Introduction
The work is reported in line with the SCARE criteria
(1). The treatment of
retroperitoneal tumor is frequently surgical resection and lymph
node dissection, which usually followed by lymphatic leakage. A
variety of approaches have been attempted to the treatment of
lymphatic leakage. However, so far none has been consistently
effective or optimal (2). This study
report a case of lymphatic leakage after retroperitoneal tumor
resection that was successfully resolved by lymphangiography and
embolization.
Case report
The study was approved by the Ethics Committee of
the Second Affiliated Hospital of Soochow University (Suzhou,
China). The patient who participated in this research provided a
signed informed consent and had complete clinical data. The patient
was a 55-year-old female patient that had abdominal mass and
complained that the mass gradually increased to affect sleep.
B-ultrasound examination revealed mixed echo zone with
approximately 243×118 mm in size in the abdominal cavity. Computed
tomography (CT) showed a large mass on the right side of the
abdomen, with the right ureter and inferior vena cava compressed to
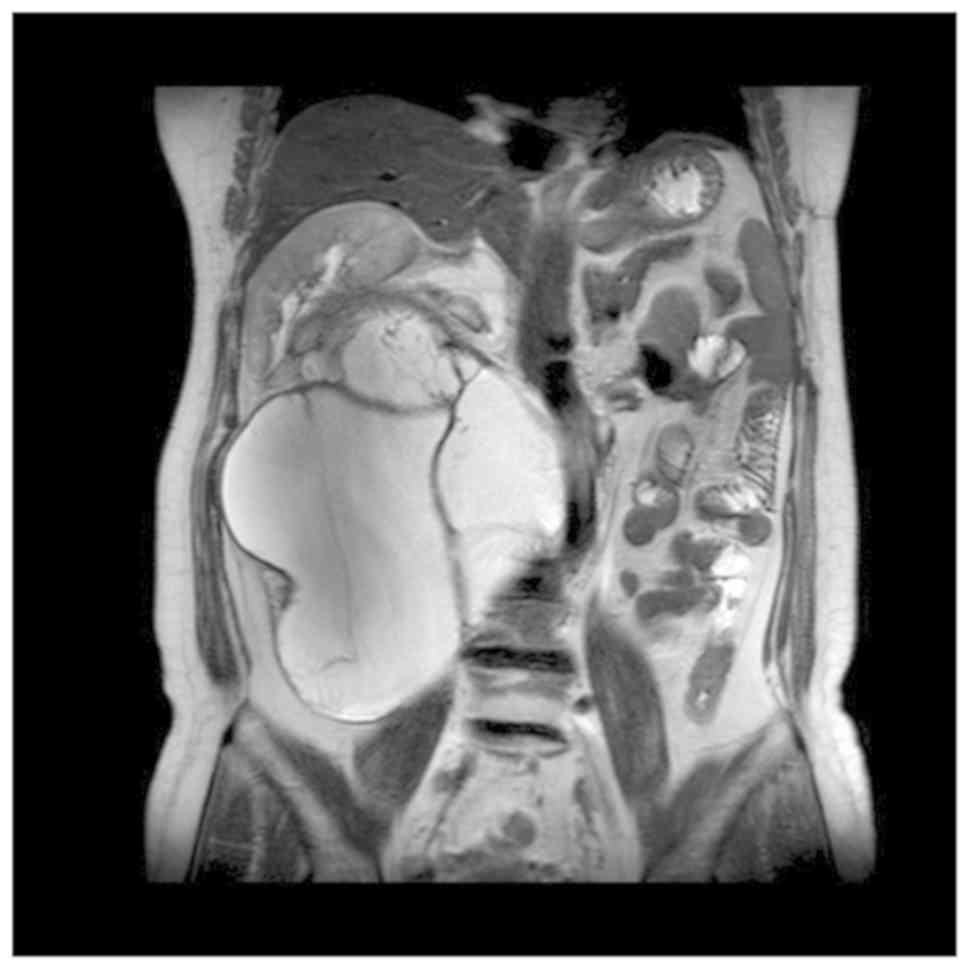
the right, indicating retroperitoneal cystadenoma. Magnetic
resonance imaging (MRI) suggested a large multilocular cystic space
in the retroperitoneal and hepatorenal space, approximately
261×181×150 mm in size, which was very likely to be epidermoid cyst
(Fig. 1). After admission, physical
examination showed a hard, local uplift in the right abdomen, about
25×12 cm in size, without tenderness, fixation or any other
positive signs. After placing the right ureteral stent, the
retroperitoneal tumor resection was performed. As a result,
intraoperative exploration revealed a large cystic solid tumor in
the right abdominal cavity, which was multilocular and lobulated.
