Introduction
Prostate cancer (PCa) ranks second for
cancer-related mortality in men, with an expected 33,330 deaths
estimated to occur in 2020 worldwide (1,2).
Treatment options for the advanced metastatic PCa are limited due
to the uncertainties in the molecular mechanisms and the serious
side effects of chemotherapy. While the advent of novel screening
methods, such as novel serum-based models like 4Kscore®
and prostate health index (PHI) (3),
and hormone ablation therapies have achieved a ~100% 5-year
survival rate for patients with localized PCa, treating patients
harboring metastatic PCa, who have a 5-year survival rate of 31%,
remains a challenge (4,5). In-depth molecular characterization to
detect novel druggable targets, identifying compounds with precise
molecular targets and limited off-target effects, and developing
novel strategies for drug delivery is critical in improving the
5-year survival rate in patients with metastatic PCa.
Our previous studies and those from other
researchers have shown that P21 (RAC1) activated kinase-1 (PAK1)
promotes PCa growth and metastasis (6–11) by
facilitating cell proliferation, cell survival, motility, invasion,
and epithelial-to-mesenchymal transition (11–14).
Previous studies have also shown that the small molecule, an
inhibitor targeting PAK1 activation-3 (IPA3) was found to be an
effective allosteric inhibitor of PAK1, which decreases PCa tumor
growth, and metastasis (12–14). However, despite the promising effect
of IPA3 on PCa, the compound has limitations related to its
pharmacokinetic properties. Specifically, IPA3 is metabolically
unstable, therefore, daily administration is required to exert its
anti-cancer effects (15), which is
not feasible in a clinical setting. Therefore, this limitation was
addressed by developing two distinct liposomal formulations of
IPA3, one based on the classical sterically stabilized
long-circulating liposomes (SSL) (16), and the other, that incorporates
lipids, which are selectively targeted by secreted phospholipase
A2 (sPLA2), an esterase overexpressed in
several types of cancer, including PCa (17).
SSL-IPA3 are long-circulating liposomes designed for
passive targeting by the enhanced permeability and retention effect
(18–20). The base formulation of these SSLs is
clinically used for the enhanced delivery of doxorubicin for the
treatment of breast cancer (21).
The mechanisms of increased efficiency for SSL stems, in part, from
the presence of the polyethylene glycol (PEG) coating, which
decreases SSL clearance by phagocytes in the reticuloendothelial
system, therefore extending their systemic circulation time and
alters the pharmacokinetics of the encapsulated drug (22). The efficacy of SSL for the treatment
of cancer is further enhanced due to the leaky vasculature of
tumors and the lack of a functional lymphatic system, which
provides access for SSL to enter and accumulate in the tumors by
the enhanced permeability and retention phenomenon (23–26).
Besides, this also provides passive targeting of SSL to the tumors,
as the intact vasculature in normal tissue limits the entry of SSL,
decreasing off-target toxicity (27–29).
sPLA2 responsive liposomes, or SPRL, are the base
formulation of SSL with alterations that include an increase in the
negatively charged glycerophospholipids (22). These alterations ensure that SPRL is
more responsive to cancers, that overexpress SPLA2,
including prostate, breast, gastric, lung, and colon cancers
(30,31). sPLA2 cleave phospholipids
at the sn-2 (a nucleophilic substitution in which the
rate-determining step involves 2 components) bond of the glycerol
backbone releasing fatty acid and lysophospholipids (31–35).
Unlike other PLA2, sPLA2 has a strong
affinity for negatively charged phospholipid head groups, in
particular, phosphatidylserine, phosphatidylglycerol, and
phosphatidylethanolamine (17,36). In
PCa, sPLA2 overexpression was associated with poor
clinical prognosis and 5-year survival rate, and the levels of
sPLA2 in PCa tissues were reportedly 10–20-fold higher
compared with that in normal tissue (31,37–39). Our
previous studies demonstrated the increased ability of SPRL, which
contained doxorubicin, to decrease PCa growth in a mouse xenograft
model (19) and validated its use as
a targeted drug delivery system (17,19).
SPRL increased their affinity to bind to the cell surface-expressed
sPLA2 on the tumor cells, due to the higher
concentrations of phosphatidylethanolamine (PE) in their
formulation (22,31,36,37).
Despite our previous success with SPRL, it has only
been tested in non-metastatic cancer models and has only been
formulated with doxorubicin, a drug that is not commonly used for
the treatment of PCa (19).
Therefore, in the present study, IPA3-encapsulated SSL and SPRL
(SSL-IPA3 and SPRL-IPA3, respectively) were created and their
efficacies in inhibiting the growth of PCa xenografts and PCa
metastasis to the lungs was investigated. The results showed that
both SSL and SPRL had increased efficacy compared with that in free
IPA3, in the treatment of the tumors, which only required
twice-weekly IP injections, as opposed to daily use.
Materials and methods
Cell culture
The human PC-3 (CRL-1435) and murine RM-1 (CRL-3310)
metastatic PCa cells were purchased from ATCC. Cells were grown in
either DMEM high-glucose medium (for PC-3 cells) or RPMI 1640
medium (for RM-1 cells) (Hyclone; GE Healthcare Life Sciences),
supplemented with 10% FBS (R&D Systems, Inc.), 100 U/ml
penicillin, and 100 mg/ml streptomycin (Themo Fisher Scientific,
Inc.) at 37°C in a humidified incubator with 5% CO2.
Cells were passaged when they were 80–90% confluent. All the other
analytical reagents were purchased from Thermo Fisher Scientific,
Inc. unless otherwise stated.
Animals
All the animal procedures were performed according
to the protocol approved by the Institutional Animal Care and Use
Committee at the Charlie Norwood Veterans Affairs Medical Center,
(GA, USA; protocol no. 19-04-114). The protocols were also in
agreement with the Animal Research: Reporting of in vivo
Experiments (ARRIVE) guidelines (40). Briefly, animals were housed 2–4 mice
per cage, in rooms maintained at 65–75°F (~18-23°C), 40–60%
humidity, a 10/14-h light/dark cycle, and ad libitum access
to food and water. Animals were handled as minimally as possible,
with minimal noise levels to avoid any stress. Athymic nude mice
(Harlan Laboratories, Inc.) were maintained in sterile cages (2
mice per cage) in a separate sterile room, with the provision of
sterile food and water. Isoflurane (3–4% in oxygen) was used to
anesthetize mice at the end of the experiment, before euthanasia by
cervical dislocation. A total of 90 male 8–10 week-old mice
weighing between 25–29 g were used for the tumor and metastasis
experiments, with 6 to 11 mice per experiment. Mice were monitored
every day for any potential infections or sickness and weighed
every 2nd day to determine any weight loss beyond 20%. No animals
died before the end of the experiment.
Preparation of liposomal encapsulated
IPA3
SSL-IPA3 liposomes were prepared as described in our
previous study (16), using the thin
lipid hydration method followed by freeze-thaw cycles and a
high-pressure extrusion (19,22).
