Introduction
Calcium (Ca2+) is sensitive to external
stimuli and participates in cellular metabolic activities.
Therefore, maintaining homeostasis of Ca2+ is crucial
for maintaining normal cell structure and cellular functions. The
intracellular calcium concentration is strictly and precisely
controlled. This specific control is critical for controlling
proteins and signaling pathways mediated by Ca2+ in the
regulation of cell proliferation, apoptosis, gene transcription,
and migration (1,2). Abnormal intracellular Ca2+
concentrations cause a number of metabolic dysfunctions. The
disorder of intracellular calcium affects the biological behaviors
of tumor cells, such as proliferation and migration. A number of
studies have shown that calcium-mediated signaling pathways are
implicated in the occurrence and development of tumors (3,4).
A previous study demonstrated that transmembrane and
coiled-coil domain 1 (TMCO1), an endoplasmic reticulum
transmembrane protein, can actively regulate intracellular calcium
concentrations (5). The present
study confirmed that TMCO1 can recognize intracellular
Ca2+ concentration and form calcium channels, actively
discharging intracellular overloaded Ca2+, providing
functions that are crucial for cellular calcium homeostasis.
Studies have demonstrated that TMCO1 is associated with skeletal
development and glaucoma (6,7). Li et al (8) revealed that TMCO1 participated in the
regulation of proliferation and migration of bladder urothelial
carcinoma via the AKT pathway, which demonstrated that TMCO1 is
involved in the development of tumors.
Lung cancer has the highest incidence of cancer
morbidity and mortality worldwide (9). Non-small cell carcinoma accounts for
84% of lung cancers and the major phenotype is lung adenocarcinoma
(10). The specific pathogenesis and
mechanism of lung adenocarcinoma development are currently unknown.
However, evidence indicated that Ca2+ channels play an
important role in proliferation and migration of lung
adenocarcinoma cells (11).
Bioinformatic analysis demonstrated that there may
be an association between TMCO1 and lung adenocarcinoma. However,
the molecular mechanism involved in the association between TMCO1
and lung adenocarcinoma has not yet been determined. Thus, the
present study aimed to investigate the underlying molecular
mechanism of TMCO1 in regulating the biological process of A549
cells.
Materials and methods
Patient and tissue samples
A total of seven patients with lung adenocarcinoma,
including three males (age, 41–59 years) and four females (age,
38–62 years), whose disease was pathologically confirmed at the
China Academy of Chinese Medical Sciences of Wangjing Hospital
(Beijing, China) were selected. Tissue samples were collected
between January 2019 and October 2019 and written informed consent
was provided by all patients. The study was approved by the Ethics
Committee of Wangjing Hospital (Beijing, China) and all patients
agreed to participate.
Antibodies
TMCO1 antibody (rabbit) was obtained from Cleveland
State University, Department of Chemistry and the Center for Gene
Regulation in Health and Disease (Cleveland, OH, USA). β-actin
antibody (rat; catalog no. CW0096) was purchased from CoWin
Biosciences (CWBio). Bcl-2 (rabbit; catalog no. BA0412), caspase-3
(rabbit; catalog no. PB0183), caspase-9 (rabbit; catalog no.
BA0690), MMP-2 (rabbit; catalog no. A00286), MMP-9 (rabbit; catalog
no. BA0573), N-cadherin (mouse; catalog no. BM1573), E-cadherin
(rabbit; catalog no. BA0475), vimentin (rabbit; catalog no.PB9359)
and calcium/calmodulin-dependent protein kinase II inhibitor 1
(CAMKII) antibodies (mouse; catalog no. M03241-1) were purchased
from Boster Biological Technology.
Bioinformatics analysis
GeneExpression Profiling Interactive Analysis
(GEPIA; http://gepia.cancer-pku.cn/) is an
online tool that provides expression level analysis functions for
The Cancer Genome Atlas (TCGA; http://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga)
and the Genotype-Tissue Expression (https://commonfund.nih.gov/GTEx) databases. In the
present study, GEPIA was used to compare the expression levels of
TMCO1 in lung adenocarcinoma (T=483) which all come from TCGA tumor
data and normal lung tissues (n=347), which were matched TCGA
normal and GTEx data, the distinct expression of different stages
of lung adenocarcinoma tissues in Tumor Node Metastasis (TNM)
standard, as well as the overall survival and progression-free
survival of the patients. In the expression level analysis of TMCO1
for the different sub-stages, log2 transcripts per million (TPM +1)
of the RNA sequencing expression level data was used for the
log-scale. One-way ANOVA was performed, using the pathological
stage as a variable to calculate the differential expression. In
the survival analysis, GEPIA online tools used the log-rank test
(also known as the Mantel-Cox test) for the hypothesis test. The
Cox proportional hazard ratio and the 95% confidence interval
information were also included in the survival plots. To
investigate survival analysis of TMCO1, patients with lung
adenocarcinoma were classified into the high expression cohort and
low expression cohort groups, according to the median expression
(the median expression threshold was 50%).
Immunohistochemical staining
The tissues (including the lung adenocarcinoma
tissues and the para-carcinoma tissues) were fixed in 10% formalin
solution (Beijing YiliFine Chemicals Co., Ltd) for 24 h at room
temperature, then they were treated with Fully-enclosed tissue
processor (ASP300s, Leica), embedded in paraffinand sectioned.
After that, the sections (3 µm) was dewaxing in a dewaxing liquid
(catalog no. BLB-01, Jiu Zhou Bailin Co., Ltd) and rehydrated in a
graded ethanol seriesat room temperature for 15 min each stage.
Tissue sections were blocked with 10% H2O2(20
ml H2O2 dissolved in 180 ml methyl alcohol,
Beijing Chemical Works) at room temperature for 10 min, followed by
treatment with 3% BSA (Beijing Solarbio Science & Technology
Co., Ltd.) at room temperature for 30 min. The sections were
incubated with rabbit polyclonal antibody TMCO1 (1:50) diluted in
2% BSA/0.1 M PBS overnight at 4°C and further incubated with goat
anti-rabbit immunoglobulin G (1:100) (IgG; catalog no. CW0103,
CWBio) at room temperature for 1 h. The sections were transferred
to an avidin-biotin-peroxidase complex solution for 30 min, then
submerged in 3, 3-diaminobenzidine (DAB; catalog no. CW0125; CWBio)
for 1 min, which produced brown staining. Hematoxylin (Beijing
Solarbio Science & Technology Co., Ltd.) was used for nuclear
staining at room temperature. After 1 min, the reaction was stopped
with 0.1 M PBS and water rinsing. The slides were fully rinsed with
0.1 M PBS between the individual steps. The tissue sections were
rinsed in water, 70% ethanol, 95% ethanol, 100% ethanol and
xylenes. Subsequently, images were captured using a light
microscope (magnification, ×400; TE2000-s, Nikon) using Image Scope
software (Nis-Elements D 2.30 software, Nikon). The number and
density of positive-stained cells were analyzed by Image-Pro Plus
6.0 (Media Cybernetics) and GraphPad Prism 8.0 software (GraphPad
Software, Inc.).
