Introduction
Surgery is the treatment of choice for patients with
breast cancer. However, the presence of circulating tumor cells
(CTCs) after surgery has been reported to be significantly
associated with early recurrence (1). The release of tumor cells into
peripheral blood as CTCs is one of the main causes of cancer
recurrence (2). Metastasis involves
the migration of cancer cells away from primary tumors and their
entry into blood circulation. Tumor metastasis is a fundamental
cellular process for maintaining tissue homeostasis by removing
displaced epithelial or endothelial cells, thus preventing them
from seeding to inappropriate sites (3). In vivo and in vitro
studies have demonstrated that the capability of cells to resist
anoikis (death after detachment) is critical for successful
metastasis (4–6).
Anoikis resistant cancer cells can often survive and
grow without adhesion to the basement membrane as aggregated
microemboli in the bloodstream and such growth represents a
hallmark of the malignant phenotype (7). A previous study has demonstrated that
anoikis resistance in CTCs was accompanied by spheroid formation
and caspase-3/9/poly (ADP ribose) polymerase (PARP) inactivation
(8). Furthermore, E-cadherin and
β-catenin are important intracellular signaling molecules
associated with cell aggregation (9), which itself is associated with colony
formation as cell clusters and indicative of poor prognosis
(10). Micrometastases resulting
from such tumor cell aggregates by β-catenin/E-cadherin activation
are thought to survive within the circulation as small cell
clusters or spheroids, thereby effecting suppression of anoikis
(11). It has suggested that the
disruption of anoikis resistance may serve as a therapeutic
strategy for the treatment of malignant cancer (12).
Monascin (MS) is a yellow pigment produced by
Monascus; it exhibits diverse pharmacological activities,
including anti-inflammatory activity and antioxidant activity
(13), and induction of cell death
in cancer cells (14). However, the
effects of MS on CTCs have not been elucidated. In the present
study, the ability of MS to induce anoikis was investigated in
murine anoikis-resistant 4T1 breast cancer cells. The biochemical
mechanisms underlying anoikis induction by MS were also determined.
The results of the present study may lead to the development of
novel strategies for the CTC metastasis treatment and
prevention.
Materials and methods
Reagents
Monascin (cat. no. 52442) and other chemicals and
reagents (unless otherwise stated) were purchased from
Sigma-Aldrich; Merck KGaA. Media, FBS (cat. no. 26140079) and
culture supplements were purchased from Invitrogen; Thermo Fisher
Scientific, Inc. Anti-PARP (cat. no. 9542), caspase-3 (cat. no.
9665), E-cadherin (cat. no. 3195), β-catenin (cat. no. 9562) and
β-actin (cat. no. 4970) were purchased from Cell Signaling
Technology, Inc.
Cell culture
The murine breast cancer 4T1 cell line was purchased
from the American Type Culture Collection and the normal murine
mammary gland (NMuMG) cell line was purchased from Bioresource
Collection and Research Center. Each cell line was cultured in DMEM
supplemented with 10% FBS and 100 U/ml streptomycin/penicillin.
Cells were incubated at 37°C in an atmosphere of 5% CO2.
For obtaining an anchorage-independent culture, cells were cultured
in the same complete medium but on a poly [2-hydroxyethyl
methacrylate (polyHEMA)]-coated plate. Briefly, polyHEMA was
dissolved in 95% ethanol to obtain 12% (w/v) stock solution.
Working solution was prepared by further dilution (1:10) of stock
solution using 95% ethanol and added to tissue culture dishes or
plates (3 ml per 10 cm dish or 0.5 ml per well in a six-well
plate). A hydrophobic surface was formed after polyHEMA solution
was evaporated at room temperature in a tissue culture hood.
Cell proliferation assay
Both 4T1 and NMuMG cell proliferation rates were
detected using the Cell Counting kit-8 (CCK-8) assay (Dojindo
Molecular Technologies, Inc.). Cells were seeded into a 96-well
plate at a density of 5×103 cells/well. After culturing
for 24 h, 48 h and 72 h at 37°C, 10 µl of CCK-8 solution was added
to the completed medium and cells were further incubated at 37°C
for 1 h. Cell viability was determined by measuring absorbance at
450 nm on a microplate reader.
Trypan blue exclusion assay
4T1 and NMuMG cells were cultured in each well
(1×106 cells/well) of a 6-well plate with or without a
polyHEMA coating. After 24 h incubation, culture medium was
discarded and cells were washed with PBS and suspended in trypan
blue stock in PBS (final concentration, 4%). Diluted trypan blue
solution (0.04%) was subsequently added to the 6-well plate (500
µl/well). After 3 min incubation at room temperature, stained cells
were observed under a light microscope at a magnification of ×10.
Dead cells were dyed blue and viable cells were colorless and
transparent.