Briefly, cholesterol (5 µmol/ml), phospholipids, including
1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) (9 µmol/m)
and DSPE-PEG (1 µmol/ml) in chloroform, and IPA3 (4 µmol/ml) in
ethanol were added into a round bottom flask. SPRL-IPA3 was also
prepared similarly using 5 µmol/ml cholesterol, 8 µmol/ml DSPC, 1
µmol/ml
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[poly(ethylene
glycol) 2000] (DSPE-PEG), 1 µmol/ml DSPE (17). The solvents were evaporated under
vacuum in a water bath at 65°C using a rotary evaporator (Buchi
Labortechnik AG). The formed thin film was then hydrated and
suspended in PBS to achieve a final lipid concentration of 10
µmol/ml. The formulation then underwent five liquid nitrogen
freeze-thaw cycles, above the phase transition temperature of the
primary lipid, before passing five times through a Lipex Extruder
(Northern Lipids, Inc.) at 65°C using double-stacked polycarbonate
membranes (80-nm; Suez Water Technologies and Solutions). Excess
unencapsulated IPA3 and lipids were eliminated using dialysis in
10% (w/v) sucrose for at least 20 h, with three changes of the
dialysis media. Liposome suspensions were stored at 4°C, protected
from light, and used within 24–48 h of preparation. Empty SPRL was
also formulated and used as vehicle controls. Quantification of
IPA3 was evaluated using methods previously described (16). The quality control used during the
formulation of SSL and SPRL included the measurement of size, zeta
potential, and drug encapsulation. The size was measured by both
dynamic light scattering and by tandem electron microscopy.
Liposomes that did not meet the minimum required characteristics of
1,000 µM IPA3 encapsulation, a size of 100 nm hydrodynamic
diameter, a poly-dispersity index of <0.3, and charge of at
least-20 mV zeta potential were not used for the experiments.
Mouse genotyping
For the genotyping of Transgenic Adenocarcinoma of
the Mouse Prostate (TRAMP) mice (Jackson Laboratory), DNA was
extracted from the ear punch of 10 to 21-day old litters. Tissues
were incubated with 50 µl of alkaline lysis buffer containing 25 ml
H2O, 62.5 µl of 10 N NaOH, and 10 µl of 0.5 M disodium
EDTA at 95°C for 90 min, followed by neutralization with 50 µl
buffer containing 24 ml H2O, 1 ml 1 M Tris-HCl (41). The TRAMP transgene (600 bp) was
amplified using the following primer sequences: Forward
5′-GCGCTGCTGACTTTCTAAACATAAG-3′ and reverse,
5′-GAGCTCACGTTAAGTTTTGATGTGT-3′, with an annealing temperature of
55°C. GAPDH was measured as an internal positive control using the
following primer seuqneces: Forward, 5′-CTAGGCCACAGAATTGAAAGATCT-3′
and reverse, 5′-GTAGGTGGAAATTCAGCATCATCC-3′. PCR was performed
using the GoTaq® M712C green master mix (Promega
Corporation) with the following thermocycling conditions: Step-1:
95°C for 3 min; Step-2: 94°C for 30 sec; Step-3: 60°C for 1 min;
Step-4: 72°C for 1 min (step-2-4 repeated for 35 cycles); Step-5:
72°C for 2 min and Step-6: Hold at 4°C until further processing.
The products were visualized using ethidium bromide containing
agarose gel (2%) under UV light.
Cell proliferation assay
Cell proliferation following IPA3 treatment was
assessed using an MTT assay (Thermo Fisher Scientific, Inc.), as
previously described (13). Briefly,
cells were seeded into 48-well cell culture plates, at a density of
5×104 cells/ml per well and incubated at 37°C in a
humidified incubator with 5% CO2 for 24 h. Cells were
treated with either 10, 20, or 30 µM IPA3 (cat. no. 3622; Tocris
BioScience) encapsulated in SSL and SPRL liposomes, empty SSL and
SPRL or dimethyl sulfoxide (DMSO) (vehicle) as controls for 24 h.
Following this, MTT reagent was added, at a final concentration of
0.25 mg/ml, and the plates were incubated at 37°C for 2 h. After
incubation, non-reduced MTT and the medium were aspirated and MTT
formazan crystals were dissolved using DMSO. Following an
additional 15 min incubation, with constant shaking
(2.8×10−3 × g using a Vari-Mix Platform Rocker (Thermo
Fisher Scientific, Inc.), plates were read at 590 nm using a Biotek
plate reader (Agilent Technologies, Inc.).
In vivo prostate tumor xenograft
assay
PC-3 cells were grown to 60–70% confluent in T75
flasks. Next, the cells were collected and suspended in sterile
normal saline. Cell suspension (3×106 cells/100 µl) was
subcutaneously injected into the right flank of 6 to 8-week-old
male athymic nude mice (Harlan Laboratories, Inc.). All treatments
(empty liposomes, SSL-IPA3, and SPRL-IPA3 (5 mg/kg) were started on
day 3 from tumor implantation and were administered two times a
week, by intraperitoneal (i.p.) injection. Tumor diameters were
measured using digital calipers on days 7, 14, 19, and 21, and the
tumor volume (mm3) was calculated by the modified
ellipsoidal formula (tumor volume=½ [length × width2]).
The average size of the tumors before treatment was 42
mm3. Mice were sacrificed on day 25 and tumors were
dissected, weighed, and snap-frozen for further analysis. We
included 12 mice in each group at the start of the experiment.
However, one mouse from the empty liposome group, one from the
SSL-IPA3 group and two mice from the SPRL-IPA3 group were later
removed due to sickness.
In vivo mouse lung colonization
(metastasis) assay
PC-3 and RM-1 cells grown to 60–70% confluence in
T75 flasks were washed once with 1X PBS, detached using trypsin,
and re-suspended in 0.9% saline. A total volume of 150 µl cell
suspension, containing 0.5×106 cells was injected
through the tail-vein into 8-week-old C57BL/6 mice, as well as
athymic nude mice. Animals in each group were injected (i.p.) with
either 5 mg/kg SSL-IPA3 or SPRL-IPA3, or vehicle control (PBS or
empty liposomes) twice weekly as previously described (18). Alternately, 27-week old TRAMP mice
were injected with the vehicle (sterile PBS), free IPA3 twice a
week, free IPA3 once daily, or SSL-IPA3 twice a week for 3 weeks.
Mice weight was monitored every 3 days, up to day 21. On day 21,
mice were euthanized, the lungs were collected and snap-frozen or
directly fixed for hematoxylin and eosin (H&E) staining. The
number of lung nodules was counted by three blinded reviewers
(individuals within the laboratory) and the average of their scores
was used for analysis. In the TRAMP mice study, 8 mice were
included in each at the start of the experiment. However, one mouse
from the twice-weekly free IPA3 group and two mice from the once
per day free IPA3 group were removed due to sickness.
Histological examination of the mouse
lungs
Tissue sections were embedded in paraffin and 5 µm
sections were cut for H&E staining. For staining, tissues were
first dehydrated twice with 95% ethanol for 30 min each, followed
by soaking in xylene for 1 h at 60–70°C. The sections were
subsequently dipped in paraffin for 12 h. Tissue sections were
stained with Harris' hematoxylin solution for 6 h at 60–70°C,
rinsed in tap water and immersed in a destaining solution
containing 10% acetic acid and 85% ethanol in water for 2 h and an
additional 10 h at room temparature. Washing slides were soaked in
a saturated lithium carbonate solution for 12 h and rinsed with tap
water. Finally, sections were stained with eosin Y in ethanol for
48 h at room temparature. Imaging was performed using a brightfield
Keyence BZ-X800 microscope (Keyence Corporation; magnification,
×4). Lung micrometastasis was analyzed using ImageJ software
(version 1.48v; National Institutes of Health) (13). Briefly, the H&E images of the
lung were converted to grayscale followed by splitting the image
into RGB channels. The area of individual channels was measured and
subtracted from the total lung area to determine the metastatic
area.
