Emergence of primary and acquired resistance to
existing conventional chemotherapies is one of the major challenges
to overcome in the clinical management of cancer (1,2).
Patients with chemoresistant cancer do not respond to conventional
drugs, which results in a poor prognosis and is often associated
with the reoccurrence of malignancies following treatment (3,4).
Therefore, understanding the underlying molecular mechanisms of
chemoresistance is necessary to improve the survival of these
patients. The cancer stem cell (CSC) phenotype is one of the
favored molecular mechanisms of chemoresistance (1). However, eradication of CSC populations
to increase the therapeutic response of cancer cells to existing
chemotherapies remains a challenge (5). Therefore, novel strategies to eliminate
CSC populations and overcome chemoresistance are key for the
successful clinical treatment of certain cancer types.
Herbal remedies have been used in traditional Asian
medicine for centuries to treat various diseases. Recently, they
have attracted global attention as potential strategies for
overcoming the chemoresistance of cancer cells, without any
observable side effects (1,6). Medicinal plant extracts have the
potential to treat human cancer either alone or synergistically
with existing chemotherapeutic drugs to inhibit resistance
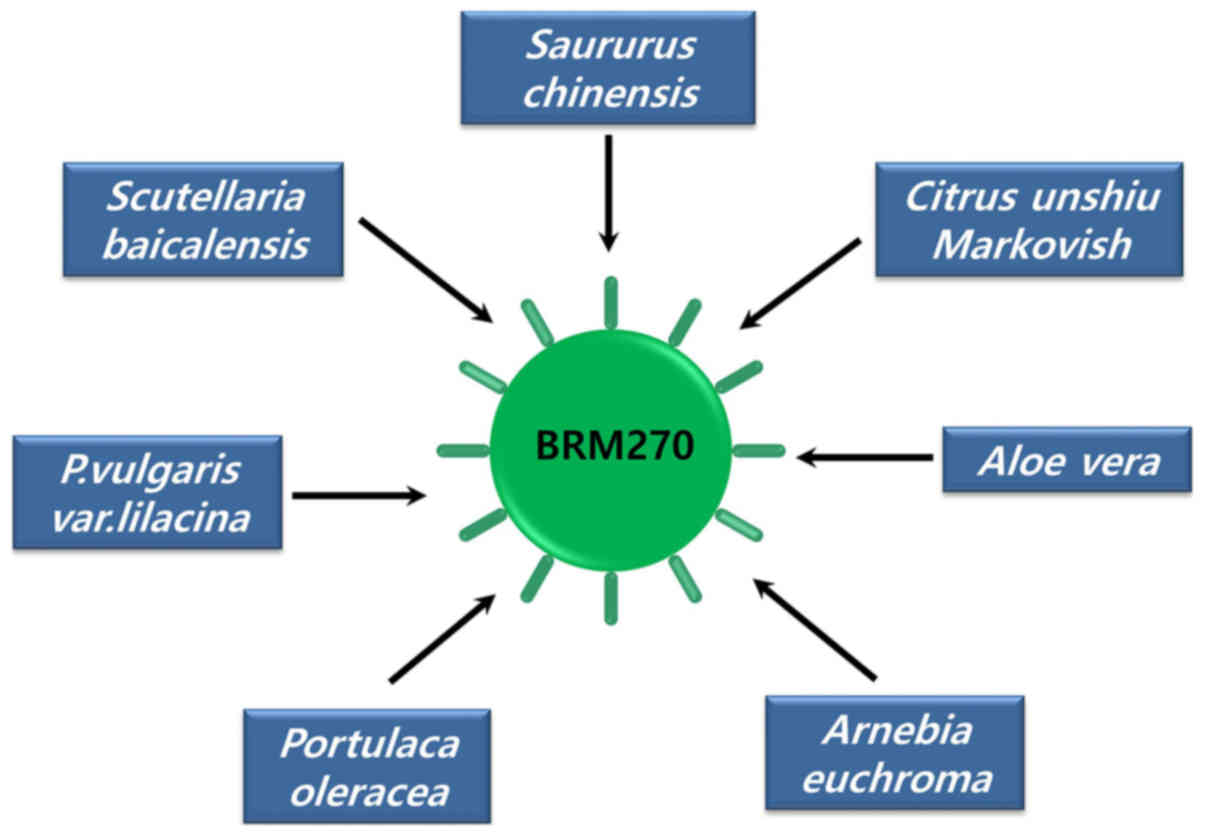
(7–11). BRM270 (BRMLife) is a
formulated extraction from seven medicinal plants used in Asian
medicine, including Saururus chinensis, Citrus unshiu Markovich,
Aloe vera, Arnebia euchroma, Portulaca oleracea, Prunella vulgaris
var. lilacina and Scutellaria bacicalensis (Fig. 1) (1).
Recently, the effectiveness of BRM270 was demonstrated as an
alternative treatment to chemotherapy on non-small cell lung cancer
(NSCLC) stem cells, which were resistant to EGFR-TKIs (epidermal
growth receptor-specific tyrosine kinase inhibitors) (1). Similarly, previous studies have
reported that BRM270 has the potential to downregulate
tumorigenesis via the following mechanisms: Suppression of NF-κB
signaling in multidrug resistance (MDR)-induced stem-like cells,
inhibition of cervical CSC proliferation via SOX2 restriction,
suppression of the recurrence and stem cell properties of
glioblastoma, suppression of pancreatic CSC proliferation, and
prevention of inflammation-promoted hepatocarcinogenesis (12–14).
