Introduction
Prostate cancer is now one of the most commonly
diagnosed cancers, with more than 1,100,000 newly diagnosed cases
worldwide in 2012 (1), and the
incidence of prostate cancer has been particularly increasing in
northeast Asian countries (2). The
increase in prostate cancer incidence primarily results from early
diagnosis following the widespread use of prostate-specific antigen
(PSA) screening. This indicates that the management of localized
prostate cancer plays an important role in its treatment.
The effectiveness of surgery, radiotherapy, and
active monitoring for localized prostate cancer remains
controversial. The first randomized trial, the Prostate Testing for
Cancer and Treatment (ProtecT) trial, indicated no significant
differences in cancer-specific survival (CSS) and overall survival
(OS) among the three treatment modalities (3). However, the prostate cancer cases in
this trial mostly had a Gleason score of 6 (77%), cT1c (21%), and
PSA <10 µg/l (median PSA, 5.8 µg/l) (4). Given that most patients had low-risk
cancer, the survival outcomes differed from those of previous
studies that used real-world data, which generally showed a
survival benefit in patients with high-risk prostate cancer
(5–10). Furthermore, the ProtecT trial
included only patients aged 50–69 years, whereas prostate cancer is
commonly detected in the elderly (those aged 70 years or older).
The number of elderly cancer patients has been increasing (11). Japan is anticipated to become a
super-aging society by 2030, with one in every three people being
65+ years and one in five people being 75+ years (12). Therefore, treatments for elderly
patients will be crucial, and clinical trials or large cohort
studies for survival outcomes of elderly patients are needed for
treatment selection.
Previous studies indicated that hormonal therapy is
relatively more efficacious and safer in Japanese men than in
Caucasian men (13,14). Nevertheless, no large cohort study on
Japanese or Asian patients has ever investigated survival after
local treatments.
For the reasons above, the present study was carried
out with two objectives: i) To compare the efficacy between surgery
and radiotherapy for clinically localized prostate cancer using
data from a Japanese regional population-based prostate cancer
database; and ii) to compare the efficacy of these two treatment
modalities between elderly and younger patients. In order to
fulfill these objectives, we conducted a coarsened exact matching
of cancer features, and patients were also categorized by age using
a cutoff point of 70 years.
Patients and methods
Study population and study design
In this study, we accessed the data of 58,894
patients diagnosed with prostate cancer between 1970 and 2014 from
the population-based cancer registry of the Kanagawa Prefecture,
Japan. The inclusion criteria were localized prostate cancer
(cT1-3N0M0), adenocarcinoma, age 50–79 years, an observation period
of ≥2 years, and either surgery or radiotherapy as the main
treatment. Patients with missing data were excluded. For those
treated with both treatment modalities, surgery was considered the
main treatment. The reason for this is that salvage radiotherapy is
common in patients with prostate cancer recurrence after surgery,
whereas salvage prostatectomy after radiotherapy is rare (15). As we used secondary data, the
requirement to obtain informed consent was waived.
The primary endpoints of this study were CSS and OS.
The patients were also categorized by the age cutoff of 70 years
(i.e., 50–69 vs. 70–79 years), and the effectiveness of treatment
in either group was analyzed in terms of survival outcomes.
This study was conducted in accordance with the
ethical standards of the Helsinki Declaration and was approved by
the institutional review board of the Kanagawa Cancer Center.
Data source
Data were collected from a population-based regional
cancer registry of the Kanagawa Prefecture. The registry stores
medical data obtained from hospitals and survival information from
the regional public health center. The primary information includes
the date of birth, age at cancer diagnosis, cancer differentiation
(well, moderate, poor and undifferentiated), clinical and
pathological stages, diagnostic methods, the main treatment
modality (surgery, laparoscopic surgery, endoscopic surgery,
radiotherapy, hormonal therapy, chemotherapy and immunotherapy),
initial symptoms, the hospital where the patient was treated, cause
of death, and date of death or the latest date of confirmed
survival. However, no information on PSA was provided.
Statistical analysis
Cancer stages were classified using the 2017 TNM
classification, and survival time was calculated from the date of
diagnosis. To analyze the patients' characteristics, we used the
Mann-Whitney U test for continuous variables and Pearson's
chi-squared tests for categorical variables. Continuous
measurements were used for analysis of age at diagnosis. To
precisely assess treatment efficacy, a 1:1 coarsened exact matching
of age at diagnosis, clinical T stage, and cancer differentiation
was made between the surgery and radiotherapy groups. Exact
matching was conducted via propensity score matching with a caliper
width of 0. The covariate balance between the two groups was
assessed using the Mann-Whitney U test and Pearson's
Chi-squared tests.
