Lung cancer ranks first in morbidity and mortality
rate among malignant tumors in both the United States and worldwide
(1). Lung cancer is subdivided into
two major categories: Non-small cell lung cancer (NSCLC), which
accounts for 80–85% of all lung cancer cases, and small-cell lung
cancer (2). The prognosis for
patients with stage IV NSCLC is extremely poor, with reported
5-year overall survival (OS) rate of 1 to 8% in the United States
in 2018 (3). Platinum-based
chemotherapy has historically been the standard first-line
treatment for metastatic NSCLC, although responses to these agents
only range between 15–30%, with a relatively short interval until
disease progression (4,5). More recently, immunotherapy has emerged
as a promising treatment alternative for patients without an
actionable driver mutation and has markedly altered the therapeutic
approach to advanced NSCLC (6). It
has been demonstrated that with immunotherapy, the 5-year survival
rate of patients with advanced NSCLC increased from 4.7 to 16% in
the United States in 2018 (3). A
recent clinical trial also indicated that nivolumab treatment
improved long-term OS rate and achieved durable responses in a
proportion of patients with pretreated advanced NSCLC (3). Unlike traditional therapies for NSCLC,
immune therapies exploit the host immune system to monitor and
destroy cancer cells via the upregulation of key immune
checkpoints; at present, NSCLC immunotherapy mainly refers to
immune checkpoint inhibitors (ICIs) and anti-programmed cell death
protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) agents (7,8).
Patients with metastatic NSCLC generally benefit
from immunotherapy; however, a number of patients may not respond
to therapy, exhibit a shorter lifetime with treatment or suffer
major life-threatening immunotoxicities (9,10).
Immune therapies lack specific biomarkers compared with the
precision of targeting genes due to the complex interactions
between tumors and the immune system. Furthermore, the response to
immunotherapy also varies according to the tumor characteristics
(9,10). Apart from immune-associated adverse
events, such as dermatitis, enteritis and hepatitis, some patients
may experience clinical manifestations such as ‘pseudoprogression’
(PP) or ‘hyperprogressive’ disease (HPD) (11). PP is connected with infiltrations of
active T cells and other immune cells within the lesion (12), whereas HPD is defined as a rapid
increase in tumor growth rate (minimum two-fold) compared with the
expected growth rate (13).
Considering both the advantages and disadvantages of
immunotherapy, it is only suitable for a small number of patients
(14). Moreover, indiscriminate
application may significantly increase the incidence of adverse
reactions. Therefore, it is crucial to establish biomarkers
predictive of the response of patients with NSCLC to immunotherapy.
As a result, studying host-tumor interactions and identifying
predictive biomarkers for the response to immune checkpoint
blockade treatment is essential for enhancing the efficacy of
immunotherapy agents. In the present article, the currently
approved biomarkers for immune checkpoint inhibition in NSCLC are
reviewed, and the emerging effective, invalid and HPD markers are
highlighted. In addition, the identification of biomarkers that
predict treatment responses, and the development of rational
therapeutic combinations that could enhance the efficacy of immune
checkpoint blockade, are discussed.
As the understanding of lung cancer has increased,
the concept of treatment has also changed. Traditional treatments
for lung cancer have some insurmountable barriers. For example,
molecular targeting drugs may not be effective when genomic testing
reveals no targetable alteration, such as epidermal growth factor
receptor (EGFR) mutations, anaplastic lymphoma kinase (ALK) or ROS1
translocation/re-arrangements (15).
As traditional methods have been shown as ineffective against
certain cancer types, the focus has shifted to immune cells, and
immunotherapy for lung cancer is attracting increasing attention.
In particular, reactivating immune cells to clear cancer cells and
stopping cancer cell immune evasion are focuses of this research
(16).
Immunotherapy is a process of continuous responses
by activating the body's immune system to attack and kill tumor
cells. First, the antitumor immune response is enhanced and
prolonged by persistent recognition and memory of tumor antigens
(16). Subsequently, certain
cytotoxic T cells differentiate into natural memory T cells, which
can provide long-term immune memory protection, even in the absence
of the original antigen stimulation (16). Therefore, immunotherapy is more
likely to achieve long-term survival compared with conventional
treatments (17).
The immunotherapy of lung cancer is subdivided into
active and passive immunotherapy. The former enhances the antitumor
effect of the body by activating the patient's own immune response,
and mainly includes vaccines and immunoregulatory agents. The
latter provides patients with products such as anti-PD-1 and
anti-PD-L1 antibodies of the immune response to enhance the body's
antitumor response, mainly through adoptive cellular immunotherapy
(18). When it comes to the most
successful immunotherapy, ICIs have been attracting increasing
attention. Immune checkpoints are important inhibitory pathways for
controlling the duration and magnitude of the immune response.
Tumors can use these pathways to resist immune responses. ICIs have
the ability to interfere with tumor resistance and enhance the
body's immune response to tumor cells, including first-generation
anti-cytotoxic T lymphocyte antigen 4 (CTLA-4) antibodies,
second-generation anti-PD-1 antibodies and anti-PD-L1 antibodies
(7). Second-generation ICIs are more
selective and safer compared with first-generation ICIs (19).
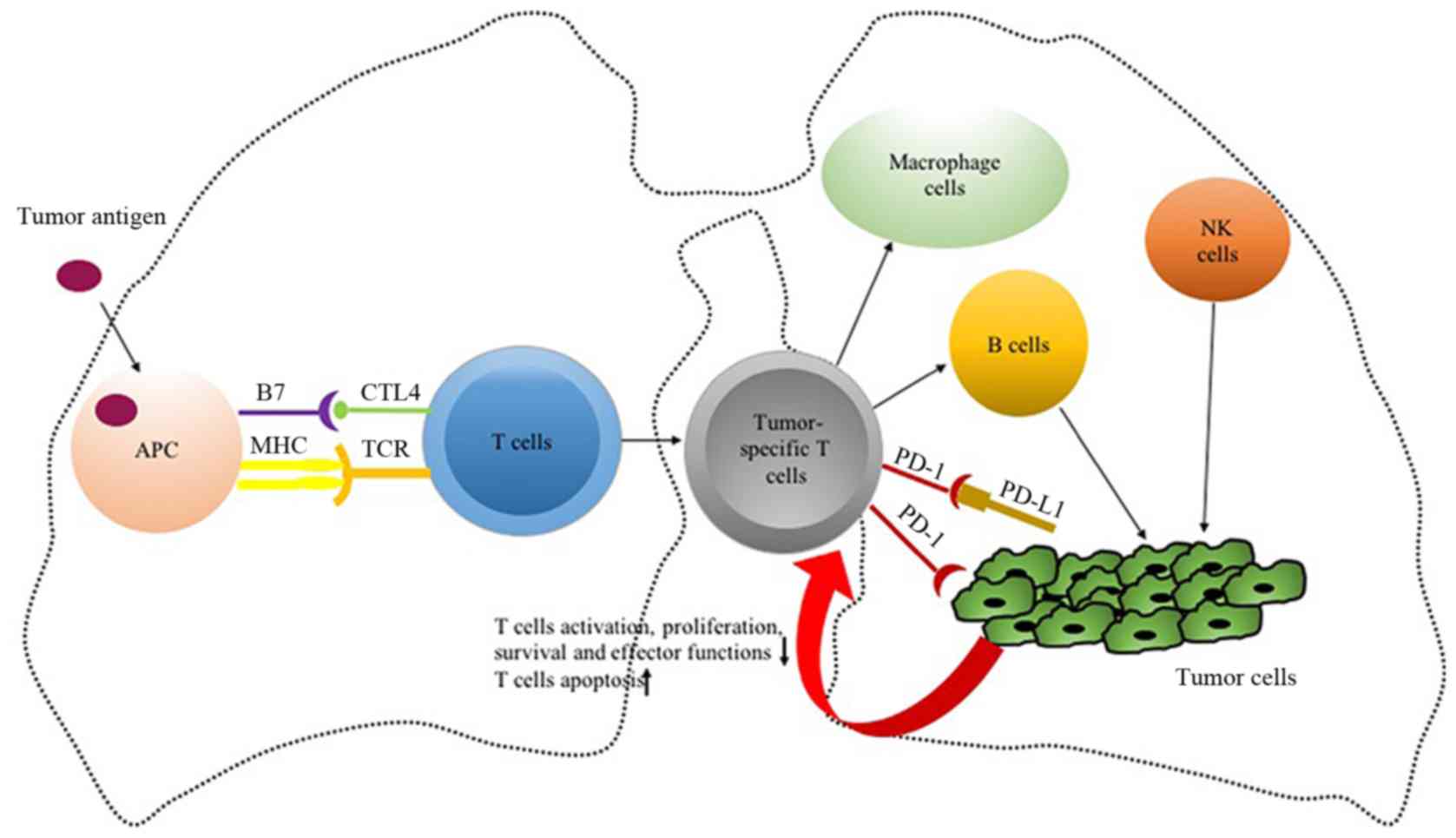
Normally, activation of T lymphocytes requires the
joint activation of two signaling pathways: The binding of the T
cell receptor to the major histocompatibility complex-antigen
peptide complex presented by antigen-presenting cells (APCs), and
the binding of the B7 molecule (B7-1 or B7-2) to CD28 on the
surface of T cells (20). CTLA-4 is
expressed exclusively on the surface of T cells, where it has a
higher affinity to B7 than CD28, and exerts the opposite function
compared with CD28 (21). CTLA-4
competes with the costimulatory receptor CD28 for binding to the
same ligands, resulting in downregulation of immune response
(22). A previous study found that
CTLA-4 was abnormally highly expressed on the surface of
tumor-infiltrating T regulatory cells (Tregs), and its expression
level in lymph nodes and tumor cells was significantly higher
compared with that of peripheral Tregs and effector T cells
(23). Tregs, a subgroup of
CD4+ T cells with notable immunosuppressive effects, can
inhibit the immune response of other cells. This inhibitory
function of Tregs depends heavily on CTLA-4. Thus, anti-CTLA-4
agents binding to CTLA-4 molecules can lead to Treg depletion or
functional blockade, thereby contributing to T cell activation and
immunological responses in cancer (24). Currently, there are two main types of
antibodies targeting CTLA-4: Ipilimumab and tremelimumab (23,24).