Cell culture and treatment
A549 cells were provided by Cleveland State
University, Department of Chemistry and the Center for Gene
Regulation in Health and Disease (Cleveland, USA), which purchased
the cells from American Type Culture Collection (ATCC). The cells
were divided into three groups: Untreated A549 cells served as the
control group, while the 29 and 33 sites of TMCO1 were knocked down
to create the A549−29− and A549−33− knockdown
groups, respectively. The 29 and 33 sites were the main functional
sites of TMCO1 as previously described (5) The cells were cultured in RPMI-1640
supplemented with 10% fetal bovine serum (both from Beijing
Solarbio Science & Technology Co., Ltd.) and cultured at 37°C,
5% CO2 in a humidified incubator (Heraeus Holding
GmbH).
Western blot analysis
The cells were lysed with radioimmunoprecipitation
assay (RIPA lysis buffer) (Beyotime Institute of Biotechnology) and
protein concentration was quantified using the bicinchoninic acid
(CWBio) method. The proteins (20 µg/lane) were separated on 10%
SDS-PAGE (CWBio) and transferred onto PVDF membranes (CWBio). The
membranes were blocked in 5% milk (Inner Mongolia Yili Industrial
Group Co., Ltd)-TBS-Tween-20 (CWbio) for 1 h at room temperature.
Then, the membranes were incubated with primary antibodies
(β-actin, 1:1,000 andTMCO1; 1:500) overnight at 4°C, then incubated
with a secondary antibody (goat anti-rabbit IgG-horseradish
peroxidase; 1:3,000, catalog no. CW0103, CWBio) for 2 h at room
temperature. Finally, the immunoreactive bands were detected using
the EasySee Western Blot kit (CWBio).
Detection of calcium ion
concentration
A549 cells were seeded (1×105) in a 20-mm
culture dish. After 24 h, 4 µm/ml of Fulo-3 solution (50 µg Fulo-3
dissolved in 44 µl DMSO, diluted with 11 ml PBS; Thermo Fisher
Scientific, Inc.) was added to the cells and incubated for 40 min
at 37°C. The cells were washed twice with PBS followed by addition
of RPMI-1640 medium (Beijing Solarbio Science & Technology Co.,
Ltd.) for 30 min. Subsequently, Hank's Balanced Salt Solution
(HBSS; Beijing Solarbio Science & Technology Co., Ltd.)
containing Ca2+ was added and the cells were scanned
under a confocal microscope (Olympus Corporation, magnification,
×400) for 300 sec. After 60 sec of scanning, 100 µl ATP (Beijing
Solarbio Science & Technology Co., Ltd.) solution (300 m/ml,
0.125 g ATP.2Na dissolved in 4 ml HBSS) was added at
room temperature. The images and ratio images were captured using
Olympus Fluoview 3.1a software (Olympus Corporation). Monitoring of
[Ca2+] required two continuous images (F1 and F2) the
ratio images R=F 1/F 2 were used to derive [Ca2+]. Data
analysis was conducted in Microsoft Excel 2010 (Microsoft
Corporation).
Cell activity analysis
A549 cells (1×104/well) were seeded into
a 96-well plate. MTT solution (Beijing Solarbio Science &
Technology Co., Ltd.) was added following 48 h of culture and the
cells were incubated for 4 h at 37°C. Subsequently, 50 µl DMSO was
added to each well and the absorbance was measured at a wavelength
of 490 nm using a microplate reader. Data analysis was performed
using GraphPad Prism 8.0 software (GraphPad Software, Inc.).
Cell migration assay by scratch test
and Transwell
A549 cells (1×105/well) were seeded into
a 24-well plate and the experiment proceeded when cells reached
100% confluency. A 200-µl pipette tip was used to make a scratch
wound in the cells, which were then cultivated in 1640 medium
without serum. Cell migration pictures were captured under a light
microscope (magnification, ×40) using Nis-Elements D 2.30 software
(Nikon) at 0, 6, 12 and 24 h after the scratch wound was made. The
migration rate was analyzed by ImageJ (National Institutes of
Health) and GraphPad Prism 8.0 (GraphPad Software, Inc.)
software.
Cell suspension (1×104/well, 100 µl) was
added into the upper chambers (Corning, Inc.). Medium containing
10% fetal bovine serum (Solarbio) was added to the lower chamber of
the 24-well plate and the cells were incubated for 24 h at 37°C.
Subsequently, cells were fixed with 90% alcohol for 15 min at room
temperature and cell migration was observed in a light microscope.
Images were captured (magnification, ×20) following Coomassie blue
staining for 1 min at room temperature using Nis-Elements D
2.30software. The number and density of positive stained cells were
analyzed by Image Pro Plus 6.0and GraphPad Prism 8.0 (GraphPad
Software, Inc.) software.
Immunocytochemical staining
A549 cells (1×105/well) were seeded in a
24-well plate and fixed at 4°C with 10% formaldehyde following 24 h
incubation. Subsequently, cells were washed twice with PBS and
incubated with 10% H2O2 (20 ml
H2O2 dissolved in 180 ml methyl alcohol,
Beijing Chemical Works) for 10 min at room temperature. Cells were
then incubated for 30 min at room temperature with 5% BSA for
blocking and antibodies (Bcl2, 1:50; caspase-3, 1:100; caspase-9,
1:100; MMP-2, 1:50; MMP-9, 1:50; N-cadherin, 1:50; E-cadherin,
1:50; vimentin, 1:100 and CAMKII, 1:50) were added for 1 h at 37°C.