The Kaplan-Meier method and univariate comparisons
using log-rank test and unadjusted Cox models were performed to
estimate CSS and OS. A two-tailed P-value of <0.05 was
considered statistically significant. All statistical analyses were
carried out with the IBM SPSS Statistics v25 (IBM Corp.). In
addition, interaction in the forest plot study was analyzed using
the statistical software ‘EZR’ (version _1.36_; Saitama Medical
Center, Jichi Medical University, Saitama, Japan), a graphical user
interface for R (version 3.4.1; The R Foundation for Statistical
Computing, Vienna, Austria) (16).
Results
Patient characteristics
The cohort included 6,805 patients, of whom 3,610
underwent surgery and 3,195 received radiotherapy as the main
treatment. After exact matching for age, clinical T stage, and
cancer differentiation, we analyzed data of 4,810 patients
(Table I), 2,405 of whom underwent
surgery as the main treatment and 2,405 who received radiotherapy.
The median observation period was 6.3 years (range, 2.0–18.7
years). Out of 4,810 patients, 43 (0.9%) and 305 (6.3%) patients
died of prostate cancer and other causes, respectively.
 | Table I.Characteristics of the entire cohort
and after matching age, clinical T stage and differentiation. |
Table I.
Characteristics of the entire cohort
and after matching age, clinical T stage and differentiation.
|
| Entire cohort | After matching |
|---|
|
|
|
|
|---|
| Characteristics | Total (n=6,805) | Surgery
(n=3,610) | Radiation
(n=3,195) | P-value | Total (n=4,810) | Surgery
(n=2,405) | Radiation
(n=2,405) | P-value |
|---|
| Age, median years
(range) | 69 (50–79) | 68 (50–79) | 71 (50–79) |
<0.001a | 69 (50–79) | 69 (50–79) | 69 (50–79) |
>0.999a |
| Age |
|
|
|
<0.001b |
|
|
|
>0.999b |
| 50-69, n
(%) | 3,519 (51.7) | 1,487 (41.2) | 1,396 (43.7) |
| 2,524 (52.5) | 1,262 (52.5) | 1,262 (52.5) |
|
| 70-79, n
(%) | 3,286 (48.3) | 2,123 (58.8) | 1,799 (56.3) |