Ipilimumab has been approved by the U.S. Food and Drug
Administration (FDA) as the first ICI for advanced melanoma based
on several clinical trials, despite poor results in lung cancer
(25). A randomized phase III trial
demonstrated that ipilimumab in combination with chemotherapy did
not markedly improve OS compared with chemotherapy alone in the
first-line treatment for patients with advanced squamous NSCLC
(26).
PD-1, also known as CD279, is a monomeric
glycoprotein and a member of the CD28 superfamily. It is expressed
on the surface of activated T cells, B cells, natural killer (NK)
cells, dendritic cells and macrophages (27). PD-L1 is a ligand of PD-1 that is
highly expressed in various tumor cells, such as lung cancer,
malignant brain tumor and melanoma cells (28). The upregulation of PD-1 ligands in
the tumor microenvironment and the connection of PD-1 to its
ligands on tumor-specific T cells are the key mechanisms of
escaping immune elimination (27–32). For
example, PD-1 expressed on CD4+ and CD8+ T
cells, as well as other immune cells, interacts with PD-L1. This
leads to decreased activation, proliferation, survival, persistence
and effector functions of T lymphocytes, induces antigen-specific T
cell apoptosis and modulates the activity of CD4+ and
CD8+ T cells, NK cells and macrophages, thereby
affecting cancer progression in vitro and in vivo
(29–31). PD-L1 can be recognized by T cells,
resulting in the release of cytokines, which not only attract other
cytotoxic immune cells, but can also induce the expression of the
checkpoints that promote immune resistance, including the metabolic
reprogramming, differentiation characteristics and promotion of
homeostatic proliferation of T cells (32–34).
In recent years, a growing body of evidence has
shown the efficacy of PD-1/PD-L1, particularly in tumor
immunotherapy. To date, the FDA has approved four immunosuppressive
agents for NSCLC: Two anti-PD-1 (nivolumab and pembrolizumab) and
two anti-PD-L1 (atezolizumab and durvalumab) agents. Four clinical
trials (CheckMate-017, CheckMate-057, KEYNOTE-010 and OAK) have
confirmed that the immunotherapy group had different benefits in
terms of efficacy and survival in the second-line treatment setting
of NSCLC compared with the chemotherapy group (14,35,36).
Moreover, a phase III trial (KEYNOTE-024) revealed that
progression-free survival (PFS) or OS with first-line pembrolizumab
treatment for NSCLC expressing PD-L1 with a tumor proportion score
≥50%, were superior to those with first-line standard
platinum-based chemotherapy (37).
Based on results of KEYNOTE-042, in 2019 the FDA approved
pembrolizumab as first-line treatment for patients with PD-L1
expression ≥1%, EGFR mutation-negative and ALK-negative advanced
NSCLC (38–40). An interim analysis from the LCMC3
multicenter study at the 2019 World Conference on Lung Cancer
confirmed that atezolizumab achieved over half of the pathological
remissions in 49% of the patients with NSCLC on neoadjuvant
therapy, suggesting the great potential of immunotherapy in the
neoadjuvant setting for lung cancer (41). The clinical studies of IMpower130 and
KEYNOTE-189 reported that the PFS or OS of lung cancer were
significantly improved with the synergistic effect of immunotherapy
combined with chemotherapy (42,43). The
PACIFIC study demonstrated that durvalumab conferred OS benefits to
patients with unresectable stage III NSCLC after chemoradiotherapy
(44). IMpower150 indicated that
immunotherapy combined with antiangiogenic agents and chemotherapy
improved survival rates as a first-line treatment for advanced
non-squamous NSCLC (45). The
addition of atezolizumab to bevacizumab plus chemotherapy
significantly improved PFS and OS among patients with metastatic
non-squamous NSCLC, regardless of PD-L1 expression and EGFR or ALK
genetic alteration status (45).
Currently, pembrolizumab, nivolumab and
atezolizumab, which target the PD-1/PD-L1 axis, are associated with
a significant improvement in OS and durable antitumor responses in
advanced NSCLC (37,51–53). For
unresectable stage III NSCLC, the PACIFIC trial established
durvalumab as a new standard for consolidation therapy, which
involves continuous maintenance therapy in patients with stable
disease and follow-up treatment (44). The preliminary data from several
ongoing trials evaluating immunotherapy in the treatment of early
and locally advanced lung cancer are promising. However, whether
utilizing immune therapy in patients with early-stage NSCLC will
improve survival remains uncertain.
For the aforementioned reasons, studying host-tumor
interactions and establishing biomarkers to predict response to
immune checkpoint inhibition are crucial steps towards using the
new panel of immunotherapy agents in the most effective manner
(Fig. 1). The aim of the present
study was to review the biomarkers for immune checkpoint
inhibition, as well as other effective, invalid and HPD markers
that may have the potential to better predict responders to
immunotherapy in NSCLC (Table
I).
The predictive value of PD-L1 and tumor mutational
burden (TMB) in lung cancer has been tested in several clinical
trials (54). Facing the challenge
of adaptability and dynamic changes of the immune system, combined
application of biomarkers and dynamic monitoring are expected to
become a popular trend of immunotherapeutic research in the future.
In addition, in order to overcome the limitations of tissue sample
testing, new test methods are emerging (55).
The expression of PD-L1 may be a better predictive
biomarker that exhibits a stronger association with the antitumor
response compared with PD-1 (56).
The results of KEYNOTE-024 suggested that patients with advanced
NSCLC with high PD-L1 expression (≥50%) had a superior OS with
pembrolizumab compared with chemotherapy (37). Data from KEYNOTE-042 indicated that
the efficacy of immunotherapy was comparable to that of
chemotherapy when patient expression of PD-L1 was 1–49%. Therefore,
the higher the expression of PD-L1, the better the treatment effect
of immunotherapy in NSCLC (38).
CheckMate017 and OAK reported that the expression
levels of PD-L1 in tumor cells may not be a suitable biomarker for
predicting the efficacy of immunotherapy (35,52).
This may be because certain signaling pathways promote the
malignant behavior of the cancer cell, such as EGFR,
mitogen-activated protein kinase (MAPK) and
phosphatidylinositol-3-kinase-protein kinase (PI3K-AKT).
Conversely, inflammatory response cytokines, particularly
interferon (IFN)-γ, induce and stimulate PD-L1 expression in tumor
cells and other types of cells in the immune microenvironment
(57). In addition, different
detection platforms and evaluation systems have different positive
critical values, and there is no consistent standard to measure the
expression of PD-L1 in tumor cells (58). Therefore, diverse biopsy sites,
primary lesions, metastatic lesions and early treatment may affect
the dynamic change in PD-L1 expression, and a single biopsy cannot
reflect the whole picture of the tumor.
The expression of PD-L1 may be a suboptimal marker
for predicting the therapeutic efficacy of NSCLC immunotherapy, but
PD-L1 is currently the most established and widely used biomarker
for the clinical immunotherapy of NSCLC (14,35,37,40,42,43–45,51–53,59,60).
TMB is defined as the total number of mutations,
including replacement and insertion/deletion, per Megabyte of
exonic regions of the evaluated genes in the tumor specimen
(mut/Mb). Mutations in somatic cells can be transcribed/expressed
into RNA/protein levels, producing neoantigens, protein fragments
or peptides; these new products are recognized by the immune system
as non-autoantigens, activating T cells and eliciting an immune
response (61). Tumors are attacked
by a large number of tumor-specific T cells in patients with a high
TMB (TMB-H) (61). The response to
anti-PD-1/PD-L1 therapy depends on the numbers of tumor-specific T
cells (61). Therefore, tumors with
TMB-H are more sensitive to anti-PD-1/PD-L1 treatment, suggesting
that TMB and neoantigen burden may be considered as therapeutic
biomarkers of immunotherapy (62).