Statistical analysis
Data are presented as the mean ± SD. The ‘n’ value
for each figure indicates the number of samples in each group. MTT
assays were performed 6 times in 3 replicates. All the data were
analyzed using parametric tests, the Student's unpaired t-test for
comparing two groups or one-way ANOVA for comparing more than two
groups, followed by Tukey's post hoc test (with pooled variance)
and the GraphPad Prism v6.01 software (GraphPad, Software, Inc.)
P<0.05 was considered to indicate a statistically significant
difference.
Results
SSL-IPA3 and SPRL-IPA3 suppress the
growth of PC-3 cell tumor xenografts in athymic nude mice with
similar efficacy
Our previous study found that SSL-IPA3 was more
effective in decreasing PC-3 tumor xenograft growth compared with
that for free IPA3 (16); however,
the ability of SPRL-IPA3 to decrease tumor growth in xenograft
models has never been investigated. Twice-weekly administration of
SSL-IPA3 (5 mg/kg) inhibited the growth of PC-3 tumor xenografts
compared with that in the control group (Fig. 1A-C). Furthermore, the same results
were demonstrated with SPRL-IPA3, administrated at the same dose
and schedule (Fig. 1A-C). The
results were found to be significantly different for tumor volume
(Fig. 1D) and weight (Fig. 1E). There was no significant
difference between empty liposomes, SSL-IPA3, and SPRL-IPA3
treatment on the total body weight of mice after 25 days (Fig. 1F). The data revealed that SSL-IPA3
and SPRL-IPA3 are effective in inhibiting the growth of prostate
tumor xenografts.
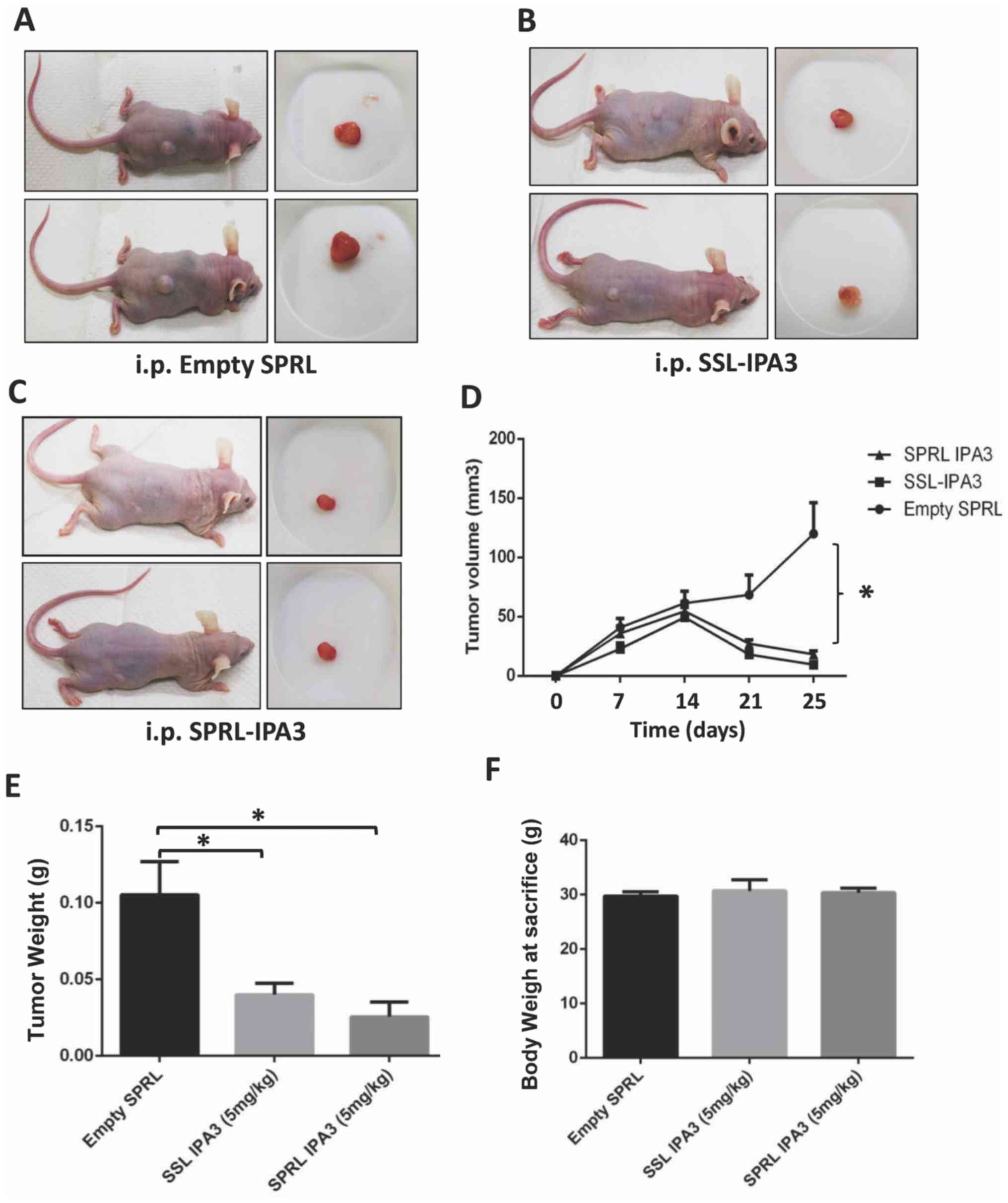 | Figure 1.SSL-IPA3 and SPRL IPA3 significantly
inhibits the growth of PC-3 cell tumor xenografts in athymic nude
mice. Images of athymic nude mice bearing PC-3 cell tumor
xenografts (left) and extracted tumors (right) treated with (A)
empty liposomes, (B) SSL-IPA3, and (C) SPRL-IPA3 (5 mg/kg). (D)
Line graph showing the volume of PC-3 cell tumor xenografts on days
7, 14, 21 and 25, following implantation in athymic nude mice
treated with empty liposomes, SSL-IPA3 and SPRL-IPA3 (5 mg/kg),
respectively. Bar graph showing the (E) weight of the tumors and
(F) body weight of PC-3 cell tumor xenograft athymic nude mice
treated with empty liposomes, SSL-IPA3 and SPRL-IPA3 (5 mg/kg),
respectively on day 25 post-tumor implantation. Data are presented
as the mean ± SD. One-way ANOVA was used to compare when there were
