Introduction
Lung cancer is the leading cause of cancer-related
death in the world, the most common of which is non-small cell lung
cancer (NSCLC) (1). At present,
smoking is considered to be the most important high risk factor for
lung cancer (2). Multi-chain
aromatic hydrocarbons and nitrosamines can cause DNA damage in
bronchial epithelial cells through a variety of mechanisms, which
can lead to cell transformation and eventually carcinogenesis.
According to the annual statistics of GLOBOCAN2018, lung cancer is
the most commonly diagnosed cancer in both sexes, with a total of
11.6% of the cases. Lung cancer accounts for 18.4% of the cancer
deaths, which is also the main cause of cancer death in men
(3). At present, the early diagnosis
of NSCLC is mainly based on computerized tomography scanning,
magnetic resonance imaging, positron emission tomography, sputum
cytology examination or histology of bronchoscopy. These techniques
are usually not practical, and are expensive, for most early NSCLC
detection (4). According to Hu et
al (5) and Chen et al
(6), serum miRNA, as a potential
biomarker for the diagnosis and prediction of prognosis of lung
cancer, and may be useful for the detection of NSCLC and provides
high sensitivity and specificity (7).
miRNA (microRNA) is a small class of non-coding RNA,
which can be used as endogenous RNA interference to regulate the
expression of target genes and is involved in the regulation of
various physiological and pathological functions (8). Bioinformatics data show that a single
miRNA can be bound to hundreds of target mRNAs to play an important
role in various biological processes (9). There is increasing evidence of the
association between miRNA and tumorigenesis (10,11).
Phospholipase and tensin homologue (PTEN) are lipid phosphatase,
which is one of the tumor inhibitor genes that are often mutated or
deleted during cancer progression. Some studies have shown that
inactivated PTEN can convert cancer genes into anti-oncogene in the
process of disease progression (12). P27 is essentially an unstructured
multifunctional protein, which can affect various biological
processes from cell cycle regulation to cell migration and
transcriptional regulation, and was initially found to be the key
regulator of cell proliferation (13).
Therefore, the expression of miRNA-21, PTEN and p27
in NSCLC patients was studied, in order to provide the basis for
the diagnosis and treatment of NSCLC.
Patients and methods
General materials
Cancer tissue and adjacent tissue specimens from 230
patients with NSCLC were collected from the Thoracic Surgery
Department of the Hubei Cancer Hospital (Wuhan, China) from March
2010 to February 2016. The samples were cryopreserved in liquid
nitrogen within 5 min of resection. The cancer tissue was treated
as the study group. There were 92 males and 23 females, aged 45–68
years, with a mean age of 51.05±10.47. Adjacent tissue was included
in the control group. The number and mean age of the study group
were consistent with those of the study group. The collection of
clinical specimens was approved by the Medical Ethics Committee of
the Hubei Cancer Hospital. All the patients signed informed consent
forms.
Inclusion and exclusion criteria
Inclusion criteria. Patients diagnosed and treated
in Hubei Cancer Hospital; cancer tissue and adjacent tissue were
obtained by resection of lung cancer in thoracic surgery of the
hospital; patients aged 35–70 years and with an education of
primary school and above; patients cooperating with the research;
patients with no other serious organ diseases; patients or lineal
consanguinity signed the informed consent forms.
Exclusion criteria: Patients who died during
treatment; patients with injury in important organs; patients
complicated with other tumors, cardiovascular and cerebrovascular
diseases; patients with physical disability; pregnant patients;
patients with other autoimmune diseases or chronic diseases;
patients transferred to other hospitals; patients with surgical
contraindications, mental diseases and language dysfunction;
patients with diseases that affect the results of this study.
Main reagent
TRIzol reagent and miRNA reverse transcriptase kit
were purchased from Invitrogen Company of the United States. SYBR
Green Master Mix was purchased from American Applied Biological
Systems Co., Ltd. ABI StepOne Plus fluorescence quantitative PCR
instrument was purchased from American Applied Biological Systems
Co., Ltd. NanoDrop 2000 spectrophotometer, KH19A desktop high speed
and high performance centrifuge were purchased from KAIDA Co., Ltd.
