Introduction
Pyropia yezoensis (Porphyra yezoensis; P
yezoensis) is an edible marine red alga containing various
biological macromolecules, such as sulfated polysaccharides, which
have antioxidant, anti-inflammatory, antitumor and immunomodulatory
activity (1–5). The anticancer bioactivities and
applications of natural polysaccharides are of considerable
interest to researchers and have been investigated using in
vivo and in vitro models. For example, Porphyra
haitanensis polysaccharide exhibited an antiproliferative
effect on the GC7901 human gastric cancer cell line via the
induction of cell apoptosis, and demonstrated an in vivo
antitumor effect on SGC7901 tumor-bearing mice (6). An ultrasound-degraded polysaccharide
from P. yezoensis also demonstrated significant inhibitory
activity in SGC7901 cells (2). In
addition, an agar-type sulfated polysaccharide derived from
Gracilaria dominguensis inhibited Ehrlich ascites carcinoma
in mice (7). Furthermore, a sulfated
polysaccharide from Champia feldmannii (Diaz-Pifferer)
inhibited sarcoma 180 tumors in mice (8). These previous studies indicate that
various sulfated polysaccharides isolated from red algae have the
potential to be used as natural antitumor agents due to their
effectiveness in inhibiting the proliferation of tumor cells in
vitro and in vivo.
There is also evidence to suggest that
oligosaccharides and polysaccharides derived from seaweed are
beneficial for human health and may have a wide range of
applications (9). In a review by
Cheong et al (10), the
notable biological activity of oligosaccharides from red seaweed
was suggested to support their development for use in functional
foods and the pharmaceutical industry. Therefore, polysaccharides,
oligosaccharides and their derivates are of great interest to
researchers. There are a number of reports concerning the use of
polysaccharides with low molecular weight (Mw) to treat cancer in
clinical trials, with these polysaccharides including glucan-based
oligosaccharides, heparan sulfate mimetics and inulin/oligofructose
(11–13). Low-Mw polysaccharides are generally
prepared by methods including acid hydrolysis, ultrasonic
degradation, an ascorbic acid/H2O2 redox
system, enzymatic degradation, microwave-assisted acid hydrolysis
and gamma-irradiation (2,14–17). The
use of different degradation methods may help to broaden the scope
of the polysaccharides.
The present study aimed to expand the antitumor
applications of sulfated polysaccharides isolated from algae, and
also to elucidate the characteristics and bioactivity of some
degraded derivatives obtained using gamma-irradiation.
Specifically, a sulfated polysaccharide was extracted from the
newly cultivated strain P. yesoensis Sookwawon 104 by dilute
hydrochloric acid extraction, and low-Mw polysaccharides were
prepared from it by gamma-irradiation (20 and 100 kGy). The in
vitro antiproliferative activity of the P. yezoensis
sulfated polysaccharide (PYSP) and its derivatives on three tumor
cell lines, namely the HeLa human cervical cancer cell line,
MDA-MB-231 human breast carcinoma cell line and Hep3B human hepatic
carcinoma cell line, and their potential mechanisms were also
investigated.
Materials and methods
Materials and chemicals
The algal specimen P. yezoensis Sookwawon 104
was collected by the National Institute of Fisheries Science (South
Korea). The specimen was identified by EJP and deposited at the
Seaweed Research Center (South Korea) with the voucher specimen
number Sookwawon 104. Thiazolyl blue tetrazolium bromide (MTT) were
purchased from BBI Life Sciences Corporation. Mannose, rhamnose,
glucuronic acid, galacturonic acid, glucose, galactose, xylose,
arabinose and fucose were obtained from the Sinopharm Chemical
Reagent Co. Ltd for the monosaccharide composition analysis.
TransScript All-in-One First-Strand cDNA Synthesis SuperMix and
TransStart® Top Green qPCR SuperMix were purchased from
Beijing TransGen Biotech Co., Ltd. Dulbecco's modified Eagle's
medium (DMEM), Leibovitz's L-15 medium and fetal bovine serum (FBS)
were purchased from Gibco (Thermo Fisher Scientific, Inc.).
Penicillin-streptomycin solution (100X) was obtained from Biosharp
Life Sciences. All other chemical reagents used were of analytical
grade.
Extraction of P. yezoensis
polysaccharide
Dried and powdered P. yezoensis Sookwawon 104
(50 g) was passed through a 40-mesh sieve. Fat and pigment were
then removed by refluxing with 250 ml 95% ethanol at 60°C for 6 h.
The residue (45 g) was extracted twice with 1 mM HCl (1.3 l) at
80°C for 2 h. After filtration, the supernatant was concentrated to
0.65 l using a rotary evaporator at 50°C. Then, 95% ethanol (2.6 l)
was added to the concentrate which was maintained at 4°C overnight.
Following centrifugation at 2,000 × g for 10 min at room
temperature, the precipitate, named PYSP, was collected and dried
in a vacuum drying oven at 70°C (18).
Preparation of degradation derivatives
by gamma-irradiation
PYSP (5% in water, w/v; pH 7.0) was degraded by
gamma-irradiation at doses of 20 and 100 kGy, respectively, as
previously described (15). The
degradation derivatives were collected, lyophilized in a vacuum and
freeze-dried. The derivatives obtained using 20 and 100 Gy were
named as PYSP-20 and PYSP-100, respectively.
Component analysis
PYSP and its degradation derivatives were subjected
to component analysis. The total sugar content was detected using
the phenol-sulfuric acid method (19). The protein content was analyzed using
the Bradford method (20). The
sulfate group content was determined using a turbidimetric method
(21).
Monosaccharide composition
analysis
The monosaccharide compositions of PYSP, PYSP-20 and
PYSP-100 were analyzed by high-performance liquid chromatography
using a 1-phenyl-3-methyl-5-pyrazolone (PMP) pre-column
derivatization method (22).
Briefly, the samples were hydrolyzed with 2 M trifluoroacetic acid
at 100°C for 4 h. Excess acid was removed by adding ethanol at
60°C, and then NaOH (0.3 M, 300 µl) and PMP (0.5 M, 300 µl) were
added to the reaction mixture, which was subsequently incubated at
70°C for another 1 h. Following neutralization by the addition of
0.3 M HCl, chloroform (1 ml) was added to the reaction mixture. The
aqueous phase of three samples (20 µl) was analyzed by Waters 1525
HPLC system (Waters Corporation; http://www.waters.com/nextgen/us/en.html) on a
Hypersil ODS-2 column (5 µm, 4.6×250 mm; Thermo Fisher Scientific
Inc.) at a flow rate of 0.8 ml/min. The mobile phases were 0.05 M
phosphate buffer solution (pH 6.8) and acetonitrile (83:17, v/v),
and the detection wavelength was 254 nm at 25°C. Different
monosaccharide standards (mannose, fucose, xylose, galactose,
glucose, arabinose, rhamnose, galacturonic acid and glucuronic
acid) were used to analyze the monosaccharide composition of PYSP
and its derivatives.