more than two groups. *P<0.001. i.p., intraperitoneal; SRPL,
secreted phospholipase A2 responsive liposomes; SSL,
sterically stabilized long-circulating liposomes; IPA3, P21 (RAC1)
activated kinase-1. Empty liposomes (n=11), SSL-IPA3 group (n=11),
SPRL-IPA3 group (n=10). |
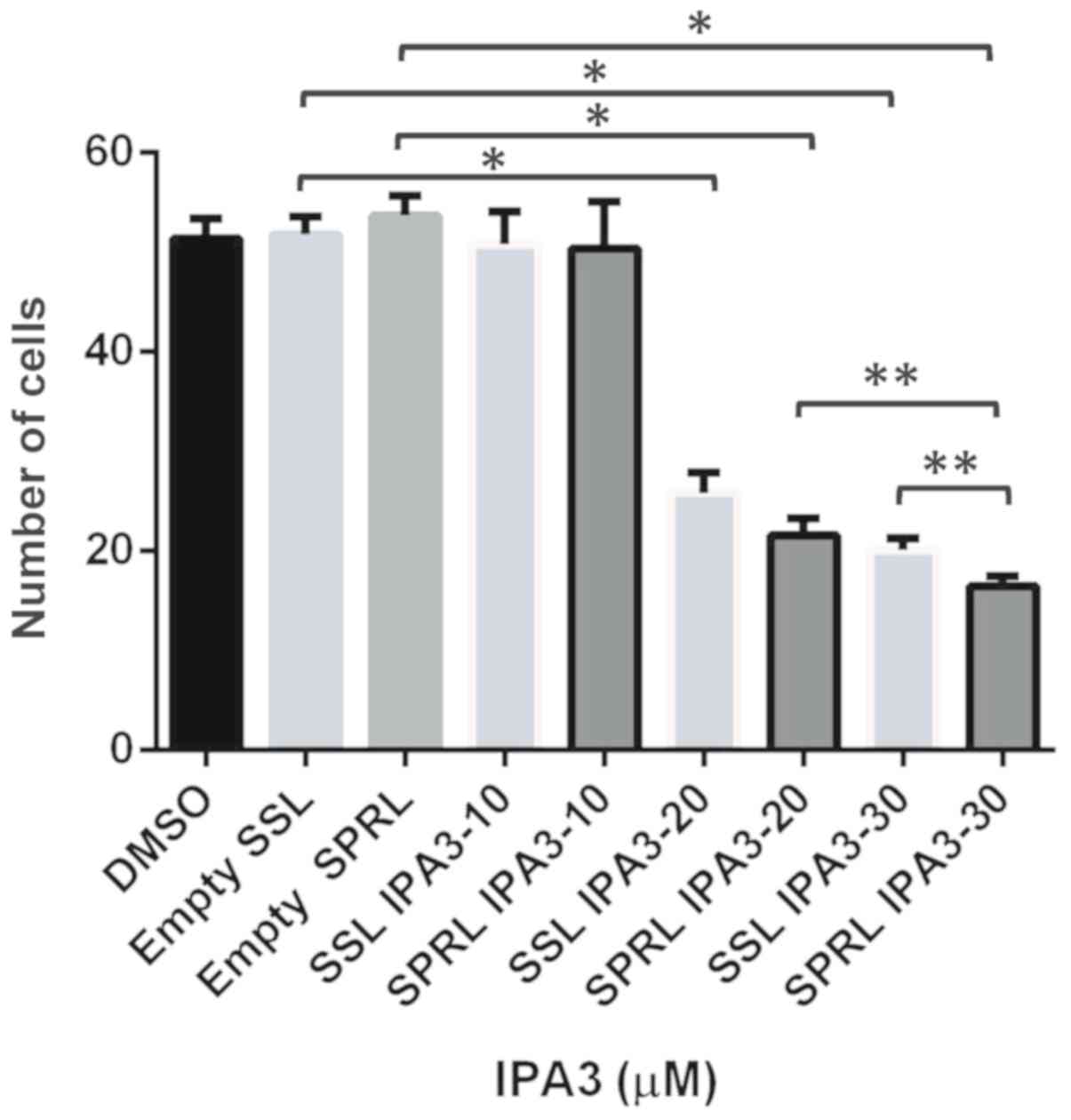
SSL-IPA3 and SPRL-IPA3 inhibit murine
RM-1 PCa cell proliferation in vitro
The lung metastasis model used in the present study
required mouse PCa cells; however, to the best of our knowledge the
effect of SSL- or SPRL-IPA3 to inhibit mouse prostate cell growth
has not been previously investigated. Therefore, the effect of this
formulation on murine prostate RM-1 metastatic PCa cell
proliferation in vitro was performed using the MTT assay. It
was found that 20 and 30 µM doses for both SSL- and SPRL-IPA3
decreased the number of cells compared with that in cells treated
with 10 µM after 24 h (Fig. 2).
There was a significant decrease in cell proliferation with 30 µM
SRPL-IPA3 compared with that in cells treated with 20 µM SPRL-IPA3,
but not between 20 and 30 µM SSL-IPA3. There was also a modest but
significant reduction in RM-1 cell proliferation in cells treated
with 30 µM SPRL-IPA3 compared with that in cells treated with 30 µM
SSL-IPA3. These data suggested that both SSL- and SPRL-IPA3 have
antiproliferative effects on RM-1 cells.
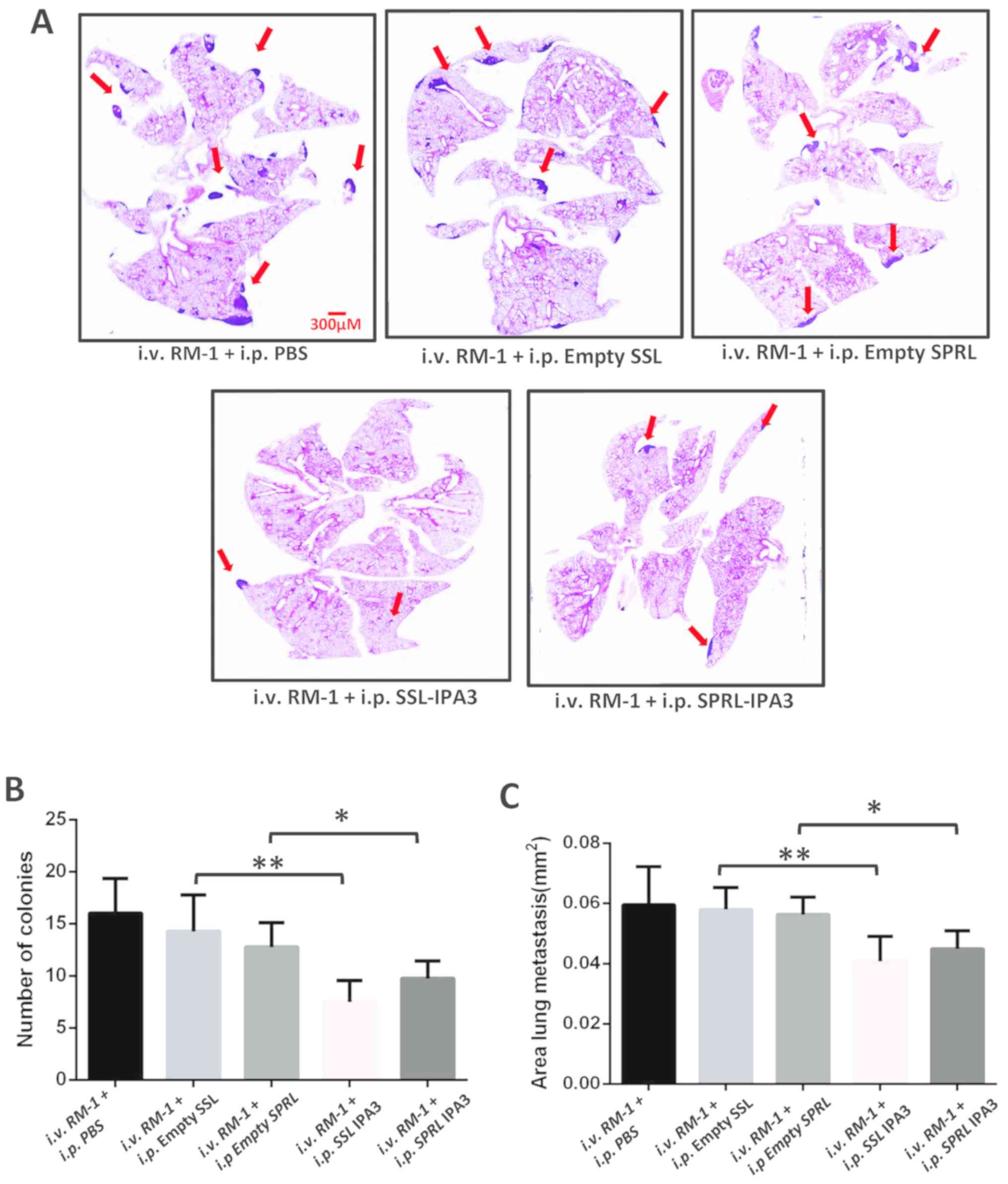
SSL-IPA3 and SPRL-IPA3 inhibit RM-1
cell metastasis to the lungs of mice
To the best of our knowledge, liposomal encapsulated
IPA3, in any formulation, has never been investigated for its
ability to inhibit cancer metastasis. Therefore, the effect of
twice-weekly administration of SSL-IPA3 and SPRL-IPA3 on lung
metastasis in C57BL/6 mice, following their intravenous injection
with RM-1 cells, was determined. Histological examination of the
mouse lungs demonstrated a significant reduction in lung
metastasis, as measured by the number of colonies and areas of lung
metastasis indicated by red arrows with intravenous RM-1 cell
injection compared with that in the vehicle group (Fig. 3A). Both SSL-IPA3 and SPRL-IPA3 (5
mg/kg) significantly reduced the number of lung nodules and
metastatic areas in the mouse lungs compared with that in the empty
liposomes or vehicle control (PBS) groups (Fig. 3B and C). SSL-IPA3 and SPRL-IPA3 were
found to be equally effective in inhibiting PCa cell lung
metastasis in mice.