Cryogenic refrigerator (−80°C) was purchased from Thermo Fisher
Scientific, Inc. The sequence of miRNA-21 primers was designed and
synthesized by Shanghai Bioengineering Co., Ltd. (Table I).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Upstream
sequence | Downstream
sequence |
|---|
| U6 |
5′-CTCGCTTCGGCAGCACA-3′ |
5′-AACGCTTCACGAATTTGCGT-3′ |
| miRNA-21 |
5′-AACGCTTCACGAATTTGCGT-3′ |
5′-TGGTGTCGTGGAGTCG-3′ |
qRT-PC detection
Total RNA was extracted in strict accordance with
the instructions using TRIzol reagent in PCa cancer tissue and PCa
adjacent tissue. The concentration and purity of extracted RNA were
detected by ultraviolet spectrophotometer. The OD value of total
RNA solution: A240/A300 was in the range of 1.8–2 1. If the
standard was not met, it would be extracted again. The integrity of
RNA was detected by 1% denatured agarose gel electrophoresis.
Configuration of reaction system and reverse transcription
synthesis of cDNA for total RNA were carried out by miRNA reverse
transcription kit (−20°C storage for use). ABI StepOne Plus
fluorescence quantitative PCR instrument (Applied Biosystems;
Thermo Fisher Scientific, Inc.) was used for determination. The
reaction system was arranged according to the specification, and
the 12.33 µl of the reaction system was filled with DEPC water to
20 µl. Reaction conditions: 95°C for 5 min; 95°C for 45 sec; 60°C
for 60 sec; 72°C for 45 sec; 45 sec for a total of 45 cycles. U6
was used as the internal parameter of the reaction. The experiment
was repeated 3 times. 2−∆∆ct method was used to analyze
the results.
Observation indicators and evaluation
criteria
miRNA-21, PTEN, p27 expression levels were compared
between the two groups; diagnostic value of miRNA-21, PTEN, p27 in
NSCLC; correlation among miRNA-21, PTEN, p27 and clinical
pathology; the prognosis of high and low expression levels of
miRNA-21, PTEN and p27 in the two groups was compared for 3-year
survival; the prognostic value of miRNA-21, PTEN and p27 in the
prognosis of death.
Follow-up
The patients were followed-up in March, June,
September and December every year for 3 years, and the survival of
the patients was recorded by telephone and outpatient medical
records.
Statistical analysis
Data were processed and analyzed using SPSS 24.0
software system (Beijing Strong-Vinda Information Technology Co.,
Ltd.). All graphics were plotted by GraphPad 8 (Shenzhen Tianruiqi
Software Technology Co., Ltd.) and the results were checked twice.
The counting data were tested by χ2. t-test was used for
detection of measurement data, which was expressed as mean ±
standard deviation (mean ± SD). ROC curve was used for diagnostic
value analysis to evaluate diagnostic effectiveness and to
calculate sensitivity and specificity. The survival rate was
calculated using the Kaplan-Meier method, and the survival rate was
compared using the log-rank test. A value of P<0.05 was
considered as indicating a statistically significant
difference.
Results
Comparison of miRNA-21, PTEN and p27
expression levels between the two groups
The expression levels of miRNA-21, PTEN and p27 of
cancer tissue in the study group were 1.35±0.46, 1.48±0.16 and
2.41±0.34, respectively. The expression levels of miRNA-21, PTEN
and p27 of adjacent tissue in the control group were 2.87±1.03,
3.10±0.09 and 4.29±1.01, respectively. The expression was low in
cancer tissue of patients with NSCLC, and there were significant
differences between the two groups (P<0.050) (Table II).
 | Table II.Comparison of miRNA-21, PTEN and p27
expression levels between the two groups. |
Table II.
Comparison of miRNA-21, PTEN and p27
expression levels between the two groups.
| Group | Case | miRNA-21 | PTEN | p27 |
|---|
| Study group | 115 | 1.35±0.46 | 1.48±0.16 | 2.41±0.34 |
| Control group | 115 | 2.87±1.03 | 3.10±0.09 | 4.29±0.19 |
| t-value |
| 14.450 | 94.630 | 51.760 |
| P-value |
| 0.001 | 0.001 | 0.001 |
Diagnostic effectiveness of miRNA-21,
PTEN and p27 in NSCLC
ROC curve analysis showed that when the cut-off
value was 1.558, the sensitivity and specificity of miRNA-21 in the
diagnosis of NSCLC were 72% and 74%, respectively, the AUC was
0.756. When the cut-off value was 1.408, the sensitivity, and
specificity of PTEN in the diagnosis of NSCLC were 82% and 56%,
respectively, the AUC was 0.687. When the cut-off value was 2.206,
the sensitivity and specificity of p27 in the diagnosis of NSCLC
were 82% and 62%, respectively, the AUC was 0.732 (Table III and Fig. 1).