A previous study summarized the association between
TMB level and the effective rate of anti-PD-1 therapy in 27
different types of tumors, and demonstrated that the level of TMB
was different among diverse tumors (63). Among those treated with
anti-PD-1/PD-L1 inhibitors, the objective response rate (ORR) of
each tumor type was positively correlated with the level of TMB.
The higher the level of TMB expression, the greater the therapeutic
effect of PD-1/PD-L1 inhibitors. Previous studies have also
reported that patients with TMB-H have a high response rate to
anti-PD-1/PD-L1 immunotherapy (61–64).
CheckMate026 revealed that the ORR of nivolumab was significantly
higher (47 vs. 28%) and the PFS was markedly prolonged (9.7 vs. 5.8
months) in the TMB-H arm compared with that of platinum-based
chemotherapy (59). CheckMate227
reported that the PFS of patients with TMB-H (≥10 mut/Mb) treated
with nivolumab and ipilimumab was superior to that of chemotherapy
(43 vs. 13%, respectively) (64).
Surprisingly, in the same clinical trial, PFS was improved in
CheckMate 227 regardless of PD-L1 expression. CheckMate012 and
CheckMate026 also observed no notable correlation between TMB and
PD-L1 expression, indicating that TMB was an independent marker of
immunotherapeutic response (65).
Another study demonstrated that PD-L1 levels combined with TMB
could better predict the efficacy of immunotherapy (66); compared with patients with both low
expression of PD-L1 and TMB-L, the clinical benefit rate among
those with high expression of PD-L1 and TMB-H was 50% (66). A similar conclusion was reached by
CheckMate026 (59).
The POPLAR study analyzed the association between
blood TMB (bTMB) and clinical benefit. For bTMB ≥10, ≥16 and ≥20,
patients treated with atezolizumab had an increased PFS and OS
compared with docetaxel, and the greatest benefits were obtained
when bTMB ≥16 (53). The OAK study
further verified that atezolizumab was associated with a PFS
benefit, with a hazard ratio of 0.65 vs. 0.98 for bTMB ≥16 vs.
<16, respectively (35).
Overall, TMB is considered as a good predictor in
immunotherapy. As an emerging biomarker, TMB may be used to screen
patients who may benefit from anti-PD-1/PD-L1 immunotherapy.
As the function of PD-1/PD-L1 inhibitors also
requires the involvement of lymphocytes near the tumor, the
abundance of TILs may also be used as a biomarker to predict the
efficacy of PD-1/PD-L1 inhibitors (67). In NSCLC, an abundance of TILs in
primary tumor tissue has been associated with a more favorable
prognosis (67). TILs, particularly
infiltration by CD8+ T cells, often indicates a good
response to immunotherapy and a favorable prognosis (68,69). It
was previously demonstrated that patients with metastatic melanoma
with high numbers of CD8+ T cells in tumor tissues and
tumor margins are more responsive to immunotherapy compared with
conventional cytotoxic chemotherapy (12). The proliferation of CD8+ T
cells has been directly associated with the shrinkage of tumors on
imaging after ICI treatment (70).
In KEYNOTE-001, the number of CD8+ T lymphocytes in the
tumor parenchyma and margins of the baseline biopsy specimen of
patients with effective pembrolizumab treatment were found to be
higher compared with those with disease progression (60).
The immune microenvironment of PD-L1 and TILs is
divided into four states as follows (72): I (TIL+/PD-L1+),
II (TIL−/PD-L1−), III
(TIL+/PD-L1−) And IV
(TIL−/PD-L1+). Based on this method, the
association between PD-L1 and CD8+ TIL density in
patients with stage III NSCLC receiving concurrent
chemoradiotherapy was evaluated (73). The PFS in patients with type I, II,
III and IV was 17.6, 13.1, not reached (NR) and 8.6 months
(P=0.02), respectively, and the OS was 35.3, 36.9, NR and 13.9
months (P=0.11), respectively. The results demonstrate that the PFS
and OS are longer in patients with high numbers of CD8+
TILs, and suggests that the abundance of TILs may be used as a
biomarker for immunotherapy.
DNA MMR (dMMR) is an important replication error
avoidance mechanism that prevents mutation and is essential for
maintenance of genetic information, since it repairs polymerase
errors during replication and prevents recombination between
closely related sequences (74). MSI
is a form of genomic instability due to reduced fidelity during the
replication of repetitive DNA (74).
The highly unstable state of microsatellites is referred to as
MSI-H or dMMR, which is easily recognized by the immune system
(74).
It was demonstrated that patients with MSI-H/dMMR
are more likely to benefit from immunotherapy (75,76).
Pembrolizumab has been approved by the FDA for use in
MSI-H/dMMR-positive solid tumors that are unresectable or
metastatic in patients who receive no other treatments (77). In addition, nivolumab and
pembrolizumab were considered as alternative second- or third-line
treatments for dMMR/MSI-H metastatic colorectal cancer (mCRC) in
the 2017 NCCN guidelines (78). The
CheckMate142 study confirmed that mCRC with MSI-H/dMMR treated with
nivolumab and pembrolizumab had an increased immune response
compared with nivolumab monotherapy (79). Moreover, nivolumab has been approved
for mCRC with MSI-H/dMMR following unsuccessful standard
chemotherapy (80). Thus, MSI-H/dMMR
has emerged as another immunotherapy-related biomarker for
screening the subpopulation of patients that are likely to benefit
from immunotherapy.
Genes and signaling pathways associated with DNA
damage repair (DDR) in tumor cells may lead to genomic instability.
A previous study suggested that the mutation status of DDR was
correlated with the level of TMB, and that patients with
co-mutation may benefit more from immunotherapy (81). A retrospective analysis also found
that the PFS and OS of patients with metastatic urothelial
carcinoma with DDR mutations were significantly improved with
anti-PD-1/PD-L1 antibody treatment (82).
KRAS mutations are found in 15–20% of patients with
NSCLC, particularly in smokers with lung adenocarcinoma (83). KRAS mutations are implicated in tumor
formation, proliferation, migration, diffusion and angiogenesis
(84). A retrospective study
demonstrated that patients with KRAS mutations exhibited low
expression of PD-L1 and a high somatic mutation load (85), whereas others also suggested that
KRAS was positively correlated with PD-L1 expression, while it did
not regulate PD-L1 expression (86).
Notably, CheckMate-057 confirmed that patients with KRAS mutations
benefited more from nivolumab compared with those without KRAS
mutations (51). The BIRCH study
also reported that patients with advanced NSCLC with KRAS mutations
receiving atezolizumab had better outcomes compared with those with
wild-type KRAS (87).
It has been described that the mutation rate of TP53
was 39–46% in adenocarcinomas, 81% in squamous cell carcinomas and
68% in large-cell carcinomas (88).
Dong et al (89) reported
that mutation of TP53 or KRAS increased the expression of PD-L1 and
infiltration by CD8+ T cells. DNA polymerase
deltacatalytic subunit gene 1 (POLD1), DNA polymerase epsilon
catalytic subunit (POLE), Breast cancer susceptibility gene 1
(BRCA1), Breast cancer susceptibility gene 2 (BRCA2), the catalytic
subunit of the DNA-activated protein kinase (PRK-DC), DNA ligase 3
(LIG3), RAD17 checkpoint clamp loader component (RAD17), RAD51
paralog C (RAD51C), FA complementation group F (FANCF),
endonuclease non-catalytic subunit of ERCC excision repair 1
(ERCC1) and other rare genetic changes associated with equilibrium
and repair of functional proteins in the process of DNA replication
also affect the efficacy of immunotherapy. These mutations lead to
an increase in the load of non-synonymous mutations and the number
of TILs, making patients more sensitive to immunotherapy (90,91).
Anti-PD-1 antibodies have been reported to be highly effective in
endometrial, bowel and lung cancer patients harboring the POLE
mutation (92–94).
Molecular targeted therapy has been found to prolong
the OS and PFS of patients with advanced NSCLC; however, it is
difficult to effectively treat this type of cancer due to the
instability of the driver genes (95). Interactions between the tumor driver
gene pathway and the PD-1/PD-L1 pathway have been demonstrated
previously (14,35,37,43–45,51). In
addition to driver gene mutations, factors in
immunotherapy-resistant pathways appear to be involved (96,97). For
example, it has been demonstrated that IFN-γ is able to recognize
the corresponding receptors on tumor cells or APCs (98), and that mutations and deletions of
the IFN-γ receptor chains, such as Janus kinase (JAK)1 and JAK2,
STATs and INF regulatory factor 1, lead to resistance to ICIs.
