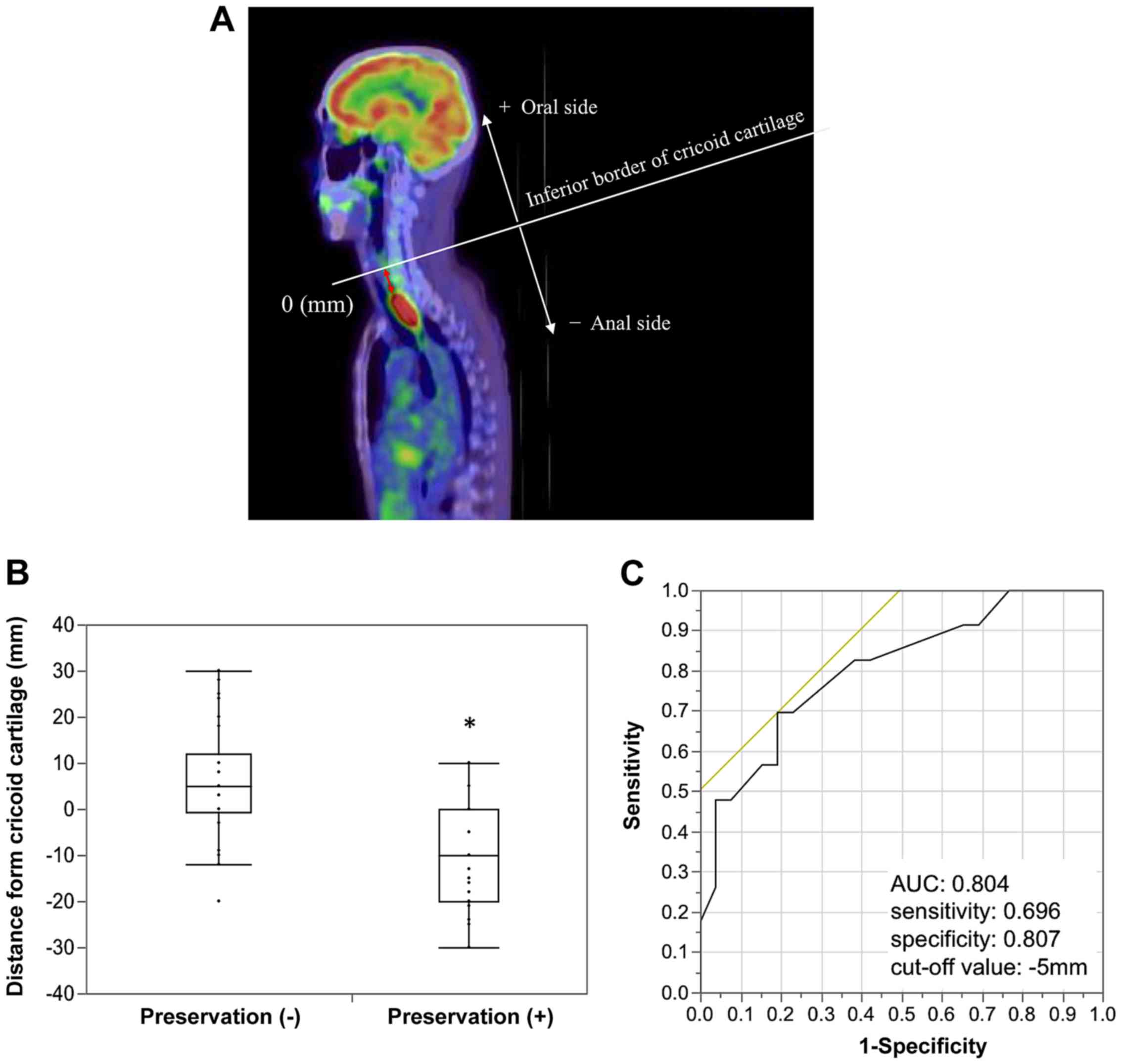
measured, as evaluated using sagittal PET-CT (Fig. 1A). No significant difference was
observed in distance from the cricoid cartilage in the surgery
group compared with the dCRT group as initial treatment (mean
distance, −0.32±14.14 vs. −6.00±14.890 mm; P=0.181; data not
shown).