The inferior vena cava and ureter were pushed up to the right
abdominal wall and the right kidney was moved up to the lower part
of the liver. After cautious separation along the tumor to protect
the blood vessels and ureters, careful irrigation was performed
before the peritoneum was closed. No active bleeding or obvious
lymphatic leakage was detected. Pelvic cavity was then placed,
followed by placing two drainage tubes at the incision.
Postoperative pathology: (Posterior peritoneal)
mature cystic teratoma showed mucinous tumor and mild to moderate
atypical hyperplasia of glandular epithelium. After the operation,
the abdominal drainage tube had a small amount in light liquid of
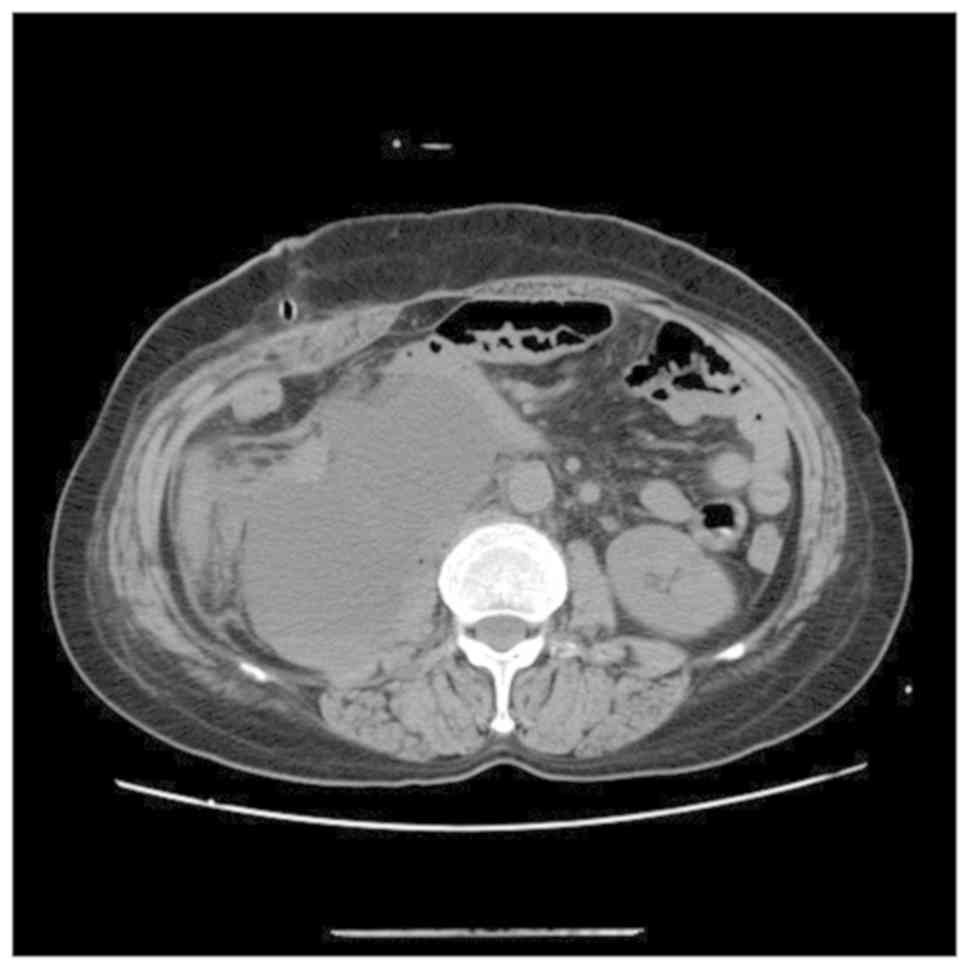
blood. CT revealed peritoneal effusion after operation on POD7
(Fig. 2), therefore,
peritoneocentesis was performed, and 200 ml of yellow-white, turbid
liquid was extracted. Two abdominal drainage tubes were removed on
the same day. After two days of observation, there was no decrease
in the amount of abdominal drainage fluid. The chylous qualitative
test of concurrent drainage fluid was performed, with positive
Sudan staining. Therefore, the patient was instructed to eat
high-calorie, high-protein, low-fat fluid, in order to improve
parenteral nutrition, and keep the drainage tube unobstructed. In
addition, electrolyte was regularly reviewed to prevent water and
electrolyte balance disorder, and the daily drainage volume was
maintained between 700-1,100 ml thereafter. Subsequently, in the
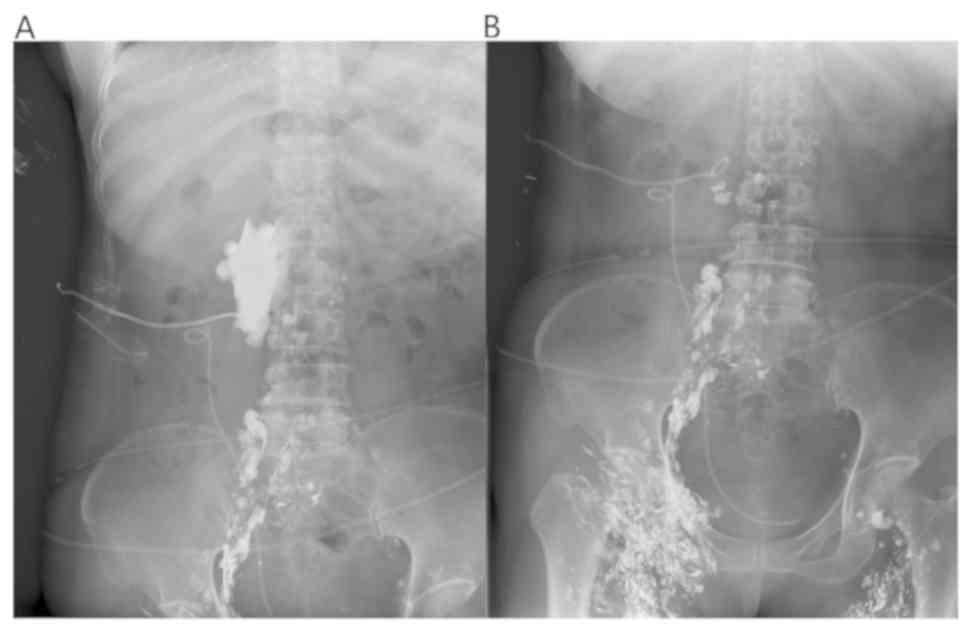