Twice-weekly administered SSL-IPA3 but
not free IPA3 inhibited lung metastasis in TRAMP mice
To further investigate the ability of SSL- and
SPRL-IPA3 to prevent metastasis the effect of twice-weekly injected
liposomal IPA3 was compared with that in free IPA3, to inhibit
spontaneous lung metastasis, that occurs in TRAMP mice. This mouse
model closely resembles the pathogenesis of human PCa and is known
to spontaneously develop metastasis at the age of 28–30 weeks
(41). TRAMP mice, at 27 weeks of
age were injected twice a week with a vehicle (sterile PBS) or free
IPA3, or once daily with free IPA3, or twice a week with SSL-IPA3
for 3 weeks (until 30 weeks of age). SPRL-IPA3 was not
investigated, as both SSL-IPA3 and SPRL-IPA3 were both efficacious
at reducing tumor growth in the aforementioned experiments.
Spontaneous lung metastasis in the TRAMP (control and treated) mice
were examined using H&E staining of lung sections (Fig. 4A), following 3 weeks of treatments.
Histological examination of the lung sections revealed lung
metastatic nodules in vehicle-treated TRAMP mice. The area of
metastatic nodules was markedly lower in TRAMP mice, administered
daily with free IPA3. Notably, twice-weekly treatment with free
IPA3 (5 mg/kg) in TRAMP mice did not inhibit PCa lung metastasis in
TRAMP mice (Fig. 4A and B). As
expected, SSL-IPA3 (5 mg/kg) administration, twice per week for 3
weeks significantly inhibited PCa lung metastasis in TRAMP mice
(Fig. 4A and B). These results
indicated that the administration of liposome-encapsulated IPA3
twice weekly for 3 weeks could significantly decrease lung
metastasis in vivo and could be developed into a future
therapeutic approach to treat metastatic PCa in humans.
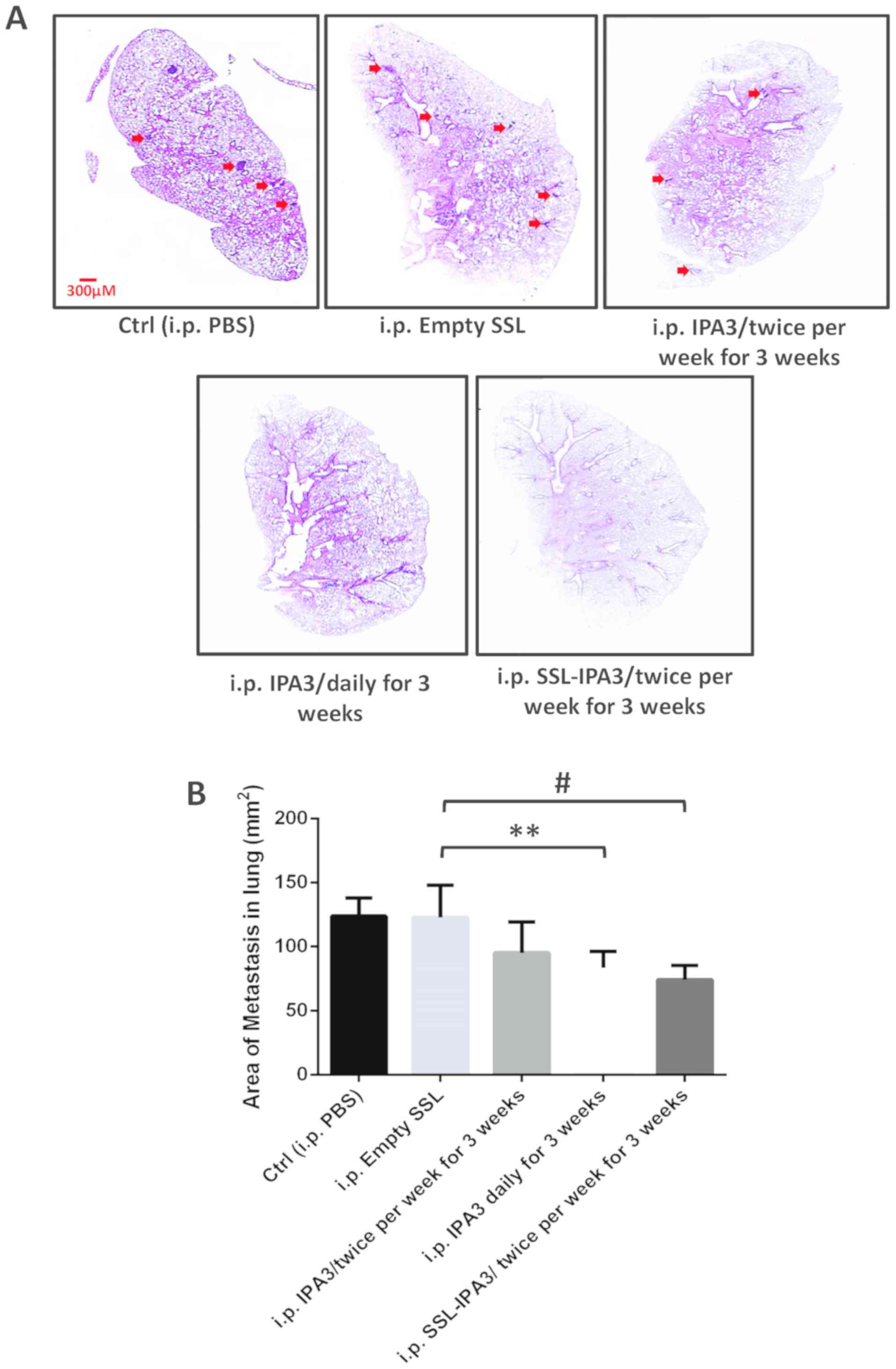 | Figure 4.SSL-IPA3 inhibits lung metastasis in
TRAMP mice. TRAMP mice, at 27 weeks of age were injected with
vehicle, empty SSL, and SSL-IPA3 (5 mg/kg) twice weekly and
compared with free IPA3 administered daily or twice weekly for 3
weeks, for lung colonization. (A) Images of H&E-stained TRAMP
lung sections showing changes in lung metastasis between the
control, free IPA3, and SSL-IPA3-treated groups for 3 weeks. The
arrows indicate the area of metastasis. (B) Bar graph indicating
reduced lung metastasis in daily administered free IPA3 and
twice-weekly administered SSL-IPA3 groups compared with that in the
control groups and twice-weekly administered free IPA3 group. Data
are presented as the mean ± SD. An unpaired Student's t-test was
used to compare between two groups and one-way ANOVA for more than
two groups. **P<0.01; #P<0.001. TRAMP, Transgenic
Adenocarcinoma of the Mouse Prostate; PBS, vehicle group; i.p.,
intraperitoneal; SRPL, secreted phospholipase A2
responsive liposomes; SSL, sterically stabilized long-circulating
liposomes; IPA3, P21 (RAC1) activated kinase-1; Ctrl, control.