Esophagectomy was performed on 48 patients, and of
these, laryngeal-preserving procedures were performed on 21
patients. The patients were divided into two groups according to
the presence or absence of laryngeal preservation and their
clinicopathological features were compared. Table II shows that there was a significant
association between BMI and laryngeal preservation (P=0.008), while
no significant differences were observed in terms of age, sex,
ASA-PS, tumor length, neoadjuvant therapy (NAT), NAT effect or
clinical T, N and stage between the two groups. Clinicopathological
factors between patients with BMI ≥20 and <20 were compared.
Table SI shows that BMI was
significantly associated with tumor length, the distance from the
cricoid cartilage and laryngeal preservation (P=0.032, P=0.018 and
P=0.008, respectively), while BMI was not associated with clinical
T or NAT.
 | Table II.Comparison between the two groups
according to laryngeal preservation in surgery cases (n=48). |
Table II.
Comparison between the two groups
according to laryngeal preservation in surgery cases (n=48).
|
|
| Laryngeal
preservation, n |
|
|---|
|
|
|
|
|
|---|
| Variable | Total, n | − | + | P-value |
|---|
| Age, years |
|
|
| >0.999 |
|
≥65 | 28 | 16 | 12 |
|
|
<65 | 20 | 11 | 9 |
|
| Sex |
|
|
| 0.185 |
|
Male | 36 | 18 | 18 |
|
|
Female | 12 | 9 | 3 |
|
| BMI |
|
|
| 0.008a |
|
≥20 | 21 | 7 | 14 |
|
|
<20 | 27 | 20 | 7 |
|
| ASA-PS |
|
|
| >0.999 |
|
1/2 | 41 | 23 | 18 |
|
|
3/4 | 7 | 4 | 3 |
|
| cT |
|
|
| 0.090 |
| T1 | 6 | 3 | 3 |
|
|
T2/T3 | 27 | 12 | 15 |
|
| T4 | 15 | 12 | 3 |
|
| Tumor length,
mm |
|
|
| 0.776 |
|
≥40 | 22 | 13 | 9 |
|
|
<40 | 26 | 14 | 12 |
|
| cN |
|
|
| 0.214 |
| N0 | 14 | 10 | 4 |
|
|
N1-N3 | 34 | 17 | 17 |
|
| cStage |
|
|
| 0.512 |
| I | 4 | 2 | 2 |
|
|
II/III | 33 | 17 | 16 |
|
| IV | 11 | 8 | 3 |
|
| NAT |
|
|
| 0.338 |
|
Present | 35 | 18 | 17 |
|
|
Absent | 13 | 9 | 4 |
|
| NAT effect |
|
|
| 0.602 |
| CR | 1 | 1 | 0 |
|
| PR | 12 | 5 | 7 |
|
| SD | 22 | 12 | 10 |
|
The distances from the cricoid cartilage with and
without laryngeal preservation were compared. Fig. 1B shows that tumors extended more to
the upper side in the non-preservation group compared with the
preservation group (mean distance, 5.44±12.54 vs. −10.81±11.37 mm;
P<0.05). Using the ROC curve, the cut-off value for laryngeal
preservation was calculated as −5 mm (AUC=0.804, sensitivity=0.696,
specificity=0.807, Youden's index; Fig.
1C). According to the cut-off value, the patients were divided
into two groups: The short group (distance from the cricoid
cartilage ≥-5 mm) and long group (distance from the cricoid
cartilage <-5 mm). The clinicopathological features of the two
groups are presented in Table III,
which shows that the distance from the cricoid cartilage was
significantly associated with laryngeal preservation, BMI and tumor
length (P=0.002, P=0.017 and P=0.038, respectively).
 | Table III.Comparison between the two groups
according to distance between the cricoid cartilage and upper tumor
edge (n=69). |
Table III.
Comparison between the two groups
according to distance between the cricoid cartilage and upper tumor
edge (n=69).