| 2,286 (47.5) | 1,143 (47.5) | 1,143 (47.5) |
|
| Clinical T stage, n
(%) |
|
|
|
<0.001b |
|
|
|
>0.999b |
| T1 | 3,083 (45.3) | 1,653 (45.8) | 1,430 (44.8) |
| 2,386 (49.6) | 1,193 (49.6) | 1,193 (49.6) |
|
| T2 | 3,014 (44.3) | 1,727 (47.8) | 1,287 (40.3) |
| 2,040 (42.4) | 1,020 (42.4) | 1,020 (42.4) |
|
| T3 | 708 (10.4) | 230 (6.4) | 478 (15.0) |
| 384 (8.0) | 192 (8.0) | 192 (8.0) |
|
| Differentiation, n
(%) |
|
|
| 0.047b |
|
|
|
>0.999b |
|
Well | 948 (13.9) | 481 (13.3) | 467 (14.6) |
| 738 (15.3) | 369 (15.3) | 369 (15.3) |
|
|
Moderate | 2,662 (39.1) | 1,459 (40.4) | 1,203 (37.7) |
| 2,024 (42.1) | 1,012 (42.1) | 1,012 (42.1) |
|
|
Poor/undifferentiated | 3,195 (47.0) | 1,670 (46.3) | 1,525 (47.7) |
| 2,048 (42.6) | 1,024 (42.6) | 1,024 (42.6) |
|
| Date of
diagnosis |
|
|
|
<0.001b |
|
|
|
<0.001b |
|
<2009 | 1,965 (28.9) | 1,127(31.2) | 838 (26.2) |
| 1,766 (36.7) | 1,029 (42.8) | 737 (30.6) |
|
|
>2010 | 4,840 (71.1) | 2,483 (68.8) | 2,357 (73.8) |
| 3,044 (63.3) | 1,376 (57.2) | 1,668 (69.4) |
|
Survival based on treatment
modality
In the surgery group, prostate cancer was the main
cause of death in 23 patients (1.0%), whereas 142 patients died of
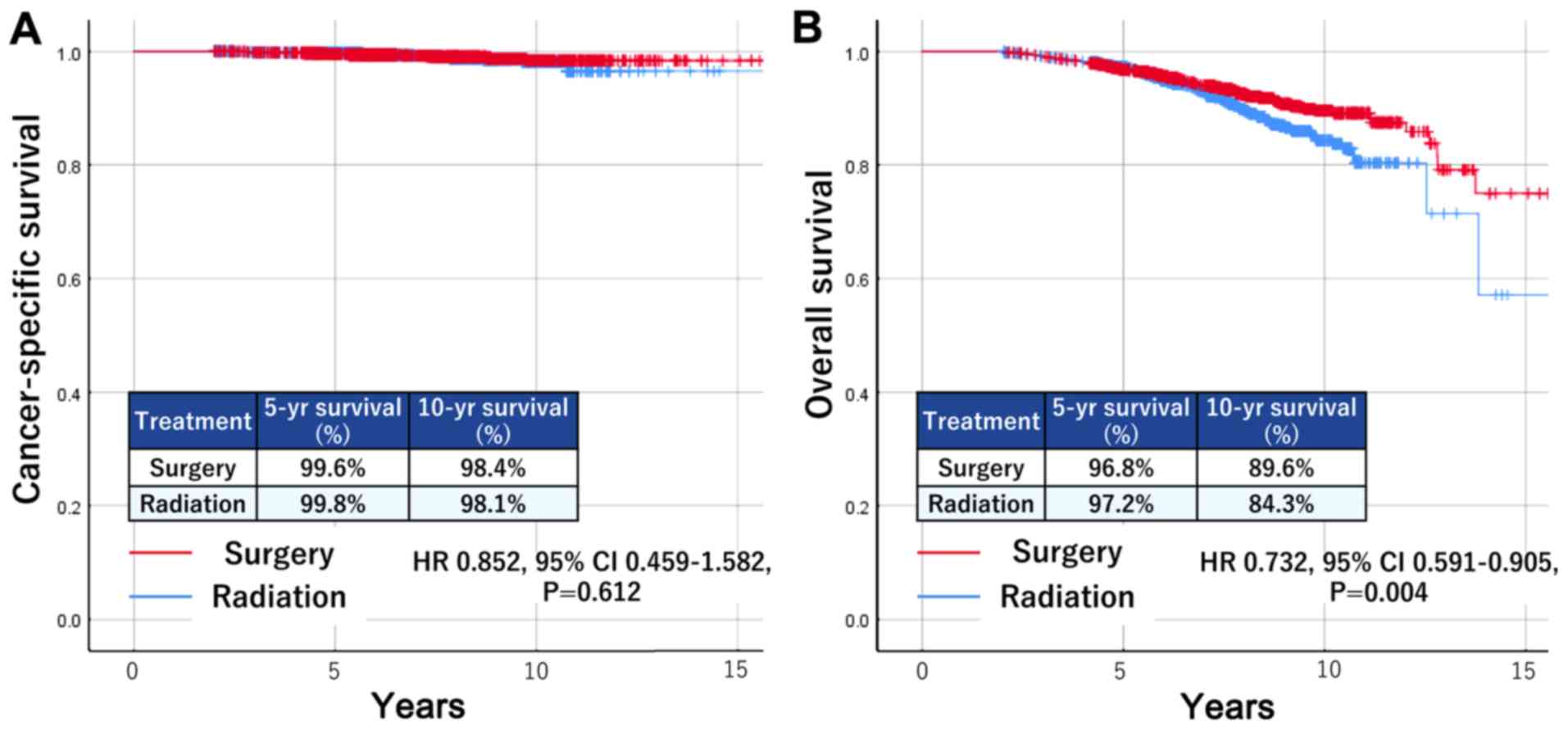
other causes (5.9%). The 5- and 10-year CSS were 99.6 and 98.4%,
respectively, and the 5- and 10-year OS were 96.8 and 89.6%,
respectively. In the radiotherapy group, there were 20 deaths due
to prostate cancer (0.8%), compared to 163 patients (6.8%) who died
of other causes. The 5-year CSS was 99.8%, and the 10-year CSS was
98.1%. The 5- and 10-year OS were 97.2 and 84.3%, respectively.
The Kaplan-Meier curves are shown in Fig. 1. There was no significant difference
in CSS between the two groups [surgery vs. radiotherapy: Hazard
ratio (HR), 0.852; 95% confidence interval (CI) 0.459–1.582;
P=0.612]; however, the surgery group had significantly better OS
than the radiotherapy group (HR, 0.732; 95% CI 0.591–0.905;
P=0.004).