Annexin V-FITC and PI double staining
assay
After 48 h treatment with 20 µM MS, 4T1 cells were
harvested via trypsinization and washed with cold PBS. Cells were
then centrifuged at 300 × g for 5 min at 4°C, after which the
supernatant was discarded and the pellet was resuspended in 1X
binding buffer. Sample solution (60 µl) was incubated with 3 µl
FITC-conjugated Annexin V (BD Pharmingen; BD Biosciences) and 1
µg/ml PI (Sigma-Aldrich; Merck KGaA; cat. no. P4170) for 15 min at
room temperature in the dark. A total of 240 µl 1X binding buffer
was added to each tube and the samples were counted (20,000 cells)
and analyzed using a CytoFLEX™ Flow Cytometer and CytExpert
software version 2.4 (Beckman Coulter, Inc.).
Western blot analysis
4T1 cells were lysed in ice-cold
radioimmunoprecipitation assay lysis buffer (Merck KGaA; cat. no.
632424) containing protease inhibitor cocktails (EMD Millipore;
cat. no. 539134). Protein concentration was determined using
Bicinchoninic Acid Protein Assay Kit (Santa Cruz Biotechnology,
Inc.; cat. no. sc-202389). Proteins (20 µg) were separated by 10%
SDS-PAGE and transferred onto PVDF membranes. Membranes were washed
with PBS supplemented with 0.1% Tween-20. After blocking with
BlockPRO™ Protein-Free Blocking Buffer (Visual Protein; cat. no.
BF01) for 1 h at room temperature, membranes were incubated with
primary antibodies against PARP (1:1,000; Cell Signaling
Technology, Inc.; cat. no. 9542), caspase-3 (1:1,000; Cell
Signaling Technology, Inc.; cat. no. 9665), E-cadherin (1:1,000;
Cell Signaling Technology, Inc.; cat. no. 3195), β-catenin
(1:1,000; Cell Signaling Technology, Inc.; cat. no. 9562) and
β-actin (1:2,000; Cell Signaling Technology, Inc.; cat. no. 4970)
primary antibodies overnight at 4°C. Membranes were then incubated
with anti-rabbit IgG HRP-linked antibody (1:5,000; Cell Signaling
Technology, Inc.; cat. no. 7074) for 1 h at room temperature.
Immobilon Western Chemiluminescent HRP Substrate (EMD Millipore;
cat. no. WBKLS0500) was used to detect the signal on the membrane
that were developed on Hyperfilm™ ECL™ film (GE Healthcare; cat.
no. 28906839).
Animal model
All animal care and experimental procedures adhered
to the guidelines of the Institutional Animal Care and Use
Committee of Wan Fang Hospital, Taipei Medical University (Taipei,
Taiwan; approval no. WAN-LAC-106-012). Female 6-week-old
BALB/cByJNar1 mice were purchased from the National Laboratory
Animal Center (Taipei, Taiwan). Animals were given a standard
laboratory diet and distilled water ad libitum under a 12-h
light/dark cycle at 22 ± 2°C and humidity (55±5%) in the animal
facility of Wan Fang Hospital. Stable 4T1-Luc cells were
established as described previously (15). A total of 20 mice were used (~18 g
weight). The tail veins of mice were injected with 1×106
4T1-Luc cells on day 0. The mice were then divided into four groups
on day 1 (n=5 per group): Vehicle control, tumor control, MS (100
mg/kg) and MS (500 mg/kg) groups. MS was fed to the mice via oral
gavage once daily, 5 days per week for a total of 4 weeks.
Prior to in vivo bioluminescence imaging,
mice were anesthetized with isoflurane in an acrylic chamber using
a 4% isoflurane/air mixture for induction. Mice were injected
intraperitoneally with substrate D-luciferin (Sigma-Aldrich; Merck
KGaA; cat. no. L9504) solution (150 mg/kg) in Dulbecco's
Phosphate-Buffered Saline (Sigma-Aldrich; Merck KGaA; cat. no.
D8537) and maintained under 2% isoflurane. After 5 min, images of
the live anesthetized mice were recorded using a bioluminescence
IVIS Spectrum System (PerkinElmer, Inc.; part no. 124262), which
included a cryogenic cooling unit and a data acquisition computer
with Living Image software version 2.5 (Xenogen Corp.). The
acquisition and overlay of pseudocolor images were conducted.
Images represented the spatial distribution of detected photons
emerging from active luciferase within the animals. Bioluminescent
signals were quantified using Living Image software 2.5 (Xenogen
Corporation) as photons/sec/region of interest on days 7, 14, 21
and 28 of treatment.
Statistical analyses
All statistics were calculated using GraphPad Prism
6 software (GraphPad Software, Inc.). P<0.05 was considered to
indicate a statistically significant difference. Data are presented
as mean ± SD of triplicate experiments. Statistical significance
for parametric data were determined using two-way ANOVA with
Tukey's multiple comparisons test to compare multiple dependent
variables against multiple independent variables. An unpaired
two-tailed t-test was used to compare one dependent variable
against one independent variable. Error bars represent the standard
error of the mean obtained from experiments performed in
triplicate.