Mw analysis
The Mw distributions of PYSP, PYSP-20 and PYSP-100
were measured by high-performance gel permeation chromatography.
Dextran standards with different molecular weights (2,000, 150,
41.1, 21.4, 7.1 and 4.6 kDa, and 180 Da) were used to calibrate the
column and establish a standard curve using linear regression
(22). Each sample, dissolved in 0.1
M Na2SO4 solution, was analyzed using a
TSK-GEL G5000 PWXL column (7.8×300 mm; Tosoh Corporation) and
Waters 2424 Refractive Index Detector (Waters Corporation), which
was eluted with 0.1 M Na2SO4 solution.
Fourier transform-infrared (FT-IR)
analysis
Each sample (4 mg) was mixed with KBr powder (0.4
g), pressed into pellets and analyzed using an Infrared
Spectrometer TENSOR 27 (Bruker Corporation) at the frequency range
from 400 to 4,000 cm−1 (23).
Cell culture
HeLa, Hep3B and MDA-MB-231 cells were purchased from
the Cell Bank of Shanghai Institute of Biochemistry and Cell
Biology. The HeLa and Hep3B cells were maintained in DMEM, and the
MDA-MB-231 cells were maintained in L-15 medium. All media were
supplemented with 10% FBS and antibiotics (100 U/ml penicillin and
100 µg/ml streptomycin). The cell cultures were incubated at 37°C
in a humidified atmosphere containing 5% CO2.
MTT assay
Hep3B, HeLa and MDA-MB-231 cells were each seeded in
96-well plates at a density of 3×103 cells/well in 200
µl medium. The cells were treated with PYSP, PYSP-20 or PYSP-100 at
concentrations of 200 or 500 µg/ml at 37°C for 48 h. Then, 20 µl
MTT (5 mg/ml) was added to each well, and the cells were incubated
for another 4 h. Finally, the cell viability was detected as
previously described (24). The
inhibition rate was calculated from the optical density (OD) at 490
nm using the following formula: Inhibition rate (%) =
(1-ODtreatment/ODuntreated) ×100.
Crystal violet assay
Hep3B, HeLa and MDA-MB-231 cells were seeded in
24-well plates at a density of 5×104 cells/well in 1 ml
medium overnight and then treated with PYSP-20 or PYSP-100 at 200
or 500 µg/ml at 37°C for 48 h. Untreated cells served as the
control. The cells were then fixed with 4% paraformaldehyde at 25°C
for 30 min, stained with 0.1% crystal violet for 30 min at room
temperature, and then washed with distilled water. Finally, 10%
acetic acid was added to each well and the absorbance at 595 nm was
measured using a Cytation 3 microplate reader (BioTek Instruments,
Inc.). The relative proliferation rate was calculated using the
following formula: Relative proliferation rate =
ODtreatment/ODuntreated.
Reverse transcription-quantitative PCR
(RT-qPCR)
Hep3B, HeLa and MDA-MB-231 cells were seeded in
24-well plates at a density of 5×104 cells/well in 1 ml
medium overnight and then treated with PYSP-20 or PYSP-100 at 200
µg/ml at 37°C for 48 h. The cells were then collected and total RNA
was extracted from them using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). RNA (2 µg/µl) was used for cDNA synthesis
using TransScript All-in-One First-Strand cDNA Synthesis SuperMix.
The cDNA samples were used as the template for the qPCR reaction
using gene-specific primers. The final reaction volume of 10 µl
contained 5 µl TransStart® Top Green qPCR SuperMix, 0.5
µl forward and reverse primers and 1 µl cDNA template. qPCR was
conducted using a LightCycler 480 Instrument II (Roche Applied
Science). The PCR thermocycling conditions were as follows: 1 cycle
at 95°C for 30 sec followed by 40 cycles at 95°C for 30 sec, 58°C
for 30 sec and 72°C for 20 sec. The relative amounts of mRNA were
calculated using the 2−ΔΔCq method (25). The primer sequences were as follows:
P53, forward: 5′-CCCCTCCTGGCCCCTGTCATCTTC-3′ and reverse:
5′-GCAGCGCCTCACAACCTCCGTCAT-3′; P21, forward:
5′-GCGGAACAAGGAGTCAGACA-3′ and reverse: 5′-GAACCAGGACACATGGGGAG-3′;
Cyclin B1, forward: 5′-CTGCTGGGTGTAGGTCCTTG-3′ and reverse:
5′-TGCCATGTTGATCTTCGCCT-3′; Cdk1, forward:
5′-TTGAAACTGCTCGCACTTGG-3′ and reverse: 5′-TCCCGGCTTATTATTCCGCG-3′;
GAPDH, forward: 5′-GCAGGGGGGAGCCAAAAGGGT-3′ and reverse:
5′-TGGGTGGCAGTGATGGCATGG-3′. GAPDH served as an internal
reference.
Statistical analysis
Data are expressed as means ± standard deviation.