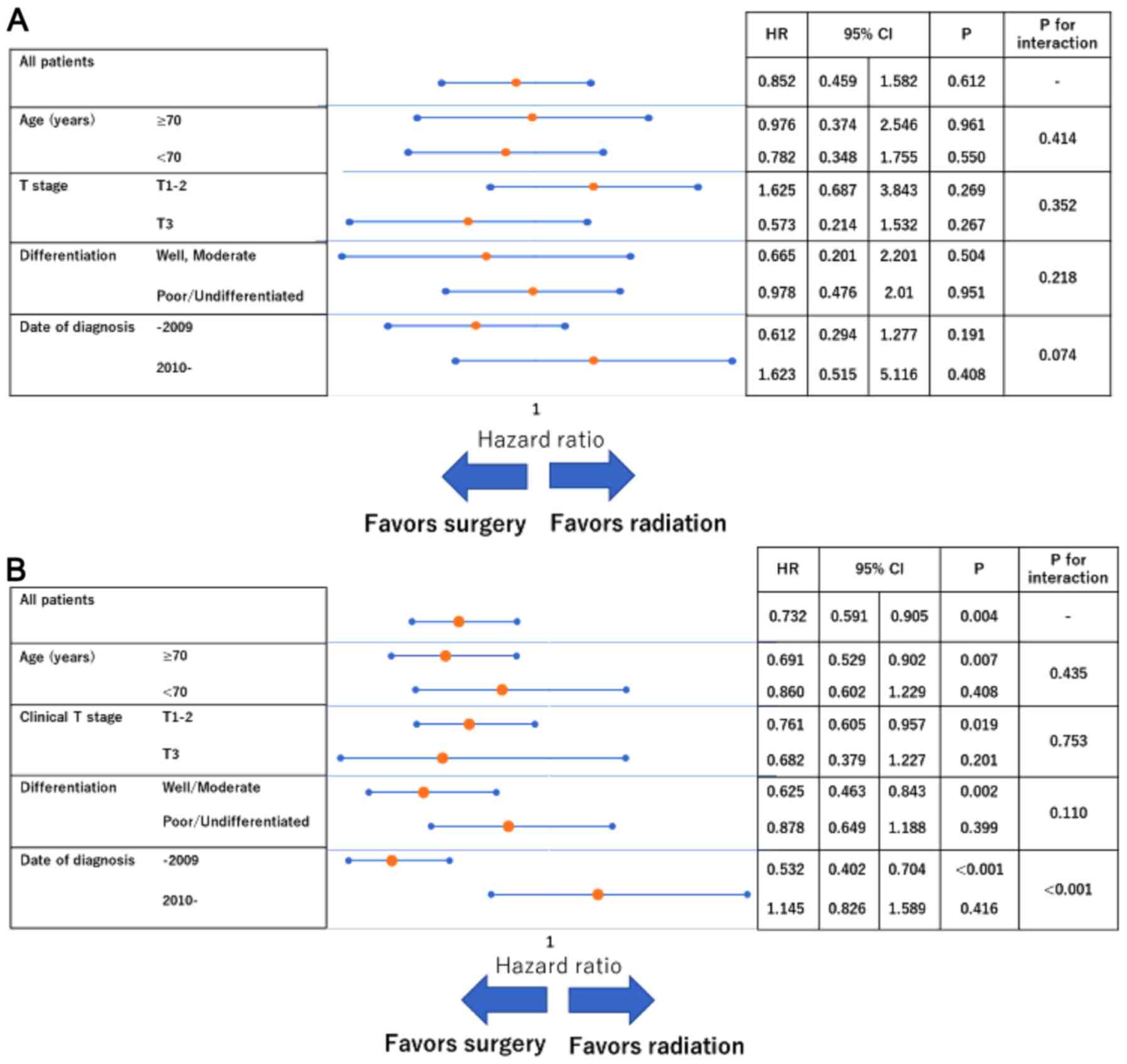
Subgroup analyses showed no significant interactions
in terms of CSS (Fig. 2A). With
respect to OS, the magnitude of the association between surgery and
improved survival was greater for patients diagnosed through 2009
(vs. 2010 onwards; P-value for interaction <0.001;
Fig. 2B).
Comparison of efficacy between elderly
and younger patients
For the secondary goal, we categorized the adjusted
cohort into two groups by age at a cutoff of 70 years. The elderly
group (aged 70–79 years) comprised 2,286 patients, and the younger
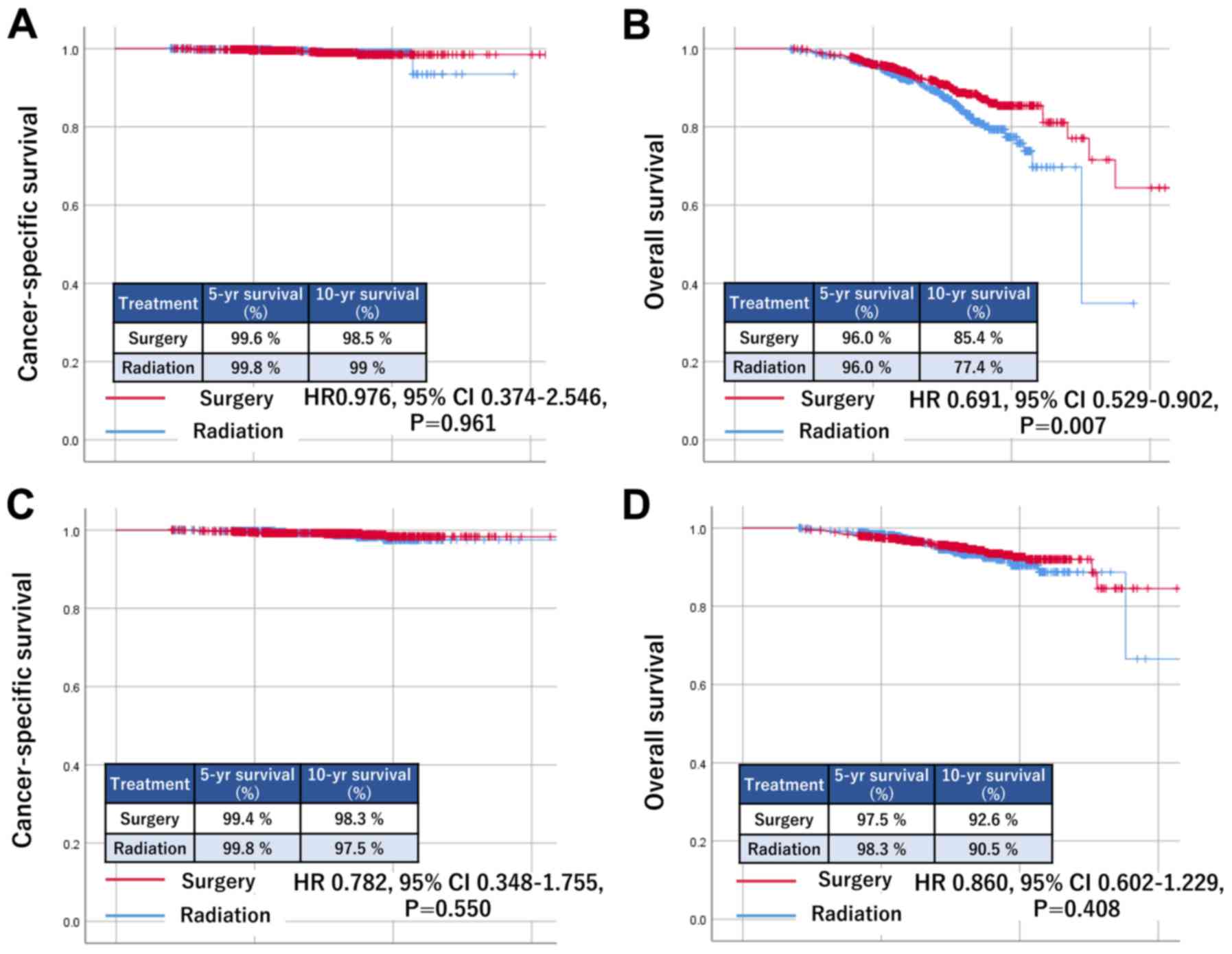
group (aged 50–69 years) comprised 2,524 patients (Table II). The Kaplan-Meier curves are
shown in Fig. 3. In the elderly
group, there was no significant difference in CSS based on
treatment modality (HR, 0.976; 95% CI 0.374–2.546; P=0.961)
(Fig. 3A). However, those who
underwent surgery had significantly better OS (HR, 0.691; 95% CI
0.529–0.902; P=0.007) (Fig. 3B). By
contrast, in the younger group, there were no significant
differences in either CSS (HR, 0.782; 95% CI 0.348–1.755; P=0.550)
(Fig. 3C) or OS (HR, 0.860; 95% CI
0.602–1.229; P=0.408) based on treatment modality (Fig. 3D).
 | Table II.Patient characteristics in the
elderly and younger group. |
Table II.
Patient characteristics in the
elderly and younger group.
|
| Elderly group | Younger group |
|---|
|
|
|
|
|---|
|
Characteristics | Total
(n=2,286) | Surgery
(n=1,143) | Radiation
(n=1,143) | P-value | Total
(n=2,524) | Surgery
(n=1,262) | Radiation