Results
Anoikis-sensitization effect of MS in
4T1 breast cancer cells
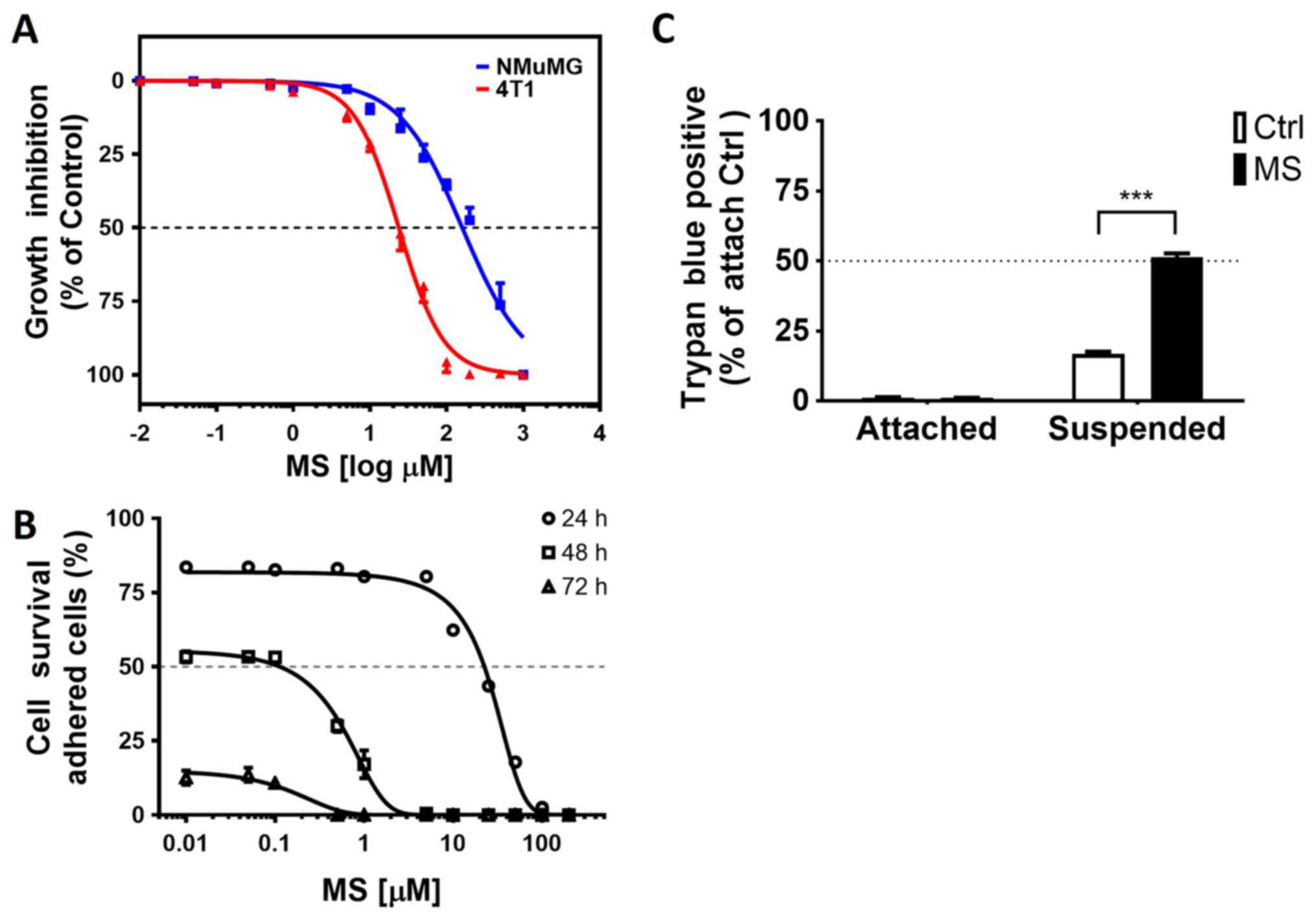
The cytotoxic effect of MS was evaluated using
murine NMuMG normal breast cells and 4T1 breast cancer cells
treated with increasing concentrations (0.1-1,000 µM) of MS for 48
h. MS markedly suppressed the survival of 4T1 breast cancer cells
without causing toxic damage to normal NMuMG cells (Fig. 1A). To further validate that MS acted
as an anoikis sensitizer, adherent and suspended breast cells were
treated with increasing concentrations (0.01–100 µM) of MS for
24–72 h. The number of dead cells in the MS-treated samples
increased with the MS concentration (10–100 µM); thus, MS exhibited
cytotoxicity in a dose- and time-dependent manner (Fig. 1B). The results were further confirmed
by using the trypan blue exclusion method to count the number of
dead cells. 4T1 cells treated with 20 µM MS for 24 h on
polyHEMA-coated culture plates (suspended cells) were more
sensitive to anoikis than attached cells (Fig. 1C).