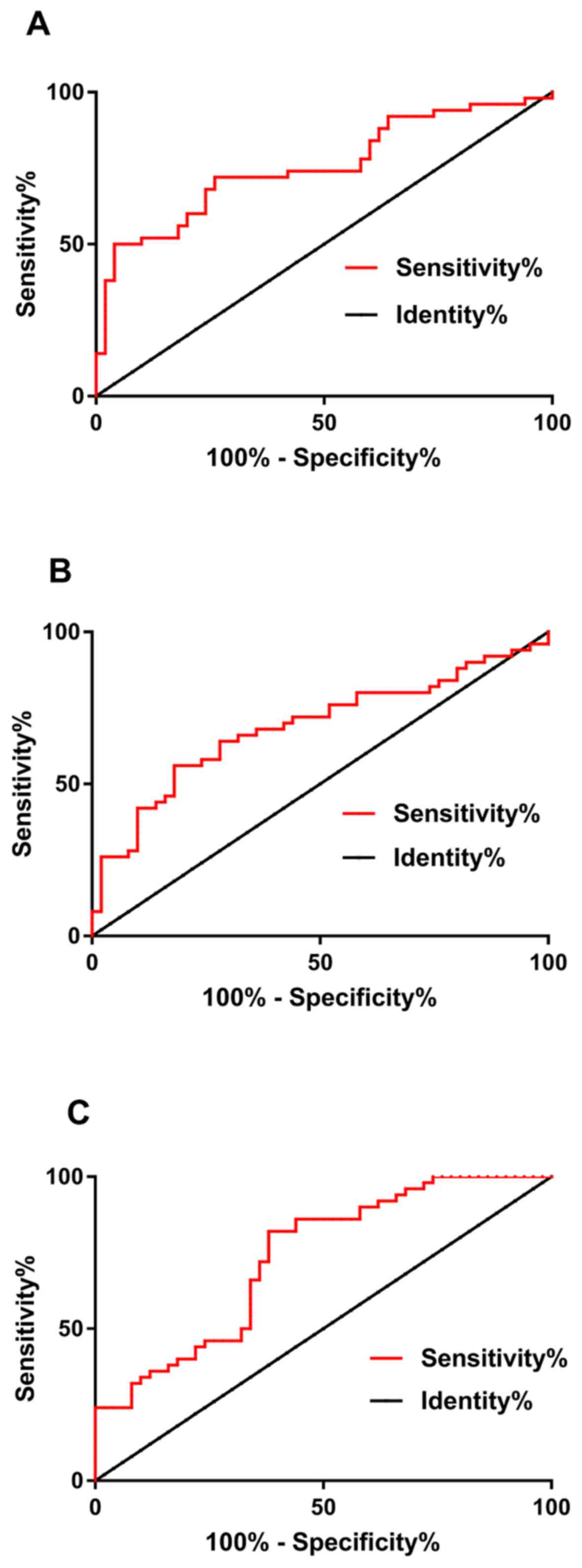 | Figure 1.Diagnostic effectiveness of miRNA-21,
PTEN and p27 for NSCLC. (A) When the cut-off value was 1.558, the
sensitivity and specificity of miRNA-21 in the diagnosis of NSCLC
were 72 and 74%, respectively, the AUC was 0.756. (B) When the
cut-off value was 1.408, the sensitivity, and specificity of PTEN
in the diagnosis of NSCLC were 82 and 56%, respectively, the AUC
was 0.687. (C) When the cut-off value was 2.206, the sensitivity
and specificity of p27 in the diagnosis of NSCLC were 82 and 62%,
respectively, the AUC was 0.732. NSCLC, non-small cell lung
cancer. |
 | Table III.Diagnostic effectiveness of miRNA-21,
PTEN and p27 in NSCLC. |
Table III.
Diagnostic effectiveness of miRNA-21,
PTEN and p27 in NSCLC.
| Index | miRNA-21 | PTEN | p27 |
|---|
| AUC | 0.756 | 0.687 | 0.732 |
| Standard error | 0.489 | 0.054 | 0.050 |
| 95% CI | 0.660–0.851 | 0.581–0.793 | 0.635–0.830 |
| P-value | 0.001 | 0.001 | 0.001 |
| Cut-off | 1.558 | 1.408 | 2.296 |
| Sensitivity | 72% | 82% | 82% |
| Specificity | 74% | 56% | 62% |
Correlation of miRNA-21, PTEN and p27
in clinicopathological features of NSCLC
There was no significant difference in age, sex and
course of disease in the study group (P>0.050), while there was
a difference in smoking, lymph node metastasis, TNM stage and
differentiation (P<0.050) (Tables
IV–VI).
 | Table IV.Correlation between miRNA-21 and
clinicopathological features in NSCLC. |
Table IV.