(n=1,262) | P-value |
|---|
| Age, median years
(range) | 73 (70–79) | 73 (70–79) | 73 (70–79) | 1.000a | 65 (50–69) | 65 (50–69) | 65 (50–69) |
>0.999a |
| Clinical T stage, n
(%) |
|
|
| 1.000b |
|
|
|
>0.999b |
| T1 | 1,074 (47.0) | 537 (47.0) | 537 (47.0) |
| 1,312 (52.0) | 656 (52.0) | 656 (52.0) |
|
| T2 | 1,050 (45.9) | 525 (45.9) | 525 (45.9) |
| 990 (39.2) | 495 (39.2) | 495 (39.2) |
|
| T3 | 162 (7.1) | 81 (7.1) | 81 (7.1) |
| 222 (8.8) | 111 (8.8) | 111 (8.8) |
|
| Differentiation, n
(%) |
|
|
| 1.000b |
|
|
|
>0.999b |
|
Well | 298 (13.0) | 149 (13.0) | 149 (13.0) |
| 440 (17.4) | 220 (17.4) | 220 (17.4) |
|
|
Moderate | 958 (41.9) | 479 (41.9) | 479 (41.9) |
| 1,066 (42.2) | 533 (42.2) | 533 (42.2) |
|
|
Poor/undifferentiated | 1,030 (45.1) | 515 (45.1) | 515 (45.1) |
| 1,018 (40.3) | 509 (40.3) | 509 (40.3) |
|
| Date of
diagnosis |
|
|
| 0.024b |
|
|
|
<0.001b |
|
<2009 | 779 (34.1) | 415 (36.3) | 364 (31.8) |
| 987 (39.1) | 614 (48.7) | 373 (29.6) |
|
|
>2010 | 1,507 (65.9) | 728 (63.7) | 779 (68.2) |
| 1,537 (60.9) | 648 (51.3) | 889 (70.4) |
|
In order to investigate which factors might lead to
the difference in OS among the elderly group, we analyzed the
causes of death. The surgery group had 101 deaths, of whom 70
(69.3%) died from intrinsic disease. Meanwhile, the radiotherapy
group had 124 deaths, including 97 (78.2%) due to intrinsic
disease. Relatively, more intrinsic deaths were seen in the
radiotherapy group than in the surgery group (P=0.128); however, no
significant difference was observed (Table III).