After rinsing twice with PBS, secondary antibody (1:100; goat
anti-rabbit IgG, catalog no. CW0103; CWBio; goat Anti-Mouse IgG,
catalog no. CW0102, CWBio) was added for 30 min at room temperature
and the cells were observed following DAB chromophore staining for
1 min at room temperature and images were obtained in a light
microscope. The integral optical density values of the images
(magnification, ×100) were analyzed using Image-Pro Plus 6.0 (Media
Cybernetics) and GraphPad Prism 8.0 (GraphPad Software, Inc.)
software.
Statistical analysis
Survival analysis was assessed using the log-rank
test. The bioinformatic expression analysis of TMCO1 used one-way
ANOVA. Cell culture experiments were repeated at least three times.
Unpaired independent samples t-test or one-way ANOVA was used for
comparisons between groups. Multiple group comparisons of Bcl-2
were analyzed by LSD and the Caspase-3 and Caspase-9 expression
were analyzed by the Duncan's test as a post-hoc test. Bonferroni's
was also used following one-way ANOVA as a post-hoc test for cell
migration, MMPs and EMT factors analyzed. All statistical analysis
was performed using SPSS for Windows (version 18.0; SPSS, Inc.).
P<0.05 was considered to indicate a statistically significant
difference. Histograms were produced using GraphPad Prism 8.0
software (GraphPad Software, Inc.).
Results
TMCO1 is associated with lung
adenocarcinoma development and high expression levels in lung
adenocarcinoma tissue
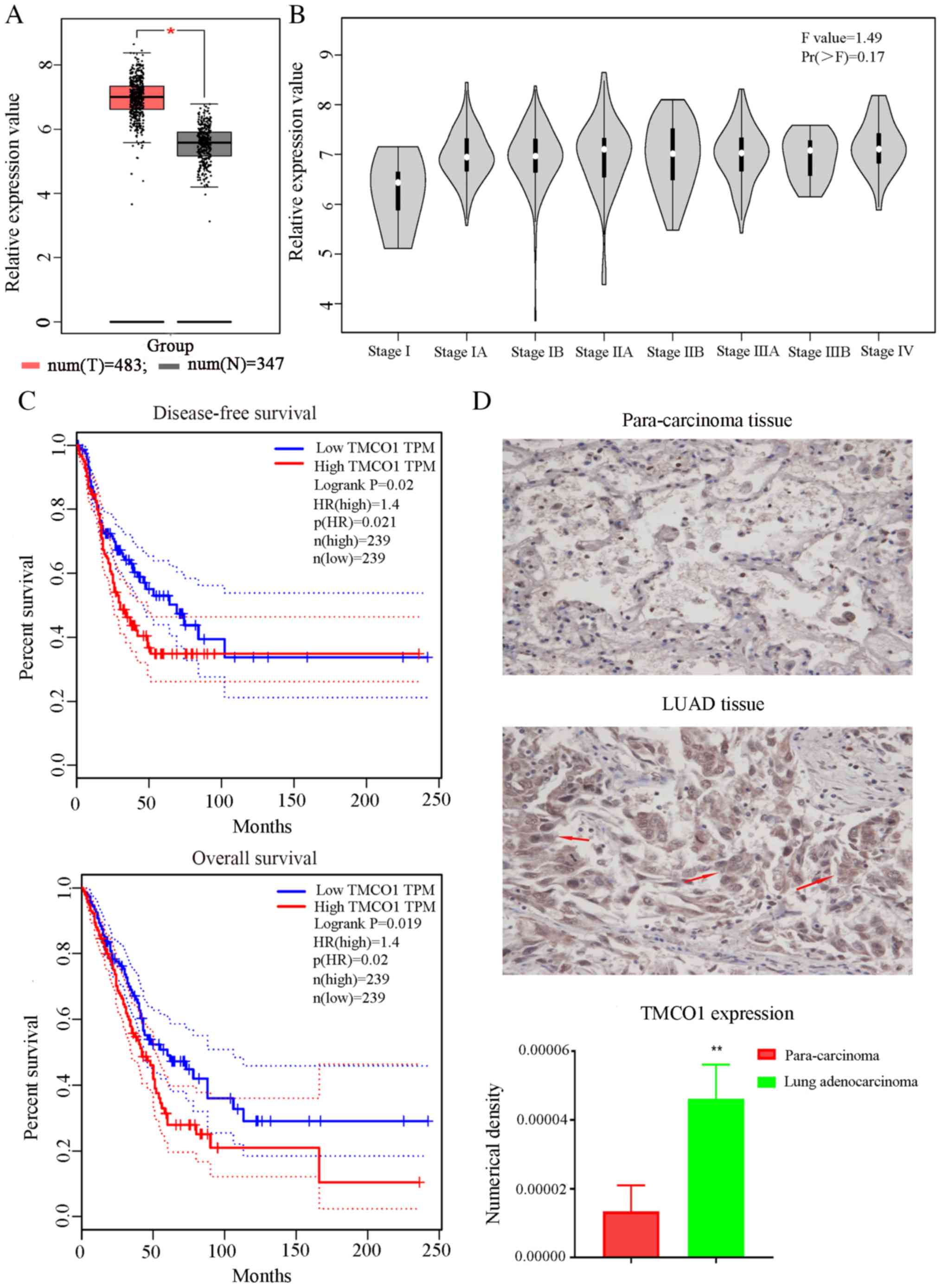
The bioinformatics analysis results showed that
TMCO1 expression levels were high in lung adenocarcinoma tissues
compared with normal tissues (Fig.
1A). Additionally, TMCO1 expression levels in stage I of the
disease was lower compared with other stages (Fig. 1B). Meanwhile, the expression levels
of TMCO1 affected the survival rate; high expression levels of
TMCO1 were associated with a significantly decreased survival rate
(dotted lines represent 95% confidence interval) (Fig. 1C). These results indicated a
potential association between TMCO1 and biological functions of
tumors. Positive staining of TMCO1 was observed in the lung
adenocarcinoma tissues and paracarcinoma tissues of these patients.
Positive TMCO1 staining in LUAD tissues was notably higher than in
paracarcinoma tissues (Fig. 1D). The
results demonstrated that TMCO1 was associated with lung
adenocarcinoma. To clarify the association between TMCO1 and lung
adenocarcinoma, A549 cells were selected for the subsequent
experiments.