Previous studies have also demonstrated the
individual effects of the seven plant extract constituents of
BRM270 (15–18). The present review discusses our
current understanding of the individual and synergistic effects of
BRM270 and highlights the important role of this compound in
disrupting the CSC phenotype and overcoming the chemoresistance of
cancer.
NSCLC includes adenocarcinomas, squamous cell
carcinomas and large-cell carcinomas, accounting for ~85% of all
lung cancer cases (19,20). The main treatment strategies for
NSCLC are chemotherapy and radiotherapy, which fail to improve the
survival rates of patients owing to primary and acquired
resistance; therefore, only 15% of patients with NSCLC have a
5-year survival rate (21,22). Inhibition of the EGFR tyrosine kinase
pathway decreases NSCLC tumorigenesis, and EGFR-TKIs, including
gefitinib and paclitaxel, are first-line treatments (1). However, the majority of patients with
initial responses to EGFR-TKI treatment become resistant after
10–16 months (1). Resistance to the
first generation of EGFR-TKIs has led to the development of novel
drug generations and combination therapies (1,22,23).
Due to the high resistance of NSCLCs to EGFR-TKIs,
studies aiming to understand the mechanisms of resistance and to
identify potential anti-resistance approaches remain necessary. Our
recent study suggested a safe and effective alternative treatment
involving the use of BRM270 for patients with EGFR-TKI-resistant
NSCLC (1). The therapeutic efficacy
of BRM270 against both non-resistant and EGFR-TKI-resistant lung
adenocarcinoma cells was demonstrated (1). Resistance was derived from repeated
exposure of lung adenocarcinoma cells to gefitinib and paclitaxel
(1). It was demonstrated that BRM270
induced the apoptosis of chemoresistant NSCLC cells and caused
G2/M cell cycle arrest via the inhibition of NF-κB/Bcl2
signaling, thereby suppressing cell proliferation. Furthermore, it
was demonstrated that BRM270 was capable of suppressing
chemoresistant NSCLC stem cells by inhibiting
epithelial-mesenchymal transition (EMT), metastasis and stemness
(1). BRM270 was also reported to
induce the expression of microRNA-128 (miR-128) in chemoresistant
NSCLC (1). MicroRNA (miR)-128 is
considered a tumor suppressor in various types of cancer (24–26), and
acts against NSCLC cells by directly regulating vascular
endothelial growth factor C (VEGF-C) (27). MiR-128 also induces lung cancer cell
apoptosis by directly targeting NIMA (never in mitosis gene
A)-related kinase 2 (28). Our
previous study reported the tumor suppressive role of miR-128 in
paclitaxel-resistant NSCLC by targeting MUC1-C and BMI-1 in CSCs
(19). Furthermore, our previous
study has suggested that miR-128 targets the c-met/PI3K/AKT pathway
in lung CSCs and reverses gefitinib resistance (29). Therefore, BRM270 serves an important
role in the prevention of the CSC phenotype, the inhibition of
cancer progression and tumor growth, and the suppression of
malignant behaviors in chemoresistant NSCLC through the induction
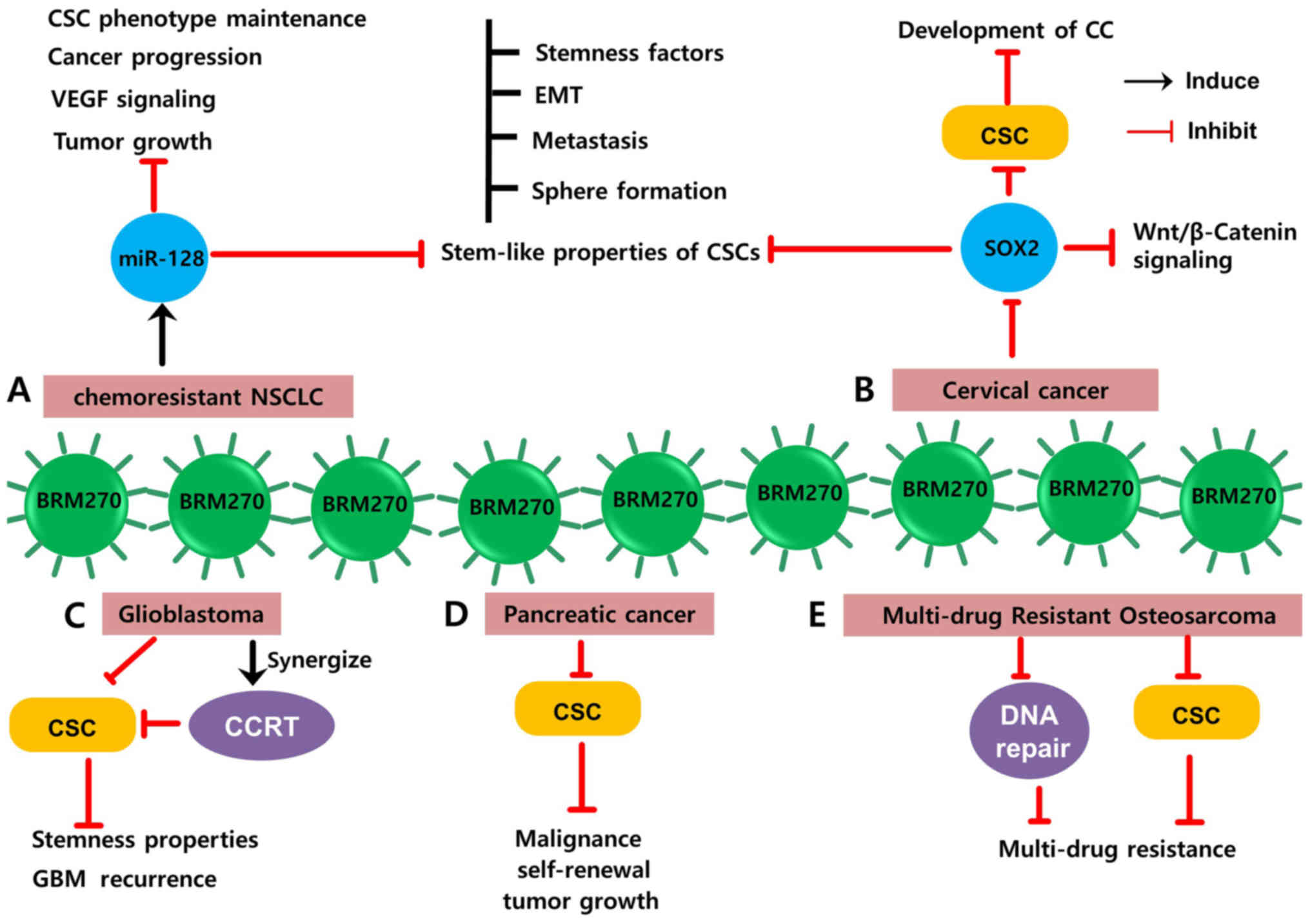
of miR-128 overexpression (Fig. 2A)
(1). Therefore, BRM270 is an
effective compound that may be used to overcome chemoresistance in
lung cancer as an alternative or addition to existing conventional
drugs.
Cervical cancer (CC) is the fourth most common cause
of cancer-associated mortality in females worldwide (30). Surgical resection alone or in
combination with adjuvant radiotherapy is commonly used to treat
advanced stages of CC. However, this approach only decreases the