MS induces caspase-dependent apoptosis
in a suspension culture of highly metastatic breast cancer
cells
4T1 breast cancer cells were plated on uncoated
(attached) or polyHEMA-coated (suspended) tissue culture plates for
48 h. To confirm the occurrence of apoptosis, an Annexin V-FITC/PI
double staining assay was performed. The percentage of apoptotic
cells (early and late apoptotic cells) in the polyHEMA-coated plate
increased significantly from 9.91 to 44.96% after treatment with 20
µM MS (Fig. 2A). As caspase
activation is considered a hallmark of apoptosis (16), western blotting was performed to
examine caspase activity. Cleaved caspase-3 expression clearly
increased in the cells in the polyHEMA-coated plates after MS
treatment. Cleaved PARP was also notably activated in 4T1 cells
treated with 20 µM MS under the suspension condition (Fig. 2B).
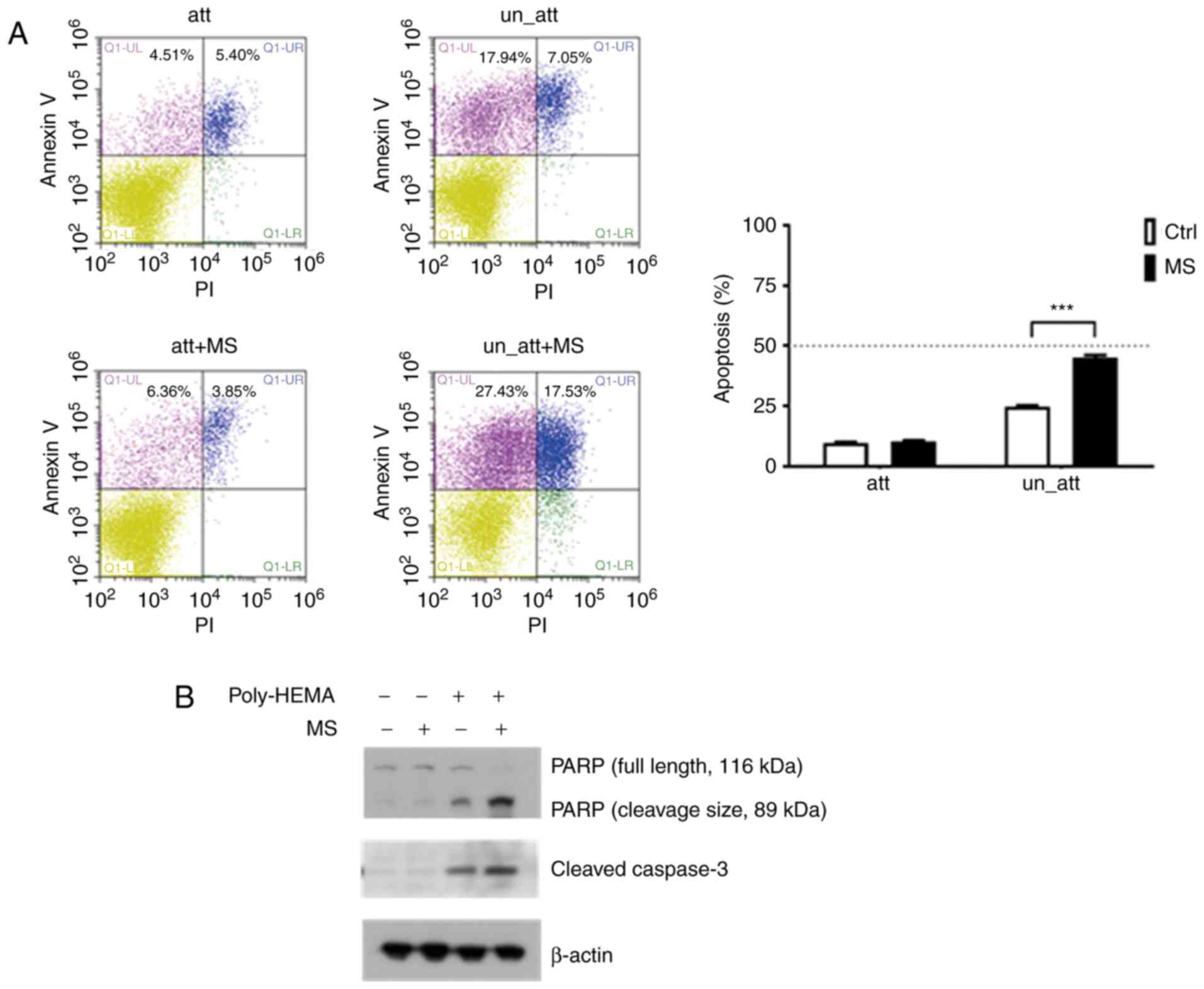 | Figure 2.Induction of apoptosis by MS in 4T1
breast cancer cells. (A) Fluorescence-activated cell sorting
analysis of Annexin V/PI staining. The results indicated early
apoptosis, defined as Annexin V-positive and PI-negative cells
(Q1-UL), and late apoptosis, defined as Annexin V-positive and