first month after operation, lymph node lipiodolography and
embolization were performed under ultrasound guidance. Meilan (1
ml) was injected between the toes of the patient, after 15 min the
lymphatic vessels on the instep were clearly blue. Target lymphatic
vessels were then selected for local anesthesia, skin incision and
lymphatic vessel separation. Then lymphatic vessels were punctured
and fixed. Lymphangiography was performed with a 2:1 mixture of
iodide oil (Guerbet) and NBCA puncture embolization (3,4). Daily
drainage was decreased after lipiodolography, and not obvious. One
week later, the lymph node lipiodolography and embolization were
re-performed (Fig. 3). As shown, the
leakage of the exudation site was reduced. One day after the
lipiodolography, 125 ml of milky liquid was drained, which
suggested obviously covered leakage area and relatively limited
scope.
Afterwards, the patient was instructed to fast and
switch to total parenteral nutrition for one week. The drainage
tube was clamped and the patient did not complain of any
discomfort. After two weeks of lipiodolography, the peritoneal
puncture drainage tube was removed, and the patient was instructed
to eat a light diet and gradually make a transition. After one week
of observation, no peritoneal effusion was found by b-ultrasound
and the patient was discharged from hospital. So far, the patient
has been reviewed every three months for one and a half years
without any discomfort. After performing lymphangiography and
embolization, there was no series of postoperative complications
such as edema or skin thickening in the lower extremities.