Control, Empty SSL (n=8), twice-weekly administered free IPA3
(n=7), daily administered free IPA3 for 3 weeks (n=6), twice-weekly
administered SSL-IPA3 for 3 weeks (n=8). |
Discussion
Despite the FDA approval of three new drugs,
abiraterone acetate, enzalutamide, and radium-223, for front-line
use in men with metastatic castration-resistant PCa in the past 5
years, the life expectancy for patients with PCa, over the past
decade has only been prolonged by one year (42,43). As
a result, patients with metastatic PCa can succumb to the disease,
which accounts for the second leading cause of cancer-associated
death in men in the US as of 2020 (1,2). Thus,
there is an urgent requirement for novel and effective therapeutic
strategies for the treatment of metastatic PCa. The higher
expression level of PAK1 protein has been found in human PCa
tissues and PCa metastasized lung lesions in patients compared with
that in the normal tissues from healthy controls (9). Our previous studies have demonstrated
that PAK1 was essential for PCa growth, epithelial-to-mesenchymal
transition and metastasis, and that the PAK1 allosteric inhibitor,
IPA3, was a potential drug to treat metastatic PCa (6,8–10,12,13,16).
These findings suggested that PAK1 could be a potential therapeutic
target to prevent PCa growth and metastasis in humans.
Several PAK inhibitors have been studied for their
anti-cancer efficacies in vitro and in vivo (10,14,15,44,45).
Several PAK1 inhibitors, such as G-5555 (46), FL172 (47), PF-3758309 (48), and AZ13705339 (49) have demonstrated PAK1 activity
suppression in vitro; however, these compounds also
exhibited off-target effects on several other kinases, such as the
Src family of kinases, Akt1, AMP Kinases, Cyclin-dependent kinase-7
and serum glucocorticoid kinase, due to their competition for the
ATP-binding site, that is conserved in several kinases (44,45).
Among these, PF-3758309 and PF-03758309 are specific inhibitors of
PAK4, a group II PAK isoform (50),
and a clinical trial on PF-03758309 for advanced solid tumors
(NCT00932126) was terminated, due to the undesirable
pharmacokinetic characteristics, such as unfavorable levels of the
drug in the plasma and the lack of a dose-response association.
Therefore, the allosteric PAK1 inhibitors, such as IPA3 (15) and NVS-PAK1-1 (51) were preferred for pharmacological
interventions in cancer research. Our previous studies have
demonstrated that IPA3, suppresses epithelial-to-mesenchymal
transition, and micro-invasion of PCa cells in vitro and
tumor growth in vivo (6,9). Daily
administration of IPA3 also attenuated PCa-induced metastasis and
bone remodeling in athymic nude mice (13). Unfortunately, IPA3 in its free form
can be rapidly metabolized in the plasma and has a very short
half-life (45), which limits its
use as a therapeutic agent for cancer. Therefore, it is not
feasible to treat patients with cancer in the clinic daily. To
overcome this, two new liposome formulations were utilized, with
distinct lipid composition to encapsulate IPA3 into nanoliposomes
to serve as a reservoir for its slow release thus, increasing the
half-life of the drug in the plasma. Due to the different lipid
composition, while both SSL-IPA3 and SPRL-IPA3 have the benefit of
high stability and long half-life once in the blood, SPRL-IPA3 has
the specificity to respond to the highly expressed sPLA2
in the tumor microenvironment (12,17,36).
In the present study, loading IPA3 into
nanoliposomes reduced the frequency of drug administration without
compromising its efficacy. The efficacy of SSL-IPA3 or SPRL-IPA3,
when administered twice a week, was comparable to the efficacy of
daily administration of free IPA3 in preventing PCa lung metastasis
in 2 different mouse models. A total of 30 µM SPRL-IPA3 exhibited
slightly higher efficacy on reducing cell survival compared with
that in cells treated with a similar dose of SSL-IPA3; however, the
efficacies of either of IPA3-containing liposomes were similar even
in inhibiting the growth of human PCa tumor xenografts implanted in
immunocompromised mice. This frequency of drug administration was
not effective with the free form of IPA3 to prevent PCa growth, as
evidenced in our previous study (16). The advantage of liposomal IPA3 was
also clear from the observation that twice a week administration of
free IPA3 did not prevent PCa metastasis to the mouse lungs thus,
presenting the liposomal-IPA3 approach as a reliable method to
treat metastatic PCa.
Several laboratories have utilized nanoparticles for
targeted drug delivery in various types of cancer, including
breast, lung, colon, and gastric cancers, which is similar to our
approach, where these nanoparticles were enriched in tumors thus,
delivering small molecule inhibitors specifically at the tumor site
(52–56). To the best of our knowledge, these
data are the first to demonstrate the ability of liposomal
formulations of IPA3 to inhibit the lung metastasis of PCa. This
was observed in two different models of PCa metastasis,
representing two different strains of mice, which supports the
rigor of our findings. Since PAK1 hyperactivation has also been
reported in other diseases, such as osteoarthritis (57), neurodevelopmental disorders (58), macrocephaly (59), intellectual disability (60), and kidney injury (61), liposomal IPA3 could have potential
therapeutic applications for several non-cancer human diseases.
The results from the present study show that the
efficacies of SSL-IPA3 and SPRL-IPA3 were very similar. The exact
reason for this is currently unknown, and requires further
investigation; however, it could be due to the specific advantage
of each of these formulations; SSL was highly stable and
long-acting, while SPRL had additional specificity in targeting the
tumor tissues. Another possibility is that both liposomes release
their contents before reaching the tumor tissue, but are still able
to maintain a constant level of IPA3 in the plasma. Furthermore, a
previous study demonstrated the increased efficacy of SPRL over SSL
using doxorubicin as the payload (19). This suggested that the differential
efficacy of these liposomes may be drug-dependent. It is also
important to note that SPRL was designed and validated against
human Group IIA sPLA2, as opposed to the mouse
sPLA2 isoform. Thus, a potential difference in the
activity of group IIA sPLA2 in mice compared with humans
may have accounted for SPRL-IPA3 not having a superior efficacy
over SSL-IPA3. Additional investigations are required to validate
this hypothesis. However, these data still provide strong evidence
that PCa growth and metastasis could be targeted effectively by the
encapsulation of IPA3, in two different lipid-based nanoparticles.