PI-positive cells (Q1-UR). 4T1 cells were cultured on a general
culture plate (att) or a polyHEMA-coated plate (un_att), treated
with 20 µM (MS) or without MS for 48 h, and then labeled with
Annexin V-FITC and PI. The results are expressed as means ± SD of
three independent experiments. ***P<0.001 vs. control.(B)
Western blot analysis for determining the effect of MS treatment
for 48 h on PARP and cleavage caspase-3 expression under attachment
and suspension conditions. MS, monascin; Ctrl, control; att,
attached; un_att, unattached; PI, propidium iodide. |
MS inhibits breast cancer cell
aggregation under the suspension condition
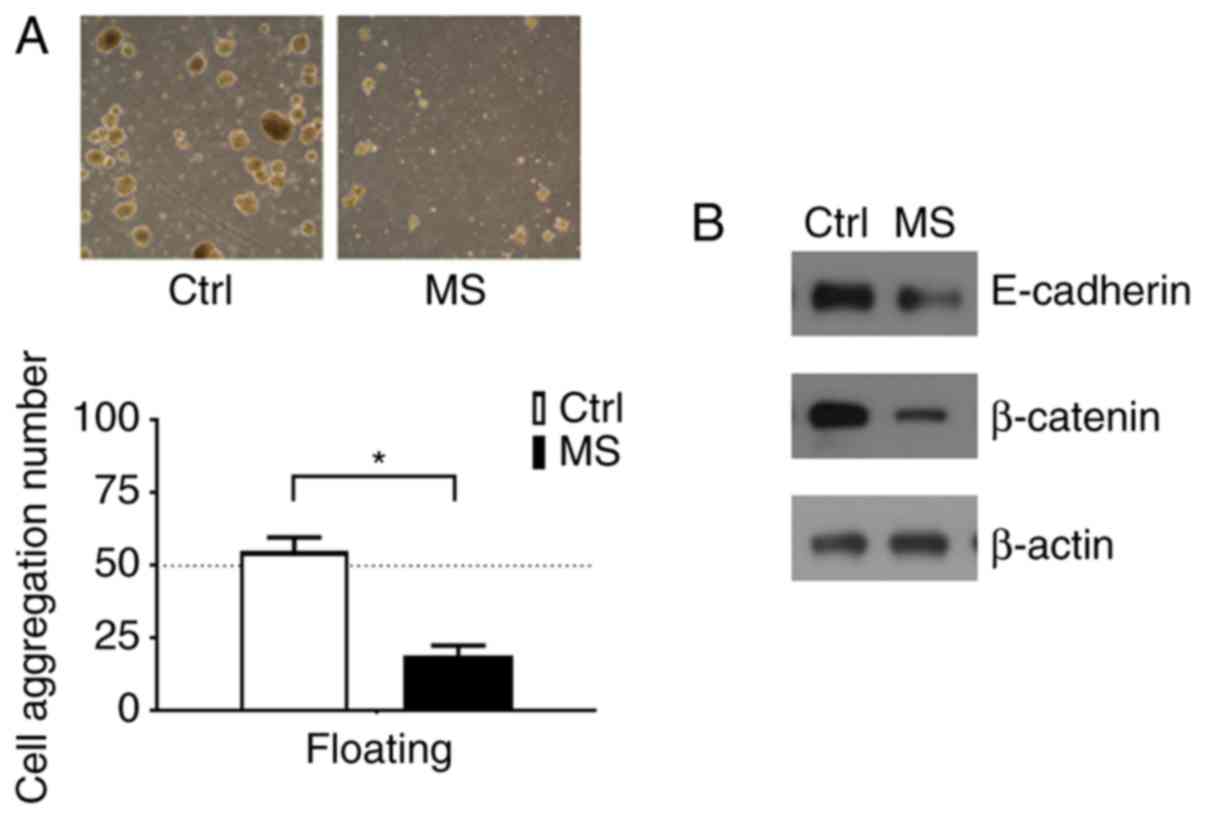
In suspension culture, 4T1 cells formed large
aggregates, but the cells did not exhibit aggregation after MS
treatment (Fig. 3A). These
observations suggest that cell aggregation provided growth signals
to the tumor cells in suspension. To understand the molecular
nature of the survival and growth signals provided by 4T1 cell
aggregation, survival pathways were analyzed. The data indicated
that MS reduced cell aggregation, which facilitated anoikis and
downregulated the expression of E-cadherin and β-catenin (Fig. 3B).