|
|
| Distance group,
n |
|
|---|
|
|
|
|
|
|---|
| Variable | Total, n | Short | Long | P-value |
|---|
| Age, years |
|
|
| 0.908 |
|
≥65 | 40 | 24 | 16 |
|
|
<65 | 29 | 17 | 12 |
|
| Sex |
|
|
| 0.562 |
|
Male | 53 | 30 | 23 |
|
|
Female | 16 | 11 | 5 |
|
| BMI |
|
|
| 0.017a |
|
≥20 | 30 | 13 | 17 |
|
|
<20 | 39 | 28 | 11 |
|
| ASA-PS |
|
|
| >0.999 |
|
1/2 | 57 | 34 | 23 |
|
|
3/4 | 12 | 7 | 5 |
|
| cT |
|
|
| 0.949 |
| 1 | 9 | 5 | 4 |
|
|
2/3 | 35 | 17 | 18 |
|
| 4 | 25 | 19 | 6 |
|
| Tumor length,
mm |
|
|
| 0.038a |
|
≥40 | 35 | 25 | 10 |
|
|
<40 | 34 | 16 | 18 |
|
| cN |
|
|
| 0.111 |
| 0 | 20 | 15 | 5 |
|
|
1-3 | 49 | 26 | 23 |
|
| cStage |
|
|
| 0.282 |
|
0/I | 7 | 3 | 4 |
|
|
II/III | 43 | 24 | 19 |
|
| IV | 19 | 14 | 5 |
|
| Laryngeal
preservation |
|
|
| 0.002a |
|
Present | 42 | 19 | 23 |
|
|
Absent | 27 | 22 | 5 |
|
The patterns of recurrence between the absence and
presence of laryngeal preservation (Table SII), and between the short and long
groups were compared (Table SIII).
No significant differences were observed in total recurrence
between the groups, but there were significantly more distant
metastases and less local recurrence in the non-preservation group
than in the preservation group (P=0.031 and 0.029, respectively;
Table SII).
Survival analysis
The 3-year overall survival rates were compared with
each clinicopathological factor. In univariate analysis, 3-year
overall survival was significantly less favorable in short group
(45.4 vs. 79.6%; P=0.009) and clinical T4 (29.2 vs. 75.7%,
P<0.0001; Table IV). In
multivariate analysis, short distance and clinical T4 were
independent prognostic risk factors for patients with CESCC [hazard
ratio (HR)=2.65; 95% CI, 1.04–8.09; P=0.039 and HR=4.22; 95% CI,
1.84–10.27; P=0.001, respectively; Table
V). A total of 48 patients who underwent surgery were also
analyzed (Table SIV). As a result,
short distance and clinical T4 were independent prognostic risk
factors in multivariate analysis (P=0.013 and P=0.011,
respectively).
 | Table IV.Three-year overall survival rates of
patients with cervical esophageal squamous cell carcinoma. |
Table IV.
Three-year overall survival rates of
patients with cervical esophageal squamous cell carcinoma.
| Variable | Total (n=69) | 3-year OS rate,
% | Univariate analysis
P-value |
|---|
| Age, years |
|
| 0.988 |
|
≥65 | 40 | 60.6 |
|
|
<65 | 29 | 58.7 |
|
| Sex |
|
| 0.539 |
|
Male | 53 | 55.7 |
|
|
Female | 16 | 74.5 |
|
| BMI |
|
| 0.747 |
|
≥20 | 30 | 60.6 |
|
|
<20 | 39 | 59.3 |
|
| ASA-PS |
|
| 0.237 |
|
1/2 | 57 | 62.9 |
|
|
3/4 | 12 | 45.5 |
|
| cT |
|
|
<0.0001a |
|
1-3 | 44 | 75.7 |
|
| 4 | 25 | 29.2 |
|
| Tumor length,
mm |
|
| 0.152 |
|
≥40 | 35 | 54.7 |
|
|
<40 | 34 | 64.0 |
|
| cN |
|
| 0.932 |
| 0 | 20 | 63.3 |
|
|
1-3 | 49 | 58.2 |
|
| Distance group |
|
| 0.009b |
|
Short | 41 | 45.4 |
|
|
Long | 28 | 79.6 |
|
| Initial
treatment |
|
| 0.360 |
|
dCRT | 31 | 54.0 |
|
|
Operation | 38 | 63.9 |
|
 | Table V.Independent prognostic factors for
cervical esophageal squamous cell carcinoma. |
Table V.
Independent prognostic factors for
cervical esophageal squamous cell carcinoma.
|
|
|
| Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Variable | Total (n=69) | 3-year OS rate,
% | HR | 95% CI | P-value |
|---|
| cT |
|
| 4.22 | 1.84–10.27 | 0.001a |
|
1-3 | 44 | 75.7 |
|
|
|
| 4 | 25 | 29.2 |
|
|
|
| Distance group |
|
| 2.65 | 1.04–8.09 | 0.039b |
|
Short | 41 | 45.4 |
|
|
|
|
Long | 28 | 79.6 |
|
|
|
Discussion
The cervical esophagus is surrounded by various
structures, such as the hypopharynx, larynx, trachea and thyroid
gland. Patients with CESCC frequently need to undergo
pharyngo-laryngo-esophagectomy and quality of life can be greatly
impaired (8). The present study
investigated which patients with CESCC needed
pharyngo-laryngo-esophagectomy and evaluated the distance between
the larynx and tumor. As a result, distance from the cricoid
cartilage ≥-5 mm was found to be an independent prognostic risk
factor. To the best of our knowledge, the present study is the
first to show that distance from the cricoid cartilage is
associated with prognosis. Doyle et al (26) reported that patients with CESCC who
underwent laryngeal-preserving surgery had an improved prognosis.