Moreover, multiple mechanisms may stimulate high expression of
PD-L1, including phosphatase and tensin homolog (PTEN) deletion or
PI3K/AKT mutation, EGFR mutation, MYC overexpression, CDK5 gene
fragmentation and 3′-untranslated region truncation of PD-L1
(99,100). It remains unknown whether the high
expression of PD-L1 affects the response to ICIs, but it may indeed
lead to a lack of therapeutic response in antitumor immunotherapies
by inhibiting the activation of antitumor T cells (99,100).
Based on the findings of KEYNOTE-024, the FDA
approved pembrolizumab for initial treatment of NSCLC with high
PD-L1 expression (≥50%) and EGFR/ALK mutation-negative, which
accounts for approximately one-third of these cancer types. In the
phase II trial of KEYNOTE-001, 64% (seven out of 11) of patients
were positive for EGFR mutations (exons 19 and 21), and 73% (eight
out of 11) of patients exhibited high PD-L1 expression (60). Among patients with NSCLC, the rate of
effectiveness of anti-PD-1 treatment was almost zero. In the
IMpower150 study, patients with advanced NSCLC with EGFR mutations
did not benefit from the combination of PD-L1 and chemotherapy
(45). Similar results were reported
by phase III of the KEYNOTE-010, CheckMate057 and OAK trials
(14,35,51).
Multiple clinical trials and retrospective studies have
demonstrated that the ORR of patients with ALK mutations treated
with anti-PD1/PD-L1 inhibitors is lower compared with that of
patients with the wild-type (14,35,45,51,69). The
reasons for the poor therapeutic effect of anti-PD-1/PD-L1 agents
in patients with EGFR/ALK mutation is that these patients may have
a lower proportion of PD-L1+/CD8+ cells, as
well as having a non-inflammatory phenotype and weak immunogenicity
(101).
Phase III clinical trials have confirmed that NSCLC
with EGFR mutations exhibited a lower response to ICIs; however,
ICIs were found to be effective against some patients with NSCLC
with EGFR mutations (98–101). Hastings et al (102) found that EGFR mutations in
different alleles may affect the response to immunosuppressive
agents. In addition, smoking may be associated with the opposite
result. Another study demonstrated that the effectiveness of
anti-PD-1 therapy, regardless of EGFR mutations, was >20% in
patients with NSCLC who were smokers (103). The latest ATLANTIC trial confirmed
that patients with EGFR mutations and PD-L1 expression ≥25% may
benefit from durvalumab (104). The
efficacy of durvalumab was the lowest in patients with NSCLC with
low expression of PD-L1 and EGFR+/ALK+ [only
one of 128 patients (4%) reached OR].
Upon tumor antigen recognition, T cells produce
IFN-γ, which leads to the expression of IFN-stimulated genes
through the IFN-γ receptors, including JAK1 and JAK2, and also
activates STAT signaling (106).
JAK1/2 mutations have been found to be associated with loss of
PD-L1 expression upon IFN-γ exposure mediated by disabling the
receptor signaling pathway (106,107).
Shin et al (107) reported
two cases with JAK1/2 loss-of-function mutations and lack of
reactive PD-L1 expression. Therefore, patients with JAK1/2
mutations may not be not suitable candidates for PD-1 blockade
therapy (108).
HPD, also known as the ‘toxic’ response, may occur
in targeted therapy and chemotherapy; however, the incidence of HPD
after immunotherapy is significantly increased to >29%,
including 10–16% in patients with NSCLC compared with those that
did not receive immunotherapy (119). Patients with HPD have a poor
overall prognosis, with an OS of only 3–4 months (120). In early 2017, HPD occurred in ~9%
of patients treated with ICIs and 19% of the patients were aged
>65 years (121). In addition,
immunotherapy-induced HPD was not correlated with tumor load, tumor
type, number of treatment lines or PD-L1 expression level, but was
associated with advanced age (>65 years) and poor OS (121). HPD is principally observed with
PD-1/PD-L1 inhibitors; however, there is no significant difference
between PD-1 inhibitors and PD-L1 inhibitors in the occurrence of
HPD (121). In the clinical
setting, patients with lung cancer with driver gene mutations have
higher rates of HPD. A retrospective study found that >500
patients with eight common lung cancer gene mutations had a high
incidence of HPD in all the mutations after using PD-1/PD-L1 alone.
Among these mutations, the incidence of EGFR, ALK and
RET mutations were 44.8, 45.5 and 43.8%, respectively
(122). In regard to the factors
and mechanisms of HPD, studies have demonstrated that certain
clinical characteristics are associated with HPD, such as age
>65 years, number of baseline metastatic sites >2 or local
recurrence, although these characteristics have inconsistent
results in different studies and are not sufficient as predictors
(13,119,123,124).
A review summarized the five biological mechanisms by which
PD-1/PD-L1 inhibitors may cause HPD; four of those formed an
immunosuppressive microenvironment to facilitate the immune escape
of tumor cells and indirectly accelerate tumor growth, while one
directly promoted tumor cell proliferation through the activation
of oncogenes (125). Gene variation
has been associated with HPD; however, basic experiments and
further investigation, including studies about mouse double minute
(MDM)2/4 amplification, DNA methyltransferase 3 α
(DNMT3A) mutation and cyclin dependent kinase inhibitor
2A/2B (CDKN2A/B) deletion, are required.
MDM2 is a critical negative regulator of p53, and
plays a key role in controlling its transcriptional activity,
protein stability and nuclear localization (126). MDM2 expression is upregulated in
numerous cancer types, resulting in a loss of p53-dependent
functions, apoptosis and cell cycle arrest (126). Previous studies have demonstrated
that the MDM2 protein has low expression levels in normal tissues,
and that amplification of the MDM2 gene may lead to
tumorigenesis (126). Kato et
al (121) reported that 67%
(four out of six) patients with MDM2/4 gene
amplification-induced HPD and that the clinical symptoms of the
other two patients rapidly deteriorated, suggesting that
MDM2/4 gene amplification may be associated with HPD. In
another article published in 2018, Kato et al (127) further explored the amplification
status of MDM2 after immunotherapy in various cancer types.
MDM2 amplification accounted for 3.5% (3,650 cases) of the
tumors, among which 99.0% (3,613 cases) had genomic co-mutations,
and the most common co-mutated genes were CDK4 (43.6%),
fibroblast growth factor receptor substrate 2 (40.8%), TP53
(20.1%) and CDKN2A (18.2%). Various pathways, including
those associated with tyrosine kinase (37.9%; 1,385/3,650), PI3K
signaling (25.4%; 926/3,650), TP53 (24.9%; 910/3,650) and MAPK
signaling (23.6%; 863/3,650) were involved. In addition,
MDM2 amplifications were less frequently associated with
TMB-H compared with the MDM2 wild-type population (2.9 vs.
6.5%, respectively; P<0.001).
Immunotherapy for NSCLC has recently evolved into a
new standard treatment modality primarily through PD-1 and PD-L1
inhibitors. However, patient selection is currently at the
discretion of the treating physician. The predictors mentioned in
the present review are based on the latest research results, and
are innovatively classified into three categories: Effective,
invalid and ‘toxic’. All the biomarkers aforementioned may be
incorporated into the prognostic bio-score systems and
decision-making algorithms to better guide the application of
clinical immunotherapy.
Despite efforts focusing on immunotherapy in NSCLC,
a number of issues remain to be addressed in future studies.
Currently available evidence indicates that PD-L1, TMB and
MSI-H/dMMR have been acknowledged for screening the population in
whom immunotherapy is effective of immune drugs, thus it is crucial
to improve drug efficiency and reduce the incidence of adverse
reactions. However, these markers allPD-L1, TMB and MSI-H/dMMR have
certain drawbacks, for example some of the predictors have not been
identified due to the limited clinical validation sample size and
contradictory research results, which requires further confirmation
by prospective studies with larger sample size. In addition,
biomarkers and their mechanisms of action remain under
investigation, therefore the role of gene mutations in
immunotherapy of lung cancer requires further clinical research and
experiments to verify in the context of precision medicine. Scoring
tools based on blood indicators or characteristic expression of
tumor gene profiles, including LIPI, TIDE and IMPRES scores, have
not been widely used due to their respective drawbacks (129–131),
and an immune prognosis assessment scale must be developed by
combining various predicted molecules. Positron emission tomography
combined with CT, dynamic contrast-enhanced CT and
diffusion-weighted magnetic resonance imaging have demonstrated
promising results for diagnosing and staging patients with lung
cancer (132), and improvement of
the evaluation criteria for immunotherapy and the risk of HPD are
expected to make these predictions more precise (133,134).
Immunotherapy has long-lasting therapeutic activity
and appears to hold promise for patients with NSCLC (135). Efforts must be focused on
identifying patients who may benefit from this type of treatment
through biomarkers, and on effectively controlling adverse
reactions.
