Introduction
Lung cancer is one of the most common types of
malignant tumor worldwide, among which non-small cell lung cancer
(NSCLC) accounts for 85–90% of cases (1–3). Despite
progress in clinical diagnosis and treatment of NSCLC over the past
several decades, the 5-year survival rate is ~15% (1,3).
Therefore, it is necessary to understand the molecular mechanisms
underlying NSCLC development and metastasis in order to improve
diagnosis and treatment of NSCLC (4).
Cell invasion and metastasis impede the treatment of
patients with NSCLC (5,6). Before acquiring these abilities, tumor
cells undergo the epithelial-to-mesenchymal transition (EMT)
(7). Normal EMT is a physiological
cell reprogramming phenomenon during development (7). However, studies have demonstrated that
deregulated EMT is associated with tumor occurrence and development
(7–9). A number of molecules, such as
E-cadherin, N-cadherin, β-catenin and SNAI1, are considered to be
key markers of EMT (8).
MicroRNAs (miRs) regulate the expression levels of
downstream target genes via binding to mRNA 3′-untranslated regions
(3′-UTRs) or coding sequences (9,10).
Multiple studies have shown that dysregulated miRNA expression
level profiles play important roles in carcinogenesis (5,10). The
expression levels of miR-30 family members (miR-30a/b/c/d/e) are
repressed in a number of types of cancer, including lung cancer
(7,11–14).
Several miR-30 family members have critical roles in EMT, migration
and invasion of NSCLC cells (7,12,13). For
example, miR-30a has been shown to inhibit EMT by targeting SNAI1
and B-cell lymphoma/leukemia 11A in NSCLC (7). Low expression levels of miR-30c can
promote invasion by inducing EMT in NSCLC (13). miR-30d can restrain NSCLC cell
motility by targeting CCNE2 (12).
XB130, a multifunctional adaptor protein, is an
oncogene that mediates cell proliferation, migration and invasion
in osteosarcoma, hepatocellular and esophageal squamous cell
carcinoma and pancreatic ductal adenocarcinoma, as well as
prostrate, breast and gastric cancer (15–22).
However, Cho et al (23)
recently suggested that XB130 acts as a tumor suppressor in skin
tumorigenesis by inhibiting inflammation, which indicates that
XB130 may serve different roles in different types of tumor. Our
previous study (24) demonstrated
that, similar to miR-30 family members, XB130 silencing can inhibit
cell migration, invasion and EMT in NSCLC. In addition, miR-30d and
miR-30e are significantly upregulated in XB130 shRNA-transfected
cells, suggesting that there may be a regulatory association
between miR-30 family members and XB130 (25).
In order to understand the effects of miR-30 family
members on the EMT of NSCLC cells and the related mechanisms, the
present study investigated the effects of miR-30 family members
overexpression and XB130 silencing on EMT in A549 and PC-9 cells.
In addition, the present study explored the regulatary association
between miR-30 family members and XB130, which may provide a novel
theoretical basis for the diagnosis and treatment of NSCLC.
Materials and methods
Cell culture and transfection
The NSCLC cell lines A549 and PC-9 were purchased
from Conservation Genetics CAS Kunming Cell Bank and FuHeng Biology
Company, respectively. Cells were cultured in RPMI-1640 medium
supplemented with 10% FBS (both Gibco; Thermo Fisher Scientific,
Inc.) and maintained in a humidified incubator containing 5%
CO2 at 37°C. miR-30 family mimics or miR-30c or miR-30d
inhibitors or XB130 siRNAs (Shanghai GenePharma Co., Ltd.) at a
final concentration of 100 nM with or without DNA plasmids were
transfected or co-transfected into cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) or Entranster™-R4000 (Engreen Biosystem Co.,
Ltd.) according to the manufacturer's instructions. Non-targeting
sequences were used for miR mimics or inhibitors or siRNAs
transfection controls. All sequences of the miRs and siRNAs used
are presented in Table I. Cells used
for western blotting and luciferase reporter assays were harvested
48 h after transfection. Wound healing and Matrigel invasion assays
were performed 24 h after transfection.
 | Table I.Sequences of primers and RNA
oligos. |
Table I.
Sequences of primers and RNA
oligos.