risk of CC progression and is associated with irreversible
morbidity (31). Due to the
aggressive nature of CC, multimodality treatments are commonly
used, including concurrent chemoradiotherapy or neoadjuvant
chemotherapy (NACT) followed by surgery (32,33).
However, the application of these approaches, especially NACT, is
restricted by the chemoresistance of CC cells to cisplatin,
paclitaxel and taxel-based chemotherapies (31). Therefore, increasing the
chemotherapeutic efficiency by targeting the underlying mechanisms
of chemoresistance is essential for the successful clinical
management of CC.
Previous studies have suggested that CC stem cells
serve a crucial role in the resistance to conventional therapies
(34–36). Our recent study reported that BRM270
inhibited CC stem cells by targeting SRY (sex determining region
Y)-box 2 (SOX2) (37). SOX2 is a
transcription factor that serves a crucial role in the maintenance
of CSC stemness and the development of resistance to existing
conventional therapies (38–41). As BRM270 has the potential to inhibit
SOX2 expression, it can block the maintenance of the CSC phenotype
from interfering with the emerging chemoresistance (37). BRM270 also negatively affects the
progression and metastasis of CC, the EMT and sphere formation of
CC stem cells, and tumor initiation (37). It also promotes the apoptosis of CC
cells by inhibiting cell proliferation. Furthermore, in CC stem
cells, BRM270 downregulates the Wnt/β-Catenin pathway, which is
essential to sustain the CSC phenotype (Fig. 2B) (37,42).
Therefore, BRM270 represents an effective
alternative to the existing conventional therapies for cervical
cancer by targeting CSCs to overcome chemoresistance, thereby
improving clinical management.
Glioblastoma multiforme (GBM) is the most common and
aggressive type of primary brain malignancy in adults (43). Vigorous multimodality treatments,
including surgeries, concurrent temozolomide chemo-irradiation and
post-radiotherapy adjuvant temozolomide are currently used to treat
patients with glioblastoma (44).
Despite these treatments, patient prognosis is poor and the 5-year
survival rate is less than 10% following the initial diagnosis.
Furthermore, the median survival time is ~14.6 months owing to the
infiltrative behavior and extreme resistance of cells to radiation
and chemotherapy (45). This
resistance also leads to the recurrence of glioblastoma following
standard cancer therapy. Accumulating evidence suggests that
glioblastoma stem cells are the major cause of chemo- and
radio-resistance and are largely responsible for tumor recurrence
(46–49). Therefore, the glioblastoma stem cell
population is an important target for the successful clinical
management of progressive and recurrent malignant glioblastoma.
A previous study demonstrated that BRM270 targets
glioblastoma stem cells, suppressing growth and viability through
the induction of apoptosis without affecting normal astrocytes
(13). The sub-G0 cell
population was significantly increased following the BRM270
treatment, indicating an expanded population of dead cells.
Notably, BRM270 treatment did not cause any difference in
G1, S or G2/M subpopulations. Furthermore,
the stemness properties of glioblastoma stem cells were also
suppressed by treatment with BRM270. While BRM270 administration
alone targeted the properties of glioblastoma stem cells, combined
treatment with concurrent chemoradiotherapy (CCRT) successfully
decreased the number of CD15-expressing stem cells and decreased
the chance of glioblastoma recurrence (Fig. 2C).