Not applicable.
This work was supported in part by the General
Project (grant nos. 81800074 and 81901454) from the National
Natural Science Foundation of China and the Youth Foundation
Project of Zhejiang Province of China (grant no. LQ18H010002).
Not applicable.
YH and XCW conceived and designed the study. LLW,
YH and SCW wrote the manuscript. XCW and JLS revised the article
for important intellectual content. All authors read and approved
the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Osmani L, Askin F, Gabrielson E and Li QK:
Current WHO guidelines and the critical role of immunohistochemical
markers in the subclassification of non-small cell lung carcinoma
(NSCLC): Moving from targeted therapy to immunotherapy. Semin
Cancer Biol. 52:103–109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gettinger S, Horn L, Jackman D, Spigel D,
Antonia S, Hellmann M, Powderly J, Heist R, Sequist LV, Smith DC,
et al: Five-year follow-up of nivolumab in previously treated
advanced non-small-cell lung cancer: Results from the CA209-003
study. J Clin Oncol. 36:1675–1684. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kelly K, Crowley J, Bunn PA Jr, Presant
CA, Grevstad PK, Moinpour CM, Ramsey SD, Wozniak AJ, Weiss GR,
Moore DF, et al: Randomized phase III trial of paclitaxel plus
carboplatin versus vinorelbine plus cisplatin in the treatment of
patients with advanced non-small-cell lung cancer: A Southwest
oncology group trial. J Clin Oncol. 19:3210–3218. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH; Eastern
Cooperative Oncology Group, : Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McNutt M: Cancer immunotherapy. Science.
342:14172013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zago G, Muller M, van den Heuvel M and
Baas P: New targeted treatments for non-small-cell lung cancer-role
of nivolumab. Biologics. 10:103–117. 2016.PubMed/NCBI
|
|
9
|
Camidge DR, Doebele RC and Kerr KM:
Comparing and contrasting predictive biomarkers for immunotherapy
and targeted therapy of NSCLC. Nat Rev Clin Oncol. 16:341–355.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suresh K, Naidoo J, Lin CT and Danoff S:
Immune checkpoint immunotherapy for non-small cell lung cancer:
Benefits and pulmonary toxicities. Chest. 154:1416–1423. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Santini FC, Rizvi H, Plodkowski AJ, Ni A,
Lacouture ME, Gambarin-Gelwan M, Wilkins O, Panora E, Halpenny DF,
Long NM, et al: Safety and efficacy of re-treating with
immunotherapy after immune-related adverse events in patients with
NSCLC. Cancer Immunol Res. 6:1093–1099. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Di Giacomo AM, Danielli R, Guidoboni M,
Calabrò L, Carlucci D, Miracco C, Volterrani L, Mazzei MA, Biagioli
M, Altomonte M and Maio M: Therapeutic efficacy of ipilimumab, an
anti-CTLA-4 monoclonal antibody, in patients with metastatic
melanoma unresponsive to prior systemic treatments: Clinical and
immunological evidence from three patient cases. Cancer Immunol
Immunother. 58:1297–1306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Champiat S, Dercle L, Ammari S, Massard C,
Hollebecque A, Postel-Vinay S, Chaput N, Eggermont A, Marabelle A,
Soria JC and Ferté C: Hyperprogressive disease is a new pattern of
progression in cancer patients treated by anti-PD-1/PD-L1. Clin
Cancer Res. 23:1920–1928. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xia L, Liu Y and Wang Y: PD-1/PD-L1
blockade therapy in advanced non-small-cell lung cancer: Current
status and future directions. Oncologist. 24 (Suppl 1):S31–S41.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang Y: Cancer immunotherapy: Harnessing
the immune system to battle cancer. J Clin Invest. 125:3335–3337.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang W, Liu J, He Y and McLeod HL:
Prospect for immune checkpoint blockade: Dynamic and comprehensive
monitorings pave the way. Pharmacogenomics. 18:1299–1304. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Almand B and Carbone DP: Biological
considerations in lung cancer. Cancer Treat Res. 105:1–30. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Assi HI, Kamphorst AO, Moukalled NM and
Ramalingam SS: Immune checkpoint inhibitors in advanced non-small
cell lung cancer. Cancer. 124:248–261. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen L and Flies DB: Molecular mechanisms
of T cell co-stimulation and co-inhibition. Nat Rev Immunol.
13:227–242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schildberg FA, Klein SR, Freeman GJ and
Sharpe AH: Coinhibitory pathways in the B7-CD28 ligand-receptor
family. Immunity. 44:955–972. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rowshanravan B, Halliday N and Sansom DM:
CTLA-4: A moving target in immunotherapy. Blood. 131:58–67. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Arce Vargas F, Furness AJS, Litchfield K,
Joshi K, Rosenthal R, Ghorani E, Solomon I, Lesko MH, Ruef N,
Roddie C, et al: Fc effector function contributes to the activity
of human anti-CTLA-4 antibodies. Cancer Cell. 33:649–663.e4. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Walker LS: Treg and CTLA-4: Two
intertwining pathways to immune tolerance. J Autoimmun. 45:49–57.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Corrales L, Scilla K, Caglevic C, Miller
K, Oliveira J and Rolfo C: Immunotherapy in lung cancer: A new age
in cancer treatment. Adv Exp Med Biol. 995:65–95. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Govindan R, Szczesna A, Ahn MJ, Schneider
CP, Gonzalez Mella PF, Barlesi F, Han B, Ganea DE, Von Pawel J,
Vladimirov V, et al: Phase III trial of ipilimumab combined with
paclitaxel and carboplatin in advanced squamous non-small-cell lung
cancer. J Clin Oncol. 35:3449–3457. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boussiotis VA, Chatterjee P and Li L:
Biochemical signaling of PD-1 on T cells and its functional
implications. Cancer J. 20:265–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang X, Teng F, Kong L and Yu J: PD-L1
expression in human cancers and its association with clinical
outcomes. Onco Targets Ther. 9:5023–5039. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Machicote A, Belén S, Baz P, Billordo LA
and Fainboim L: Human CD8+HLA-DR+ regulatory T cells, similarly to
classical CD4+Foxp3+ cells, suppress immune responses via
PD-1/PD-L1 axis. Front Immunol. 9:27882018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ventriglia J, Paciolla I, Pisano C, Cecere
SC, Di Napoli M, Tambaro R, Califano D, Losito S, Scognamiglio G,
Setola SV, et al: Immunotherapy in ovarian, endometrial and
cervical cancer: State of the art and future perspectives. Cancer
Treat Rev. 59:109–116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yahata T, Mizoguchi M, Kimura A, Orimo T,
Toujima S, Kuninaka Y, Nosaka M, Ishida Y, Sasaki I, Fukuda-Ohta Y,
et al: Programmed cell death ligand 1 disruption by clustered
regularly interspaced short palindromic repeats/Cas9-genome editing
promotes antitumor immunity and suppresses ovarian cancer
progression. Cancer Sci. 110:1279–1292. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Boussiotis VA: Molecular and biochemical
aspects of the PD-1 checkpoint pathway. N Engl J Med.
375:1767–1778. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gajewski TF, Schreiber H and Fu YX: Innate
and adaptive immune cells in the tumor microenvironment. Nat
Immunol. 14:1014–1022. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Spranger S, Spaapen RM, Zha Y, Williams J,
Meng Y, Ha TT and Gajewski TF: Up-regulation of PD-L1, IDO, and
T(regs) in the melanoma tumor microenvironment is driven by CD8(+)
T cells. Sci Transl Med. 5:200ra1162013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rittmeyer A, Barlesi F, Waterkamp D, Park
K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols
MC, et al: Atezolizumab versus docetaxel in patients with
previously treated non-small-cell lung cancer (OAK): A phase 3,
open-label, multicentre randomised controlled trial. Lancet.
389:255–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vokes EE, Ready N, Felip E, Horn L, Burgio
MA, Antonia SJ, Arén Frontera O, Gettinger S, Holgado E, Spigel D,
et al: Nivolumab versus docetaxel in previously treated advanced
non-small-cell lung cancer (CheckMate 017 and CheckMate 057):
3-Year update and outcomes in patients with liver metastases. Ann
Oncol. 29:959–965. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Reck M, Rodriguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nosaki K, Saka H, Hosomi Y, Baas P, de
Castro G Jr, Reck M, Wu YL, Brahmer JR, Felip E, Sawada T, et al:
Safety and efficacy of pembrolizumab monotherapy in elderly
patients with PD-L1-positive advanced non-small-cell lung cancer:
Pooled analysis from the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042
studies. Lung Cancer. 135:188–195. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lopes G, Wu YL, Kudaba I, Kowalski D, Cho
BC, Bondarenko I, Kubota K, Lubiniecki GM, Zhang J, Kush DA and Mok
T: Pembrolizumab (pembro) versus platinum-based chemotherapy
(chemo) as first-line therapy for advanced/metastatic NSCLC with a
PD-L1 tumor proportion score (TPS) >= 1%: Open-label, phase 3
KEYNOTE-042 study. J Clin Oncol. 36 (18 Suppl):LBA4. 2018.