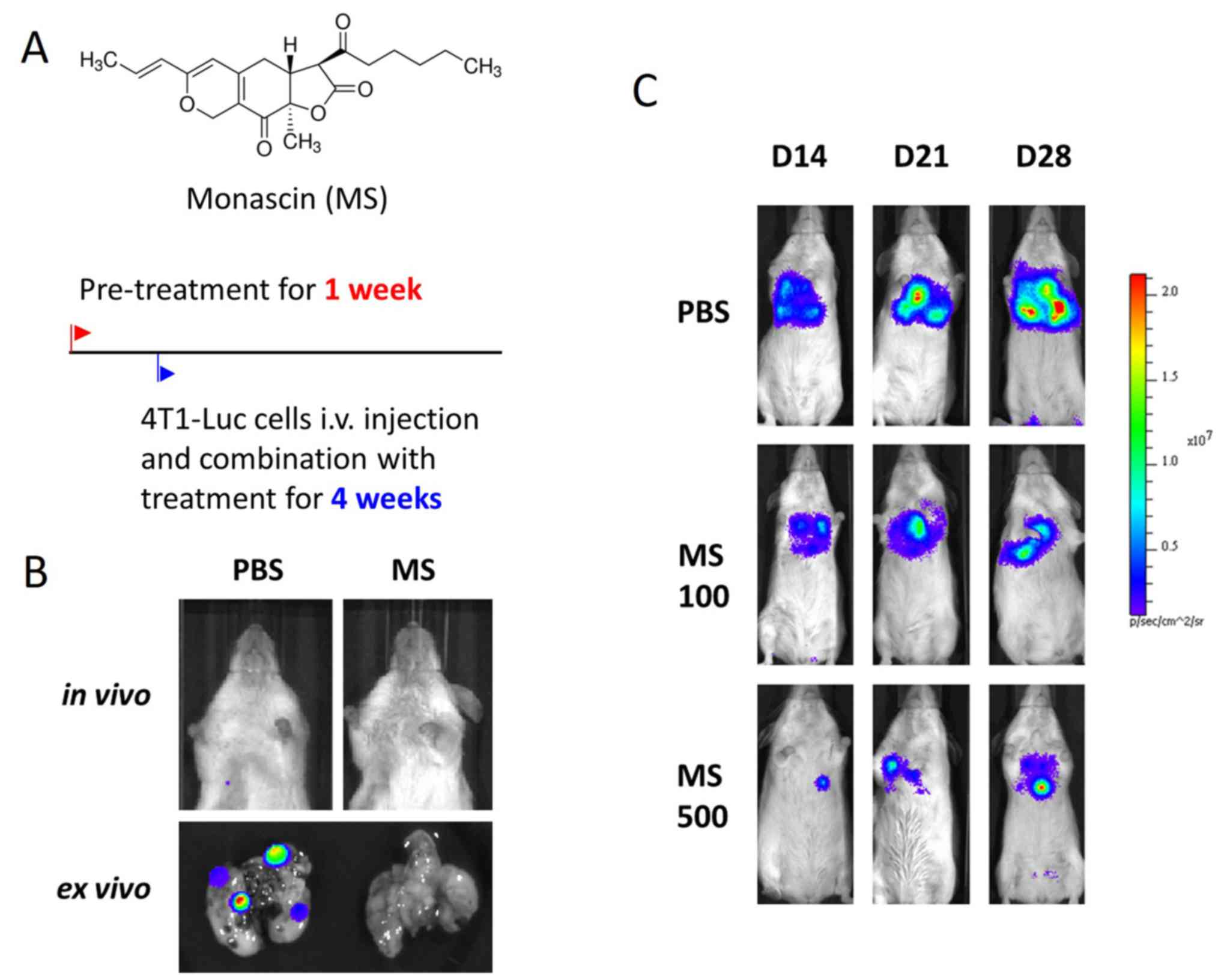
MS treatment reduces lung metastasis
in 4T1-Luc animal model
Considering that 4T1 aggregation was inhibited by MS
treatment in vitro (Fig. 3A),
the in vivo effect of MS on lung metastasis was determined.
4T1 cells were transfected with the firefly luciferase gene (Luc)
in an experimental metastasis model. 4T1-Luc cells were injected
into the tail vein of the mice. Non-invasive bioluminescence
imaging enabled the early detection of cancer metastasis. Weekly
bioluminescence imaging was conducted for 4 weeks, and the radiance
antemortem was used as a surrogate measurement of tumor burden. The
mice treated with MS (100–500 mg/kg daily) exhibited a notable
reduction in both the number and size of pulmonary metastases,
compared with the vehicle-treated mice (Fig. 4).
Discussion
Anchorage independence is a well-known
characteristic of CTCs that allows floating cancer cell metastasis
(1). Based on this assumption,
anoikis resistance may be a crucial early characteristic of
malignant cancer, because cancer cells are either deprived of
extracellular matrix or exposed to foreign matrix components during
metastasis (17). Moreover, anoikis
resistance is associated with a high degree of tumor metastasis and
advanced stage of cancers (18).
Therefore, targeting anoikis-resistance pathways represents a
promising strategy for antimetastatic therapy. In previous report,
we have descripted that the count of CTCs is a significant
predictor for liver metastasis within six months of surgery
(19). Due to the difficulty in
establishing the primary tumor circulating cells, the 4T1 breast
cancer cell line was used to develop CTC-like model. Similarly, in
another study performed by Park et al (20), stable CTC-like cells derived from
human breast cancer MDA-MB-468 cell line were established.
Therefore, the effects of MS on 4T1 cells aggregation was examined
via poly-HEMA coating culture. The present results indicated the
suspended cells, forced to grow under anchorage-independent
conditions, exhibited upregulated β-catenin/E-cadherin expression
and downregulated expression of the intrinsic apoptosis pathway,
via inhibiting PARP/caspase-3 cleaved as a mechanism for
suppressing anoikis. To the best of our knowledge, this was the
first report of MS resensitizing breast cancer cells to anoikis and
reducing colony-forming ability under a suspension condition.