| Name | Sequence
(5′→3′) |
|---|
| WT-30 | F:
CCGCTCGAGATTAAAGTGACTCTTTACT |
|
| R:
CGGGATCCGAGAATGAACATTAAACAGA |
| XB130 | F:
CTAGCTAGCATGGAGCGGTACAAAGCCCTG |
|
| R:
CCGGAATTCCTAACTTGCTCCTTTCTTCTCCCATT |
| hsa-miR-30a | F:
UGUAAACAUCCUCGACUGGAAG |
|
| R:
CUUCCAGUCGAGGAUGUUUACA |
| hsa-miR-30b | F:
UGUAAACAUCCUACACUCAGCU |
|
| R:
AGCUGAGUGUAGGAUGUUUACA |
| hsa-miR-30c | F:
UGUAAACAUCCUACACUCUCAGC |
|
| R:
GCUGAGAGUGUAGGAUGUUUACA |
| hsa-miR-30d | F:
UGUAAACAUCCCCGACUGGAAG |
|
| R:
CUUCCAGUCGGGGAUGUUUACA |
| hsa-miR-30e | F:
UGUAAACAUCCUUGACUGGAAG |
|
| R:
CUUCCAGUCAAGGAUGUUUACA |
| Anti-30c |
GCUGAGAGUGUAGGAUGUUUACA |
| Anti-30d |
CUUCCAGUCGGGGAUGUUUACA |
| XB130 siRNA-1 | F:
GGAGCUAAAGGAAACCCUACU |
|
| R:
AGUAGGGUUUCCUUUAGCUCC |
| XB130 siRNA-2 | F:
GAUUCUUGACCAGGAGAAC |
|
| R:
GUUCUCCUGGUCAAGAAUC |
| NC
siRNA/miR-cont | F:
UUCUCCGAACGUGUCACGUTT |
|
| R:
ACGUGACACGUUCGGAGAATT |
| Anti-cont |
CAGUACUUUUGUGUAGUACAA |
Plasmid construction
miR-30 family binding sites in XB130 3′UTR were
predicted using TargetScan (http://www.targetscan.org/vert_71/) and PicTar
(https://pictar.mdc-berlin.de/) target
prediction databases. In order to construct the dual-luciferase
reporter plasmid, a fragment containing the miR-30 family binding
sites was amplified from XB130 3′-UTR with primers WT-30-For and
WT-30-Rev (Table I). Then, the
fragment was cloned into psiCHECK-2 vector (Promega Corporation)
and the recombinant was named WT-30. The XB130 open reading frame
(ORF) was amplified from PC-9 cDNA using primers XB130-For and
XB130-Rev (Table I), and then
inserted to the vector pcDNA3.1(+) (Invitrogen; Thermo Fisher
Scientific, Inc.), which was named pcDNA3.1-XB130 ORF. To obtain
PC-9 cDNA, total RNA in PC-9 cells was extracted using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. Then total RNA was reverse
transcribed using a PrimeScript™ RT reagent Kit with gDNA Eraser
(Takara Biomedical Technology Co., Ltd.), with removing gDNA at
42°C for 2 min followed by reverse transcription at 37°C for 15
min. The synthesized cDNA was used as a template for XB130 3′-UTR
and ORF amplifications. PCR reaction was performed using a
PrimeSTAR® Max DNA Polymerase kit (Takara Biomedical
Technology Co., Ltd.) and the reaction mixture included 25 µl 2X
PrimeSTAR Max Premix, 1 µl upstream primers, 1 µl downstream
primers, 1 µl cDNA template and 22 µl ddH2O. PCR
thermocycling were as follows: Denaturation at 98°C for 10 sec,
annealing at 55°C for 15 sec and extension at 72°C for 15 sec for
35 cycles. All primers were synthesized at the Sangon Biotech Co.
Ltd. All constructs were confirmed via DNA sequencing.
Western blotting analysis
Cells were lysed in RIPA buffer containing 1 mM
phenylmethylsulfonyl fluoride (PMSF) (both Beyotime Institute of
Biotechnology). The prepared protein samples were quantified using
a bicinchoninic protein assay kit (Beijing Solarbio Science and
Technology Co., Ltd.). Equal amounts of protein (40 µg/lane) were
separated by 12% SDS-PAGE and then transferred to a PVDF membrane.
The membranes were firstly blocked with 5% bovine serum albumin
(Beijing Solarbio Science and Technology Co., Ltd.) at room
temperature for 1 h and then incubated with primary antibodies
against XB130 (1:1,000; cat. no. ab106433; Abcam), N-cadherin
(1:5,000; cat. no. 22018-1-AP; ProteinTech Group, Inc.), SNAI1
(1:2,000; cat. no. 26183-1-AP; ProteinTech Group, Inc.), β-catenin
(1:5,000; cat. no. 51067-2-AP; ProteinTech Group, Inc.) and GAPDH
(1:5,000; cat. no. 10494-1-AP; ProteinTech Group, Inc.) overnight
at 4°C, and finally with horseradish peroxidase conjugated
secondary antibodies (1:5,000; cat. no. SA00001-2; ProteinTech
Group, Inc.) at room temperature for 1 h. The specific protein
bands were visualized using an ECL reagent (EMD Millipore).
Wound healing assay
Transfected cells proliferated 12-well plates as
confluent monolayers were mechanically scratched using a 200 µl
pipette tip to create a straight wound. Cells were washed twice
with PBS to remove the debris and then cultured with RPMI-1640
medium containing 4% FBS for 48 h to allow wound healing Images
were captured at 0 and 48 h to determine cell migration using a
light microscope (magnification, ×100). Healing distance between
the wound (%) was expressed as follows: [(Gap distance at 0 h-Gap
distance at 48 h)/Gap distance at 0 h] ×100%.
Matrigel invasion assay
A 24-well Matrigel transwell chamber (Costar;
Corning, Inc.) was used to measure cell invasion. Briefly,
transwell chamber with 8-µm pore size was precoated with 100 µl
Matrigel matrix (1:8 dilution) for 1 h at 37°C (BD Biosciences).
The upper chamber was plated with 2×105 cells in
serum-free RPMI-1640 medium. The chambers were then inserted into a
24-well plate with 0.6 ml complete RPMI-1640 medium containing 10%
FBS in each well. After incubation at 37°C for 48 h, the cells
remaining on the upper chamber were removed; cells adhering to the
lower surface were fixed using methanol for 30 min and stained
using crystal violet for 1 h at room temperature. Images were
captured using a light microscope (magnification, ×200) and counted
using ImageJ software version 1.48 (National Institutes of
Health).
Luciferase reporter assay
Reporter plasmids and RNA oligos were transiently
co-transfected into PC-9 cells. After 48 h, the luciferase
activities were measured using a Luc-Pair™ Duo-Luciferase HS Assay
kit (GeneCopoeia, Inc.) on a BioTek Synergy2 Multimode Microplate
Reader (BioTek Instruments, Inc.). Firefly luciferase was
used for normalization.