Discussion
Lymphatic leakage is a well-known complication
following retroperitoneal tumor resection (5). Many attempts have been made in the
treatment of lymphatic leakage, as listed: i) Abdominal drainage:
In spite of no direct evidence supporting that abdominal drainage
can promote the healing of fistula, it can provide clinical
diagnostic basis and alleviate a series of clinical symptoms such
as abdominal pain and distension to a certain extent. In addition,
the therapeutic treatment plan can be adjusted according to the
amount of drainage fluid. ii) Antisecosis: Mid-chain triglyceride
diet with parenteral nutrition can reduce the amount of lymphatic
leakage, which is because the short-chain triacylglycerol contained
in food can be absorbed directly into the blood through the
intestinal tract, while the long-chain triacylglycerol should be
transported and absorbed through the lymphatic pathway (6). iii) Octreotide and somatostatin: It has
been reported that the addition of octreotide in food exert a
significantly early scavenging effect on postoperative lymphatic
drainage of patients, mainly to prevent the conversion of
triglycerides in the diet into free fatty acids in the intestinal
tract, thereby reducing the absorption of fatty acids (7,8). In
recent years, surgical intervention has been reported to be guided
by near-infrared fluorescence imaging technology, and indocyanine
green can be used to locate leakage hot spots, providing high
sensitivity and real-time imaging to help surgeons perform
preventive ligation in cases where it is needed. This technique may
have the potential to more accurately diagnose and treat lymphatic
leakage during surgery (9).
Nevertheless, some leaks still persist despite
conservative treatment, therefore, more effective treatments are
needed (10). Currently,
lymphangiography in conjunction with embolization is a relatively
safe, powerful, and reliable interventional method (11).
Lymphatic intervention is less invasive compared
with surgery, involving injection of ethiodized oil into the
lymphatic system to obtain a lymphangiogram (12). In addition to its diagnostic value,
lymphangiography has also been reported to have therapeutic effects
(11). This is possibly due to the
high viscosity of contrast medium such as ethiodized oil, which can
stimulate the growth of local new granulation tissue, triggering a
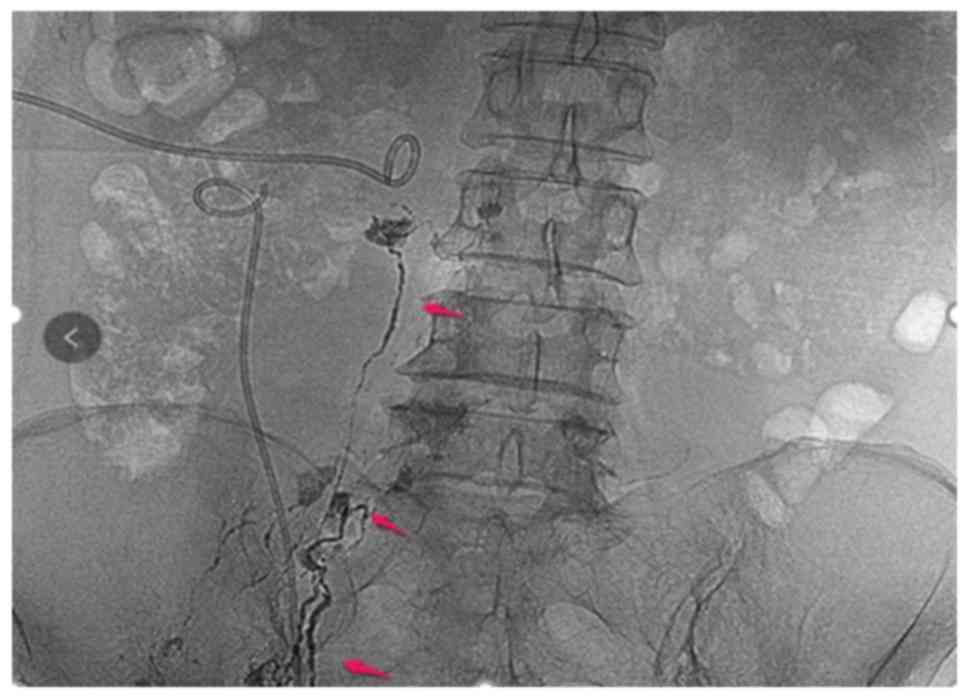
series of inflammatory reactions to decrease leakage. In this case
report, two procedures of lymphangiography and embolization were
performed, in which thin strips of lymphatic vessels and leakage
development were observed, however, without immediate effect
(Fig. 4). The second attempt was
successful and the amount of drainage was decreased from a maximum
output of 700 to 125 ml/day after lymphangiography.