Correlation between miRNA-21 and
clinicopathological features in NSCLC.
| Clinicopathological
feature | n (115) | miRNA-21 | F | P-value |
|---|
| Age, years |
|
| 0.480 | 0.632 |
|
>50 | 62 | 1.36±0.45 |
|
|
| ≤50 | 53 | 1.32±0.44 |
|
|
| Sex |
|
| 0.206 | 0.837 |
|
Male | 92 | 1.33±0.42 |
|
|
|
Female | 23 | 1.31±0.40 |
|
|
| Course of disease,
weeks |
|
| 1.032 | 0.304 |
|
>5 | 60 | 1.41±0.23 |
|
|
| ≤5 | 55 | 1.37±0.18 |
|
|
| Smoking |
|
| 2.582 | 0.011 |
|
Yes | 104 | 1.72±0.69 |
|
|
| No | 11 | 1.18±0.12 |
|
|
| Lymph node
metastasis |
|
| 3.487 | 0.007 |
|
Yes | 69 | 1.48±0.75 |
|
|
| No | 46 | 1.09±0.13 |
|
|
| TNM stage |
|
| 3.410 | 0.009 |
|
I–II | 41 | 1.06±0.17 |
|
|
|
III–IV | 74 | 1.45±0.72 |
|
|
| Differentiation
degree |
|
| 5.073 | 0.001 |
| Poorly
differentiated | 77 | 1.08±0.20 |
|
|
|
Moderately and highly
differentiated | 38 | 1.51±0.69 |
|
|
 | Table VI.Correlation between p27 and
clinicopathological features in NSCLC. |
Table VI.
Correlation between p27 and
clinicopathological features in NSCLC.
| Clinicopathological
feature | n (115) | p27 | F | P-value |
|---|
| Age, years |
|
| 0.446 | 0.656 |
|
>50 | 62 | 2.42±0.31 |
|
|
|
≤50 | 53 | 2.39±0.41 |
|
|
| Sex |
|
| 0.518 | 0.605 |
|
Male | 92 | 2.43±0.42 |
|
|
|
Female | 23 | 2.38±0.39 |
|
|
| Course of disease,
weeks |
|
| 0.755 | 0.451 |
|
>5 | 60 | 2.45±0.33 |
|
|
| ≤5 | 55 | 2.40±0.38 |
|
|
| Smoking |
|
| 3.950 | 0.001 |
|
Yes | 104 | 2.92±0.57 |
|
|
| No | 11 | 2.23±0.29 |
|
|
| Lymph node
metastasis |
|
| 7.238 | 0.004 |
|
Yes | 69 | 2.89±0.63 |
|
|
| No | 46 | 2.19±0.22 |
|
|
| TNM stage |
|
| 5.284 | 0.001 |
|
I–II | 41 | 2.28±0.31 |
|
|
|
III–IV | 74 | 2.75±0.52 |
|
|
| Differentiation
degree |
|
| 6.730 | 0.001 |
| Poorly
differentiated | 77 | 2.31±0.26 |
|
|
|
Moderately and highly
differentiated | 38 | 2.78±0.49 |
|
|
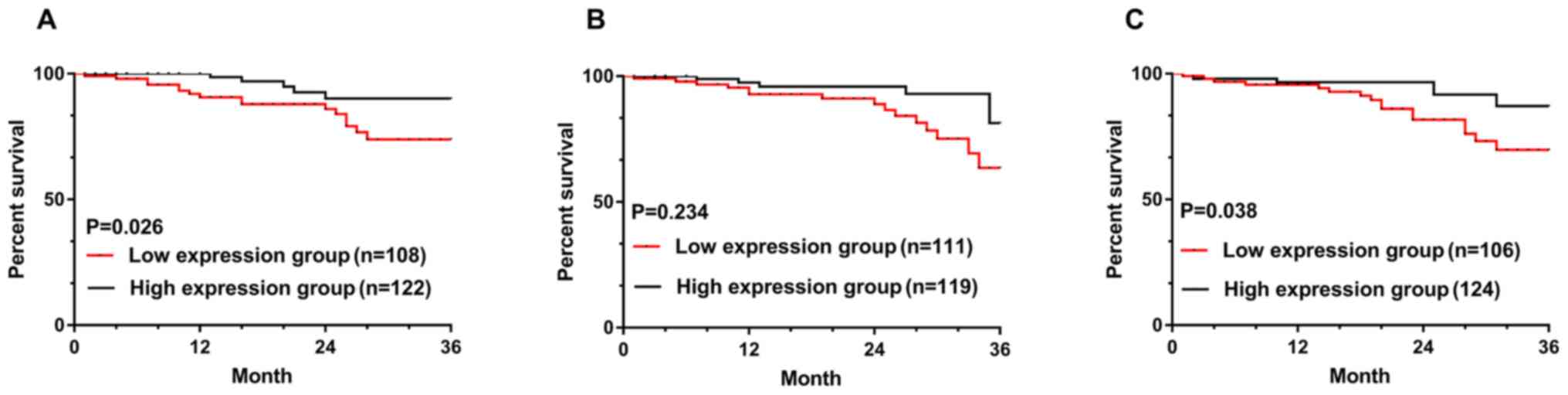
Survival rate at 3 years in patients
with high and low expressions of miRNA-21, PTEN and p27
According to the median value of miRNA-21, PTEN and
p27 expression levels, there were 108 cases in miRNA-21 low
expression group (≥0.82) and 122 cases in group with high