Statistical analysis
Data are expressed as the mean ± SD of three
independent experiments. Differences among groups were analyzed by
one-way ANOVA followed by the post hoc Tukey's test using SPSS
software (version 20; SPSS, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
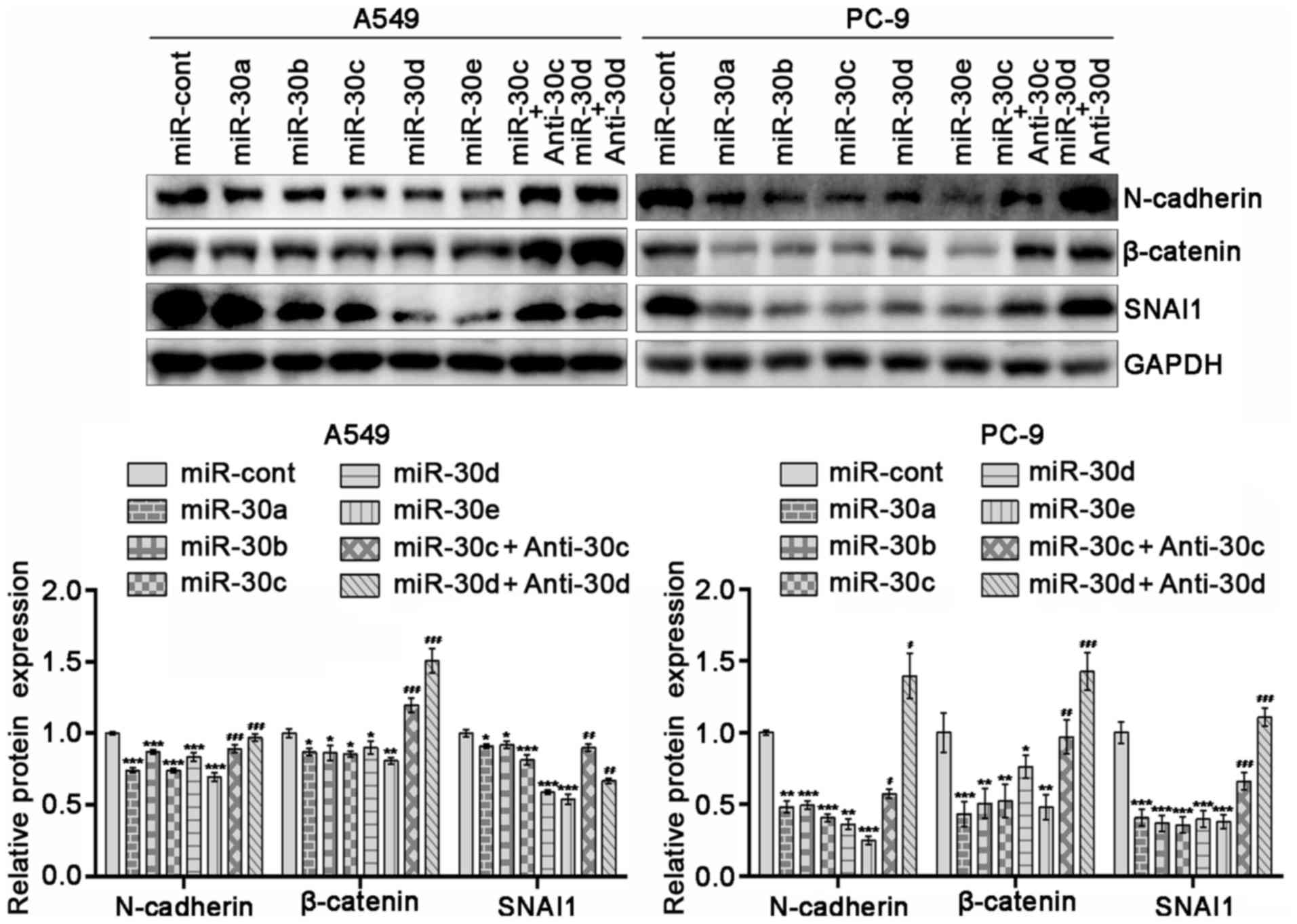
miR-30 family members suppress
expression levels of N-cadherin, β-catenin and SNAI1 in NSCLC
cells
In order to determine the effects of miR-30 family
members on the EMT of NSCLC cells, the expression levels of EMT
markers, including N-cadherin, β-catenin and SNAI1, in A549 and
PC-9 cells overexpressing miR-30 family members were determined.
The results revealed that overexpression of miR-30 family members
decreased the expression levels of EMT markers in these cells
(P<0.05). Due to the similarity of the miR-30a-e sequences,
miR-30c or miR-30d inhibitors were randomly selected. miR-30c or
miR-30d inhibitors reversed the effect of miR-30c or
miR-30d-overexpression on the expression levels of EMT markers
(Fig. 1; P<0.05). These results
indicated that upregulation of miR-30 family members may prevent
the EMT of NSCLC cells.