In the present study, the 3-year overall survival rate was improved
in the laryngeal preservation group compared with the
non-preservation group in 48 patients with CESCC who underwent
surgery (73.0 vs. 51.8%, P=0.120; Fig.
S1). Patients who underwent laryngeal preservation surgery
tended to have an improved prognosis, but this was associated with
tumor size, depth, location and length. Of these factors, it was
hypothesized that tumor location was associated with prognosis, as
distance from the cricoid cartilage ≥-5 mm was an independent
prognostic risk factor in multivariate analysis (Table SIV).
Table SII showed
that there were more patients with distant metastasis in the
non-preservation group than in the preservation group, and in the
short group than in the long group. Liu et al (32) reported that the incidence of distant
metastasis was 7.3% in hypopharynx cancer, which is relatively high
among head and neck cancers. In addition, some studies have
reported that positive N-stage is a predictor of distant metastasis
in head and neck cancers (33,34). The
reason for these results may be that distant metastasis tends to
occur from the lymphatic flow around the pharynx. The
aforementioned results are consistent with the present data, which
revealed that non-preserving larynx cases had numerous distant
metastases.
It was hypothesized that the worse prognosis for
tumors that extended to the oral side was due to a positive
surgical margin, and 48 patients who underwent surgery were
analyzed (Table SIII). As a result,
pathological R0 or R1/R2 and laryngeal preservation had no
significant association with prognosis, while short group was an
independent prognostic risk factor. These results suggested that
the worse prognosis in short group was not due to a positive
surgical margin.
Table II shows that
there was a significant association between BMI and laryngeal
preservation in surgical cases. Additionally, Table SI revealed that BMI was
significantly associated with the distance from the cricoid
cartilage and laryngeal preservation, while BMI was not associated
with clinical T or NAT. Therefore, it was considered that patients
whose tumor had spread to the oral side had a lower BMI due to poor
oral intake; however, BMI was not associated with prognosis.
Previous studies investigating esophageal cancer
have been based on thoracic esophageal cancer data, and few reports
have compared prognosis following surgery and dCRT in patients with
CESCC. Takebayashi et al (9)
demonstrated that surgery as the initial treatment for CESCC tended
to be improved compared with dCRT (5-year overall survival rate,
60.6 vs. 51.4%). On the other hand, Valmasoni et al
(8) reported that surgery tended to
be worse compared with dCRT (5-year overall survival rate, 12.6 vs.
26.7%). Therefore, the strategy for CESCC treatment is
controversial, and it is usually treated based on the strategy for
thoracic esophageal cancer. In the present study, surgery as the
initial treatment, which included NAC, NACRT and surgery alone,
tended to result in an improved prognosis compared with dCRT
(3-year overall survival rate, 63.9 vs. 54.0%, P=0.360; Fig. S2). In addition, salvage surgery when
dCRT was non-CR or recurred after CR had an improved prognosis than
without salvage surgery (Fig. S3).
These results suggested that CESCC should be treated in the same
way as thoracic esophageal cancer.
The present study has some limitations. There may be
some bias because it was a retrospective study and the sample size
was not large enough to identify potential differences between the
two groups. Another limitation was that patients received different
treatments, for example surgery as the initial treatment group
included patients who underwent NAC, NACRT and surgery alone. The
results of the present study need to be validated in prospective
studies with larger sample sizes, and further analysis of tumor
status, such as tumor size, tracheal invasion and effects of NAC or
CRT are needed.