Increased intracellular calcium
concentration following TMCO1 knockdown
The present study examined the expression levels of
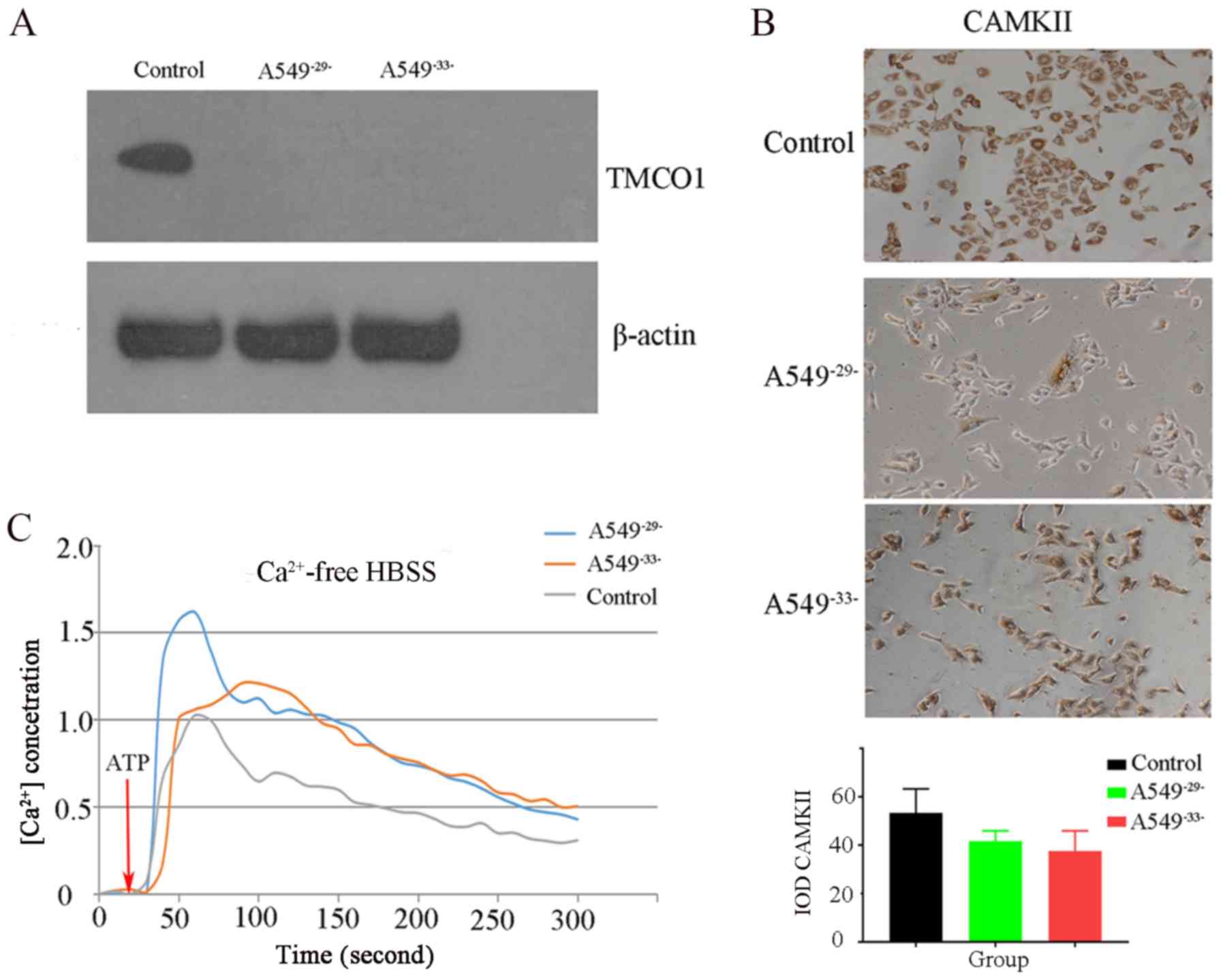
TMCO1 protein in each group of A549 cells using western blotting.
The results demonstrated that the expression levels of TMCO1 in the
A549−29− and A549−33− groups were notably
decreased compared with the control group, indicating that TMCO1
expression levels were successfully knocked down (Fig. 2A). CAMKII protein expression levels
in the A549−29− and A549−33− group were
slightly decreased compared with the control group (Fig. 2B), indicating that CAMKII expression
was inhibited after TMCO1 knockdown. In addition, confocal
microscopy revealed that the intracellular calcium concentration
after TMCO1 knockdown in the A549−29− and
A549−33− groups was higher compared with the control
group (Fig. 2C). These assays
demonstrated that knocking down TMCO1 could cause Ca2+
overload in A549 cells, decreasing CAMKII expression levels.
TMCO1 knockdown decreases cell
activity and affects cell apoptosis
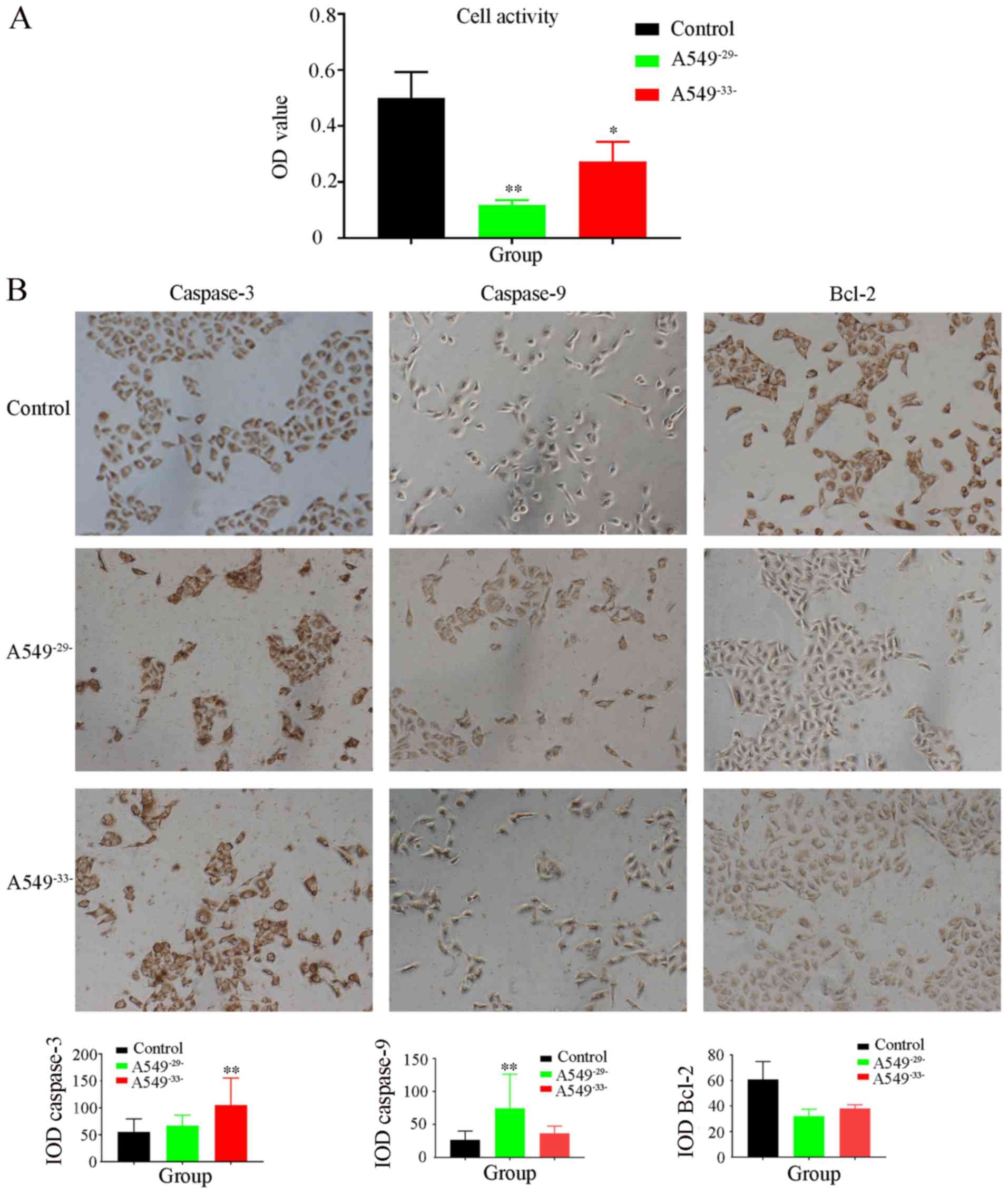
MTT results showed that the activity of the
A549−29−knockdown cells was three-fold lower compared
with control group, and the cell activity of A549−33−
group was also significantly decreased. The results indicated that
A549 cell activity was significantly inhibited by TMCO1 knockdown
(Fig. 3A). Meanwhile, Bcl-2
expression levels markedly decreased in the A549−29−
group compare to the control. By contrast, the expression levels of
caspase-3 were significantly increased in the A549−33−
group compared with the control group. Caspase-9 protein expression
levels were significantly upregulated in the
A549−29−group compared with the control group (Fig. 3B). The results suggest that TMCO1
regulated cell apoptosis by affecting Bcl-2, caspase-3 and
caspase-9 expression levels.
Cell migration ability decreases
following TMCO1 knockdown
Following the scratch test, the migration of the
A549−29−cells and A549−33−cellsweresuppressed
after 12 h compared with the control group. The migration rate of
the A549−29− cells significantly decreased compared with
the control group over time, indicating that TMCO1 exerted a
certain regulatory effect on the A549 cell migration process
(Fig. 4A). The Transwell assay
demonstrated that the cell migration rate in the
A549−29− group was significantly lower compared with the
control group after 24 h and the migration of cells in the
A549−33− group was also markedly lower compared with the
control group (Fig. 4B). Examination
of migration-associated factors showed that MMP-2 expression levels
were significantly lower in the A549−33− group compared
with controls, and MMP-9 protein expression levels in the
A549−29− group significantly decreased compared with the
control group (Fig. 4C). In
addition, knockdown of TMCO1 downregulated N-cadherin and vimentin
expression levels, which were decreased in the A549−33−
group, whereas E-cadherin expression levels were significantly
increased in the A549−33− group compared with the
control group (Fig. 4D). The results
showed that MMP-2 and MMP-9 expression levels were decreased by
inhibiting TMCO1 expression levels, accompanied by decreases in