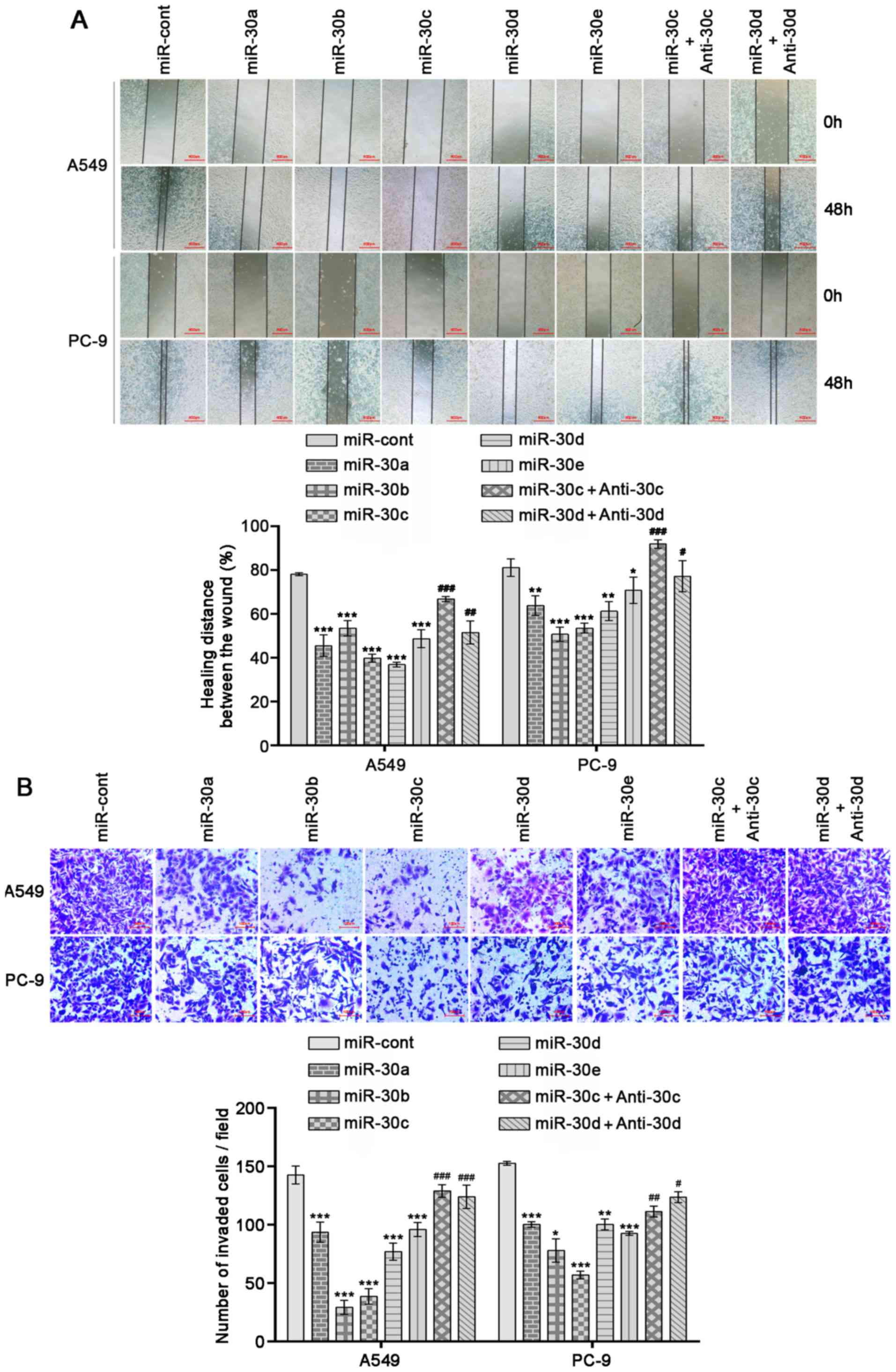
miR-30 family members inhibit the
migration and invasion of NSCLC cells
Since miR-30 family members suppressed the
expression levels of EMT markers, the effects of miR-30 family
members on cell migration and invasion were further investigated
using wound healing and Matrigel Transwell assays. A549 and PC-9
cells overexpressing miR-30 family members exhibited significant
decreases in invasion and migration abilities compared with cells
transfected with negative control mimics (Fig. 2A and B; P<0.05). Moreover, the
overexpression of miR-30c or miR-30d inhibitors reversed the
effects of miR-30c or miR-30d mimics (Fig. 2A and B; P<0.05). These results
indicated that increased expression levels of miR-30 family members
inhibit NSCLC cell invasion and migration by impeding the EMT
process.
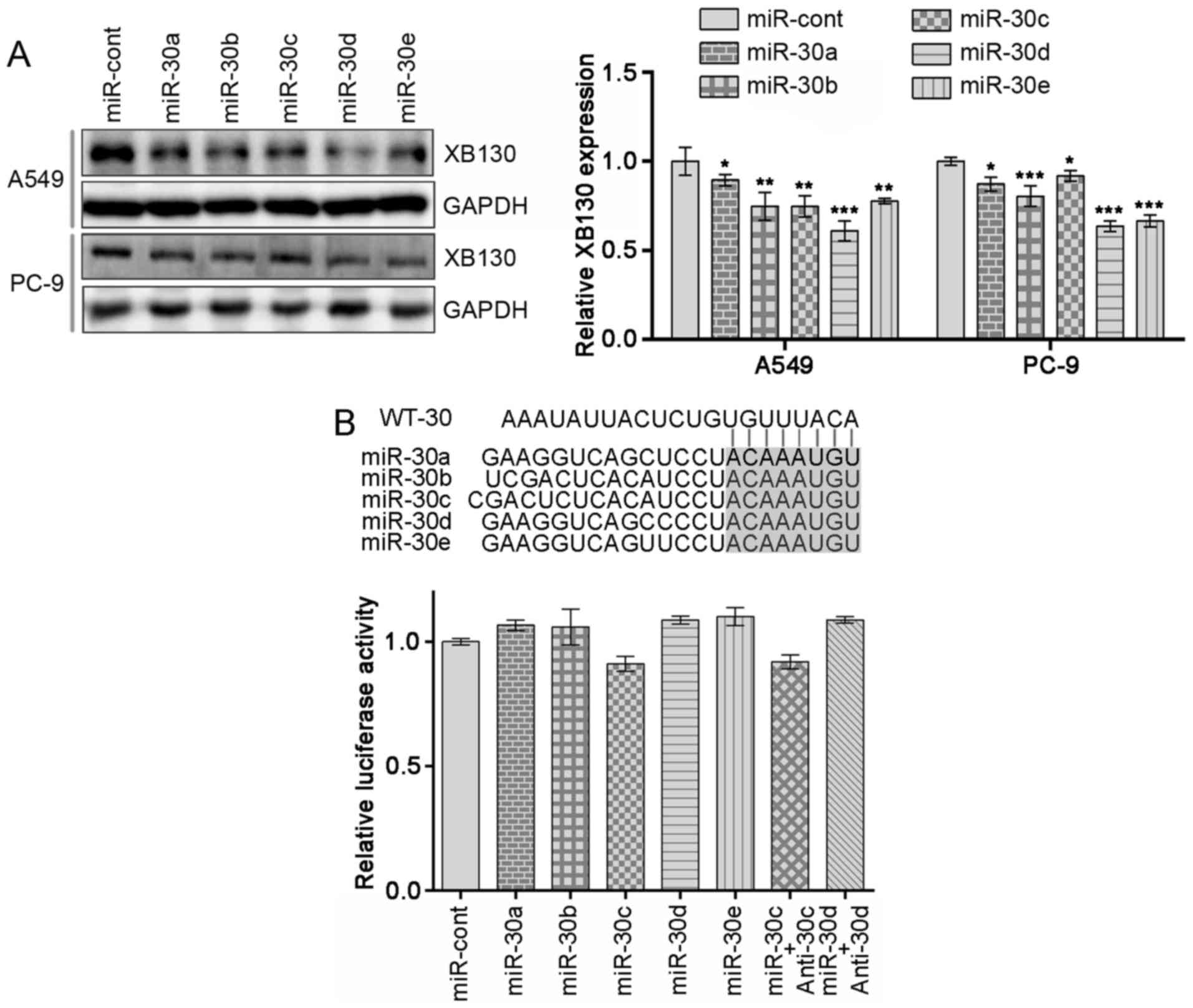
miR-30 family members regulate XB130
expression levels in NSCLC
Next, the molecular mechanisms underlying the
functions of miR-30 family members were determined. Cells
transfected with miR-30 family members exhibited decreased
expression levels of endogenous XB130 protein compared with the
control (Fig. 3A; P<0.05). In
order to confirm whether XB130 is a direct target of miR-30 family
members, miR-30 family binding sites in XB130 3′UTR were identified
using publicly available databases (TargetScan and PicTar).
However, co-transfection of miR-30 family members into PC-9 cells
did not inhibit the activity of Renilla luciferase in
plasmid WT-30 compared with the control (Fig. 3B). Together, these data suggested
that miR-30 family members negatively regulate the expression
levels of XB130 but do not directly target its 3′UTR.
XB130 is involved in the EMT induced
by miR-30 family members in NSCLC cells
In order to evaluate whether miR-30 family members
inhibit NSCLC cell EMT partially by suppressing XB130 expression
levels, siRNAs silencing XB130 were transfected into A549 and PC-9
cells. siRNA treatment led to notable decreases in the protein
expression levels of XB130, N-cadherin, β-catenin and SNAI1