GraphPad Prism 5.0 (GraphPad Software, Inc.) and Origin 8.5
(OriginLab Corporation) were used to prepare graphs and for
analysis of the data using one-way and two-way ANOVA analysis of
variance followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Characterization of the
polysaccharides
Extraction of 50 g dried P. yezoensis
Sookwawon 104 using diluted hydrochloric acid extraction and
ethanol precipitation yielded 5 g PYSP. The carbohydrate content,
Mw and chemical composition of PYSP and its degradation products
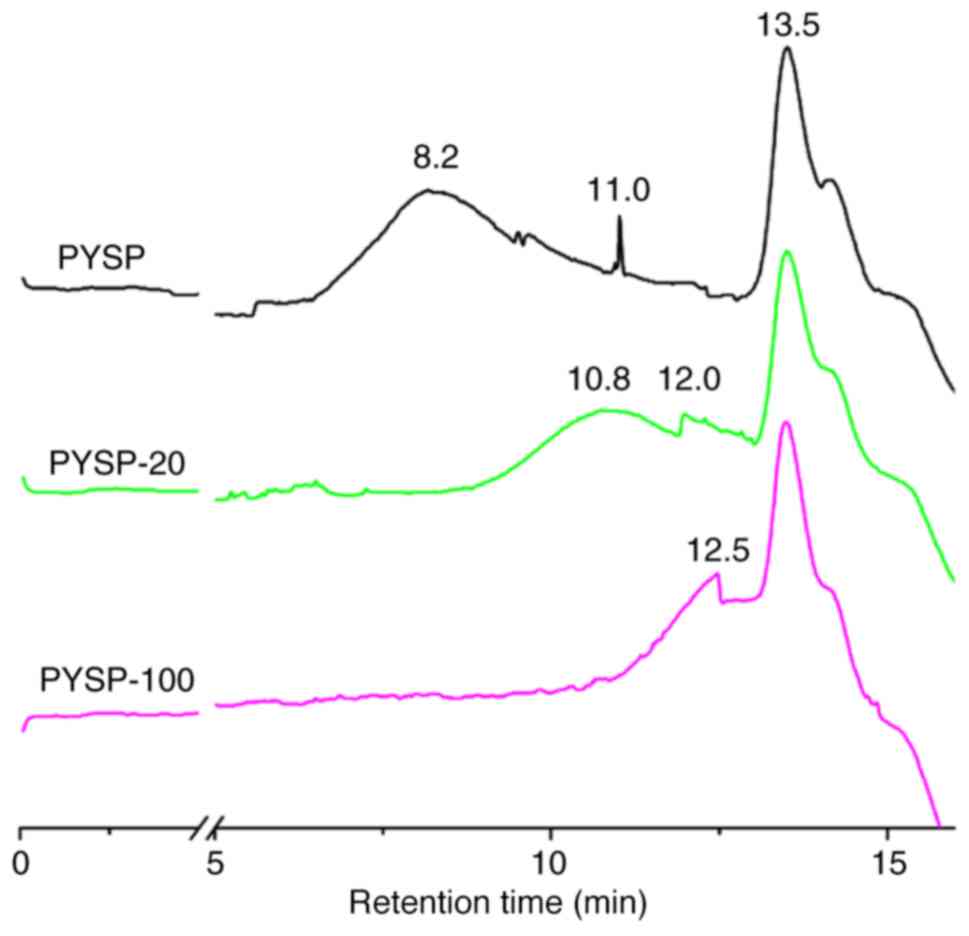
are shown in Table I. PYSP, PYSP-20
and PYSP-100 were composed of galactose, fucose and glucose in a
molar ratio of 1.6:17.9:1.0, 2.0:17.6:1.0 and 1.7:13.5:1.0,
respectively. The chemical compositions of PYSP, PYSP-20, and
PYSP-100 were not markedly different; however, the Mw distribution
was clearly reduced when the dose of gamma-irradiation was
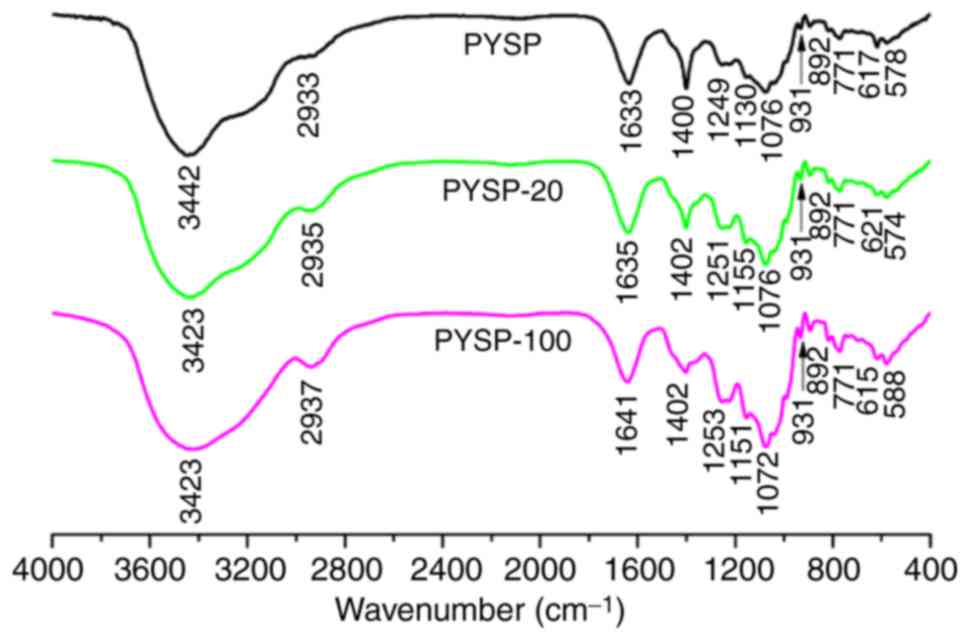
increased (Fig. 1). The FT-IR
spectrum (Fig. 2) of each sample
revealed a major broad stretching peak at ~3,430 cm−1
for the hydroxyl group, and a weak band at ~2,930 cm−1
for the C-H stretching vibration. The peak at 931 cm−1
indicated the existence of an ether bond (-C-O-C-), suggesting all
samples contained 3,6-anhydro-α-L-galactose (14). The signals presented at ~1,250 and
890 cm−1 were respectively caused by the stretching
vibrations of S=O and C-O-S groups (26,27),
indicating that all samples contained sulfate groups. The peaks
near 1,635 and 1,400 cm−1 observed for PYSP, PYSP-20 and
PYSP-100 were the stretching vibrations of carboxyl and carbonyl
groups (28). Together, the
composition analysis and FT-IR spectra confirmed that PYSP and its
derivatives did not exhibit any marked differences, with the
exception of Mw distribution.
 | Table I.Mw distributions and chemical
compositions of PYSP and its derivatives. |
Table I.
Mw distributions and chemical
compositions of PYSP and its derivatives.