This case demonstrates postoperative lymphatic
leakage that was successfully treated by performing repeated
lymphangiography and embolization. This technique should be
considered for further application and study of lymphatic leakage
after abdominal surgery and lymphadenectomy. However, this case has
certain limitations. First, the patient is followed up for a short
period of time. Second, there is a lack of multi-sample
retrospective analysis to compare the effects of different
treatment methods on the prognosis of patients with postoperative
lymphatic leakage. Nowadays, intranodal lymphangiography and
ultrasound-guided lymphangiography are also used in clinical
practice, resulting in less trauma to patients and better
positioning accuracy, which can be further studied (11).
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JG wrote the manuscript, interpreted and analyzed
the data. WC designed the study and performed the experiments. YJ
was responsible for the analysis and discussion of the data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Second Affiliated Hospital of Soochow University (Suzhou,
China). The patient who participated in this research provided a
signed informed consent and had complete clinical data.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report and any
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Agha RA, Borrelli MR, Farwana R, Koshy K,
Fowler AJ and Orgill DP; SCARE Group, : The SCARE 2018 Statement:
Updating consensus Surgical CAse REport (SCARE) guidelines. Int J
Surg. 60:132–136. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim EA, Park H, Jeong SG, Lee C, Lee JM
and Park CT: Octreotide therapy for the management of refractory
chylous ascites after a staging operation for endometrial
adenocarcinoma. J Obstet Gynaecol Res. 40:622–626. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nadolski GJ, Chauhan NR and Itkin M:
Lymphangiography and lymphatic embolization for the treatment of
refractory chylous ascites. Cardiovasc Intervent Radiol.
41:415–423. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Inoue M, Nakatsuka S, Yashiro H, Tamura M,
Suyama Y, Tsukada J, Ito N, Oguro S and Jinzaki M: Lymphatic
intervention for various types of lymphorrhea: Access and
treatment. Radiographics. 36:2199–2211. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Capocasale E, Iaria M, Vistoli F, Signori
S, Mazzoni MP, Dalla Valle R, De Lio N, Perrone V, Amorese G, Mosca
F, et al: Incidence, diagnosis, and treatment of chylous leakage
after laparoscopic live donor nephrectomy. Transplantation.
93:82–86. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Steven BR and Carey S: Nutritional
management in patients with chyle leakage: A systematic review. Eur
J Clin Nutr. 69:776–780. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weniger M, D'Haese JG, Angele MK,
Kleespies A, Werner J and Hartwig W: Treatment options for chylous
ascites after major abdominal surgery: A systematic review. Am J
Surg. 211:206–213. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Seow C, Murray L and McKee RF: Surgical
pathology is a predictor of outcome in post-operative lymph
leakage. Int J Surg. 8:636–638. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang F, Zhou J, Li H, Yang F, Xiao R, Chi
C, Tian J and Wang J: Near-infrared fluorescence-guided
thoracoscopic surgical intervention for postoperative chylothorax.
Interact Cardiovasc Thorac Surg. 26:171–175. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kortes N, Radeleff B, Sommer CM, Bellemann
N, Ott K, Richter GM, Kauczor HU and Stampfl U: Therapeutic
lymphangiography and CT-guided sclerotherapy for the treatment of
refractory lymphatic leakage. J Vasc Interv Radiol. 25:127–132.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee EW, Shin JH, Ko HK, Park J, Kim SH and
Sung KB: Lymphangiography to treat postoperative lymphatic leakage:
A technical review. Korean J Radiol. 15:724–732. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iwai T, Uchida J, Matsuoka Y, Kosoku A,
Shimada H, Nishide S, Kabei K, Kuwabara N, Yamamoto A, Naganuma T,
et al: Experience of lymphangiography as a therapeutic tool for
lymphatic leakage after kidney transplantation. Transplant Proc.
50:2526–2530. 2018. View Article : Google Scholar : PubMed/NCBI
|