N-cadherin and vimentin expression levels and increases in
E-cadherin expression levels, resulting in a significant decrease
to the migration ability of the A549 cells.
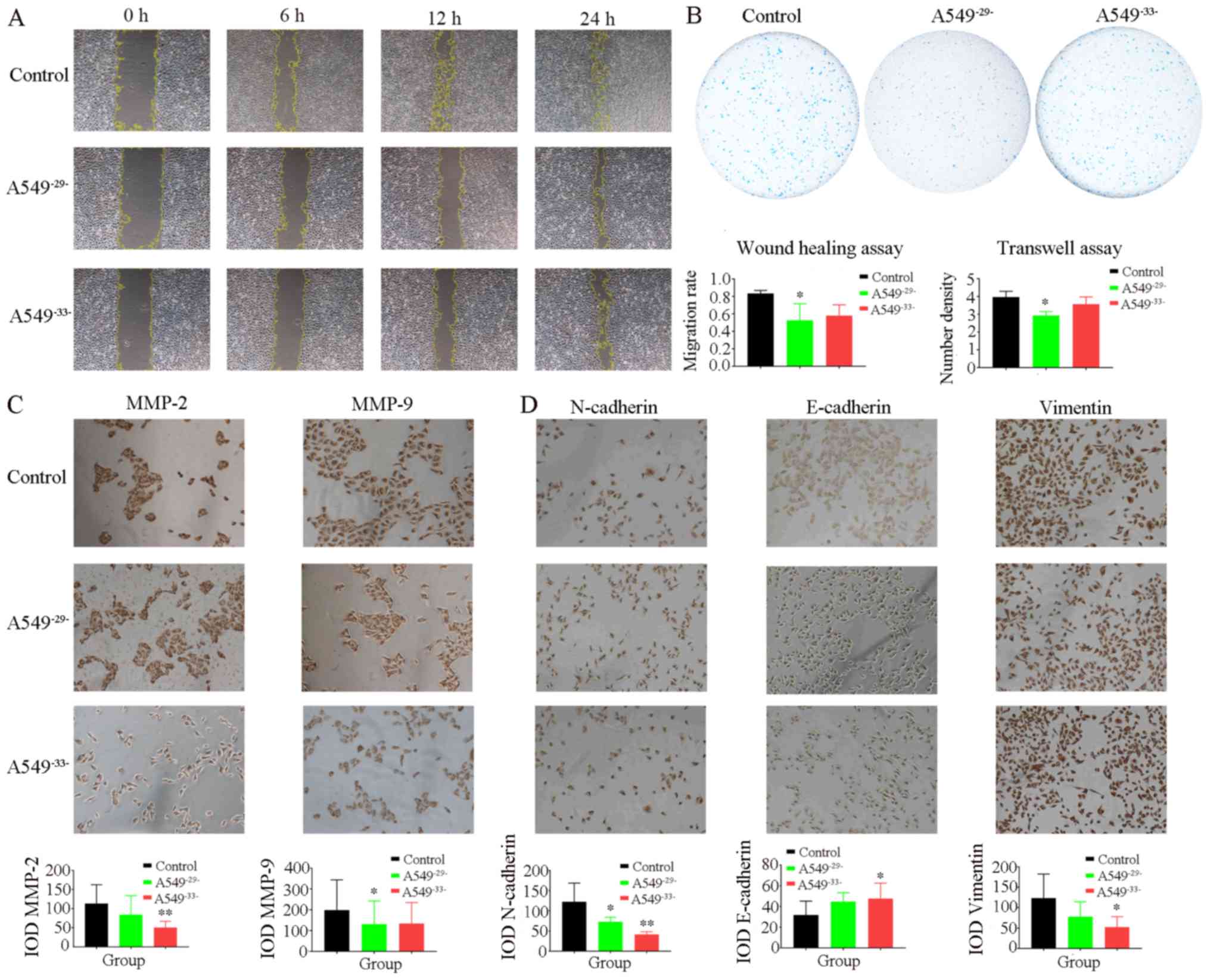 | Figure 4.Detection of migration activity and
expression levels of MMP-2, MMP-9, N-cadherin, E-cadherin and
Vimentin in A549 cells. (A) Scratch test showed that the migration
of A549−29−and A549−33−cells were suppressed
after 12 h compared with the control group. The migration rate of
the A549−29− cells significantly decreased compared with
the control group over time. The migratory rate slightly decreased
in the A549−33− group compared with the control group
(magnification, ×40). (B) The Transwell results showed that the
cell migration rate in the A549−29− group was
significantly lower compared with the control group after 24 h and
the migration of cells in the A549−33− group was also
lower compared with the control group (magnification, ×20). (C) The
results showed that MMP-2 expression levels were significantly
lower in the A549−33− group compared with control and
its expression was inhibited in the A549−29− group
compare with the control. The MMP-9 protein expression levels in
the A549−29− group significantly decreased compared with
the control group and its expression in A549−33− was
also less than the control (magnification, 100X). (D) The results
showed that the expressions of N-cadherin and Vimentin in the
A549−29− and A549−33− groups were lower than
the control group. In addition, the expression of E-cadherin in the
A549−29− and A549−33−groups was higher than
the control group (magnification, ×100). Image Pro Plus software
was used to analyze the images. Data are expressed as the mean ±
standard deviation. *P<0.05, **P<0.01 vs. control. MMP,
matrix metalloproteinase; IOD, integrated optical density. |
Discussion
Proliferation and migration are the primary
characteristics of cancer. Therefore, identification of their
pathological mechanism is an important theoretical basis for
clinical treatment. Lung adenocarcinoma is a multifactorial disease
and its proliferation and migration processes involve numerous
signaling pathways and factors, such as Ca2+ (12,13).
Studies have shown that active calcium ions can stimulate tumor
cell proliferation and trigger drug resistance (14,15).
Yang et al (16),
demonstrated that decreasing intracellular calcium concentrations
decreased the proliferation and migration of A549 cells. However,
none of these studies demonstrated whether intracellular calcium
overload affected the biological function of A549 cells. Moreover,
to the best of our knowledge, no studies have ever clarified that
TMCO1 was associated with lung adenocarcinoma biological processes.
In the present study, bioinformatics analysis showed that TMCO1
expression levels in tissue from patients with lung adenocarcinoma
were higher compared with normal tissue samples. The results of the
present study also demonstrated that TMCO1 expression levels were
inversely proportional to the survival rate. These results
indicated that TMCO1 may be associated with lung
adenocarcinoma.
Previous studies have demonstrated that
Ca2+ are active in cancer cells at abnormally elevated