Therefore, BRM270 alone and as a combined treatment
with CCRT is effective in suppressing the CSC phenotype, overcoming
resistance to radiotherapy and chemotherapy, and preventing the
recurrence of malignant glioblastoma.
Pancreatic ductal adenocarcinoma (PDAC) is
considered one of the deadliest types of carcinoma, with a median
survival time of less than 5–8 months (11). Gemcitabine is used as the first-line
drug for PDAC, though intrinsic and acquired resistances remain
major challenges. CSCs have been identified in previous studies as
crucial players in the resistance of PDAC to existing conventional
chemotherapy (11,50).
Our recent study established that BRM270 was capable
of inhibiting malignancy and the self-renewal capacity of
CD44+ PDAC cells (14).
CD44 is a marker of the CSC population and the initiation of
cancer, suggesting that BRM270 is able to inhibit CSC populations
and stem-like properties of PDAC (51). BRM270 treatment also suppresses PDAC
CSC-derived tumor growth via the downregulation of sonic hedgehog
signaling in PDAC stem cells (14).
The aberrant activation of the sonic hedgehog pathway is one of the
major fundamental drivers of PDAC stem cell self-renewal and is
associated with decreased survival rates in patients (Fig. 2D) (52–54).
Therefore, BRM270 is an effective treatment for PDAC
and has the potential to reduce its resistance to existing
chemotherapies by inhibiting PDAC stem cells, a crucial player in
chemoresistance.
Osteosarcoma is an aggressive malignant tumor that
primarily affects the skeletal system, and leads to a 5-year
survival rate below 20% (55). It
remains a challenge for current therapeutic strategies to
effectively treat osteosarcoma due to the MDR of cancer cells to
existing chemotherapeutic drugs, including doxorubicin and
cisplatin (56,57). The presence of stem cell populations
and altered DNA repair mechanisms have been identified as the major
underlying methods of MDR in osteosarcoma (57,58).
Therefore, targeting these mechanisms would likely inhibit MDR and
sensitize osteosarcoma cells to existing drugs.
The capability of BRM270 to circumvent MDR with
minimal adverse side effects has been reported (12). BRM270 impacts osteosarcoma MDR by
damaging DNA repair mechanisms and negatively affecting CSC
populations (12). Our previous
study demonstrated that BRM270 disrupts the formation of the
microtubule cytoskeleton in doxorubicin-resistant osteosarcoma stem
cells, inducing chromosomal condensation and nuclear fragmentation
(12). BRM270 also induces
programmed cell death and mitotic catastrophe in osteosarcoma stem
cells by promoting irreversible DNA damage in the premature
apoptosis stage and inhibiting cell proliferation selectively,
without affecting the functions of normal cells (12). BRM270 treatment also induces the
expression of key pro-apoptotic proteins, including structural
maintenance of chromosome 2 (SMC2), Caspase-8, Interleukin-6
(IL-6), Cyclin-dependent kinase 6 (CDK6) and p65 (Fig. 2E) (12).
Therefore, BRM270 is a safe and effective natural
compound to treat MDR osteosarcoma by promoting unrepairable DNA
damage and cytotoxicity in osteosarcoma stem cells.
Hepatitis B virus (HBV), hepatitis C virus (HCV),
non-alcoholic fatty liver disease, alcoholism or aflatoxin exposure
are common causes of chronic hepatic injury (59). Persistent inflammation, as a result
of chronic liver injury, is strongly associated with
hepatocarcinogenesis, leading to the development of hepatocellular
carcinoma (HCC) after years of inflammation (59). For example, the inability to clear
HCV leads to chronic hepatitis C infection, resulting in
inflammation-induced lesions in the liver, hepatic fat
accumulation, and progressive fibrosis followed by HCC or cirrhosis
(60–63). Chronic liver inflammation includes
phorbal-12-myristate-13-acetate (PMA)-induced inflammation, which
promotes hepatocarcinogenesis and HCC progression. This
inflammation induces the expression of numerous inflammatory
cytokines, including tumor necrosis factor alpha (TNFα),
Interleukin 1 (IL-1), IL-23, IL-6, cyclooxygenase-2 (COX-2), and
lymphotoxins (LT) α and β (59,64).