|
|
|
|
|
| Molar ratio of
monosaccharides |
|---|
|
|
|
|
|
|
|
|---|
| Sample | Carbohydrate
(%) | Mw (kDa) | Protein (%) | Sulfate (%) | Glc | Gal | Fuc |
|---|
| PYSP | 83.6±1.63 | 3,315; 137; 8 | 0.83±0.03 | 12.2±0.07 | 1.6 | 17.9 | 1.0 |
| PYSP-20 | 83.0±1.66 | 172; 44; 8 | 0.42±0.07 | 12.7±0.15 | 2.0 | 17.6 | 1.0 |
| PYSP-100 | 83.0±1.55 | 25.8; 8 | 0.38±0.08 | 12.6±0.37 | 1.7 | 13.5 | 1.0 |
Antiproliferative activity
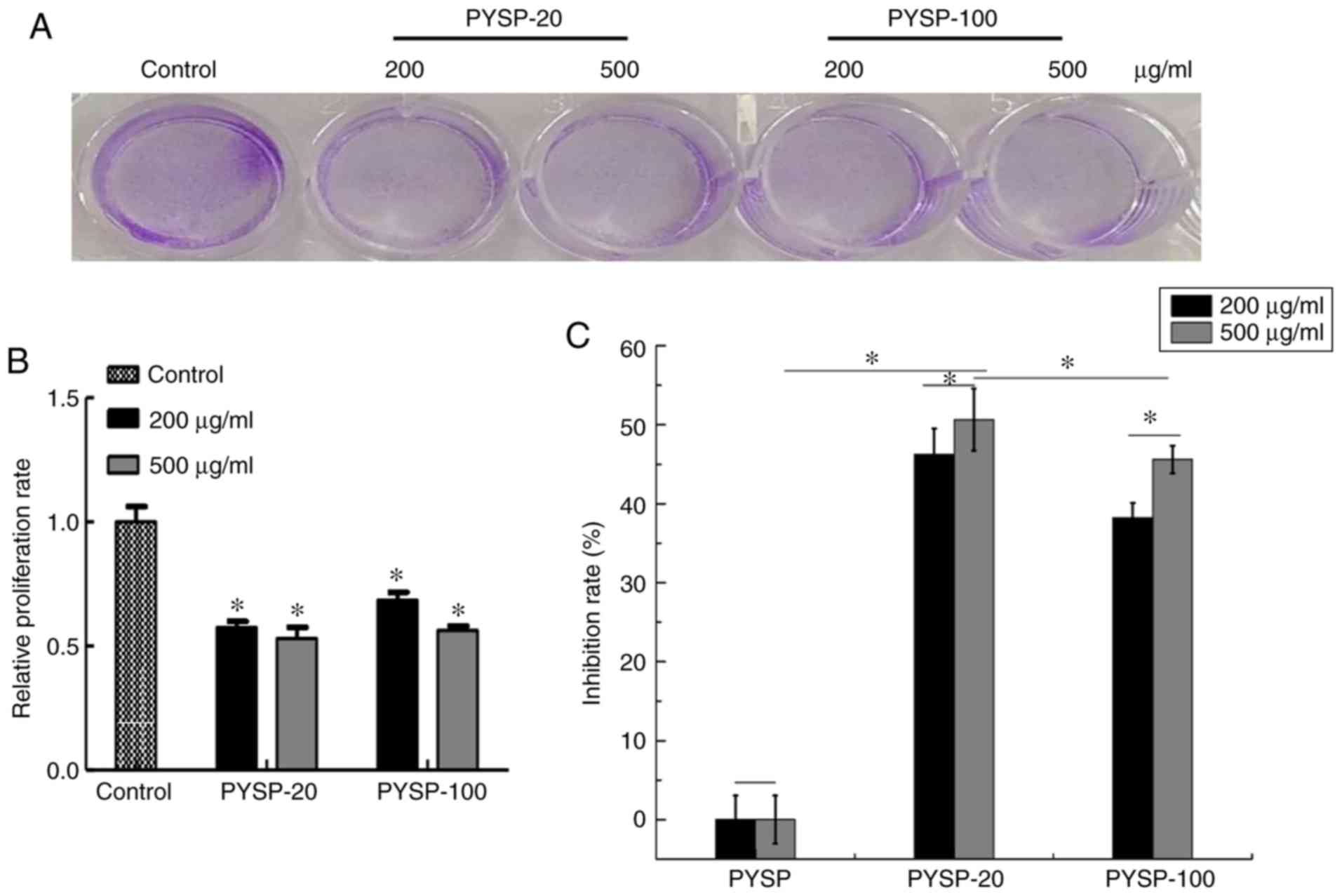
The in vitro antiproliferative effects of
PYSP, PYSP-20 and PYSP-100 on Hep3B, HeLa and MDA-MB-231 cells were
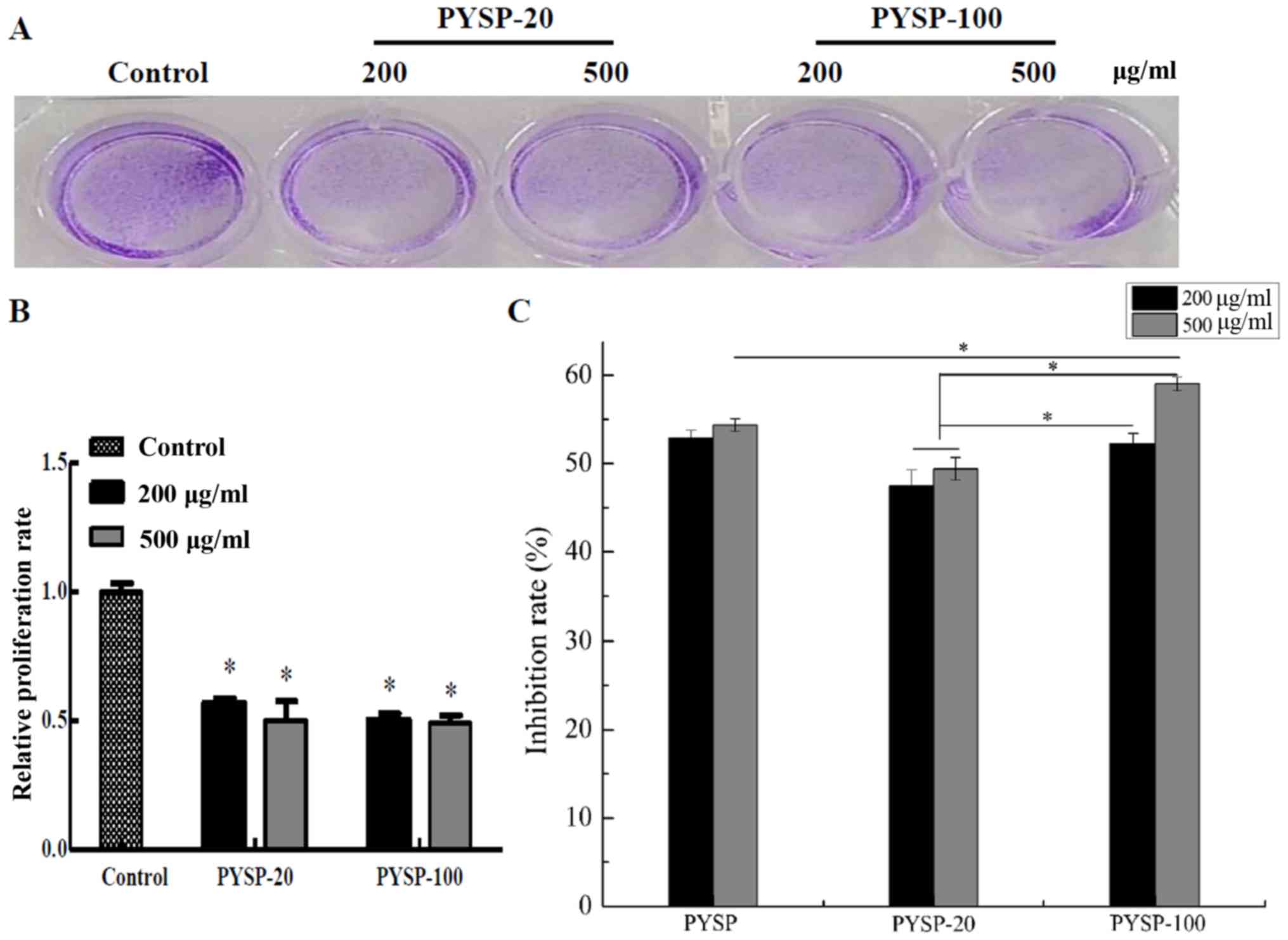
analyzed using MTT and crystal violet assays. As shown in Fig. 3, PYSP-20 and PYSP-100 exhibited
marked antiproliferative effects on MDA-MB-231 cells, whereas PYSP
had weaker antiproliferative activity. PYSP-20 and PYSP-100
displayed inhibition rates of 40–50% in MDA-MB-231 cells. Notably,
the inhibition rate for PYSP-20 at a concentration of 500 µg/ml
reached 50.6% (Fig. 3C). According
to the results of the crystal violet assay, the relative
proliferation rate of the cells decreased by almost half following
treatment with PYSP-20 or PYSP-100 (Fig.