View Article : Google Scholar
|
|
40
|
Mok TSK, Wu YL, Kudaba I, Kowalsk DM, Cho
BC, Turna HZ, de Castro G Jr, Srimuninnimit V, Laktionov KK,
Bondarenko I, et al: Final analysis of the phase III KEYNOTE-042
study: Pembrolizumab (Pembro) versus platinum-based chemotherapy
(Chemo) as first-line therapy for patients (Pts) with
PD-L1-positive locally advanced/metastatic NSCLC. Ann Oncol. 30
(Suppl 2):ii38–ii68. 2019. View Article : Google Scholar
|
|
41
|
Oezkan F, He K, Owen D, Pietrzak M, Cho
Jh, Kitzler R, Pearson R, Rusch V, Chaft J, Suh R, et al: OA13.07
Neoadjuvant atezolizumab in resectable NSCLC patients:
Immunophenotyping results from the interim analysis of the
multicenter trial LCMC3. J Thorac Oncol. 14 (10 Suppl):S242–S243.
2019. View Article : Google Scholar
|
|
42
|
Gandhi L, Rodriguez-Abreu D, Gadgeel S,
Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ,
Powell SF, et al: Pembrolizumab plus chemotherapy in metastatic
non-small-cell lung cancer. N Engl J Med. 378:2078–2092. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
West H, McCleod M, Hussein M, Morabito A,
Rittmeyer A, Conter HJ, Kopp HG, Daniel D, McCune S, Mekhail T, et
al: Atezolizumab in combination with carboplatin plus
nab-paclitaxel chemotherapy compared with chemotherapy alone as
first-line treatment for metastatic non-squamous non-small-cell
lung cancer (IMpower130): A multicentre, randomised, open-label,
phase 3 trial. Lancet Oncol. 20:924–937. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Antonia SJ, Villegas A, Daniel D, Vicente
D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, et
al: Overall survival with durvalumab after chemoradiotherapy in
stage III NSCLC. N Engl J Med. 379:2342–2350. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Socinski MA, Jotte RM, Cappuzzo F, Orlandi
F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D,
Thomas CA, Barlesi F, et al: Atezolizumab for first-line treatment
of metastatic nonsquamous NSCLC. N Engl J Med. 378:2288–2301. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Buchbinder EI and Desai A: CTLA-4 and PD-1
pathways: Similarities, differences, and implications of their
inhibition. Am J Clin Oncol. 39:98–106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wei SC, Levine JH, Cogdill AP, Zhao Y,
Anang NAS, Andrews MC, Sharma P, Wang J, Wargo JA, Pe'er D and
Allison JP: Distinct cellular mechanisms underlie anti-CTLA-4 and
anti-PD-1 checkpoint blockade. Cell. 170:1120–1133.e17. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Curran MA, Montalvo W, Yagita H and
Allison JP: PD-1 and CTLA-4 combination blockade expands
infiltrating T cells and reduces regulatory T and myeloid cells
within B16 melanoma tumors. Proc Natl Acad Sci USA. 107:4275–4280.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Das R, Verma R, Sznol M, Boddupalli CS,
Gettinger SN, Kluger H, Callahan M, Wolchok JD, Halaban R,
Dhodapkar MV and Dhodapkar KM: Combination therapy with anti-CTLA-4
and anti-PD-1 leads to distinct immunologic changes in vivo. J
Immunol. 194:950–959. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Reck M, Schenker M, Lee KH, Provencio M,
Nishio M, Lesniewski-Kmak K, Sangha R, Ahmed S, Raimbourg J, Feeney
K, et al: Nivolumab plus ipilimumab versus chemotherapy as
first-line treatment in advanced non-small-cell lung cancer with
high tumour mutational burden: Patient-reported outcomes results
from the randomised, open-label, phase III CheckMate 227 trial. Eur
J Cancer. 116:137–147. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. New Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fehrenbacher L, Spira A, Ballinger M,
Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D,
Artal-Cortes A, Lewanski C, et al: Atezolizumab versus docetaxel
for patients with previously treated non-small-cell lung cancer
(POPLAR): A multicentre, open-label, phase 2 randomised controlled
trial. Lancet. 387:1837–1846. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yu Y, Zeng D, Ou Q, Liu S, Li A, Chen Y,
Lin D, Gao Q, Zhou H, Liao W and Yao H: Association of survival and
immune-related biomarkers with immunotherapy in patients with
non-small cell lung cancer: A meta-analysis and individual
patient-level analysis. JAMA Netw Open. 2:e1968792019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chan TA, Yarchoan M, Jaffee E, Swanton C,
Quezada SA, Stenzinger A and Peters S: Development of tumor
mutation burden as an immunotherapy biomarker: Utility for the
oncology clinic. Ann Oncol. 30:44–56. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Salmaninejad A, Valilou SF, Shabgah AG,
Aslani S, Alimardani M, Pasdar A and Sahebkar A: PD-1/PD-L1
pathway: Basic biology and role in cancer immunotherapy. J Cell
Physiol. 234:16824–16837. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Patel SP and Kurzrock R: PD-L1 expression
as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther.
14:847–856. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gibney GT, Weiner LM and Atkins MB:
Predictive biomarkers for checkpoint inhibitor-based immunotherapy.
Lancet Oncol. 17:e542–e551. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Carbone DP, Reck M, Paz-Ares L, Creelan B,
Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F,
et al: First-line nivolumab in stage IV or recurrent non-small-cell
lung cancer. New Engl J Med. 376:2415–2426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Garon EB, Rizvi NA, Hui RN, Leighl N,
Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L,
et al: Pembrolizumab for the treatment of non-small-cell lung
cancer. New Engl J Med. 372:2018–2028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Fumet JD, Truntzer C, Yarchoan M and
Ghiringhelli F: Tumour mutational burden as a biomarker for
immunotherapy: Current data and emerging concepts. Eur J Cancer.
131:40–50. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Johnson DB, Frampton GM, Rioth MJ, Yusko
E, Xu Y, Guo X, Ennis RC, Fabrizio D, Chalmers ZR, Greenbowe J, et
al: Targeted next generation sequencing identifies markers of
response to PD-1 blockade. Cancer Immunol Res. 4:959–967. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yarchoan M, Hopkins A and Jaffee EM: Tumor
mutational burden and response rate to PD-1 inhibition. N Engl J
Med. 377:2500–2501. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hellmann MD, Ciuleanu TE, Pluzanski A, Lee
JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers
S, Salman P, et al: Nivolumab plus ipilimumab in lung cancer with a
high tumor mutational burden. N Engl J Med. 378:2093–2104. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bylicki O, Barazzutti H, Paleiron N,
Margery J, Assié JB and Chouaid C: First-line treatment of
non-small-cell lung cancer (NSCLC) with immune checkpoint
inhibitors. BioDrugs. 33:159–171. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Rizvi H, Sanchez-Vega F, La K, Chatila W,
Jonsson P, Halpenny D, Plodkowski A, Long N, Sauter JL, Rekhtman N,
et al: Molecular determinants of response to anti-programmed cell
death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in
patients with non-small-cell lung cancer profiled with targeted
next-generation sequencing. J Clin Oncol. 36:633–641. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zeng DQ, Yu YF, Ou QY, Li XY, Zhong RZ,
Xie CM and Hu QG: Prognostic and predictive value of
tumor-infiltrating lymphocytes for clinical therapeutic research in
patients with non-small cell lung cancer. Oncotarget.
7:13765–13781. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Geng Y, Shao Y, He W, Hu W, Xu Y, Chen J,
Wu C and Jiang J: prognostic role of tumor-infiltrating lymphocytes
in lung cancer: A meta-analysis. Cell Physiol Biochem.
37:1560–1571. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Dong ZY, Wu SP, Liao RQ, Huang SM and Wu
YL: Potential biomarker for checkpoint blockade immunotherapy and
treatment strategy. Tumour Biol. 37:4251–4261. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Tumeh PC, Harview CL, Yearley JH, Shintaku
IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu
V, et al: PD-1 blockade induces responses by inhibiting adaptive
immune resistance. Nature. 515:568–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Sun R, Limkin EJ, Vakalopoulou M, Dercle
L, Champiat S, Han SR, Verlingue L, Brandao D, Lancia A, Ammari S,
et al: A radiomics approach to assess tumour-infiltrating CD8 cells
and response to anti-PD-1 or anti-PD-L1 immunotherapy: An imaging
biomarker, retrospective multicohort study. Lancet Oncol.