expression of miRNA-21 (<0.82). There were 111 cases in low
expression group of PTEN (≥2.52) and 119 cases in group with high
expression of PTEN (<2.52). There were 106 cases in p27 low
expression group (≥3.18) and 124 cases in p27 high expression group
(<3.18). Patients were randomly visited for 36 months, and the
termination time and the termination event were March 2018 and the
death of patients. The success rate of follow-up in 36 months was
95.22% (219/230). A total of 16 patients died in the miRNA-21 low
expression group, and the 36-month survival rate was 85.19%. A
total of 5 patients died in miRNA-21 high expression group, and the
36-month survival rate was 95.90%. A total of 16 patients died in
the PTEN low expression group, and the 36-month survival rate was
85.59%. A total of 6 patients died in TEN high expression group,
and the 36-month survival rate was 94.96%. A total of 16 patients
died in the p27 low expression group, and the 36-month survival
rate was 84.91%. A total of 7 patients died in the p27 high
expression group, and the 36-month survival rate was 94.35%
(Fig. 2).
Prognostic value of miRNA-21, PTEN and
p27 in the prognosis of survival
When the cut-off value was 1.905, the sensitivity
and specificity of miRNA-21 were 85.24 and 60%, respectively, and
the AUC was 0.786. When the cut-off value was 1.724, the
sensitivity and specificity of PTEN were 95.24 and 55%,
respectively, and the AUC was 0.776. When the cut-off value was
2.739, the sensitivity and specificity of p27 were 81.5 and 55%,
respectively, the AUC was 0.682 (Table
VII and Fig. 3).
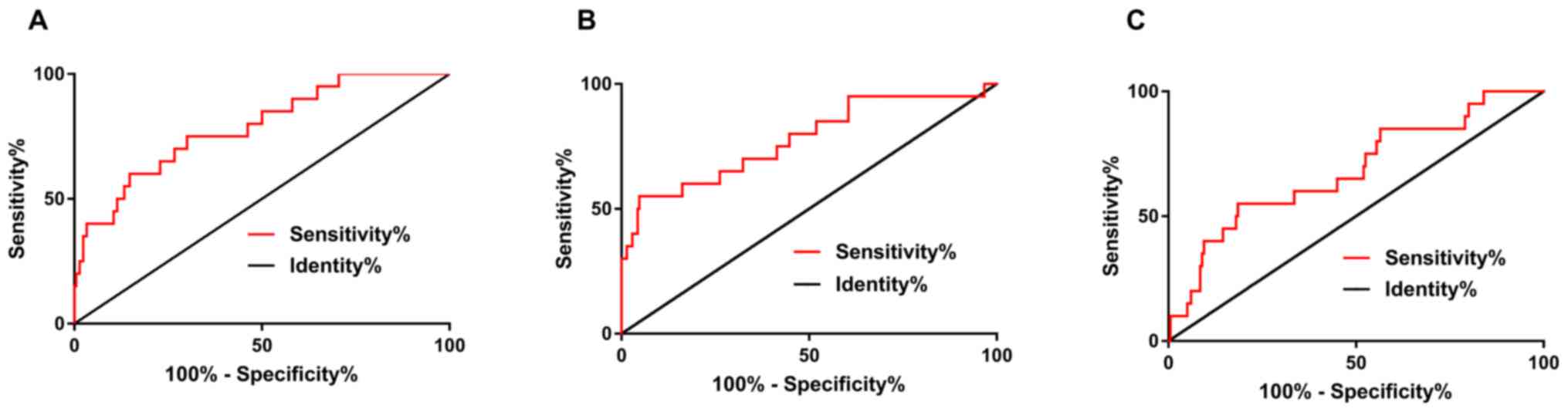 | Figure 3.Prognostic value of miRNA-21, PTEN and
p27 in the prognosis of death. (A) When the cut-off value was
1.905, the sensitivity and specificity of miRNA-21 were 85.24 and
60%, respectively, the AUC was 0.786. (B) When the cut-off value
was 1.724, the sensitivity and specificity of miRNA-21 were 95.24
and 55%, respectively, the AUC was 0.776. (C) When the cut-off
value was 2.739, the sensitivity and specificity of p27 were 81.5
and 55.%, respectively, the AUC was 0.682. |
 | Table VII.The prognostic value of miRNA-21,
PTEN and p27 for survival. |
Table VII.