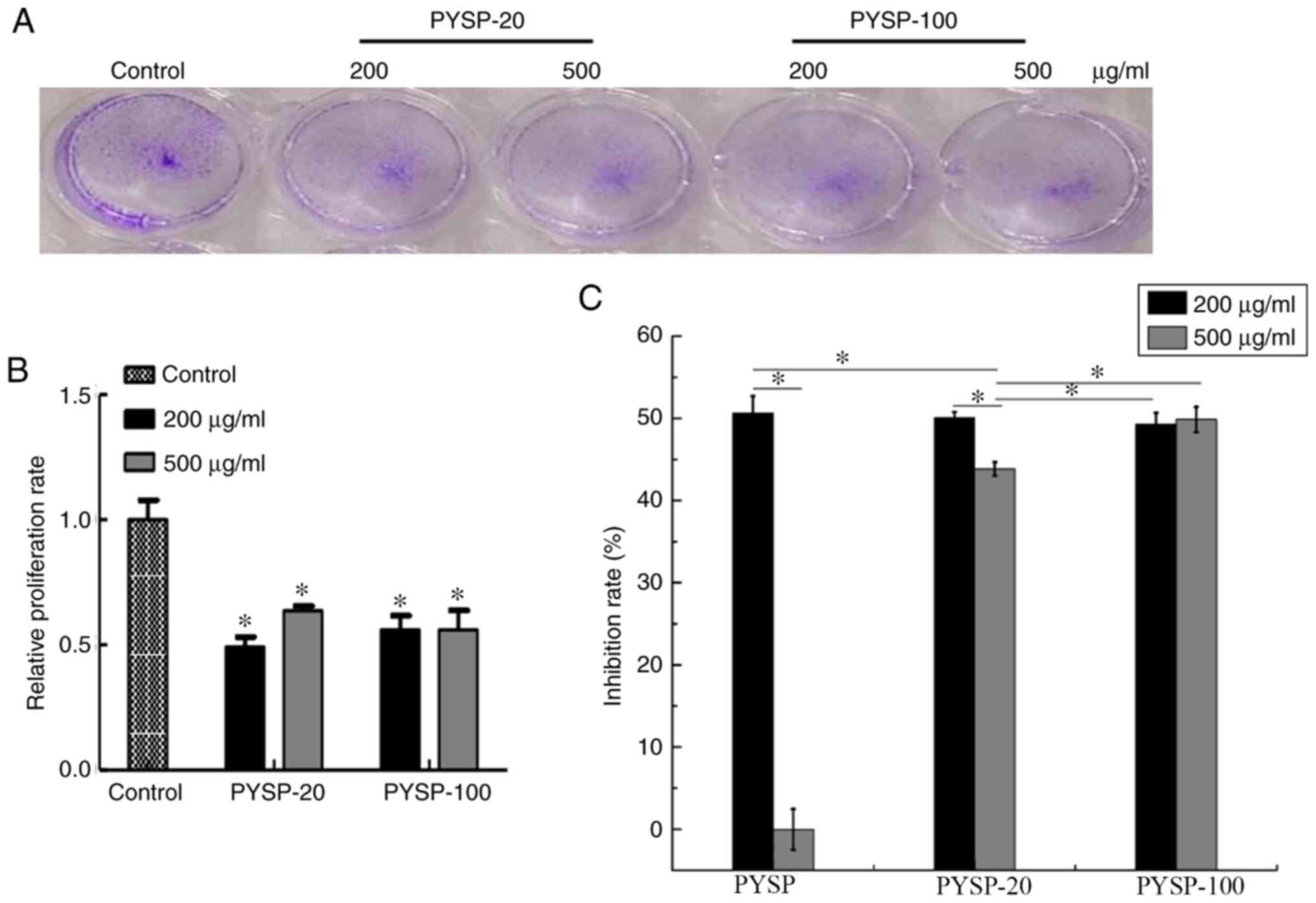
3A and B). The effects of PYSP, PYSP-20 and PYSP-100 on HeLa
cells are shown in Fig. 4. PYSP-20
and PYSP-100 at concentrations of 200 and 500 µg/ml exhibited
notable antiproliferative effects against HeLa cells, with a
maximum inhibition rate of ~50% (Fig.
4C). However, PYSP exhibited an antiproliferative effect only
at 200 µg/ml. Consistent with the results of the MTT assay, the
relative proliferation rates of HeLa cells treated with PYSP-20 or
PYSP-100 determined using the crystal violet assay (Fig. 4A and B) exhibited a similar
inhibitory trend as those of the MTT assay in Fig. 4C. In Hep3B cells, PYSP, PYSP-20 and
PYSP-100 exhibited antiproliferative effects at concentrations of
200 and 500 µg/ml. and their inhibition rate reached ~50%. The
inhibition rate of PYSP-100 was significantly higher than that of
PYSP or PYSP-20 at the concentration of 500 µg/ml (Fig. 5C). Also, the relative proliferation
rates of PYSP-20 and PYSP-100 were been reduced by almost half
compared with those in the control group (Fig. 5A and B).