 | Table III.Cause of death in the elderly
group. |
Table III.
Cause of death in the elderly
group.
| Cause of death | Total (n=225) | Surgery
(n=101) | Radiation
(n=124) | P-value |
|---|
| Prostate cancer
death, n (%) | 19 (8.4) | 11 (10.9) | 8 (6.5) | 0.234 |
| Intrinsic death, n
(%) | 167 (74.2) | 70 (69.3) | 97 (78.2) | 0.128 |
| Extrinsic death, n
(%) | 5 (2.2) | 2 (2.0) | 3 (2.4) | 0.824 |
| Unknown death, n
(%) | 34 (15.1) | 18 (17.8) | 16 (12.9) | 0.306 |
Discussion
The question of whether surgery is more efficacious
(i.e., having better survival outcomes) than radiotherapy for
localized prostate cancer has remained inconsistently answered.
Most previous studies showed that surgery yielded better prognosis
than radiotherapy (5–10). According to a meta-analysis of 19
studies with 118,830 patients, those treated with radiotherapy were
at higher risk of overall mortality (HR, 1,63; 95% CI, 1.54–1.73;
P<0.001) and cancer-specific mortality (HR, 2.08; 95% CI,
1.76–2.47; P<0.001) than those undergoing surgery (5). A population-based study of 68,665
patients conducted between 1992 and 2005 by Abdollah et al
pointed out an association between radiotherapy and decreased CSS
at all risk levels of prostate cancer (P<0.001) (6). However, in our study, both treatment
modalities offered good CSS, and no significant difference in CSS
was observed between patients undergoing surgery and radiotherapy.
This result was similar to that from the ProtecT trial. Further,
the latest nationwide population-based study of 41,503 patients in
Sweden showed a lower difference in CSS between patients undergoing
surgery and radiotherapy than those in previous studies
(radiotherapy vs. surgery: Low- and intermediate-risk: HR, 1.24;
95% CI, 0.97–1.58; high-risk: HR, 1.03; 95% CI, 0.81–1.31)
(17).
Unlike in the younger group, there was a significant
difference in OS based on the treatment modality in the elderly
group, leading to that same tendency in the entire cohort. This
result may come from a bias in treatment selection; the reason for
treatment selection was not indicated in the database. More
intrinsic deaths were seen among elderly patients treated with
radiotherapy than those treated with surgery. We could predict that
patients with heavy comorbidities and low performance status had a
tendency to be treated with radiotherapy. Therefore, considering
that there was no significant difference in CSS, we could expect
radiotherapy to be a good treatment selection for elderly patients
because of its lower invasiveness.
Interestingly, the forest-plot subanalysis showed a
significant interaction among treatment type with respect to OS
based on the date of diagnosis. This might result from advances in
radiotherapy modalities, such as intensity-modulated radiotherapy
(IMRT), which have lower adverse events than three-dimensional
conformal radiotherapy (18–20). Additionally, the combination of IMRT
and brachytherapy has been shown to achieve a good cancer control
rate with lower toxicity (21,22).
Moreover, as combination androgen deprivation therapy has been
proved to improve survival, particularly in intermediate- and
high-risk localized prostate cancer (23,24),
combination hormonal therapy has become widely used worldwide.
Finally, these improvements in survival might have led patients
with good general condition to choose radiotherapy, particularly
after 2010, thereby contributing to better OS in this group.
This study also has some limitations that need to be
considered when interpreting the results. First, there was a lack
of clinicopathological data related to prostate cancer. In
particular, PSA values, which are crucial to determine the risk of
localized prostate cancer, were unavailable in the database, making
it impossible for us to stratify the patients by risk group.