This treatment strategy would allow for the administration of the
drug less frequently and improve drug efficacy while minimizing its
side-effects.
Acknowledgements
Not applicable.
Funding
The work was primarily funded by the Department of
Defense Prostate Cancer Research Program Idea Development Award
(grant no. PC150431 GRANT11996600). Partial financial support was
also provided by the National Heart Lung and Blood Institute (grant
no. R01HL103952), National Center for Advancing Translational
Sciences (grant no. UL1TR002378), Wilson Pharmacy Foundation
(intramural), and Translational Research Initiative grant
(intramural). This work has been accomplished using the resources
and facilities at the VA Medical Center in Augusta, GA (USA).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Author's contributions
AV, WNM, BSC, and PRS contributed to the design and
conception of the study and acquired, analyzed, and/or interpreted
the data. AV, BSC, WNM, and PRS drafted the initial manuscript. All
authors approved the final version of the manuscript.
Ethics approval and consent to
participate
Ethics approval was granted by the Institutional
Animal Care and Use Committee at the Charlie Norwood Veterans
Affairs Medical Center (Georgia, USA; approval no. ACORP
#19-04-114).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
American Cancer Society. Cancer Facts and
Figures 2020. Atlanta: American Cancer Society;
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tan GH, Nason G, Ajib K, Woon DT,
Herrera-Caceres J, Alhunaidi O and Perlis N: Smarter screening for
prostate cancer. World J Urol. 37:991–999. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Malvezzi M, Carioli G, Bertuccio P,
Boffetta P, Levi F, La Vecchia C and Negri E: European cancer
mortality predictions for the year 2018 with focus on colorectal
cancer. Ann Oncol. 29:1016–1022. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Al-Azayzih A, Gao F and Somanath PR: P21
activated kinase-1 mediates transforming growth factor β1-induced
prostate cancer cell epithelial to mesenchymal transition. Biochim
Biophys Acta. 1853:1229–1239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Al-Maghrabi J, Emam E, Gomaa W, Al-Qaydy
D, Al-Maghrabi B, Buhmeida A, Abuzenadah A, Al-Qahtani M and
Al-Ahwal M: Overexpression of PAK-1 is an independent predictor of
disease recurrence in colorectal carcinoma. Int J Clin Exp Pathol.
8:15895–15902. 2015.PubMed/NCBI
|
|
8
|
Goc A, Abdalla M, Al-Azayzih A and
Somanath PR: Rac1 activation driven by 14-3-3ζ dimerization
promotes prostate cancer cell-matrix interactions, motility and
transendothelial migration. PLoS One. 7:e405942012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goc A, Al-Azayzih A, Abdalla M, Al-Husein
B, Kavuri S, Lee J, Moses K and Somanath PR: P21 activated kinase-1
(Pak1) promotes prostate tumor growth and microinvasion via
inhibition of transforming growth factor β expression and enhanced
matrix metalloproteinase 9 secretion. J Biol Chem. 288:3025–3035.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kichina JV, Goc A, Al-Husein B, Somanath
PR and Kandel ES: PAK1 as a therapeutic target. Expert Opin Ther
Targets. 14:703–725. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park J, Kim JM, Park JK, Huang S, Kwak SY,
Ryu KA, Kong G, Park J and Koo BS: Association of p21-activated
kinase-1 activity with aggressive tumor behavior and poor prognosis
of head and neck cancer. Head Neck. 37:953–963. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Najahi-Missaoui W, Quach ND, Jenkins A,
Dabke I, Somanath PR and Cummings BS: Effect of P21-activated
kinase 1 (PAK-1) inhibition on cancer cell growth, migration, and
invasion. Pharmacol Res Perspect. 7:e005182019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Verma A, Artham S, Alwhaibi A, Adil MS,
Cummings BS and Somanath PR: PAK1 inhibitor IPA-3 mitigates
metastatic prostate cancer-induced bone remodeling. Biochem
Pharmacol. 177:1139432020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ong CC, Gierke S, Pitt C, Sagolla M, Cheng
CK, Zhou W, Jubb AM, Strickland L, Schmidt M, Duron SG, et al:
Small molecule inhibition of group I p21-activated kinases in
breast cancer induces apoptosis and potentiates the activity of
microtubule stabilizing agents. Breast Cancer Res. 17:592015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deacon SW, Beeser A, Fukui JA, Rennefahrt
UE, Myers C, Chernoff J and Peterson JR: An isoform-selective,
small-molecule inhibitor targets the autoregulatory mechanism of
p21-activated kinase. Chem Biol. 15:322–331. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Al-Azayzih A, Missaoui WN, Cummings BS and
Somanath PR: Liposome-mediated delivery of the p21 activated
kinase-1 (PAK-1) inhibitor IPA-3 limits prostate tumor growth in
vivo. Nanomedicine. 12:1231–1239. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Quach ND, Mock JN, Scholpa NE, Eggert MW,
Payré C, Lambeau G, Arnold RD and Cummings BS: Role of the
phospholipase A2 receptor in liposome drug delivery in
prostate cancer cells. Mol Pharm. 11:3443–3451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Adamina M, Bolli M, Albo F, Cavazza A,
Zajac P, Padovan E, Schumacher R, Reschner A, Feder C, Marti WR, et
al: Encapsulation into sterically stabilised liposomes enhances the
immunogenicity of melanoma-associated Melan-A/MART-1 epitopes. Br J
Cancer. 90:263–269. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mock JN, Costyn LJ, Wilding SL, Arnold RD
and Cummings BS: Evidence for distinct mechanisms of uptake and
antitumor activity of secretory phospholipase A2
responsive liposome in prostate cancer. Integr Biol (Camb).
5:172–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Woodle MC and Lasic DD: Sterically
stabilized liposomes. Biochim Biophys Acta. 1113:171–199. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou R, Mazurchuk R and Straubinger RM:
Antivasculature effects of doxorubicin-containing liposomes in an
intracranial rat brain tumor model. Cancer Res. 62:2561–2566.
2002.PubMed/NCBI
|
|
22
|
Zhu G, Mock JN, Aljuffali I, Cummings BS
and Arnold RD: Secretory phospholipase A2 responsive
liposomes. J Pharm Sci. 100:3146–3159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fenske DB and Cullis PR: Liposomal
nanomedicines. Expert Opin Drug Deliv. 5:25–44. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sakakibara T, Chen FA, Kida H, Kunieda K,
Cuenca RE, Martin FJ and Bankert RB: Doxorubicin encapsulated in
sterically stabilized liposomes is superior to free drug or
drug-containing conventional liposomes at suppressing growth and
metastases of human lung tumor xenografts. Cancer Res.
56:3743–3746. 1996.PubMed/NCBI
|
|
25
|
Maruyama K: Intracellular targeting
delivery of liposomal drugs to solid tumors based on EPR effects.