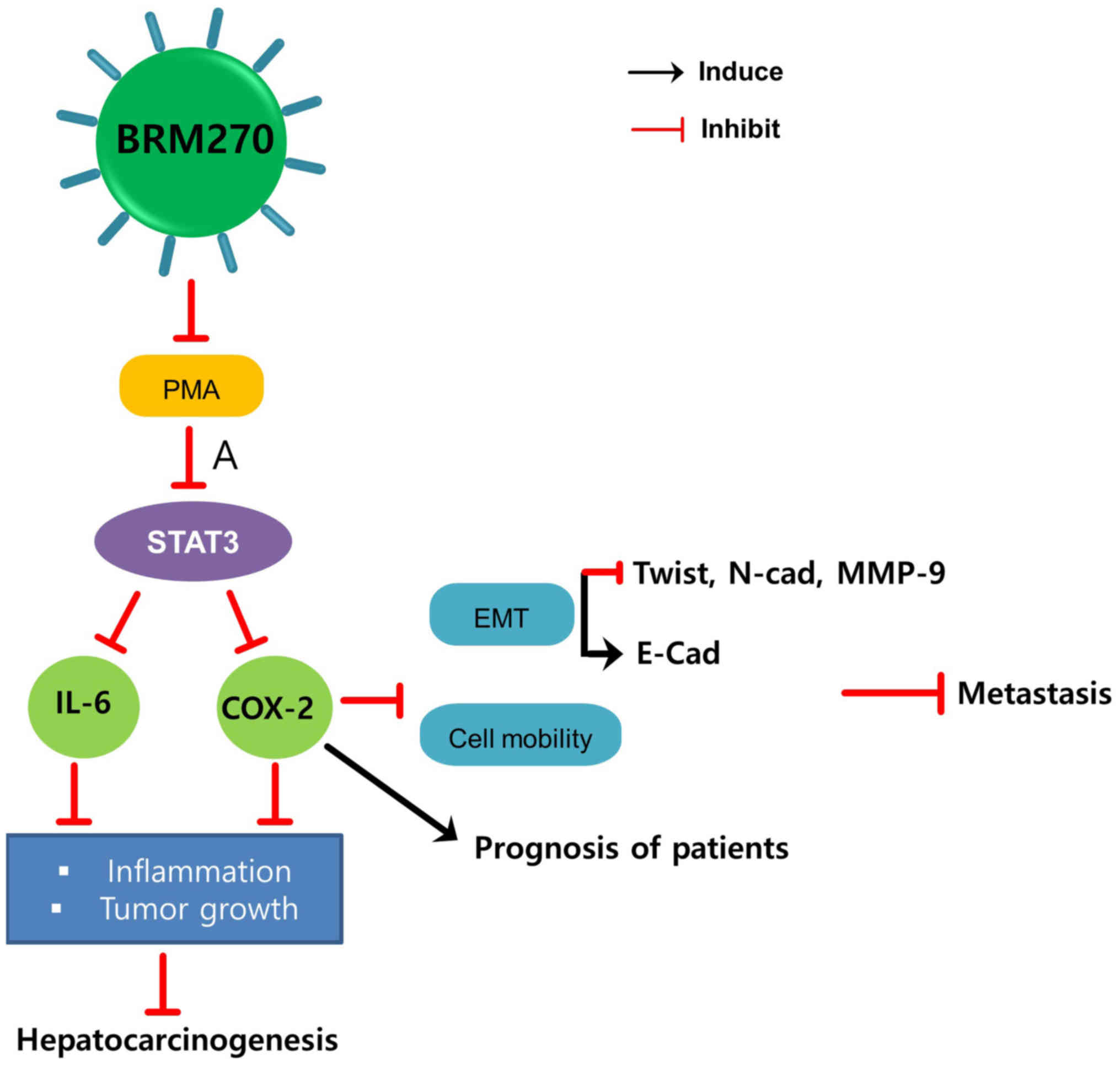
Our recent study reported that this enhanced BRM270
prevents PMA-induced inflammation through suppression of signal
transducer and activator of transcription 3 (STAT3) signaling
(14). As PMA is a COX-2 inducer and
a mediator of inflammation, inhibition of PMA-induced inflammation
by BRM270 decreases the expression of IL-6 and COX-2, leading to
the suppression of hepatocarcinogenesis progression (14). BRM270 also inhibits hepatic tumor
growth and HCC cell metastasis and improves the prognosis of
patients with HCC via the inhibition of IL-6 and COX-2 expression
(Fig. 3) (14).
Therefore, BRM270 is a natural compound treatment
that has the potential to prevent hepatocarcinogenesis induced by
inflammation through the downregulation of the IL-6/STAT3/COX-2
axis.
BRM270, a compound made of seven herbal plant
extracts, is capable of negatively regulating the resistance of
cancer cells to existing conventional therapies by inhibiting the
CSC phenotype. BRM270 exerts its antitumor effects against
chemoresistance via the suppression of CSC populations and the
stem-like properties of CSCs. The compound can be used either as a
standalone treatment or synergistically with existing drugs.
Furthermore, the functionally enhanced product of BRM270 prevents
inflammation-induced hepatocarcinogenesis. BRM270 does not cause
any adverse side effects and does not affect normal human cells,
but selectively targets cancer cells. Future studies should focus
on further understanding the effects of BRM270 on CSCs and
chemoresistance and investigate the possibility of BRM270 in
clinical use.
Not applicable.
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
funded by the Ministry of Education, Korea (grant no.
2020R1I1A2052417), the Korean Research Institute of Bioscience and
Biotechnology Research Initiative Program, Korea (grant nos.
KGM5162021 and RBM0112011), and the Scientific Research Team
Support Plan of Heilongjiang Bayi Agricultural University, China
(grant no. TDJH201904).
Not applicable.
NC, HK and TK contributed toward the conception of
the study, writing the manuscript and performing the literature
search. JK, JL and YHP conducted analysis and revised the
manuscript. HNS and TK performed analysis and the quality
assessment of the study. All authors read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Kwon T, Chandimali N, Huynh DL, Zhang JJ,
Kim N, Bak Y, Yoon DY, Yu DY, Lee JC, Gera M, et al: BRM270
inhibits cancer stem cell maintenance via microRNA regulation in
chemoresistant A549 lung adenocarcinoma cells. Cell Death Dis.