19:1180–1191. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Teng MW, Ngiow SF, Ribas A and Smyth MJ:
Classifying cancers based on T-cell infiltration and PD-L1. Cancer
Res. 75:2139–2145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Tokito T, Azuma K, Kawahara A, Ishii H,
Yamada K, Matsuo N, Kinoshita T, Mizukami N, Ono H, Kage M and
Hoshino T: Predictive relevance of PD-L1 expression combined with
CD8+ TIL density in stage III non-small cell lung cancer patients
receiving concurrent chemoradiotherapy. Eur J Cancer. 55:7–14.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Baretti M and Le DT: DNA mismatch repair
in cancer. Pharmacol Ther. 189:45–62. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Nebot-Bral L, Coutzac C, Kannouche PL and
Chaput N: Why is immunotherapy effective (or not) in patients with
MSI/MMRD tumors? Bull Cancer. 106:105–113. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Quiroga D, Lyerly HK and Morse MA:
Deficient mismatch repair and the role of immunotherapy in
metastatic colorectal cancer. Curr Treat Options Oncol. 17:412016.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Le DT, Kim TW, Van Cutsem E, Geva R, Jäger
D, Hara H, Burge M, O'Neil B, Kavan P, Yoshino T, et al: Phase II
open-label study of pembrolizumab in treatment-refractory,
microsatellite instability-high/mismatch repair-deficient
metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol. 38:11–19.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Faivre JC, Adam V, Block V, Metzger M,
Salleron J and Dauchy S: Clinical practice guidelines of the French
association for supportive care in cancer and the French society
for psycho-oncology: Refusal of treatment by adults afflicted with
cancer. Support Care Cancer. 25:3425–3435. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Overman MJ, McDermott R, Leach JL, Lonardi
S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, et al:
Nivolumab in patients with metastatic DNA mismatch repair-deficient
or microsatellite instability-high colorectal cancer (CheckMate
142): An open-label, multicentre, phase 2 study. Lancet Oncol.
18:1182–1191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Morse MA, Overman MJ, Hartman L, Khoukaz
T, Brutcher E, Lenz HJ, Atasoy A, Shangguan T, Zhao H and El-Rayes
B: Safety of nivolumab plus low-dose ipilimumab in previously
treated microsatellite instability-high/mismatch repair-deficient
metastatic colorectal cancer. Oncologist. 24:1453–1461. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wang Z, Zhao J, Wang G, Zhang F, Zhang Z,
Zhang F, Zhang Y, Dong H, Zhao X, Duan J, et al: Comutations in DNA
damage response pathways serve as potential biomarkers for immune
checkpoint blockade. Cancer Res. 78:6486–6496. 2018.PubMed/NCBI
|
|
82
|
Teo MY, Seier K, Ostrovnaya I, Regazzi AM,
Kania BE, Moran MM, Cipolla CK, Bluth MJ, Chaim J, Al-Ahmadie H, et
al: Alterations in DNA damage response and repair genes as
potential marker of clinical benefit from PD-1/PD-L1 blockade in
advanced urothelial cancers. J Clin Oncol. 36:1685–1694. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Rodenhuis S, van de Wetering ML, Mooi WJ,
Evers SG, van Zandwijk N and Bos JL: Mutational activation of the
K-ras oncogene. A possible pathogenetic factor in adenocarcinoma of
the lung. N Engl J Med. 317:929–935. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Slebos RJ, Kibbelaar RE, Dalesio O,
Kooistra A, Stam J, Meijer CJ, Wagenaar SS, Vanderschueren RG, van
Zandwijk N, Mooi WJ, et al: K-ras oncogene activation as a
prognostic marker in adenocarcinoma of the lung. N Engl J Med.
323:561–565. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ji M, Liu Y, Li Q, Li X, Ning Z, Zhao W,
Shi H, Jiang J and Wu C: PD-1/PD-L1 expression in non-small-cell
lung cancer and its correlation with EGFR/KRAS mutations. Cancer
Biol Ther. 17:407–413. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Calles A, Liao X, Sholl LM, Rodig SJ,
Freeman GJ, Butaney M, Lydon C, Dahlberg SE, Hodi FS, Oxnard GR, et
al: Expression of PD-1 and Its ligands, PD-L1 and PD-L2, in smokers
and never smokers with KRAS-mutant lung cancer. J Thorac Oncol.
10:1726–1735. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Peters S, Gettinger S, Johnson ML, Jänne
PA, Garassino MC, Christoph D, Toh CK, Rizvi NA, Chaft JE,
Carcereny Costa E, et al: Phase II trial of atezolizumab as
first-line or subsequent therapy for patients with programmed
death-ligand 1-selected advanced non-small-cell lung cancer
(BIRCH). J Clin Oncol. 35:2781–2789. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Kato S, Han SY, Liu W, Otsuka K, Shibata
H, Kanamaru R and Ishioka C: Understanding the function-structure
and function-mutation relationships of p53 tumor suppressor protein
by high-resolution missense mutation analysis. Proc Natl Acad Sci
USA. 100:8424–8429. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Dong ZY, Zhong WZ, Zhang XC, Su J, Xie Z,
Liu SY, Tu HY, Chen HJ, Sun YL, Zhou Q, et al: Potential predictive
value of TP53 and KRAS mutation status for response to PD-1
blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res.
23:3012–3024. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Chabanon RM, Pedrero M, Lefebvre C,
Marabelle A, Soria JC and Postel-Vinay S: Mutational landscape and
sensitivity to immune checkpoint blockers. Clin Cancer Res.
22:4309–4321. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Strickland KC, Howitt BE, Shukla SA, Rodig
S, Ritterhouse LL, Liu JF, Garber JE, Chowdhury D, Wu CJ, D'Andrea
AD, et al: Association and prognostic significance of
BRCA1/2-mutation status with neoantigen load, number of
tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high
grade serous ovarian cancer. Oncotarget. 7:13587–13598. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Jacome AA and Eng C: Role of immune
checkpoint inhibitors in the treatment of colorectal cancer: Focus
on nivolumab. Expert Opin Biol Ther. 19:1247–1263. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Song Z, Cheng G, Xu C, Wang W, Shao Y and
Zhang Y: Clinicopathological characteristics of POLE mutation in
patients with non-small-cell lung cancer. Lung Cancer. 118:57–61.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Mehnert JM, Panda A, Zhong H, Hirshfield
K, Damare S, Lane K, Sokol L, Stein MN, Rodriguez-Rodriquez L,
Kaufman HL, et al: Immune activation and response to pembrolizumab
in POLE-mutant endometrial cancer. J Clin Invest. 126:2334–2340.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Sharma P, Hu-Lieskovan S, Wargo JA and
Ribas A: Primary, adaptive, and acquired resistance to cancer
immunotherapy. Cell. 168:707–723. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Syn NL, Teng MWL, Mok TSK and Soo RA:
De-novo and acquired resistance to immune checkpoint targeting.
Lancet Oncol. 18:e731–e741. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Knutson KL and Disis ML: Tumor
antigen-specific T helper cells in cancer immunity and
immunotherapy. Cancer Immunol Immunother. 54:721–728. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Cha JH, Chan LC, Li CW, Hsu JL and Hung
MC: Mechanisms controlling PD-L1 expression in cancer. Mol Cell.
76:359–370. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Lin H, Wei S, Hurt EM, Green MD, Zhao L,
Vatan L, Szeliga W, Herbst R, Harms PW, Fecher LA, et al: Host
expression of PD-L1 determines efficacy of PD-L1 pathway
blockade-mediated tumor regression. J Clin Invest. 128:17082018.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Gainor JF, Shaw AT, Sequist LV, Fu X,
Azzoli CG, Piotrowska Z, Huynh TG, Zhao L, Fulton L, Schultz KR, et
al: EGFR mutations and ALK rearrangements are associated with low
response rates to PD-1 pathway blockade in non-small cell lung
cancer: A retrospective analysis. Clin Cancer Res. 22:4585–4593.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Hastings K, Yu HA, Wei W, Sanchez-Vega F,
DeVeaux M, Choi J, Rizvi H, Lisberg A, Truini A, Lydon CA, et al:
EGFR mutation subtypes and response to immune checkpoint blockade
treatment in non-small-cell lung cancer. Ann Oncol. 30:1311–1320.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Garassino MC, Gelibter AJ, Grossi F,
Chiari R, Soto Parra H, Cascinu S, Cognetti F, Turci D, Blasi L,
Bengala C, et al: Italian nivolumab expanded access program in
nonsquamous non-small cell lung cancer patients: Results in
never-smokers and EGFR-mutant patients. J Thorac Oncol.