(Fig. 4A; P<0.05). In addition,
ectopic overexpression of XB130 in A549 and PC-9 cells
overexpressing miR-30c or miR-30d counteracted the inhibitory
effects of miR-30c or miR-30d mimics on EMT markers (Fig. 4B; P<0.05). These observations
indicated that XB30 may be involved in miR-30 family-induced EMT in
NSCLC.
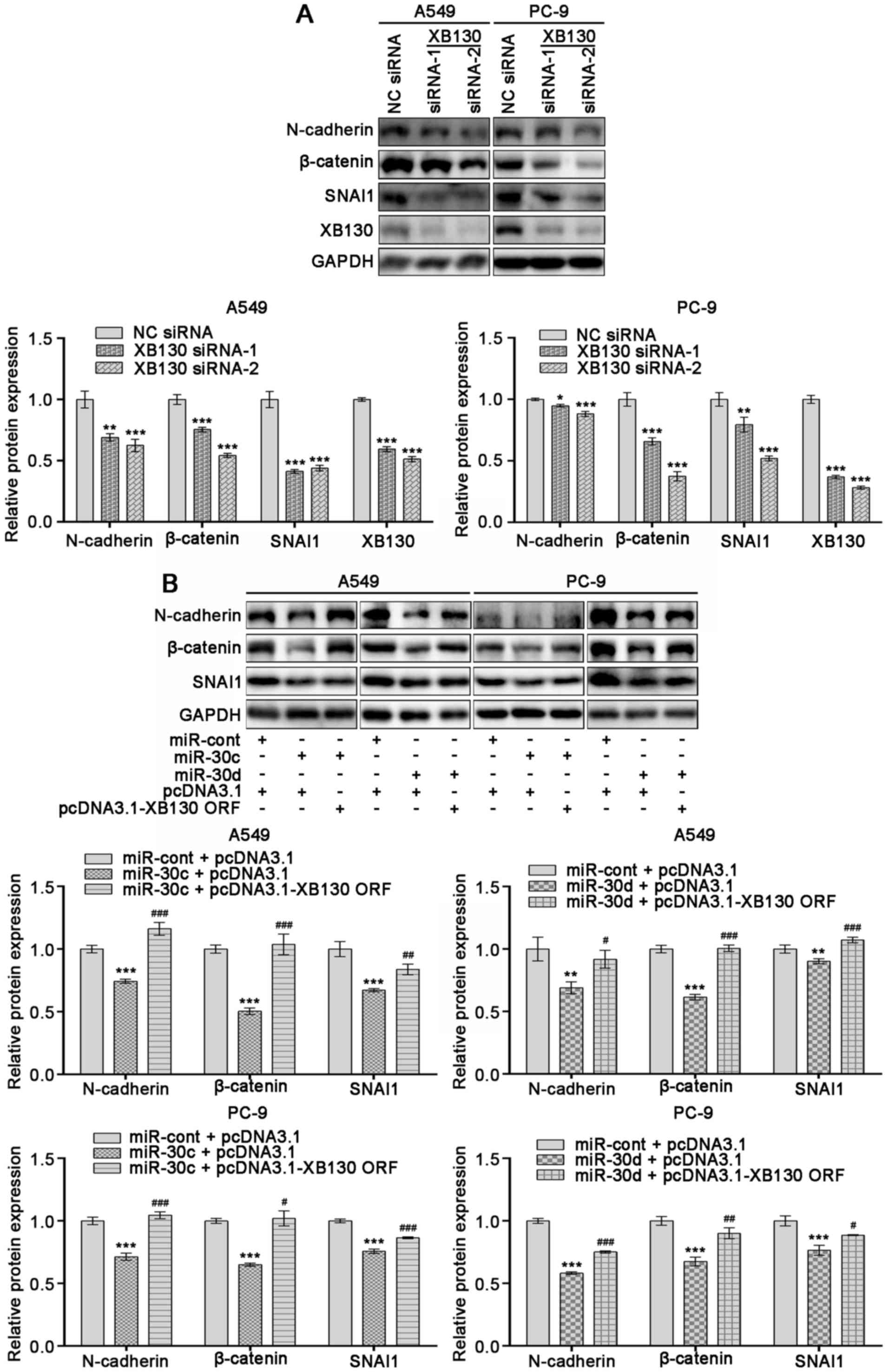 | Figure 4.XB130 is involved in miR-30
family-induced epithelial-to-mesenchymal transition of non-small
cell lung cancer cells. A549 and PC-9 cells were transfected with
(A) XB130 siRNAs (siRNA-1 and −2) or NC siRNA or (B) co-transfected
with miR mimics and DNA plasmids. After 48 h of transfection, total
proteins were obtained and the expression levels of XB130,
N-cadherin, β-catenin and SNAI1 were determined using western
blotting. GAPDH was used as a loading control. Results are
representative of the mean ± standard deviation of three
independent experiments. *P<0.05, **P<0.01 and ***P<0.001
vs. NC siRNA or miR-cont and pcDNA3.1; #P<0.05,
##P<0.01 and ###P<0.001 vs. miR-30c or
miR-30d and pcDNA3.1. miR, microRNA; siRNA, small interfering RNA;
NC, negative control; cont, control; ORF, open reading frame. |
Discussion
EMT is a key process during tumor development and
metastasis (26). miRs are involved
in a number of essential biological processes, including EMT, and
their dysregulation is associated with tumorigenesis (10,27).
miR-30 family members are downregulated in NSCLC and are associated
with the development and metastasis of NSCLC (7,11–14). The
present study confirmed that overexpression of miR-30 family
members in A549 and PC-9 cells reversed NSCLC EMT by inhibiting
XB130 expression levels.
Previously, miR-30a and miR-30c have been
demonstrated to regulate the EMT of NSCLC cells (7,13).
However, the roles of other miR-30 family members in the EMT of
NSCLC cells have not been fully elucidated. The present study
demonstrated that overexpression of miR-30 family members
significantly reversed EMT by decreasing N-cadherin, β-catenin and
SNAI1 expression levels, and also attenuated migration and
invasion. It was confirmed that XB130 protein expression levels