9:2442018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Toge M, Yokoyama S, Kato S, Sakurai H,
Senda K, Doki Y, Hayakawa Y, Yoshimura N and Saiki I: Critical
contribution of MCL-1 in EMT-associated chemo-resistance in A549
non-small cell lung cancer. Int J Oncol. 46:1844–1848. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu X, Han X, Wan X, He C, Wang Y, Mao A,
Yu F, Zhou T, Feng L, Zhang P, et al: SPZ1 is critical for
chemoresistance and aggressiveness in drug-resistant breast cancer
cells. Biochem Pharmacol. 156:43–51. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hudson AL, Weir C, Moon E, Harvie R, Klebe
S, Clarke SJ, Pavlakis N and Howell VM: Establishing a panel of
chemo-resistant mesothelioma models for investigating
chemo-resistance and identifying new treatments for mesothelioma.
Sci Rep. 4:61522014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roy L and Cowden Dahl KD: Can stemness and
chemoresistance be therapeutically targeted via signaling pathways
in ovarian cancer? Cancers (Basel). 10:2412018. View Article : Google Scholar
|
|
6
|
Kim TW, Lee SY, Kim M, Cheon C, Jang BH,
Shin YC and Ko SG: DSGOST regulates resistance via activation of
autophagy in gastric cancer. Cell Death Dis. 9:6492018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Y, Zhu J and Zhang W: Antitumor
effect of traditional Chinese herbal medicines against lung cancer.
Anticancer Drugs. 25:983–991. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ko JK and Auyeung KK: Target-oriented
mechanisms of novel herbal therapeutics in the chemotherapy of
gastrointestinal cancer and inflammation. Curr Pharm Des. 19:48–66.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Q, Acharya N, Liu Z, Zhou X, Cromie
M, Zhu J and Gao W: Enhanced anticancer effects of Scutellaria
barbata D. Don in combination with traditional Chinese medicine
components on non-small cell lung cancer cells. J Ethnopharmacol.
217:140–151. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang H, Wang F, Wu S, Liu Z, Li T, Mao L,
Zhang J, Li C, Liu C and Yang Y: Traditional herbal
medicine-derived sulforaphene promotes mitophagic cell death in
lymphoma cells through CRM1-mediated p62/SQSTM1 accumulation and
AMPK activation. Chem Biol Interact. 281:11–23. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chandimali N, Huynh DL, Jin WY and Kwon T:
Combination effects of hispidin and gemcitabine via inhibition of
stemness in pancreatic cancer stem cells. Anticancer Res.
38:3967–3975. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mongre RK, Sodhi SS, Ghosh M, Kim JH, Kim
N, Park YH, Kim SJ, Heo YJ, Sharma N and Jeong DK: The novel
inhibitor BRM270 downregulates tumorigenesis by suppression of
NF-KB signaling cascade in MDR-induced stem like cancer-initiating
cells. Int J Oncol. 46:2573–2585. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jeon HY, Park CG, Ham SW, Choi SH, Lee SY,
Kim JY, Seo S, Jin X, Kim JK, Eun K, et al: BRM270, a compound from
natural plant extracts, inhibits glioblastoma stem cell properties
and glioblastoma recurrence. J Med Food. 20:838–845. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huynh DL, Koh H, Chandimali N, Zhang JJ,
Kim N, Kang TY, Ghosh M, Gera M, Park YH, Kwon T and Jeong DK:
BRM270 inhibits the proliferation of CD44 positive pancreatic
ductal adenocarcinoma cells via downregulation of sonic hedgehog
signaling. Evid Based Complement Alternat Med. 2019:86204692019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi BY, Joo JC, Lee YK, Jang IS, Park SJ
and Park YJ: Anti-cancer effect of Scutellaria baicalensis
in combination with cisplatin in human ovarian cancer cell. BMC
Complement Altern Med. 17:2772017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu C, Wang H, Chen S, Yang R, Li H and
Zhang G: Baicalein inhibits cell growth and increases cisplatin
sensitivity of A549 and H460 cells via miR-424-3p and targeting
PTEN/PI3K/Akt pathway. J Cell Mol Med. 22:2478–2487. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Wang H, Zhou R, Zhong W, Lu S, Ma
Z and Chai Y: Baicalin inhibits human osteosarcoma cells invasion,
metastasis, and anoikis resistance by suppressing the transforming
growth factor-β1-induced epithelial-to-mesenchymal transition.
Anticancer Drugs. 28:581–587. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao R, Gao X, Cai Y, Shao X, Jia G, Huang
Y, Qin X, Wang J and Zheng X: Antitumor activity of Portulaca
oleracea L. polysaccharides against cervical carcinoma in vitro
and in vivo. Carbohydr Polym. 96:376–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koh H, Park H, Chandimali N, Huynh DL,
Zhang JJ, Ghosh M, Gera M, Kim N, Bak Y, Yoon DY, et al:
MicroRNA-128 suppresses paclitaxel-resistant lung cancer by
inhibiting MUC1-C and BMI-1 in cancer stem cells. Oncotarget.