The prognostic value of miRNA-21,
PTEN and p27 for survival.
| Index | miRNA-21 | PTEN | p27 |
|---|
| AUC | 0.786 | 0.776 | 0.682 |
| Standard error | 0.054 | 0.063 | 0.065 |
| 95% CI | 0.680–0.891 | 0.653–0.899 | 0.554–0.809 |
| P-value | 0.001 | 0.001 | 0.007 |
| Cut-off | 1.905 | 1.724 | 2.739 |
| Sensitivity | 85.24% | 95.24% | 81.5% |
| Specificity | 60.00% | 55.00% | 55.00% |
Discussion
NSCLC accounts for 80–85% of all lung cancer and is
still one of the leading causes of cancer-related death in the
world (14). The World Health
Organization (WHO) estimates that lung cancer is the cause of 1.37
million deaths worldwide each year and that 71% of these deaths are
caused by smoking, indicating that ~400,000 people die of lung
cancer each year (15). Because the
etiology and pathogenesis of PHC have not been determined yet, some
studies (16–18) have shown that the diagnosis of NSCLC
by genetic index is more reliable and the detection method is
convenient. Therefore, it is very important to find an index that
can accurately reflect the occurrence, progression and change of
NSCLC and a convenient means of detection. Therefore, the
expression of miRNA-21, PTEN and p27 in NSCLC patients were
explored, which is of great significance for the early screening of
NSCLC.
miRNA is a class of endogenous non-coding
short-stranded RNA, which can degrade the target gene and inhibit
the translation of the target gene, thus completing the silencing
after gene transcription (19). It
has been reported (20) that nearly
30% of the coding proteins in human body are affected and regulated
by miRNAs. miRNA is differentially expressed in tumors, which can
inhibit or promote the occurrence and progression of tumors by
regulating the target genes.
In this study, the expression of miRNA-21, PTEN and
p27 in cancer tissue of 230 patients with NSCLC was detected by
qRT-PCR, and it was found that the expression levels of miRNA-21,
PTEN and p27 in cancer tissue were significantly lower than those
in adjacent tissue. In the study results of Zhu (21), Marsit et al (22) and Catzavelos et al (23), the expression was also decreased,
which can further confirm the results of the present study.
According to the ROC curve of miRNA-21, PTEN and p27, the AUC was,
respectively, 0.756, 0.687 and 0.732, with high sensitivity. This
showed that miRNA-21, PTEN and p27 had very good diagnostic value
in NSCLC. Through the analysis of the correlation between the low
expression of miRNA-21, PTEN, p27 and clinical medical records, it
was found that smoking, lymphoid metastasis, TNM analysis and
differentiation of tissue types were correlated with the expression
of miRNA-21, PTEN and p27. In the study of Liu et al
(24), miR-21 significantly
inhibited the growth, migration and invasion of NSCLC cells.
miRNA-21 was associated with TNM stage and lymph node metastasis,
and may be an independent prognostic factor in NSCLC patients.