The secondary metabolite MS produced by
Monascus species has been reported to exhibit multiple
biological effects, including anti-inflammatory effects (21). In the present study, the effects of
MS on tumor growth and metastasis in 4T1 metastatic tumor-bearing
mice were investigated, and the effects on cell adhesion, anoikis
resistance and cell migration in 4T1 breast cancer cells were
examined. In the current study, it was revealed that MS restored
anoikis sensitivity in suspended 4T1 cancer cells. Notably, MS
inhibited the proliferation of 4T1 cells but was not cytotoxic to
normal NMuMG cells. Also, MS selectively induced cell death in
suspended 4T1 cells and MS-induced cell death was demonstrated to
be due to apoptosis, as demonstrated by the high levels of
proapoptotic caspase activation and PARP cleavage. Finally, MS
treatment prevented the growth of floating 4T1-Luc cells injected
into the tail vein of the experimental mice and inhibited the
migration of the cells to the lungs.
However, there were a few limitations to the present
study. The current results were not derived from isolated primary
circulating tumor cells, and clinical data was not available to
support the in vitro and in vivo findings. Further
studies are required to investigate the effect of MS treatment on
clinical breast cancer metastasis, and this should be confirmed in
a clinical trial. Multiple animal models have been developed
recently, but these models are not designed appropriately to study
the step-by-step progression of metastasis (22). In this complicated metastasis
process, the present study only describes the anoikis resistance on
circulating tumor cells. Despite having certain limitations, such
as the step of intravasation from primary site into the
bloodstream, the poly-HEMA coating culture-induced cell cluster
formation may be an in vitro experimental model for anoikis
resistance investigation.
Previous studies on tumor metastasis and the
epithelial-mesenchymal transition (EMT) have reported that EMT
enables cancer cells to migrate away from the primary tumor
(23–25). However, whether cell detachment
additionally triggers EMT remains unclear. In the present study,
high levels of β-catenin expression following detachment may be
associated with cell aggregation and proliferation. β-Catenin is a
versatile protein that serves multiple fundamental functions in
cells, such as controlling intercellular junction integrity and
regulating transcriptional processes as a co-transcription factor,
mediating the canonical Wnt signaling pathway (26). Thus, targeting β-catenin activity and
reducing anoikis resistance may represent strategies for preventing
the development and progression of breast cancer. A previous study
has revealed that disruption of E-cadherin-mediated adhesion
sensitizes multicellular spheroids of tumor cells to treatment with
chemotherapeutic drugs (27). MS,
with the function to downregulate E-cadherin, may therefore have
potential as a neoadjuvant for clinical metastatic prevention after
surgery.
In conclusion, the current results demonstrate that
MS targeting anti-anoikis may increase the killing of breast tumor
cells persisting in the circulation as anchorage-independent
micrometastasis. Future studies are needed on MS, including
evaluation of anoikis resistance as a clinical application.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from Wan
Fang Hospital, Taipei Medical University (grant nos. 106-swf-05,
106-swf-11 and 108-wf-eva-09) and the Ministry of Science and
Technology, Taiwan (grant no. MOST 109-2314-B-038-039).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KYC, YTT and BYH conceived and designed the study.
KYC, JAL and BYH performed animal experiments. HYY and ACH assisted
in the animal experiments. YTT and BYH directed the project,
analyzed and interpreted the data and wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal care and experimental procedures adhered
to the guidelines of the Institutional Animal Care and Use
Committee of Wan Fang Hospital, Taipei Medical University (approval
no. WAN-LAC-106-012, Taipei, Taiwan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Aceto N, Bardia A, Miyamoto DT, Donaldson
MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, et al:
Circulating tumor cell clusters are oligoclonal precursors of
breast cancer metastasis. Cell. 158:1110–1122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cristofanilli M, Budd GT, Ellis MJ,
Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ,
Terstappen LW and Hayes DF: Circulating tumor cells, disease
progression, and survival in metastatic breast cancer. N Engl J
Med. 351:781–791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Dalum G, Holland L and Terstappen LW:
Metastasis and circulating tumor cells. EJIFCC. 23:87–97.
2012.PubMed/NCBI
|
|
4
|
Sirimangkalakitti N, Chamni S,
Suwanborirux K and Chanvorachote P: Renieramycin M Sensitizes
Anoikis-resistant H460 lung cancer cells to anoikis. Anticancer
Res. 36:1665–1671. 2016.PubMed/NCBI
|
|
5
|
Zhang X, Yang L, Chien S and Lv Y:
Suspension state promotes metastasis of breast cancer cells by
up-regulating cyclooxygenase-2. Theranostics. 8:3722–3736. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zou G, Ren B, Liu Y, Fu Y, Chen P, Li X,
Luo S, He J, Gao G, Zeng Z, et al: Inhibin B suppresses anoikis
resistance and migration through the transforming growth factor-β
signaling pathway in nasopharyngeal carcinoma. Cancer Sci.