8:110540–110551. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pazhouhandeh M, Samiee F, Boniadi T,
Khedmat AF, Vahedi E, Mirdamadi M, Sigari N, Siadat SD, Vaziri F,
Fateh A, et al: Comparative network analysis of patients with
non-small cell lung cancer and smokers for representing potential
therapeutic targets. Sci Rep. 7:138122017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lam WK and Watkins DN: Lung cancer: Future
directions. Respirology. 12:471–477. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kwon T, Rho JK, Lee JC, Park YH, Shin HJ,
Cho S, Kang YK, Kim BY, Yoon DY and Yu DY: An important role for
peroxiredoxin II in survival of A549 lung cancer cells resistant to
gefitinib. Exp Mol Med. 47:e1652015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ham SY, Kwon T, Bak Y, Yu JH, Hong J, Lee
SK, Yu DY and Yoon DY: Mucin 1-mediated chemo-resistance in lung
cancer cells. Oncogenesis. 5:e1852016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin Y and Wu Z: MicroRNA-128 inhibits
proliferation and invasion of glioma cells by targeting COX-2.
Gene. 658:63–69. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang X, Shangguan W, Zhang M, Mei S, Wang
L and Yang R: MiR-128 enhances dendritic cell-mediated anti-tumor
immunity via targeting of p38. Mol Med Rep. 16:1307–1313. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu X, Liang Z, Gao K, Li H, Zhao G, Wang
S and Fang J: MicroRNA-128 inhibits EMT of human osteosarcoma cells
by directly targeting integrin α2. Tumour Biol. 37:7951–7957. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu J, Cheng Y, Li Y, Jin Z, Pan Y, Liu G,
Fu S, Zhang Y, Feng K and Feng Y: MicroRNA-128 plays a critical
role in human non-small cell lung cancer tumourigenesis,
angiogenesis and lymphangiogenesis by directly targeting vascular
endothelial growth factor-C. Eur J Cancer. 50:2336–2350. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao D, Han W, Liu X, Cui D and Chen Y:
MicroRNA-128 promotes apoptosis in lung cancer by directly
targeting NIMA-related kinase 2. Thorac Cancer. 8:304–311. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang J, Feng X, Zhou W, Wu Y and Yang Y:
MiR-128 reverses the gefitinib resistance of the lung cancer stem
cells by inhibiting the c-met/PI3K/AKT pathway. Oncotarget.
7:73188–73199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sikander M, Hafeez BB, Malik S, Alsayari
A, Halaweish FT, Yallapu MM, Chauhan SC and Jaggi M: Cucurbitacin D
exhibits potent anti-cancer activity in cervical cancer. Sci Rep.
6:365942016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang J, Zhang Y, Liu S, Zhang Q, Wang Y,
Tong L, Chen X, Ji Y, Shang Q, Xu B, et al: Metadherin confers
chemoresistance of cervical cancer cells by inducing autophagy and
activating ERK/NF-κB pathway. Tumour Biol. 34:2433–2440. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Burzawa J, Gonzales N and Frumovitz M:
Challenges in the diagnosis and management of cervical
neuroendocrine carcinoma. Expert Rev Anticancer Ther. 15:805–810.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Colombo N and Peiretti M: Critical review
of neoadjuvant chemotherapy followed by surgery for locally
advanced cervical cancer. Int J Gynecol Cancer. 20 (11 Suppl
2):S47–S48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chhabra R: Cervical cancer stem cells:
Opportunities and challenges. J Cancer Res Clin Oncol.
141:1889–1897. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang R and Rofstad EK: Cancer stem cells
(CSCs), cervical CSCs and targeted therapies. Oncotarget.
8:35351–35367. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang L, Guo H, Lin C, Yang L and Wang X:
Enrichment and characterization of cancer stemlike cells from a
cervical cancer cell line. Mol Med Rep. 9:2117–2123. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chandimali N, Sun HN, Park YH and Kwon T:
BRM270 suppresses cervical cancer stem cell characteristics and
progression by inhibiting SOX2. In Vivo. 34:1085–1094. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Balahmar RM, Boocock DJ, Coveney C, Ray S,
Vadakekolathu J, Regad T, Ali S and Sivasubramaniam S:
Identification and characterisation of NANOG+/
OCT-4high/SOX2+ doxorubicin-resistant stem-like cells
from transformed trophoblastic cell lines. Oncotarget. 9:7054–7065.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Piva M, Domenici G, Iriondo O, Rábano M,
Simões BM, Comaills V, Barredo I, López-Ruiz JA, Zabalza I, Kypta R
and Vivanco Md: Sox2 promotes tamoxifen resistance in breast cancer
cells. EMBO Mol Med. 6:66–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Y, Chen K, Li L, Li R, Zhang J and Ren
W: Overexpression of SOX2 is involved in paclitaxel resistance of
ovarian cancer via the PI3K/Akt pathway. Tumour Biol. 36:9823–9828.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jin Y, Jiang Z, Guan X, Chen Y, Tang Q,
Wang G and Wang X: miR-450b-5p suppresses stemness and the
development of chemoresistance by targeting SOX2 in colorectal
cancer. DNA Cell Biol. 35:249–256. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Manzo-Merino J, Contreras-Paredes A,
Vazquez-Ulloa E, Rocha-Zavaleta L, Fuentes-Gonzalez AM and Lizano
M: The role of signaling pathways in cervical cancer and molecular
therapeutic targets. Arch Med Res. 45:525–539. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Safa AR, Saadatzadeh MR, Cohen-Gadol AA,
Pollok KE and Bijangi-Vishehsaraei K: Emerging targets for
glioblastoma stem cell therapy. J Biomed Res. 30:19–31. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu JJ and Wong ET: Personalized medicine
for glioblastoma: Current challenges and future opportunities. Curr
Mol Med. 13:358–367. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Garrido W, Rocha JD, Jaramillo C,
Fernandez K, Oyarzun C, San Martin R and Quezada C: Chemoresistance
in high-grade gliomas: Relevance of adenosine signalling in
stem-like cells of glioblastoma multiforme. Curr Drug Targets.