In summary, the distance between the inferior border
of the cricoid cartilage and upper tumor edge ≥-5 mm was an
independent prognostic factor for CESCC. This may be due to
lymphatic flow around the cervical esophagus.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
KK, AS and EO designed the present study. AS, HF,
HK, MK, KS, TA, TKo, RM, YM, YK, HI, TKu, MN, KO and EO performed
the surgeries. KK, AS and HF analyzed the data. KK and AS wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and national research committee and with the 1964
Helsinki declaration and its later amendments or comparable ethical
standards. Informed consent was provided by all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Davies L and Welch HG: Epidemiology of
head and neck cancer in the United States. Otolaryngol Head Neck
Surg. 135:451–457. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tachimori Y, Ozawa S, Numasaki H,
Fujishiro M, Matsubara H, Oyama T, Shinoda M, Toh Y, Udagawa H and
Uno T; Registration Committee for Esophageal Cancer of the Japan
Esophageal Society, : Comprehensive registry of esophageal cancer
in Japan, 2009. Esophagus. 13:110–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tachimori Y, Ozawa S, Numasaki H, Ishihara
R, Matsubara H, Muro K, Oyama T, Toh Y, Udagawa H and Uno T;
Registration Committee for Esophageal Cancer of the Japan
Esophageal Society, : Comprehensive registry of esophageal cancer
in Japan, 2010. Esophagus. 14:189–214. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tachimori Y, Ozawa S, Numasaki H, Ishihara
R, Matsubara H, Muro K, Oyama T, Toh Y, Udagawa H and Uno T;
Registration Committee for Esophageal Cancer of the Japan
Esophageal Society, : Comprehensive registry of esophageal cancer
in Japan, 2011. Esophagus. 15:127–152. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Popescu B, Popescu CR, Grigore R, Mogoanta
CA, Ionita E, Moculescu C and Bertesteanu SV: Morphology and
morphopathology of hypopharyngo-esophageal cancer. Rom J Morphol
Embryol. 53:243–248. 2012.PubMed/NCBI
|
|
7
|
Popescu CR, Bertesteanu SV, Mirea D,
Grigore R, lonescu D and Popescu B: The epidemiology of hypopharynx
and cervical esophagus cancer. J Med Life. 3:396–401.
2010.PubMed/NCBI
|
|
8
|
Valmasoni M, Pierobon ES, Zanchettin G,
Briscolini D, Moletta L, Ruol A, Salvador R and Merigliano S:
Cervical esophageal cancer treatment strategies: A cohort study
appraising the debated role of surgery. Ann Surg Oncol.
25:2747–2755. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takebayashi K, Tsubosa Y, Matsuda S,
Kawamorita K, Niihara M, Tsushima T, Yokota T, Sato H, Onozawa Y,
Ogawa H, et al: Comparison of curative surgery and definitive
chemoradiotherapy as initial treatment for patients with cervical
esophageal cancer. Dis Esophagus. 30:1–5. 2017.
|
|
10
|
Hoeben A, Polak J, Van De Voorde L,
Hoebers F, Grabsch HI and de Vos-Geelen J: Cervical esophageal
cancer: A gap in cancer knowledge. Ann Oncol. 27:1664–1674. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tong DK, Law S, Kwong DL, Wei WI, Ng RW
and Wong KH: Current management of cervical esophageal cancer.
World J Surg. 35:600–607. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chou SH, Li HP, Lee JY, Huang MF, Lee CH
and Lee KW: Radical resection or chemoradiotherapy for cervical
esophageal cancer? World J Surg. 34:1832–1839. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Adelstein DJ, Rice TW, Tefft M, Koka A,
Van Kirk MA, Kirby TJ and Taylor ME: Aggressive concurrent
chemoradiotherapy and surgical resection for proximal esophageal
squamous cell carcinoma. Cancer. 74:1680–1685. 1994.PubMed/NCBI
|
|
14
|
Gkika E, Gauler T, Eberhardt W, Stahl M,
Stuschke M and Pöttgen C: Long-term results of definitive
radiochemotherapy in locally advanced cancers of the cervical
esophagus. Dis Esophagus. 27:678–684. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hermans R: Imaging of hypopharyngeal and
cervical oesophageal cancer. Cancer Imaging. 4:7–9. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ott K, Lordick F, Molls M, Bartels H,
Biemer E and Siewert JR: Limited resection and free jejunal graft
interposition for squamous cell carcinoma of the cervical
oesophagus. Br J Surg. 96:258–266. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ferahkose Z, Bedirli A, Kerem M, Azili C,
Sozuer EM and Akin M: Comparison of free jejunal graft with gastric
pull-up reconstruction after resection of hypopharyngeal and
cervical esophageal carcinoma. Dis Esophagus. 21:340–345. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Daiko H, Hayashi R, Saikawa M, Sakuraba M,
Yamazaki M, Miyazaki M, Ugumori T, Asai M, Oyama W and Ebihara S:
Surgical management of carcinoma of the cervical esophagus. J Surg
Oncol. 96:166–172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kelley DJ, Wolf R, Shaha AR, Spiro RH,
Bains MS, Kraus DH and Shah JP: Impact of clinicopathologic
parameters on patient survival in carcinoma of the cervical
esophagus. Am J Surg. 170:427–431. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Haguenauer JP and Pignat JC: Total
pharyngo-laryngo-esophagectomy and reconstruction by gastric or
colic pull up. Auris Nasus Larynx. 12 (Suppl 2):S41–S43. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Condon HA: Anaesthesia for
pharyngo-laryngo-oesophagectomy with pharyngo-gastrostomy. Br J
Anaesth. 43:1061–1065. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marmuse JP, Koka VN, Guedon C and Benhamou
G: Surgical treatment of carcinoma of the proximal esophagus. Am J
Surg. 169:386–390. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao L, Zhou Y, Mu Y, Chai G, Xiao F, Tan
L, Lin SH and Shi M: Patterns of failure and clinical outcomes of
definitive radiotherapy for cervical esophageal cancer. Oncotarget.