concentrations and stimulate the proliferation and migration of
cancer cells (17,18). Using immunohistochemical staining,
the present study demonstrated that the positive staining of TMCO1
expression levels was higher in lung adenocarcinoma tissues
compared with paracarcinoma tissues. When the concentration of
calcium ions in the lung adenocarcinoma cells is high, it may
activate TMCO1 to regulate intracellular calcium.
Classical lung adenocarcinoma A549 cells were used
to demonstrate that knockdown of the 29 or 33 sites of TMCO1
triggered intracellular calcium overload, consistent with the
research results of Wang et al (5), demonstrating that TMCO1 can prevent
intracellular calcium overload. As a mediator of Ca2+
signaling, CAMKII can affect numerous biological cell functions,
including cell proliferation and migration, by regulating multiple
steps of the calcium cycle (19).
The present study demonstrated that inhibiting TMCO1 expression
levels in A549 cells could cause Ca2+ overload, leading
to decreased CAMKII expression levels. The present study
demonstrated that TMCO1 expression levels are active in lung
adenocarcinoma tissues and participate in regulating calcium
concentration in A549 cells, indicating that there is an
association between TMCO1 and lung adenocarcinoma.
In order to determine the regulatory mechanism of
TMCO1 in the A549 cell activity, the present study examined A549
cell proliferation using MTT. The results showed that the activity
of A549 cells decreased significantly after inhibiting TMCO1
expression levels, suggesting that TMCO1 knockdown could slow down
A549 cell proliferation. Byun et al (20) also found that A549 cell activity was
decreased by KCP10043F treatment to inhibit Ca2+
expression levels, which was consistent with the results of the
present study. Expression levels of apoptotic proteins were
measured, which demonstrated that the knockdown of TMCO1 inhibited
the expression level of Bcl-2. Previous studies showed that Bcl-2
and A549 cell proliferation were inversely associated and
Ca2+ was closely associated with Bcl-2 through multiple
targets and pathways in cell proliferation and apoptotic processes
(21,22). The present study demonstrated that,
following the knockdown of TMCO1 in A549 cells, intracellular
calcium overload was provoked, resulting in a decrease in Bcl-2
protein expression levels. In addition, as a second messenger in
cells, calcium ions are associated with other factors, such as
caspases (23) involved in cell
apoptosis. In order to investigate this, the present study examined
the expression levels of caspase-3 and caspase-9. Li et al
(24) demonstrated that the
apoptosis regulators caspase-3 and caspase-9 are active when A549
cell proliferation was decreased by angelicin treatment. Similarly,
the present study demonstrated that the expression levels of
apoptotic factors caspase-3 and caspase-9 were higher following
TMCO1 knockdown compared with the control group. Therefore, the
present study indicated that TMCO1 plays a central role in
regulating the apoptosis of A549 cells. This function may be
achieved by downregulating Bcl-2 expression levels and upregulating
caspase-3 and caspase-9 protein expression levels in A549
cells.