Due to the greater in vitro antiproliferative
activity of PYSP-20 and PYSP-100 when compared with PYSP, their
potential antiproliferative mechanism was further explored through
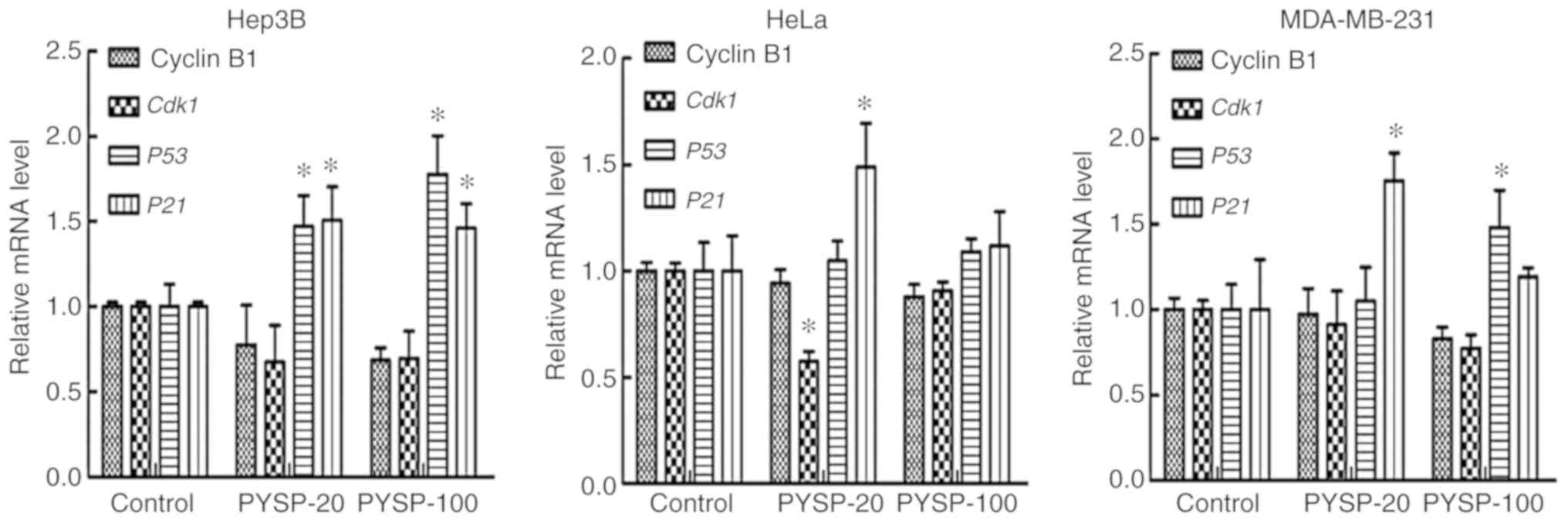
measuring the expression of genes regulating the cell cycle, namely
Cyclin B1, Cdk1, P53 and P21 (Fig. 6). The treatment of Hep3B cells with
PYSP-20 or PYSP-100 appeared to reduce the mRNA levels of cyclin B1
and Cdk1 compared with those in the control group, while the
expression levels of P53 and P21 significantly
increased. In HeLa cells, Cdk1 was significantly decreased
after PYSP-20 treatment. Furthermore, P53 and P21 in
HeLa cells appeared to be upregulated following PYSP-20 or PYSP-100
treatment, with P21 exhibiting a significant increase in
response to treatment with PYSP-20. In the MDA-MB-231 cells, Cyclin
B1 and Cdk1 appeared to be slightly downregulated after
PYSP-20 or PYSP-100 treatment. Moreover, PYSP-20 exposure
significantly increased the mRNA levels of P21, while
PYSP-100 significantly increased the mRNA levels of P53
expression in MDA-MB-231 cells.
Discussion
Red algae is an abundant marine resource that
comprises various species, including Gracilaria gracili and
P. yezoensis. P. yezoensis contains multiple
bioactive macromolecules, including polysaccharides, proteins and
polyunsaturated fatty acids (29).
In the present study, the polysaccharide PYSP was extracted from a
new red alga strain P. yezoensis Sookwawon 104 and the
degradation derivatives PYSP-20 and PYSP-100 were prepared by
gamma-irradiation. The antiproliferative activities of these
polysaccharides were investigated in vitro against Hep3B,
HeLa and MDA-MB-231 cells. In the Mw analysis, the elution curve
indicated that PYSP mainly comprises high-Mw polysaccharide. Since
the available evidence shows that the reduction of Mw may improve
the bioavailability of polysaccharides (30), a degradation method using
gamma-irradiation was used to prepare low-Mw polysaccharides from
PYSP using the irradiation doses 20 and 100 kGy, according to our
previous studies (15,31,32). In
the present study, PYSP-20 and PYSP-100 exhibited a significant
reduction in Mw compared with PYSP, but the monosaccharide
composition and sulfate group content did not change markedly,
consistent with our previous study (32). In addition, in the FT-IR spectra,
there was also no clear difference in the characteristic absorption
bands among these polysaccharides, the only exception being that
PYSP-20 and PYSP-100 exhibited a slight difference in the
stretching vibrations of carboxyl and carbonyl groups, possibly due
to the breaking of those chemical bonds by the gamma-irradiation
(32).
Polysaccharides have been shown to exhibit lower
inhibition rates on tumor cells when used at low concentrations.
For example, a polysaccharide from Cordyceps gunnii mycelia
demonstrated only weak inhibitory activity against tumor cells when
used at a low concentration, such as 50 or 100 µg/ml (33). Zhang et al (34) also demonstrated that low
concentrations of polysaccharide, ranging from 25 to 100 µg/ml, had
only a weak inhibitory effect on tumor cell viability. Therefore,
with consideration of these previous studies, the sample
concentrations used in the present study were selected as 200 and
500 µg/ml. PYSP and its derivatives exhibited different Mw
distribution ranges, and PYSP with a higher Mw exhibit weaker
antitumor capability compared with its low-Mw derivatives (PYSP-20
and PYSP-100). Therefore, we speculate that Mw is a key factor
affecting the distinct antiproliferative activity of PYSP and its
derivatives. This is consistent with a previous study (35), in which the Mw of sulfated
Artemisia sphaerocephala polysaccharides was highly
associated with their antitumor activity, and low-Mw polysaccharide
demonstrated a greater inhibitory ability against A549, HepG2 and
HeLa cells in vitro. However, for the HeLa cells in the
present study, the high-Mw polysaccharide PYSP exhibited
antiproliferative activity only at the lower concentration,
indicating that the antiproliferative activity of high-Mw
polysaccharide might also be affected by the dosage. Choromanska
et al (36) demonstrated that
high-Mw β-glucan had stronger growth inhibitory activity against
A549 and H69AR cells at a low concentration (200 µg/ml) compared
with other higher concentrations. Although these results indicate
that the low-Mw polysaccharides in the present study have a
promising in vitro antiproliferative effect on cancer cell
lines, validation of their antitumor effect and evaluation of
toxicity are required in further studies. Also, previous studies
have demonstrated that the upregulation of P53 and
P21, together with the downregulation of Cyclin B1 and
Cdk1, serve important roles in blocking the cell cycle,
which is the potential antitumor mechanism of a variety of clinical
anticancer medicines (37–40). The present data demonstrate that
PYSP-20 and PYSP-100 are able to regulate the expression of P53,
P21, Cyclin B1 and Cdk1 and so may induce cell cycle
arrest. As was shown above the studies have shown that
polysaccharides have antitumor activity. Meanwhile, it was reported
that polysaccharide was also a kind chemotherapeutic assistant
drug. For example, one study reported that when low-Mw
polysaccharide was used as a carrier for 5-fluorouracil (5-FU), the
antitumor activity of 5-FU against transplanted S180 tumors in mice
was enhanced (41). Thus, the
synergistic effects of polysaccharide with conventional
chemotherapeutic drugs, as a combination therapy against cancer,
are of considerable interest.