109:3416–3427. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cominetti MR, Altei WF and
Selistre-de-Araujo HS: Metastasis inhibition in breast cancer by
targeting cancer cell extravasation. Breast Cancer (Dove Med
Press). 11:165–178. 2019.PubMed/NCBI
|
|
8
|
Laguinge LM, Samara RN, Wang W, El-Deiry
WS, Corner G, Augenlicht L, Mishra L and Jessup JM: DR5 receptor
mediates anoikis in human colorectal carcinoma cell lines. Cancer
Res. 68:909–917. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Steadman K, Stein WD, Litman T, Yang SX,
Abu-Asab M, Dutcher SK and Bates S: PolyHEMA spheroids are an
inadequate model for the drug resistance of the intractable solid
tumors. Cell Cycle. 7:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hong Y, Fang F and Zhang Q: Circulating
tumor cell clusters: What we know and what we expect (Review). Int
J Oncol. 49:2206–2216. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang HG, Jenabi JM, Zhang J, Keshelava N,
Shimada H, May WA, Ng T, Reynolds CP, Triche TJ and Sorensen PH:
E-cadherin cell-cell adhesion in ewing tumor cells mediates
suppression of anoikis through activation of the ErbB4 tyrosine
kinase. Cancer Res. 67:3094–3105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Giannoni E, Fiaschi T, Ramponi G and
Chiarugi P: Redox regulation of anoikis resistance of metastatic
prostate cancer cells: Key role for Src and EGFR-mediated
pro-survival signals. Oncogene. 28:2074–2086. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee BH, Hsu WH, Huang T, Chang YY, Hsu YW
and Pan TM: Effects of monascin on anti-inflammation mediated by
Nrf2 activation in advanced glycation end product-treated THP-1
monocytes and methylglyoxal-treated wistar rats. J Agric Food Chem.
61:1288–1298. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su NW, Lin YL, Lee MH and Ho CY:
Ankaflavin from Monascus-fermented red rice exhibits selective
cytotoxic effect and induces cell death on Hep G2 cells. J Agric
Food Chem. 53:1949–1954. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ho BY, Lin CH, Apaya MK, Chao WW and Shyur
LF: Silibinin and paclitaxel cotreatment significantly suppress the
activity and lung metastasis of triple negative 4T1 mammary tumor
cell in mice. J Tradit Complement Med. 2:301–311. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mohamed MS, Bishr MK, Almutairi FM and Ali
AG: Inhibitors of apoptosis: Clinical implications in cancer.
Apoptosis. 22:1487–1509. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim YN, Koo KH, Sung JY, Yun UJ and Kim H:
Anoikis resistance: An essential prerequisite for tumor metastasis.
Int J Cell Biol. 2012:3068792012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Piyush T, Rhodes JM and Yu LG: MUC1
O-glycosylation contributes to anoikis resistance in epithelial
cancer cells. Cell Death Discov. 3:170442017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tien YW, Kuo HC, Ho BI, Chang MC, Chang
YT, Cheng MF, Chen HL, Liang TY, Wang CF, Huang CY, et al: A high
circulating tumor cell count in portal vein predicts liver
metastasis from periampullary or pancreatic cancer: A high portal
venous CTC count predicts liver metastases. Medicine (Baltimore).
95:e34072016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park JY, Jeong AL, Joo HJ, Han S, Kim SH,
Kim HY, Lim JS, Lee MS, Choi HK and Yang Y: Development of
suspension cell culture model to mimic circulating tumor cells.
Oncotarget. 9:622–640. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng CF and Pan TM: Monascus-fermented
red mold dioscorea protects mice against alcohol-induced liver
injury, whereas its metabolites ankaflavin and monascin regulate
ethanol-induced peroxisome proliferator-activated receptor-gamma
and sterol regulatory element-binding transcription factor-1
expression in HepG2 cells. J Sci Food Agric. 98:1889–1898. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khanna C and Hunter K: Modeling metastasis
in vivo. Carcinogenesis. 26:513–523. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bill R and Christofori G: The relevance of
EMT in breast cancer metastasis: Correlation or causality? FEBS
Lett. 589:1577–1587. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alix-Panabieres C, Mader S and Pantel K:
Epithelial-mesenchymal plasticity in circulating tumor cells. J Mol
Med (Berl). 95:133–142. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Melzer C, von der Ohe J and Hass R: Breast
carcinoma: From initial tumor cell detachment to settlement at
secondary sites. Biomed Res Int. 2017:85343712017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao C, Xiao G and Hu J: Regulation of
Wnt/β-catenin signaling by posttranslational modifications. Cell
Biosci. 4:132014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Green SK, Francia G, Isidoro C and Kerbel
RS: Antiadhesive antibodies targeting E-cadherin sensitize
multicellular tumor spheroids to chemotherapy in vitro. Mol Cancer
Ther. 3:149–159. 2004.PubMed/NCBI
|