15:931–942. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bischof J, Westhoff MA, Wagner JE,
Halatsch ME, Trentmann S, Knippschild U, Wirtz CR and Burster T:
Cancer stem cells: The potential role of autophagy, proteolysis,
and cathepsins in glioblastoma stem cells. Tumour Biol.
39:10104283176922272017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Uribe D, Torres A, Rocha JD, Niechi I,
Oyarzún C, Sobrevia L, San Martín R and Quezada C: Multidrug
resistance in glioblastoma stem-like cells: Role of the hypoxic
microenvironment and adenosine signaling. Mol Aspects Med.
55:140–151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Safari M and Khoshnevisan A: Cancer stem
cells and chemoresistance in glioblastoma multiform: A review
article. J Stem Cells. 10:271–285. 2015.PubMed/NCBI
|
|
50
|
Cioffi M, Trabulo SM, Sanchez-Ripoll Y,
Miranda-Lorenzo I, Lonardo E, Dorado J, Reis Vieira C, Ramirez JC,
Hidalgo M, Aicher A, et al: The miR-17-92 cluster counteracts
quiescence and chemoresistance in a distinct subpopulation of
pancreatic cancer stem cells. Gut. 64:1936–1948. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ju SY, Chiou SH and Su Y: Maintenance of
the stemness in CD44(+) HCT-15 and HCT-116 human colon cancer cells
requires miR-203 suppression. Stem Cell Res. 12:86–100. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Rosow DE, Liss AS, Strobel O, Fritz S,
Bausch D, Valsangkar NP, Alsina J, Kulemann B, Park JK, Yamaguchi
J, et al: Sonic hedgehog in pancreatic cancer: From bench to
bedside, then back to the bench. Surgery. 152 (3 Suppl 1):S19–S32.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Song L, Chen X, Wang P, Gao S, Qu C and
Liu L: Effects of baicalein on pancreatic cancer stem cells via
modulation of sonic hedgehog pathway. Acta Biochim Biophys Sin
(Shanghai). 50:586–596. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li SH, Fu J, Watkins DN, Srivastava RK and
Shankar S: Sulforaphane regulates self-renewal of pancreatic cancer
stem cells through the modulation of Sonic hedgehog-GLI pathway.
Mol Cell Biochem. 373:217–227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhou W, Hao M, Du X, Chen K, Wang G and
Yang J: Advances in targeted therapy for osteosarcoma. Discov Med.
17:301–307. 2014.PubMed/NCBI
|
|
56
|
Kun-Peng Z, Xiao-Long M and Chun-Lin Z:
Overexpressed circPVT1, a potential new circular RNA biomarker,
contributes to doxorubicin and cisplatin resistance of osteosarcoma
cells by regulating ABCB1. Int J Biol Sci. 14:321–330. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang ZD, Wang RZ, Xia YZ, Kong LY and Yang
L: Reversal of multidrug resistance by icaritin in
doxorubicin-resistant human osteosarcoma cells. Chin J Nat Med.
16:20–28. 2018.PubMed/NCBI
|
|
58
|
Qi XT, Li YL, Zhang YQ, Xu T, Lu B, Fang
L, Gao JQ, Yu LS, Zhu DF, Yang B, et al: KLF4 functions as an
oncogene in promoting cancer stem cell-like characteristics in
osteosarcoma cells. Acta Pharmacol Sin. 40:546–555. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Vescovo T, Refolo G, Vitagliano G, Fimia
GM and Piacentini M: Molecular mechanisms of hepatitis C
virus-induced hepatocellular carcinoma. Clin Microbiol Infect.
22:853–861. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wu Q, Chen JX, Chen Y, Cai LL, Wang XZ,
Guo WH and Zheng JF: The chemokine receptor CCR10 promotes
inflammation-driven hepatocarcinogenesis via PI3K/Akt pathway
activation. Cell Death Dis. 9:2322018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yan HX, Wu HP, Zhang HL, Ashton C, Tong C,
Wu H, Qian QJ, Wang HY and Ying QL: p53 promotes
inflammation-associated hepatocarcinogenesis by inducing HMGB1
release. J Hepatol. 59:762–768. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Huan HB, Wen XD, Chen XJ, Wu L, Wu LL,
Zhang L, Yang DP, Zhang X, Bie P, Qian C and Xia F: Sympathetic
nervous system promotes hepatocarcinogenesis by modulating
inflammation through activation of alpha1-adrenergic receptors of
Kupffer cells. Brain Behav Immun. 59:118–134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Li C, Deng M, Hu J, Li X, Chen L, Ju Y,
Hao J and Meng S: Chronic inflammation contributes to the
development of hepatocellular carcinoma by decreasing miR-122
levels. Oncotarget. 7:17021–17034. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jung IH, Choi JH, Chung YY, Lim GL, Park
YN and Park SW: Predominant activation of JAK/STAT3 pathway by
interleukin-6 is implicated in hepatocarcinogenesis. Neoplasia.
17:586–597. 2015. View Article : Google Scholar : PubMed/NCBI
|