Using scratch and Transwell experiments, the present
study also demonstrated that knockdown of TMCO1 inhibited the
migration of A549 cells. The migration ability of the knockdown
group cells exhibited different degrees of attenuation compared
with the control group. Further tests showed that knockdown of
TMCO1 could downregulate the expression levels of MMP-2 and MMP-9.
The MMP family is one type of calcium-dependent proteolytic enzyme
that is directly involved in lung adenocarcinoma migration
(25). Shi et al (26), showed that decreasing the expression
levels of MMP-2 and MMP-9 could hinder lung adenocarcinoma cell
migration, consistent with the results of the present study.
Furthermore, epithelial-to-mesenchymal transition (EMT) has a
critical role in tumor metastasis (27). As EMT markers, the downregulation of
adhesion factor E-cadherin and the upregulation of N-cadherin
expression levels are accompanied by migration and invasion in lung
adenocarcinoma cells (28,29). The present study demonstrated that
TMCO1 knockdown could downregulate the expression levels of
N-cadherin and vimentin and upregulate the expression level of
E-cadherin, indicating that the knockdown of TMCO1 inhibited the
EMT process in A549 cells. As an important marker of EMT, vimentin
expression levels increase significantly during tumor migration
(30). The results of the present
study indicated that TMCO1 can participate in regulating A549 cell
migration by mediating MMP-2, MMP-9 and EMT activity.
In conclusion, the present study demonstrated that
TMCO1 was closely associated with the cell activity, apoptosis and
migration of lung adenocarcinoma cells. TMCO1 affected the
apoptosis of lung adenocarcinoma cells by regulating the expression
levels of Bcl-2, caspase-3 and caspase-9. Furthermore, it also
regulated the expression levels of MMP-2, MMP-9 and EMT processes
to influence the migration of lung adenocarcinoma cells. The
results demonstrated that TMCO1 had a relationship with A549 cells
viability and migration. TMCO1, as a regulator of cellular calcium
ions, was shown to affect numerous growth factors, which was in
accordance with previous reports demonstrating that calcium ions
participate in the regulation of numerous biological functions
through complex networks (31,32). In
the present study, immunostaining was performed to observe changes
in the expression levels of associated proteins, which suggested
that TMCO1 participated in A549 cell biological processes. Future
studies will verify changes in the expression levels of relevant
factors at the gene level and determine the regulatory mechanism
and regulatory targets of TMCO1 in lung adenocarcinoma to construct
a complete network of TMCO1 regulatory mechanisms. To the best of
our knowledge, the present study was the first to demonstrate an
association between the proliferation, apoptosis and migration
processes of lung adenocarcinoma, and TMCO1, which provides a new
theoretical basis and potential clinical target for lung
adenocarcinoma treatment and prognosis.
Acknowledgements
The authors would like to thank Dr Aimin Zhou
(Cleveland State University, Department of Chemistry and the Center
for Gene Regulation in Health and Disease, Cleveland, OH, USA) for
providing the A549 cells.
Funding
The present study was funded by international
cooperation projects of China Academy of Chinese Medical Sciences
(grant no. GH2017-04-04) and TCM Clinical Base project of State
Administration of Traditional Chinese Medicine (grant no.
JDZX2015275).
Availability of data and materials
The datasets used and/or analyzed in the study are
available from the corresponding author upon reasonable
request.
Authors' contributions
PZ designed the study. CY and JQB performed western
blot experiments. PYH collected the patient tissue samples. JRZ and
QO performed and analyzed the IHC results. YW performed the cell
culture and MTT experiments. HMS and QYL performed the Transwell
assays. YZ collected and analyzed all the data. All authors
participated in writing the manuscript and approved the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
The Code of Ethics of the World Medical Association. All patients
agreed to participate in the present study and the study was
approved by the Ethics Committee of Wangjing Hospital (Beijing,
China; approval no. WJEC-KT-2019-019).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviation
Abbreviations:
|
TMCO
|
transmembrane and coiled-coil
domain
|
References
|
1
|
Moe AM, Golding AE and Bement WM: Cell
healing: Calcium, repair and regeneration. Semin Cell Dev Biol.
45:18–23. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cui C, Merritt R, Fu L and Pan Z:
Targeting calcium signaling in cancer therapy. Acta Pharm Sin B.
7:1–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tennakoon S, Aggarwal A and Kállay E: The
calcium-sensing receptor and the hallmarks of cancer. Biochim
Biophys Acta. 1863:1398–1407. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bong AHL and Monteith GR: Calcium
signaling and the therapeutic targeting of cancer cells. Biochim
Biophys Acta Mol Cell Res. 1865:1786–1794. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang QC, Zheng Q, Tan H, Zhang B, Li X,
Yang Y, Yu J, Liu Y, Chai H, Wang X, et al: TMCO1 is an ER Ca(2+)
load-activated Ca(2+) channel. Cell. 165:1454–1466. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Michael Yates T, Ng OH, Offiah AC,
Willoughby J, Berg JN; DDD Study, ; Johnson DS:
Cerebrofaciothoracic dysplasia: Four new patients with a recurrent
TMCO1 pathogenic variant. Am J Med Genet A. 179:43–49. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kondkar AA, Mousa A, Azad TA, Sultan T,
Alawad A, Altuwaijri S, Al-Obeidan SA and Abu-Amero KK:
Polymorphism rs7555523 in transmembrane and coiled-coil domain 1
(TMCO1) is not a risk factor for primary open angle glaucoma in a
Saudi cohort. J Negat Results Biomed. 15:172016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li CF, Wu WR, Chan TC, Wang YH, Chen LR,
Wu WJ, Yeh BW, Liang SS and Shiue YL: Transmembrane and coiled-coil
domain 1 impairs the AKT signaling pathway in urinary bladder
urothelial carcinoma: A characterization of a tumor suppressor.
Clin Cancer Res. 23:7650–7663. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou BO, Nie J, Yang W, Huang C, Huang YE
and Zhao H: Effect of hydrothorax EGFR gene mutation and EGFR-TKI
targeted therapy on advanced non-small cell lung cancer patients.