were downregulated by miR-30 family overexpression. miR-30 family
binding sites in XB130 mRNA 3′UTR were identified via
bioinformatics tools; however, the expression levels of
Renilla luciferase in the reporter plasmid were not
suppressed by the overexpression of miR-30 family members. It was
hypothesized that miR-30 family members may suppress XB130
expression levels via other binding sites in XB130 mRNA
(28). Alternatively, the secondary
structure of XB130 3′UTR transcript from the reporter plasmid or
certain RNA-binding proteins binding with the XB130 3′UTR may have
prevented miR-30 family members binding (29,30).
However, these hypotheses require further verification. Another
possible explanation is that XB130 may not be a direct target of
miR-30 family members but an indirect mediator of this family,
regulating EMT in NSCLC cells (25).
XB130, also known as actin filament associated
protein 1-like 2, is a member of AFAP family (31). As a tumor promotor, XB130 expression
levels are upregulated in numerous types of cancer tissues and can
mediate cell proliferation, migration, invasion and EMT by
crosslinking actin filaments, or by downstream activation of
associated signaling pathways, such as PI3K/AKT (15–18,20,22,32–34).
XB130 mRNA is a good predictor of 5-year disease-free
survival rate for patients with NSCLC, as well as a marker to
distinguish adenocarcinoma from squamous cell carcinoma (35). To the best of our knowledge, the
expression level profile of the XB130 protein in NSCLC tissues has
not previously been elucidated. Shiozaki et al (33) demonstrated that XB130 interference
decreased cell proliferation in NSCLC. Our previous study (24) confirmed that XB130 silencing
inhibited NSCLC cell migration, invasion and EMT, similar to the
functions of miR-30 family members. Moreover, ectopic
overexpression of XB130 in the present study reversed the effects
of miR-30c or miR-30d overexpression on the levels of
EMT-associated proteins, indicating that XB130 is involved in
mechanism by which miR-30 family members mediate the EMT
process.