In summary, a sulfated polysaccharide from P.
yezoensis Sookwawon 104 and its low-Mw derivatives obtained by
gamma irradiation were investigated in the present study. Gamma
irradiation did not cause significant changes in the sulfate group
content and monosaccharide composition, although changes in the Mw
distribution were observed. The in vitro antiproliferation
assays indicated that Mw had a significant influence on the
antitumor activity of the sulfated polysaccharides. The low-Mw
polysaccharides exhibited stronger antiproliferative effects than
PYSP, and the potential mechanisms may involve cell cycle arrest.
Prior to the further research and development of PYSP and its
degradation derivatives, strong supporting data from in vivo
antitumor assays are urgently required. However, the current
findings promote the exploitation and utilization of polysaccharide
from P. yezoensis Sookwawon 104 as a promising candidate for
cancer adjuvant therapy.
Acknowledgements
The authors would like to thank Dr Alan K Chang
(Wenzhou University; Wenzhou, China) for helpful discussion and for
revising the language of the manuscript.
Funding
This study was financially supported by the National
Natural Science Foundation of China (grant nos. 41876197 and
81872952), the National Key Research and Development Project (grant
no. 2018YFD0901503), the Natural Science Foundation of Zhejiang
Province (grant nos. LY18C020006 and LGN18C020004), the Scientific
Foundation of Education Department of Zhejiang Province (grant no.
Y201737374), the National Research Foundation of Korea (grant no.
NRF-2018R1D1A1B07049359) and a Golden Seed Project Grant funded by
Ministry of Oceans and Fisheries (grant no. 213008-05-4-SB910).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DH, LY, YC, XM, SW, JZ and EJP performed the
experiments. JL designed the experiments used to evaluate the
physicochemical properties. DH, HT, MW and JIC designed the study;
DH and HT analyzed the data. DH wrote the original draft of the
manuscript, and DH, LY and HT revised it. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang Q, Li N, Zhou G, Lu X, Xu Z and Li
Z: In vivo antioxidant activity of polysaccharide fraction from
Porphyra haitanensis (Rhodephyta) in aging mice. Pharmacol
Res. 48:151–155. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu X, Zhou C, Yang H, Huang X, Ma H, Qin X
and Hu J: Effect of ultrasonic treatment on the degradation and
inhibition cancer cell lines of polysaccharides from Porphyra
yezoensis. Carbohyd Polym. 117:650–656. 2015. View Article : Google Scholar
|
|
3
|
Liu QM, Xu SS, Li L, Pan TM, Shi CL, Liu
H, Cao MJ, Su WJ and Liu GM: In vitro and in vivo immunomodulatory
activity of sulfated polysaccharide from Porphyra
haitanensis. Carbohyd Polym. 165:189–196. 2017. View Article : Google Scholar
|
|
4
|
Varela-Álvarez E, Paulino C and Serrão EA:
Development and characterization of twelve microsatellite makers
for Porphyra linearis Greville. Genetica. 145:127–130. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Venkatraman KL and Mehta A: Health
benefits and pharmacological effects of Porphyra Species.
Plant Foods Hum Nutr. 74:10–17. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen YY and Xue YT: Optimization of
microwave assisted extraction, chemical characterization and
antitumor activities of polysaccharides from Porphyra
haitanensis. Carbohyd Polym. 206:179–186. 2019. View Article : Google Scholar
|
|
7
|
Fernández LE, Valiente OG, Mainardi V,
Bello JL, Vélez H and Rosado A: Isolation and characterization of
an antitumor active agar-type polysaccharide of Gracilaria
dominguensis. Carbohyd Res. 190:77–83. 1989. View Article : Google Scholar
|
|
8
|
Lins KO, Bezerra DP, Alves AP, Alencar NM,
Lima MW, Torres VM, Farias WR, Pessoa C, de Moraes MO and
Costa-Lotufo LV: Antitumor properties of a sulfated polysaccharide
from the red seaweed Champia feldmannii (Diaz-Pifferer). J
Appl Toxicol. 29:20–26. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brown ES, Allsopp PJ, Magee PJ, Gill CI,
Nitecki S, Strain CR and McSorley EM: Seaweed and human health.
Nutr Rev. 72:205–216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheong KL, Qiu HM, Du H, Liu Y and Khan
BM: Oligosaccharides derived from red seaweed: Production,
properties, and potential health and cosmetic application.
Molecules. 23:24512018. View Article : Google Scholar
|
|
11
|
Taper HS and Roberfroid MB:
Inulin/oligofructose and anticancer therapy. Br J Nutr. 87 (Suppl
2):S283–S286. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ferro V, Dredge K, Liu L, Hammond E,
Bytheway I, Li C, Johnstone K, Karoli T, Davis K, Copeman E and
Gautam A: PI-88 and novel heparan sulfate mimetics inhibit
angiogenesis. Semin Thromb Hemost. 33:557–568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vetvicka V: Synthetic
oligosaccharides-clinical application in cancer therapy. Anticancer
Agents Med Chem. 13:720–724. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao T, Zhang Q, Qi H, Zhang H, Niu X, Xu
Z and Li Z: Degradation of porphyran from Porphyra
haitanensis and the antioxidant activities of the degraded
porphyrans with different molecular weight. Int J Biol Macromol.