13:1146–1155. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Garassino MC, Cho BC, Kim JH, Mazières J,
Vansteenkiste J, Lena H, Corral Jaime J, Gray JE, Powderly J,
Chouaid C, et al: Durvalumab as third-line or later treatment for
advanced non-small-cell lung cancer (ATLANTIC): An open-label,
single-arm, phase 2 study. Lancet Oncol. 19:521–536. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Su S, Dong ZY, Xie Z, Yan LX, Li YF, Su J,
Liu SY, Yin K, Chen RL, Huang SM, et al: Strong programmed death
ligand 1 expression predicts poor response and de novo resistance
to EGFR tyrosine kinase inhibitors among NSCLC patients with EGFR
mutation. J Thorac Oncol. 13:1668–1675. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Briscoe J, Guschin D, Rogers NC, Watling
D, Müller M, Horn F, Heinrich P, Stark GR and Kerr IM: JAKs, STATs
and signal transduction in response to the interferons and other
cytokines. Philos Trans R Soc Lond B Biol Sci. 351:167–171. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Shin DS, Zaretsky JM, Escuin-Ordinas H,
Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, Grasso CS, Hugo W,
Sandoval S, Torrejon DY, et al: Primary resistance to PD-1 blockade
mediated by JAK1/2 mutations. Cancer Discov. 7:188–201. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Garcia-Diaz A, Shin DS, Moreno BH, Saco J,
Escuin-Ordinas H, Rodriguez GA, Zaretsky JM, Sun L, Hugo W, Wang X,
et al: Interferon receptor signaling pathways regulating PD-L1 and
PD-L2 expression. Cell Rep. 29:37662019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Massacesi C, di Tomaso E, Fretault N and
Hirawat S: Challenges in the clinical development of PI3K
inhibitors. Ann N Y Acad Sci. 1280:19–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Mendes RD, Canté-Barrett K, Pieters R and
Meijerink JP: The relevance of PTEN-AKT in relation to
NOTCH1-directed treatment strategies in T-cell acute lymphoblastic
leukemia. Haematologica. 101:1010–1017. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Peng W, Chen JQ, Liu C, Malu S, Creasy C,
Tetzlaff MT, Xu C, McKenzie JA, Zhang C, Liang X, et al: Loss of
PTEN promotes resistance to T cell-mediated immunotherapy. Cancer
Discov. 6:202–216. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Roh MR, Gupta S, Park KH, Chung KY, Lauss
M, Flaherty KT, Jönsson G, Rha SY and Tsao H: Promoter methylation
of PTEN Is a significant prognostic factor in melanoma survival. J
Invest Dermatol. 136:1002–1011. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Biton J, Mansuet-Lupo A, Pécuchet N,
Alifano M, Ouakrim H, Arrondeau J, Boudou-Rouquette P, Goldwasser
F, Leroy K, Goc J, et al: TP53, STK11, and EGFR mutations predict
tumor immune profile and the response to Anti-PD-1 in lung
adenocarcinoma. Clin Cancer Res. 24:5710–5723. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Skoulidis F, Goldberg ME, Greenawalt DM,
Hellmann MD, Awad MM, Gainor JF, Schrock AB, Hartmaier RJ, Trabucco
SE, Gay L, et al: STK11/LKB1 mutations and PD-1 inhibitor
resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov.
8:822–835. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Jamme P, Fernandes M, Copin MC,
Descarpentries C, Escande F, Morabito A, Grégoire V, Jamme M,
Baldacci S, Tulasne D, et al: Alterations in the PI3K pathway drive
resistance to MET inhibitors in NSCLC harboring MET exon 14
skipping mutations. J Thorac Oncol. 15:741–751. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Saigi M, Alburquerque-Bejar JJ and
Sanchez-Cespedes M: Determinants of immunological evasion and
immunocheckpoint inhibition response in non-small cell lung cancer:
The genetic front. Oncogene. 38:5921–5932. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Zaretsky JM, Garcia-Diaz A, Shin DS,
Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY,
Abril-Rodriguez G, Sandoval S, Barthly L, et al: Mutations
associated with acquired resistance to PD-1 blockade in melanoma. N
Engl J Med. 375:819–829. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Gettinger S, Choi J, Hastings K, Truini A,
Datar I, Sowell R, Wurtz A, Dong W, Cai G, Melnick MA, et al:
Impaired HLA class I antigen processing and presentation as a
mechanism of acquired resistance to immune checkpoint inhibitors in
lung cancer. Cancer Discov. 7:1420–1435. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
S Saâda-Bouzid E, Defaucheux C,
Karabajakian A, Coloma VP, Servois V, Paoletti X, Even C, Fayette
J, Guigay J, Loirat D, et al: Hyperprogression during
anti-PD-1/PD-L1 therapy in patients with recurrent and/or
metastatic head and neck squamous cell carcinoma. Ann Oncol.
28:1605–1611. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Fuentes-Antrás J, Provencio M and
Diaz-Rubio E: Hyperprogression as a distinct outcome after
immunotherapy. Cancer Treat Rev. 70:16–21. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Kato S, Goodman A, Walavalkar V,
Barkauskas DA, Sharabi A and Kurzrock R: Hyperprogressors after
immunotherapy: Analysis of genomic alterations associated with
accelerated growth rate. Clin Cancer Res. 23:4242–4250. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Mazieres J, Drilon A, Lusque A, Mhanna L,
Cortot AB, Mezquita L, Thai AA, Mascaux C, Couraud S, Veillon R, et
al: Immune checkpoint inhibitors for patients with advanced lung
cancer and oncogenic driver alterations: Results from the
IMMUNOTARGET registry. Ann Oncol. 30:1321–1328. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Ferrara R, Mezquita L, Texier M, Lahmar J,
Audigier-Valette C, Tessonnier L, Mazieres J, Zalcman G, Brosseau
S, Le Moulec S, et al: Hyperprogressive disease in patients with
advanced non-small cell lung cancer treated with PD-1/PD-L1
inhibitors or with single-agent chemotherapy. JAMA Oncol.
4:1543–1552. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Kim CG, Kim KH, Pyo KH, Xin CF, Hong MH,
Ahn BC, Kim Y, Choi SJ, Yoon HI, Lee JG, et al: Hyperprogressive
disease during PD-1/PD-L1 blockade in patients with non-small-cell
lung cancer. Ann Oncol. 30:1104–1113. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Champiat S, Ferrara R, Massard C, Besse B,
Marabelle A, Soria JC and Ferté C: Hyperprogressive disease:
Recognizing a novel pattern to improve patient management. Nat Rev
Clin Oncol. 15:748–762. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Oliner JD, Saiki AY and Caenepeel S: The
role of MDM2 amplification and overexpression in tumorigenesis.
Cold Spring Harb Perspect Med. 6:a0263362016. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Kato S, Ross JS, Gay L, Dayyani F, Roszik
J, Subbiah V and Kurzrock R: Analysis of MDM2 amplification:
Next-generation sequencing of patients with diverse malignancies.
JCO Precis Oncol 2018. 10.1200/PO.17.00235. 2018. View Article : Google Scholar
|
|
128
|
Giusti R, Mazzotta M, Filetti M, Marinelli
D, Di Napoli A, Scarpino S, Scafetta G, Mei M, Vecchione A, Ruco L
and Marchetti P: CDKN2A/B gene loss and MDM2 alteration as a
potential molecular signature for hyperprogressive disease in
advanced NSCLC: A next-generation-sequencing approach. J Clin
Oncol. 37 (15 Suppl):e20628. 2019. View Article : Google Scholar
|
|
129
|
Auslander N, Zhang G, Lee JS, Frederick
DT, Miao B, Moll T, Tian T, Wei Z, Madan S, Sullivan RJ, et al:
Robust prediction of response to immune checkpoint blockade therapy
in metastatic melanoma. Nat Med. 24:1545–1549. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Jiang P, Gu S, Pan D, Fu J, Sahu A, Hu X,
Li Z, Traugh N, Bu X, Li B, et al: Signatures of T cell dysfunction
and exclusion predict cancer immunotherapy response. Nat Med.
24:1550–1558. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Mezquita L, Auclin E, Ferrara R, Charrier
M, Remon J, Planchard D, Ponce S, Ares LP, Leroy L,
Audigier-Valette C, et al: Association of the lung immune
prognostic index with immune checkpoint inhibitor outcomes in
patients with advanced non-small cell lung cancer. JAMA Oncol.
4:351–357. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Harders SW, Balyasnikowa S and Fischer BM:
Functional imaging in lung cancer. Clin Physiol Funct Imaging.
34:340–355. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Rossi S, Castello A, Toschi L and Lopci E:
Immunotherapy in non-small-cell lung cancer: Potential predictors
of response and new strategies to assess activity. Immunotherapy.
10:797–805. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Vrankar M and Unk M: Immune RECIST
criteria and symptomatic pseudoprogression in non-small cell lung
cancer patients treated with immunotherapy. Radiol Oncol.
52:365–369. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Andrews MC and Wargo JA: Cancer evolution
during immunotherapy. Cell. 171:740–742. 2017. View Article : Google Scholar : PubMed/NCBI
|