Gleason scores, which play an important role in evaluating the
prognosis of men with prostate cancer, were also unavailable. Only
a limited number of factors were included in our analyses; this
enabled us to analyze exactly matched data in a relatively large
cohort. However, PSA value and Gleason scores are needed for a more
accurate analysis. Second, the median observation period was
relatively short for localized prostate cancer. To have more
precise survival outcome data for the sake of comparison, the
observation should have lasted 15–20 years. Third, as previously
mentioned, neither the performance status nor the comorbidity was
known; therefore, patient selection bias could not be eliminated.
Fourth, the database contained no data on the timing of treatment
after cancer diagnosis and the administration of combination
hormonal therapy. Therefore, our study included patients treated
with surgery or radiotherapy after active monitoring. Fifth, the
prognosis of patients receiving combination therapy could not be
evaluated in this study. We categorized patients who underwent both
surgery and radiotherapy into the surgery group because salvage
radiotherapy is a common treatment for patients with biochemical
recurrence after surgery in clinical practice, as mentioned in the
Methods section. Although it would have been better to compare the
results between combination therapy and surgery because surgery and
adjuvant radiotherapy could yield better outcomes than surgery
alone, the precise timing of each therapy in this study is unknown.
Further, we could not determine whether the patients were treated
with adjuvant or salvage radiotherapy. As such, other combination
therapies, such as surgery and hormone deprivation or surgery and
chemotherapy, could not be evaluated. Finally, the biological
effective dose, which could strongly affect oncological outcomes,
was unavailable in patients treated with radiotherapy.
In conclusion, our study showed no significant
difference in CSS between surgery and radiotherapy, but surgery
yielded significantly better OS, particularly in elderly patients.
However, as mentioned, the study results should be interpreted with
caution, given that some important factors were unavailable in this
study. Further, the study found no significant difference in terms
of both CSS and OS among younger patients, although we did observe
a tendency toward improved survival in patients treated with
radiotherapy after 2010. This implied that radiotherapy was less
invasive, and it should therefore be considered for use in elderly
patients.
As the registration of prostate cancer data in the
National Cancer Database was initiated in April 2018, these
limitations are expected to be overcome. Despite the limitations,
our study has hitherto been the largest cohort study in Japan or
any Asian country. Furthermore, to our best knowledge, this is the
very first study to compare treatment modalities using the
coarsened exact matching method. Our study results provide
important data relating to Asian patients, particularly Japanese
patients. They could inform the selection of the appropriate
treatment for localized prostate cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MY, MS, HN and TK conceived and designed the current
study, acquired, analyzed and interpreseted the data, and drafted
the manuscript. RJ, ST, TT, GN, SU, KK and HU contributed to the
conception and design of the present study, and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the ethical standards of the Helsinki Declaration and was approved
by the Institutional Review Board of Kanagawa Cancer Center
(approval no. EKI-048).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CSS
|
cancer-specific survival
|
|
OS
|
overall survival
|
|
PSA
|
prostate-specific antigen
|
|
ProtecT
|
Prostate Testing for Cancer and
Treatment
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
|
IMRT
|
intensity-modulated radiotherapy
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kimura T and Egawa S: Epidemiology of
prostate cancer in Asian countries. Int J Urol. 25:524–531. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hamdy FC, Donovan JL, Lane JA, Mason M,
Metcalfe C, Holding P, Davis M, Peters TJ, Turner EL, Martin RM, et
al: 10-Year Outcomes after monitoring, surgery, or radiotherapy for
localized prostate cancer. N Engl J Med. 375:1415–1424. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lane JA, Donovan JL, Davis M, Walsh E,
Dedman D, Down L, Turner EL, Mason MD, Metcalfe C, Peters TJ, et
al: Active monitoring, radical prostatectomy, or radiotherapy for
localised prostate cancer: Study design and diagnostic and baseline
results of the ProtecT randomised phase 3 trial. Lancet Oncol.
15:1109–1118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wallis CJD, Saskin R, Choo R, Herschorn S,
Kodama RT, Satkunasivam R, Shah PS, Danjoux C and Nam RK: Surgery
Versus radiotherapy for clinically-localized prostate cancer: A
systematic review and meta-analysis. Eur Urol. 70:21–30. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abdollah F, Schmitges J, Sun M, Jeldres C,
Tian Z, Briganti A, Shariat SF, Perrotte P, Montorsi F and
Karakiewicz PI: Comparison of mortality outcomes after radical
prostatectomy versus radiotherapy in patients with localized
prostate cancer: A population-based analysis. Int J Urol.