miR-21 significantly inhibited the growth migration and invasion of
NSCLC cells. In the study of Zhang et al (25), it was shown that the cells
transfected with miR-21 inhibitor showed significantly decreased
cell growth and invasion. This is basically consistent with the
results of this study. A previous study shows that PTEN is a tumor
inhibitor gene, and its protein product is inversely proportional
to the phosphorylated Akt in endometria and breast cancer cell
lines (26). In the study of Marsit
et al (22), PTEN was often
lost in lung tumors, but PTEN loss may also be a favorable
prognostic marker. This suggests that miRNA-21, PTEN and p27 can be
used as potential biomarkers for the diagnosis of NSCLC. The human
epidermal growth factor receptor (EGFR) belongs to the ErbB family
of receptor tyrosine kinases (RTKs) and plays an important role in
the pathogenesis and progression of different cancers, thus it has
become a major subject for scholars (27,28). In
the study by Wang et al (29), EGFR mutation-induced drug resistance
has become a major threat to the treatment of NSCLC; the resistance
mechanism involves the modification of intracellular signaling
pathways. According to Xu et al (30), molecular genetic analysis showed that
KRAS mutations were frequent in NSCLC. However, there is no
previous study on the relationship between EGFR mutation, KRAS
mutation and expression of miRNA-21, PTEN and p27.
The clinical value of miRNA-21, PTEN and p27 was
preliminarily proven through the above experiments, but for
example, we did not conduct cell-based experiments. Therefore,
further research is still required.
In conclusion, the expression of miRNA-21, PTEN and
p27 in cancer tissue of NSCLC patients were low. ROC curve analysis
shows that the three indexes have good diagnostic efficacy and are
expected to be excellent indexes for early clinical diagnosis and
prognosis of NSCLC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LY analyzed and interpreted the general data of the
patients. JY performed PCR and was responsible for the analysis of
the observation indicators. LY wrote the manuscript. Both authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Hubei Cancer Hospital (Wuhan, China). Patients who participated in
this research, signed an informed consent and had complete clinical
data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jamal-Hanjani M, Wilson GA, McGranahan N,
Birkbak NJ, Watkins TBK, Veeriah S, Shafi S, Johnson DH, Mitter R,
Rosenthal R, et al TRACERx Consortium, : Tracking the evolution of
non-small-cell lung cancer. N Engl J Med. 376:2109–2121. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wakelee HA, Chang ET, Gomez SL, Keegan TH,
Feskanich D, Clarke CA, Holmberg L, Yong LC, Kolonel LN, Gould MK,
et al: Lung cancer incidence in never smokers. J Clin Oncol.
25:472–478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferlay J, Colombet M, Soerjomataram I,
Mathers C, Parkin DM, Piñeros M, Znaor A and Bray F: Estimating the
global cancer incidence and mortality in 2018: GLOBOCAN sources and
methods. Int J Cancer. 144:1941–1953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu Z, Chen X, Zhao Y, Tian T, Jin G, Shu
Y, Chen Y, Xu L, Zen K, Zhang C, et al: Serum microRNA signatures
identified in a genome-wide serum microRNA expression profiling
predict survival of non-small-cell lung cancer. J Clin Oncol.
28:1721–1726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K,
Guo J, Zhang Y, Chen J, Guo X, et al: Characterization of microRNAs
in serum: A novel class of biomarkers for diagnosis of cancer and
other diseases. Cell Res. 18:997–1006. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Casal-Mouriño A, Valdés L, Barros-Dios JM
and Ruano-Ravina A: Lung cancer survival among never smokers.
Cancer Lett. 451:142–149. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vitsios DM, Davis MP, van Dongen S and
Enright AJ: Large-scale analysis of microRNA expression,
epi-transcriptomic features and biogenesis. Nucleic Acids Res.
45:1079–1090. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iorio MV and Croce CM: MicroRNA
dysregulation in cancer: Diagnostics, monitoring and therapeutics.
A comprehensive review. EMBO Mol Med. 4:143–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin X, Chen Y, Chen H, Fei S, Chen D, Cai
X, Liu L, Lin B, Su H, Zhao L, et al: Evaluation of tumor-derived
exosomal miRNA as potential diagnostic biomarkers for early-stage
non-small cell lung cancer using next-generation sequencing. Clin
Cancer Res. 23:5311–5319. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xia H, Li Y and Lv X: MicroRNA-107
inhibits tumor growth and metastasis by targeting the BDNF-mediated
PI3K/AKT pathway in human non-small lung cancer. Int J Oncol.
49:1325–1333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xie Y, Naizabekov S, Chen Z and Tokay T:
Power of PTEN/AKT: Molecular switch between tumor suppressors and
oncogenes. Oncol Lett. 12:375–378. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sharma SS and Pledger WJ: The
non-canonical functions of p27 (Kip1) in normal and tumor biology.