In conclusion, the present study demonstrated that
miR-30 family members decreased the EMT of NSCLC cells by
suppressing XB130 expression levels. However, the molecular
mechanism by which the miR-30 family inhibited XB130 expression
need further investigation. For wound healing assay, culturing
cells with medium containing 4% FBS to allow wound healing is a
limitation of the present study. In addition, further experiments
that shed light on XB130 protein expression in cancer and
paracancerous tissues and perform correlations of the expression
levels of the miR-30 family and XB130 in cancer tissues from
patients with NSCLC are required to verify the findings of the
present study. Combined with the low expression levels of miR-30
family members exhibited by patients with NSCLC (7,11–14), the
present results supported the hypothesis that enhancing miR-30
family expression levels or silencing XB130 may provide improved
survival benefit for patients with NSCLC (13,31,36–39).
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81660474), Guizhou
Provincial Natural Science Foundation [grant no. (2019)1274], Joint
Foundation of Collaboration Project between Scientific and
Technological Bureau of Guizhou Province and Universities of
Guizhou Province [grant no. LH(2016)7347], Natural Science
Foundation of Guizhou Provincial Health Commission (grant no.
gzwjkj2019-1-035), Project of Science and Technology of Guiyang
[grant no. ZhuKeHe(2017)30-4], Regional Common Diseases and Adult
Stem Cell Transformation Research and Innovation Platform of
Guizhou Provincial Department of Science and Technology [grant no.
(2019)4008] and Program of Scientific and Technological Innovation
Team of Guizhou Province [grant no. (2017)5652].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QW, WY and JZ designed the study. KS and YJ
performed the experiments. YZ and YX analyzed the data. QW wrote
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
American Cancer Society, . Global cancer
facts & figures. 4th. Atlanta: American Cancer Society;
2018
|
|
2
|
Mao Y, Yang D, He J and Krasna MJ:
Epidemiology of lung cancer. Surg Oncol Clin N Am. 25:439–445.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stankovic B, Bjørhovde HAK, Skarshaug R,
Aamodt H, Frafjord A, Müller E, Hammarström C, Beraki K, Bækkevold
ES, Woldbæk PR, et al: immune cell composition in human non-small
cell lung cancer. Front Immunol. 9:31012019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rong B and Yang S: Molecular mechanism and
targeted therapy of Hsp90 involved in lung cancer: New discoveries
and developments (Review). Int J Oncol. 52:321–336. 2018.PubMed/NCBI
|
|
5
|
Li S, Gao M, Li Z, Song L, Gao X, Han J,
Wang F, Chen Y, Li W, Yang J and Han X: Role of microRNAs in
metastasis of non-small cell lung cancer. Front Biosci (Landmark
Ed). 21:998–1005. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wood SL, Pernemalm M, Crosbie PA and
Whetton AD: The role of the tumor-microenvironment in lung
cancer-metastasis and its relationship to potential therapeutic
targets. Cancer Treat Rev. 40:558–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kumarswamy R, Mudduluru G, Ceppi P,
Muppala S, Kozlowski M, Niklinski J, Papotti M and Allgayer H:
MicroRNA-30a inhibits epithelial-to-mesenchymal transition by
targeting Snai1 and is downregulated in non-small cell lung cancer.
Int J Cancer. 130:2044–2053. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iderzorig T, Kellen J, Osude C, Singh S,
Woodman JA, Garcia C and Puri N: Comparison of EMT mediated
tyrosine kinase inhibitor resistance in NSCLC. Biochem Biophys Res
Commun. 496:770–777. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li J, Wang Q, Wen R, Liang J, Zhong X,
Yang W, Su D and Tang J: MiR-138 inhibits cell proliferation and
reverses epithelial-mesenchymal transition in non-small cell lung
cancer cells by targeting GIT1 and SEMA4C. J Cell Mol Med.
19:2793–2805. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vishnoi A and Rani S: MiRNA biogenesis and
regulation of diseases: An overview. Methods Mol Biol. 1509:1–10.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhong K, Chen K, Han L and Li B:
MicroRNA-30b/c inhibits non-small cell lung cancer cell
proliferation by targeting Rab18. BMC Cancer. 14:7032014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen D, Guo W, Qiu Z, Wang Q, Li Y, Liang
L, Liu L, Huang S, Zhao Y and He X: MicroRNA-30d-5p inhibits tumour
cell proliferation and motility by directly targeting CCNE2 in
non-small cell lung cancer. Cancer Lett. 362:208–217. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhong Z, Xia Y, Wang P, Liu B and Chen Y:
Low expression of microRNA-30c promotes invasion by inducing
epithelial mesenchymal transition in non-small cell lung cancer.
Mol Med Rep. 10:2575–2579. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu G, Cai J, Wang L, Jiang L, Huang J, Hu
R and Ding F: MicroRNA-30e-5p suppresses non-small cell lung cancer
tumorigenesis by regulating USP22-mediated Sirt1/JAK/STAT3
signaling. Exp Cell Res. 362:268–278. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J, Sun W, Wei H, Wang X, Li H and Yi Z:
Expression of XB130 in human ductal breast cancer. Int J Clin Exp
Pathol. 8:5300–5308. 2015.PubMed/NCBI
|
|
16
|
Wang X, Wang R, Liu Z, Hao F, Huang H and
Guo W: XB130 expression in human osteosarcoma: A clinical and
experimental study. Int J Clin Exp Pathol. 8:2565–2573.
2015.PubMed/NCBI
|
|
17
|
Shiozaki A, Kosuga T, Ichikawa D, Komatsu
S, Fujiwara H, Okamoto K, Iitaka D, Nakashima S, Shimizu H,
Ishimoto T, et al: XB130 as an independent prognostic factor in
human esophageal squamous cell carcinoma. Ann Surg Oncol.