8:21852–21860. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grass GD, Cooper SL, Armeson K,
Garrett-Mayer E and Sharma A: Cervical esophageal cancer: A
population-based study. Head Neck. 37:808–814. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamada K, Murakami M, Okamoto Y, Okuno Y,
Nakajima T, Kusumi F, Takakuwa H and Matsusue S: Treatment results
of radiotherapy for carcinoma of the cervical esophagus. Acta
Oncol. 45:1120–1125. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Doyle DJ, Goyal A, Bansal P and Garmon EH:
American Society of Anesthesiologists Classification (ASA Class).
StatPearls Treasure Island (FL): StatPearls Publishing: 2020
|
|
27
|
Japan Esophageal Society: Japanese
classification of esophageal cancer, 11th edition: Part II and III.
Esophagus. 14:37–65. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Makino T, Yamasaki M, Miyazaki Y,
Takahashi T, Kurokawa Y, Nakajima K, Takiguchi S, Mori M and Doki
Y: Short- and long-term outcomes of larynx-preserving surgery for
cervical esophageal cancer: Analysis of 100 consecutive cases. Ann
Surg Oncol. (Suppl 5):23:S858–S865. 2016. View Article : Google Scholar
|
|
29
|
Ando N, Kato H, Igaki H, Shinoda M, Ozawa
S, Shimizu H, Nakamura T, Yabusaki H, Aoyama N, Kurita A, et al: A
randomized trial comparing postoperative adjuvant chemotherapy with
cisplatin and 5-fluorouracil versus preoperative chemotherapy for
localized advanced squamous cell carcinoma of the thoracic
esophagus (JCOG9907). Ann Surg Oncol. 19:68–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hara H, Tahara M, Daiko H, Kato K, Igaki
H, Kadowaki S, Tanaka Y, Hamamoto Y, Matsushita H, Nagase M and
Hosoya Y: Phase II feasibility study of preoperative chemotherapy
with docetaxel, cisplatin, and fluorouracil for esophageal squamous
cell carcinoma. Cancer Sci. 104:1455–1460. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sai H, Mitsumori M, Yamauchi C, Araki N,
Okumura S, Nagata Y, Nishimura Y and Hiraoka M: Concurrent
chemoradiotherapy for esophageal cancer: Comparison between
intermittent standard-dose cisplatin with 5-fluorouracil and daily
low-dose cisplatin with continuous infusion of 5-fluorouracil. Int
J Clin Oncol. 9:149–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu JC, Bhayani M, Kuchta K, Galloway T
and Fundakowski C: Patterns of distant metastasis in head and neck
cancer at presentation: Implications for initial evaluation. Oral
Oncol. 88:131–136. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuperman DI, Auethavekiat V, Adkins DR,
Nussenbaum B, Collins S, Boonchalermvichian C, Trinkaus K, Chen L
and Morgensztern D: Squamous cell cancer of the head and neck with
distant metastasis at presentation. Head Neck. 33:714–718. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Senft A, Hoekstra OS, Witte BI, Leemans CR
and de Bree R: Screening for distant metastases in head and neck
cancer patients using FDG-PET and chest CT: Validation of an
algorithm. Eur Arch Otorhinolaryngol. 273:2643–2650. 2016.
View Article : Google Scholar : PubMed/NCBI
|