Cell Cycle. 15:1189–1201. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fenchel K, Sellmann L and Dempke WC:
Overall survival in non-small cell lung cancer - what is clinically
meaningful? Transl Lung Cancer Res. 5:115–119. 2016.PubMed/NCBI
|
|
15
|
Shepherd L, Ryom L, Law M, Petoumenos K,
Hatleberg CI, d'Arminio Monforte A, Sabin C, Bower M, Bonnet F,
Reiss P, et al Data Collection on Adverse Events of Anti-HIV Drugs
(D:A:D) Study Group, : Cessation of cigarette smoking and the
impact on cancer incidence in human immunodeficiency virus-infected
persons: The data collection on adverse events of anti-HIV drugs
study. Clin Infect Dis. 68:650–657. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kozub M, Gachewicz B, Kasprzyk M, Roszak
M, Gasiorowski L and Dyszkiewicz W: Impact of smoking history on
postoperative complications after lung cancer surgery - a study
based on 286 cases. Kardiochir Torakochirurgia Pol. 16:13–18.
2019.(In Polish). PubMed/NCBI
|
|
17
|
Sozzi G, Conte D, Leon M, Ciricione R, Roz
L, Ratcliffe C, Roz E, Cirenei N, Bellomi M, Pelosi G, et al:
Quantification of free circulating DNA as a diagnostic marker in
lung cancer. J Clin Oncol. 21:3902–3908. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao W, Zhao JJ, Zhang L, Xu QF, Zhao YM,
Shi XY and Xu AG: Serum miR-21 level: A potential diagnostic and
prognostic biomarker for non-small cell lung cancer. Int J Clin Exp
Med. 8:14759–14763. 2015.PubMed/NCBI
|
|
19
|
Ha M and Kim VN: Regulation of microRNA
biogenesis. Nat Rev Mol Cell Biol. 15:509–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carthew RW: Gene regulation by microRNAs.
Curr Opin Genet Dev. 16:203–208. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu H, Chen W, Xu J, Yin L, Zhu H, Liu J,
Wang T and He X: MiR-21 expression significance in non-small cell
lung cancer tissue and plasma. Int J Clin Exp Med. 10:2918–2924.
2017.
|
|
22
|
Marsit CJ, Zheng S, Aldape K, Hinds PW,
Nelson HH, Wiencke JK and Kelsey KT: PTEN expression in
non-small-cell lung cancer: Evaluating its relation to tumor
characteristics, allelic loss, and epigenetic alteration. Hum
Pathol. 36:768–776. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Catzavelos C, Tsao MS, DeBoer G,
Bhattacharya N, Shepherd FA and Slingerland JM: Reduced expression
of the cell cycle inhibitor p27Kip1 in non-small cell lung
carcinoma: A prognostic factor independent of Ras. Cancer Res.
59:684–688. 1999.PubMed/NCBI
|
|
24
|
Liu ZL, Wang H, Liu J and Wang ZX:
MicroRNA-21 (miR-21) expression promotes growth, metastasis, and
chemo- or radioresistance in non-small cell lung cancer cells by
targeting PTEN. Mol Cell Biochem. 372:35–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang JG, Wang JJ, Zhao F, Liu Q, Jiang K
and Yang GH: MicroRNA-21 (miR-21) represses tumor suppressor PTEN
and promotes growth and invasion in non-small cell lung cancer
(NSCLC). Clin Chim Acta. 411:846–852. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
David O: Akt and PTEN: New diagnostic
markers of non-small cell lung cancer? J Cell Mol Med. 5:430–433.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Arteaga CL: The epidermal growth factor
receptor: From mutant oncogene in nonhuman cancers to therapeutic
target in human neoplasia. J Clin Oncol. 19 (Suppl):S32–S40.
2001.
|
|
28
|
Yarden Y and Sliwkowski MX: Untangling the
ErbB signalling network. Nat Rev Mol Cell Biol. 2:127–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang DD, Ma L, Wong MP, Lee VH and Yan H:
Contribution of EGFR and ErbB-3 heterodimerization to the EGFR
mutation-induced gefitinib- and erlotinib-resistance in
non-small-cell lung carcinoma treatments. PLoS One.
10:e01283602015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu Y, Zong S, Gao X, Zhang H, Wang B, Li
P, Liu T and Li S: Combined treatment of ABT199 and irinotecan
suppresses KRAS-mutant lung cancer cells. Gene. 688:1–6. 2019.
View Article : Google Scholar : PubMed/NCBI
|