Oncol Lett. 11:1413–1417. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pascoe HM, Knipe HC, Pascoe D and Heinze
SB: The many faces of lung adenocarcinoma: A pictorial essay. J Med
Imaging Radiat Oncol. 62:654–661. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y, Yu WK, Chen L, Chan YS, Liu D, Fong
CC, Xu T, Zhu G, Sun D and Yang M: Electrotaxis of tumor-initiating
cells of H1975 lung adenocarcinoma cells is associated with both
activation of stretch-activated cation channels (SACCs) and
internal calcium release. Bioelectrochemistry. 124:80–92. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Déliot N and Constantin B: Plasma membrane
calcium channels in cancer: Alterations and consequences for cell
proliferation and migration. Biochim Biophys Acta. 1848:2512–2522.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan H, Shen YX and Yuan YF: Expression and
Prognostic Roles of TRPV5 and TRPV6 in Non-small cell lung cancer
after curative resection. Asian Pac J Cancer Prev. 6:2559–2563.
2014. View Article : Google Scholar
|
|
14
|
Ma X, Cai Y, He D, Zou C, Zhang P, Lo CY,
Xu Z, Chan FL, Yu S, Chen Y, et al: Transient receptor potential
channel TRPC5 is essential for P-glycoprotein induction in
drug-resistant cancer cells. Proc Natl Acad Sci USA.
109:16282–16287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Capiod T: Extracellular calcium has
multiple targets to control cell proliferation. Adv Exp Med Biol.
898:133–156. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang LL, Liu BC, Lu XY, Yan Y, Zhai YJ,
Bao Q, Doetsch PW, Deng X, Thai TL, Alli AA, et al: Inhibition of
TRPC6 reduces non-small cell lung cancer cell proliferation and
invasion. Oncotarget. 8:5123–5134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Racioppi L, Nelson ER, Huang W, Mukherjee
D, Lawrence SA, Lento W, Masci AM, Jiao Y, Park S, Liu Y, et al:
CaMKK2 in myeloid cells is a key regulator of the
immune-suppressive microenvironment in breast cancer. Nat Commun.
10:24502019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vaz CV, Rodrigues DB, Socorro S and Maia
CJ: Effect of Extracellular calcium on regucalcin expression and
cell viability in neoplastic and non-neoplastic human prostate
cells. Biochim Biophys Acta. 1853:2621–2628. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yuan K, Chung LW, Siegal GP and Zayzafoon
M: Alpha-CaMKII controls the growth of human osteosarcoma by
regulating cell cycle progression. Lab Invest. 87:938–950. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Byun JS, Sohn JM, Leem DG, Park B, Nam JH,
Shin DH, Shin JS, Kim HJ, Lee KT and Lee JY: In vitro synergistic
anticancer activity of the combination of T-type calcium channel
blocker and chemotherapeutic agent in A549 cells. Bioorg Med Chem
Lett. 26:1073–1079. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He Y, Lin G, Han D, Shi D, Liu T, Gao Y,
Guan W and Cheng G: Aclidinium Bromide holds promising inhibitory
effects in A549 lung cancer cells potentials by regulating PI3K/AKT
signaling pathway. J BUON. 24:560–565. 2019.PubMed/NCBI
|
|
22
|
Vervliet T, Parys JB and Bultynck G: Bcl-2
proteins and calcium signaling: Complexity beneath the surface.
Oncogene. 35:5079–5092. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Annunziato L, Amoroso S, Pannaccione A,
Cataldi M, Pignataro G, D'Alessio A, Sirabella R, Secondo A, Sibaud
L and Di Renzo GF: Apoptosis induced in neuronal cells by oxidative
stress: Role played by caspases and intracellular calcium ions.
Toxicol Lett. 139:125–133. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li G, He Y, Yao J, Huang C, Song X, Deng
Y, Xie S, Ren J, Jin M and Liu H: Angelicin inhibits human lung
carcinoma A549 cell growth and migration through regulating JNK and
ERK pathways. Oncol Rep. 36:3504–3512. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin X, Li HR, Lin XF, Yu ME, Tu XW, Hua
ZD, Lin M, Xu NL, Han LL and Chen YS: Silencing of Livin inhibits
tumorigenesis and metastasis via VEGF and MMPs pathway in lung
cancer. Int J Oncol. 47:657–667. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shi S, Luo W, Zhang R, Wang C, Zheng Y,
Song Y, Wang R, Zhang L, Zhang L, Li W and Luo Z: CRTC2 promotes
non-small cell lung cancer A549 migration and invasion in vitro.
Thorac Cancer. 9:136–141. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pastushenko I and Blanpain C: EMT
Transition states during tumor progression and metastasis. Trends
Cell Biol. 29:212–226. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Daugaard I, Sanders KJ, Idica A,
Vittayarukskul K, Hamdorf M, Krog JD, Chow R, Jury D, Hansen LL,
Hager H, et al: MiR-151a induces partial EMT by regulating
E-cadherin in NSCLC cells. Oncogenesis. 6:e3662017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gloushankova NA, Rubtsova SN and Zhitnyak
IY: Cadherin-mediated cell-cell interactions in normal and cancer
cells. Tissue Barriers. 5:e13569002017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tadokoro A, Kanaji N, Liu D, Yokomise H,
Haba R, Ishii T, Takagi T, Watanabe N, Kita N, Kadowaki N and
Bandoh S: Vimentin regulates invasiveness and is a poor prognostic
marker in non-small cell lung cancer. Anticancer Res. 36:1545–1551.
2016.PubMed/NCBI
|
|
31
|
Sun Z, Zhang H, Wang X, Wang QC, Zhang C,
Wang JQ, Wang YH, An CQ, Yang KY, Wang Y, et al: TMCO1 is essential
for ovarian follicle development by regulating ER Ca 2+
Store of Granulosa cells. Cell Death Differ. 9:1686–1701. 2018.
View Article : Google Scholar
|
|
32
|
Alanay Y, Ergüner B, Utine E, Haçariz O,
Kiper PO, Taşkıran EZ, Perçin F, Uz E, Sağiroğlu MŞ, Yuksel B, et
al: TMCO1 Deficiency causes autosomal recessive
cerebrofaciothoracic dysplasia. Am J Med Genet A. 164:291–304.
2014. View Article : Google Scholar
|