20:3140–3150. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen B, Liao M, Wei Q, Liu F, Zeng Q, Wang
W and Liu J, Hou J, Yu X and Liu J: XB130 is overexpressed in
prostate cancer and involved in cell growth and invasion.
Oncotarget. 7:59377–59387. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi M, Zheng D, Sun L, Wang L, Lin L, Wu
Y, Zhou M and Liao W, Liao Y, Zuo Q and Liao W: XB130 promotes
proliferation and invasion of gastric cancer cells. J Transl Med.
12:12014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li GM, Liang CJ, Zhang DX, Zhang LJ, Wu JX
and Xu YC: XB130 knockdown inhibits the proliferation,
invasiveness, and metastasis of hepatocellular carcinoma cells and
sensitizes them to TRAIL-induced apoptosis. Chin Med J (Engl).
131:2320–2331. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shiozaki A, Lodyga M, Bai XH, Nadesalingam
J, Oyaizu T, Winer D, Asa SL, Keshavjee S and Liu M: XB130, a novel
adaptor protein, promotes thyroid tumor growth. Am J Pathol.
178:391–401. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xie T, Jiang C, Dai T, Xu R, Zhou X, Su X
and Zhao X: Knockdown of XB130 restrains cancer stem cell-like
phenotype through inhibition of Wnt/β-Catenin signaling in breast
cancer. Mol Carcinog. 58:1832–1845. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cho HR, Wang Y, Bai X, Xiang YY, Lu C,
Post A, Al Habeeb A and Liu M: XB130 deficiency enhances
carcinogen-induced skin tumorigenesis. Carcinogenesis.
40:1363–1375. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Q, Yang G, Jiang Y, Luo M, Li C, Zhao
Y, Xie Y, Song K and Zhou J: XB130, regulated by miR-203, miR-219,
and miR-4782-3p, mediates the proliferation and metastasis of
non-small-cell lung cancer cells. Mol Carcinog. 59:557–568. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takeshita H, Shiozaki A, Bai XH, Iitaka D,
Kim H, Yang BB, Keshavjee S and Liu M: XB130, a new adaptor
protein, regulates expression of tumor suppressive microRNAs in
cancer cells. PLoS One. 8:e590572013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fazilaty H, Rago L, Kass Youssef K, Ocaña
OH, Garcia-Asencio F, Arcas A, Galceran J and Nieto MA: A gene
regulatory network to control EMT programs in development and
disease. Nat Commun. 10:51152019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shukla V, Adiga D, Jishnu PV, Varghese VK,
Satyamoorthy K and Kabekkodu SP: Role of miRNA clusters in
epithelial to mesenchymal transition in cancer. Front Biosci (Elite
Ed). 12:48–78. 2020.PubMed/NCBI
|
|
28
|
Atambayeva S, Niyazova R, Ivashchenko A,
Pyrkova A, Pinsky I, Akimniyazova A and Labeit S: The binding sites
of miR-619-5p in the mRNAs of human and orthologous genes. BMC
Genomics. 18:4282017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kelly TJ, Suzuki HI, Zamudio JR, Suzuki M
and Sharp PA: Sequestration of microRNA-mediated target repression
by the Ago2-associated RNA-binding protein FAM120A. RNA.
25:1291–1297. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zheng Z, Reichel M, Deveson I, Wong G, Li
J and Millar AA: Target RNA secondary structure is a major
determinant of miR159 efficacy. Plant Physiol. 174:1764–1778. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bai XH, Cho HR, Moodley S and Liu M:
XB130-a novel adaptor protein: Gene, function, and roles in
tumorigenesis. Scientifica (Cairo). 2014:9030142014.PubMed/NCBI
|
|
32
|
Zhang J, Jiang X and Zhang J: Prognostic
significance of XB130 expression in surgically resected pancreatic
ductal adenocarcinoma. World J Surg Oncol. 12:492014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shiozaki A, Shen-Tu G, Bai X, Iitaka D, De
Falco V, Santoro M, Keshavjee S and Liu M: XB130 mediates cancer
cell proliferation and survival through multiple signaling events
downstream of Akt. PLoS One. 7:e436462012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yamanaka D, Akama T, Chida K, Minami S,
Ito K, Hakuno F and Takahashi S: Phosphatidylinositol
3-kinase-associated protein (PI3KAP)/XB130 crosslinks actin
filaments through its actin binding and multimerization properties
in vitro and enhances endocytosis in HEK293 cells. Front Endocrinol
(Lausanne). 7:892016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lodyga M, Xhi C, Anraku M, Liu N, Tsao M
and Liu M: P-080 Prognostic expression of a novel adaptor protein
XB130 in non-small-cell lung cancer. Lung Cancer. 49 (Suppl
2):S1352005. View Article : Google Scholar
|
|
36
|
Luan N, Wang Y and Liu X: Absent
expression of miR-30a promotes the growth of lung cancer cells by
targeting MEF2D. Oncol Lett. 16:1173–1179. 2018.PubMed/NCBI
|
|
37
|
Li G, Fang J, Wang Y, Wang H and Sun CC:
MiRNA-based therapeutic strategy in lung cancer. Curr Pharm Des.
23:6011–6018. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shiozaki A and Liu M: Roles of XB130, a
novel adaptor protein, in cancer. J Clin Bioinforma. 1:102011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu J, Bai XH, Lodyga M, Han B, Xiao H,
Keshavjee S, Hu J, Zhang H, Yang BB and Liu M: XB130, a novel
adaptor protein for signal transduction. J Biol Chem.
282:16401–16412. 2007. View Article : Google Scholar : PubMed/NCBI
|