38:45–50. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi JI and Kim HJ: Preparation of low
molecular weight fucoidan by gamma-irradiation and its anticancer
activity. Carbohyd Polym. 97:358–362. 2013. View Article : Google Scholar
|
|
16
|
Yanagido A, Ueno M, Jiang Z, Cho K,
Yamaguchi K, Kim D and Oda T: Increase in anti-inflammatory
activities of radical-degraded porphyrans isolated from discolored
nori (Pyropia yezoensis). Int J Biol Macromol. 117:78–86.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saruchi Kumar V, Mittal H and Alhassan SM:
Biodegradable hydrogels of tragacanth gum polysaccharide to improve
water retention capacity of soil and environment-friendly
controlled release of agrochemicals. Int J Biol Macromol.
132:1252–1261. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma XL, Song FF, Zhang H, Huan X and Li SY:
Compositional monosaccharide analysis of Morus nigra Linn by HPLC
and HPCE quantitative determination and comparison of
polysaccharide from Morus nigra Linn by HPCE and HPLC. Curr Pharma
Anal. 13:433–437. 2017. View Article : Google Scholar
|
|
19
|
Dubois M, Gilles KA, Hamilton JK, Rebers
PA and Smith F: Colorimetric method for determination of sugars and
related substances. Anal Chem. 28:350–356. 1956. View Article : Google Scholar
|
|
20
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dodgson KS and Price RG: A note on the
determination of the ester sulphate content of sulphated
polysaccharides. Biochem J. 84:106–110. 1962. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu S, Zhang X, Liu J, Song J, Yu P, Chen
P, Liao Z, Wu M and Tong H: Physicochemical characterization of
Sargassum fusiforme fucoidan fractions and their
antagonistic effect against P-selectin-mediated cell adhesion. Int
J Biol Macromol. 133:656–662. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen L, Chen P, Liu J, Hu C, Yang S, He D,
Yu P, Wu M and Zhang X: Sargassum fusiforme polysaccharide
SFP-F2 activates the NF-κB signaling pathway via CD14/IKK and P38
Axes in raw264.7 cells. Mar Drugs. 16:2642018. View Article : Google Scholar
|
|
24
|
Chen P, He D, Zhang Y, Yang S, Chen L,
Wang S, Zou H, Liao Z, Zhang X and Wu M: Sargassum fusiforme
polysaccharides activate antioxidant defense by promoting
Nrf2-dependent cytoprotection and ameliorate stress insult during
aging. Food Funct. 7:4576–4588. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alboofetileh M, Rezaei M, Tabarsa M, Rittà
M, Donalisio M, Mariatti F, You S, Lembo D and Cravotto G: Effect
of different non-conventional extraction methods on the
antibacterial and antiviral activity of fucoidans extracted from
Nizamuddinia zanardinii. Int J Biol Macromol. 124:131–137.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xia YG, Wang TL, Yu SM, Liang J and Kuang
HX: Structural characteristics and hepatoprotective potential of
Aralia elata root bark polysaccharides and their effects on
SCFAs produced by intestinal flora metabolism. Carbohyd Polym.
207:256–265. 2019. View Article : Google Scholar
|
|
28
|
Liu C, Omer AM and Ouyang XK: Adsorptive
removal of cationic methylene blue dye using carboxymethyl
cellulose/k-carrageenan/activated montmorillonite composite beads:
Isotherm and kinetic studies. Int J Biol Macromol. 106:823–833.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cao J, Wang J, Wang S and Xu X:
Porphyra species: A mini-review of its pharmacological and
nutritional properties. J Med Food. 19:111–119. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Babin JL, Traylor KL and Witt DM:
Laboratory monitoring of low-molecular-weight heparin and
fondaparinux. Semin Thromb Hemost. 43:261–269. 2017.PubMed/NCBI
|
|
31
|
Choi JI, Kim HJ and Lee JW: Structural
feature and antioxidant activity of low molecular weight laminarin
degraded by gamma irradiation. Food Chem. 129:520–523. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Choi JI, Lee SG, Han SJ, Cho MH and Lee
PC: Effect of gamma irradiation on the structure of fucoidan.
Radiat Phys Chem. 100:54–58. 2014. View Article : Google Scholar
|
|
33
|
Zhu ZY, Dong F, Liu X, Lv Q, YingYang, Liu
F, Chen L, Wang T, Wang Z and Zhang Y: Effects of extraction
methods on the yield, chemical structure and anti-tumor activity of
polysaccharides from Cordyceps gunnii mycelia. Carbohydr
Polym. 140:461–471. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Y, Wang Z, Li D, Zang W, Zhu H, Wu
P, Mei Y and Liang Y: A polysaccharide from Antrodia
cinnamomea mycelia exerts antitumor activity through blocking
of TOP1/TDP1-mediated DNA repair pathway. Int J Biol Macromol.
120B:1551–1560. 2018. View Article : Google Scholar
|
|
35
|
Wang J, Bao A, Meng X, Guo H, Zhang Y,
Zhao Y, Kong W, Liang J, Yao J and Zhang J: An efficient approach
to prepare sulfated polysaccharide and evaluation of anti-tumor
activities in vitro. Carbohyd Polyms. 184:366–375. 2018. View Article : Google Scholar
|
|
36
|
Choromanska A, Kulbacka J, Harasym J,
Oledzki R, Szewczyk A and Saczko J: High- and low-molecular weight
oat Beta-glucan reveals antitumor activity in human epithelial lung
cancer. Pathol Oncol Res. 24:583–592. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pletenpol JA and Stewart ZA: Cell cycle
checkpoint signaling: Cell cycle arrest versus apoptosis.
Toxicology 181-182. 475–481. 2002. View Article : Google Scholar
|
|
38
|
Wang J, Zhang YS, Thakur K, Hussain SS,
Zhang JG, Xiao GR and Wei ZJ: Licochalcone A from licorice root, an
inhibitor of human hepatoma cell growth via induction of cell
apoptosis and cell cycle arrest. Food Chem Toxicol. 120:407–417.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao H, Li S, Wang G, Zhao W, Zhang D,
Wang F, Li W and Sun L: Study of the mechanism by which dinaciclib
induces apoptosis and cell cycle arrest of lymphoma Raji cells
through a CDK1-involved pathway. Cancer Med. 8:4348–4358. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Khan T, Date A, Chawda H and Patel K:
Polysaccharides as potential anticancer agents-A review of their
progress. Carbohyd Polyms. 210:412–428. 2019. View Article : Google Scholar
|
|
41
|
Wang X and Zhang Z: The antitumor activity
of a red alga polysaccharide complexes carrying 5-fluorouracil. Int
J Biol Macromol. 69:542–545. 2014. View Article : Google Scholar : PubMed/NCBI
|