19:836–844. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sooriakumaran P, Nyberg T, Akre O,
Haendler L, Heus I, Olsson M, Carlsson S, Roobol MJ, Steineck G and
Wiklund P: Comparative effectiveness of radical prostatectomy and
radiotherapy in prostate cancer: Observational study of mortality
outcomes. BMJ. 348:g15022014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abdollah F, Sun M, Thuret R, Jeldres C,
Tian Z, Briganti A, Shariat SF, Perrotte P, Rigatti P, Montorsi F
and Karakiewicz P: A competing-risks analysis of survival after
alternative treatment modalities for prostate cancer patients:
1988–2006. Eur Urol. 59:88–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Merino T, San Francisco IF, Rojas PA,
Bettoli P, Zúñiga A and Besa P: Intensity-modulated radiotherapy
versus radical prostatectomy in patients with localized prostate
cancer: Long-term follow-up. BMC Cancer. 13:5302013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun M, Sammon JD, Becker A, Roghmann F,
Tian Z, Kim SP, Larouche A, Abdollah F, Hu JC, Karakiewicz PI and
Trinh QD: Radical prostatectomy vs radiotherapy vs observation
among older patients with clinically localized prostate cancer: A
comparative effectiveness evaluation. BJU Int. 113:200–208. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bluethmann SM, Mariotto AB and Rowland JH:
Anticipating the ‘silver tsunami’: Prevalence trajectories and
comorbidity burden among older cancer survivors in the United
States. Cancer Epidemiol Biomarkers Prev. 25:1029–1036. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Muramatsu N and Akiyama H: Japan:
Super-aging society preparing for the future. Gerontologist.
51:425–432. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fukagai T, Namiki TS, Carlile RG, Yoshida
H and Namiki M: Comparison of the clinical outcome after hormonal
therapy for prostate cancer between Japanese and Caucasian men. BJU
Int. 97:1190–1193. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Namiki M, Ueno S, Kitagawa Y, Fukagai T
and Akaza H: Effectiveness and adverse effects of hormonal therapy
for prostate cancer: Japanese experience and perspective. Asian J
Androl. 14:451–457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mohler JL, Armstrong AJ, Bahnson RR,
D'Amico AV, Davis BJ, Eastham JA, Enke CA, Farrington TA, Higano
CS, Horwitz EM, et al: Prostate Cancer, Version 1.2016. J Natl
Compr Canc Netw. 14:19–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Robinson D, Garmo H, Lissbrant IF, Widmark
A, Pettersson A, Gunnlaugsson A, Adolfsson J, Bratt O, Nilsson P
and Stattin P: Prostate cancer death after radiotherapy or radical
prostatectomy: A nationwide population-based observational study.
Eur Urol. 73:502–511. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Viani GA, Viana BS, Martin JE, Rossi BT,
Zuliani G and Stefano EJ: Intensity-modulated radiotherapy reduces
toxicity with similar biochemical control compared with
3-dimensional conformal radiotherapy for prostate cancer: A
randomized clinical trial. Cancer. 122:2004–2011. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu T, Zhang Q, Zheng T, Shi H, Liu Y, Feng
S, Hao M, Ye L, Wu X and Yang C: The effectiveness of intensity
modulated radiation therapy versus three-dimensional radiation
therapy in prostate cancer: A meta-analysis of the literatures.
PLoS One. 11:e01544992016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shimizuguchi T, Nihei K, Okano T,
Machitori Y, Ito K and Karasawa K: A comparison of clinical
outcomes between three-dimensional conformal radiotherapy and
intensity-modulated radiotherapy for prostate cancer. Int J Clin
Oncol. 22:373–379. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Löser A, Beyer B, Carl CO, Löser B,
Nagaraj Y, Frenzel T, Petersen C, Krüll A, Graefen M and Schwarz R:
Toxicity and risk factors after combined high-dose-rate
brachytherapy and external beam radiation therapy in men ≥75 years
with localized prostate cancer. Strahlenther Onkol. 195:374–382.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fang FM, Wang YM, Wang CJ, Huang HY and
Chiang PH: Comparison of the outcome and morbidity for localized or
locally advanced prostate cancer treated by high-dose-rate
brachytherapy plus external beam radiotherapy (EBRT) versus EBRT
alone. Jpn J Clin Oncol. 38:474–479. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
D'Amico AV, Manola J, Loffredo M, Renshaw
AA, DellaCroce A and Kantoff PW: 6-month androgen suppression plus
radiation therapy vs radiation therapy alone for patients with
clinically localized prostate cancer: A randomized controlled
trial. JAMA. 292:821–827. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hanks GE, Pajak TF, Porter A, Grignon D,
Brereton H, Venkatesan V, Horwitz EM, Lawton C, Rosenthal SA,
Sandler HM, et al: Phase III trial of long-term adjuvant androgen
deprivation after neoadjuvant hormonal cytoreduction and
radiotherapy in locally advanced carcinoma of the prostate: The
radiation therapy oncology group protocol 92-02. J Clin Oncol.
21:3972–3978. 2003. View Article : Google Scholar : PubMed/NCBI
|