Adv Drug Deliv Rev. 63:161–169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fang J, Nakamura H and Maeda H: The EPR
effect: Unique features of tumor blood vessels for drug delivery,
factors involved, and limitations and augmentation of the effect.
Adv Drug Deliv Rev. 63:136–151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deshpande PP, Biswas S and Torchilin VP:
Current trends in the use of liposomes for tumor targeting.
Nanomedicine (Lond). 8:1509–1528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sawant RR and Torchilin VP: Challenges in
development of targeted liposomal therapeutics. AAPS J. 14:303–315.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shuler TE, Riddle CD Jr and Potts DW:
Polymicrobic septic arthritis caused by Kingella kingae and
enterococcus. Orthopedics. 13:254–256. 1990.PubMed/NCBI
|
|
30
|
Yamashita S, Ogawa M, Sakamoto K, Abe T,
Arakawa H and Yamashita J: Elevation of serum group II
phospholipase A2 levels in patients with advanced
cancer. Clin Chim Acta. 228:91–99. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dong Q, Patel M, Scott KF, Graham GG,
Russell PJ and Sved P: Oncogenic action of phospholipase
A2 in prostate cancer. Cancer Lett. 240:9–16. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Belinsky GS, Rajan TV, Saria EA, Giardina
C and Rosenberg DW: Expression of secretory phospholipase
A2 in colon tumor cells potentiates tumor growth. Mol
Carcinog. 46:106–116. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang M, Hao FY, Wang JG and Xiao W: Group
IIa secretory phospholipase A2 (sPLA2IIa) and
progression in patients with lung cancer. Eur Rev Med Pharmacol
Sci. 18:2648–2654. 2014.PubMed/NCBI
|
|
34
|
Yamashita S, Yamashita J and Ogawa M:
Overexpression of group II phospholipase A2 in human
breast cancer tissues is closely associated with their malignant
potency. Br J Cancer. 69:1166–1170. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang C, Yu H, Xu H and Yang L: Expression
of secreted phospholipase A2-Group IIA correlates with
prognosis of gastric adenocarcinoma. Oncol Lett. 10:3050–3058.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Quach ND, Arnold RD and Cummings BS:
Secretory phospholipase A2 enzymes as pharmacological
targets for treatment of disease. Biochem Pharmacol. 90:338–348.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dong Z, Liu Y, Scott KF, Levin L, Gaitonde
K, Bracken RB, Burke B, Zhai QJ, Wang J, Oleksowicz L and Lu S:
Secretory phospholipase A2-IIa is involved in prostate
cancer progression and may potentially serve as a biomarker for
prostate cancer. Carcinogenesis. 31:1948–1955. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiang J, Neubauer BL, Graff JR, Chedid M,
Thomas JE, Roehm NW, Zhang S, Eckert GJ, Koch MO, Eble JN and Cheng
L: Expression of group IIA secretory phospholipase A2 is
elevated in prostatic intraepithelial neoplasia and adenocarcinoma.
Am J Pathol. 160:667–671. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lu S and Dong Z: Overexpression of
secretory phospholipase A2-IIa supports cancer stem cell
phenotype via HER/ERBB-elicited signaling in lung and prostate
cancer cells. Int J Oncol. 50:2113–2122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kilkenny C, Browne W, Cuthill IC, Emerson
M and Altman DG; NC3Rs Reporting Guidelines Working Group, : Animal
research: Reporting in vivo experiments: The ARRIVE guidelines. Br
J Pharmacol. 160:1577–1579. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gao F, Alwhaibi A, Sabbineni H, Verma A,
Eldahshan W and Somanath PR: Suppression of Akt1-β-catenin pathway
in advanced prostate cancer promotes TGFβ1-mediated epithelial to
mesenchymal transition and metastasis. Cancer Lett. 402:177–189.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sonnenburg DW and Morgans AK: Emerging
therapies in metastatic prostate cancer. Curr Oncol Rep. 20:462018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Teo MY, Rathkopf DE and Kantoff P:
Treatment of advanced prostate cancer. Annu Rev Med. 70:479–499.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Semenova G and Chernoff J: Targeting PAK1.
Biochem Soc Trans. 45:79–88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rudolph J, Crawford JJ, Hoeflich KP and
Wang W: Inhibitors of p21-activated kinases (PAKs). J Med Chem.
58:111–129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ndubaku CO, Crawford JJ, Drobnick J,
Aliagas I, Campbell D, Dong P, Dornan LM, Duron S, Epler J, Gazzard
L, et al: Design of Selective PAK1 Inhibitor G-5555: Improving
Properties by Employing an Unorthodox Low-pK a Polar Moiety. ACS
Med Chem Lett. 6:1241–1246. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Maksimoska J, Feng L, Harms K, Yi C,
Kissil J, Marmorstein R and Meggers E: Targeting large kinase
active site with rigid, bulky octahedral ruthenium complexes. J Am
Chem Soc. 130:15764–15765. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Murray BW, Guo C, Piraino J, Westwick JK,
Zhang C, Lamerdin J, Dagostino E, Knighton D, Loi CM, Zager M, et
al: Small-molecule p21-activated kinase inhibitor PF-3758309 is a
potent inhibitor of oncogenic signaling and tumor growth. Proc Natl
Acad Sci USA. 107:9446–9451. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
McCoull W, Hennessy EJ, Blades K, Chuaqui
C, Dowling JE, Ferguson AD, Goldberg FW, Howe N, Jones CR, Kemmitt
PD, et al: Optimization of highly kinase selective bis-anilino
pyrimidine PAK1 Inhibitors. ACS Med Chem Lett. 7:1118–1123. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shao YG, Ning K and Li F: Group II
p21-activated kinases as therapeutic targets in gastrointestinal
cancer. World J Gastroenterol. 22:1224–1235. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Karpov AS, Amiri P, Bellamacina C,
Bellance MH, Breitenstein W, Daniel D, Denay R, Fabbro D, Fernandez
C, Galuba I, et al: Optimization of a dibenzodiazepine hit to a
potent and selective allosteric PAK1 inhibitor. ACS Med Chem Lett.
6:776–781. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hu Q, Shang L, Wang M, Tu K, Hu M, Yu Y,
Xu M, Kong L, Guo Y and Zhang Z: Co-Delivery of paclitaxel and
interleukin-12 regulating tumor microenvironment for cancer
immunochemotherapy. Adv Healthc Mater. 9:19018582020. View Article : Google Scholar
|
|
53
|
Unal O, Akkoc Y, Kocak M, Nalbat E,
Dogan-Ekici AI, Yagci Acar H and Gozuacik D: Treatment of breast
cancer with autophagy inhibitory microRNAs carried by
AGO2-conjugated nanoparticles. J Nanobiotechnology. 18:652020.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhao H, Mu X, Zhang X and You Q: Lung
cancer inhibition by betulinic acid nanoparticles via adenosine
5′-Triphosphate (ATP)-binding cassette transporter G1 gene
downregulation. Med Sci Monit. 26:e9220922020.PubMed/NCBI
|
|
55
|
Azimee S, Rahmati M, Fahimi H and Moosavi
MA: TiO2 nanoparticles enhance the chemotherapeutic effects of
5-fluorouracil in human AGS gastric cancer cells via autophagy
blockade. Life Sci. 248:1174662020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tan L, Han S, Ding S, Xiao W, Ding Y, Qian
L, Wang C and Gong W: Chitosan nanoparticle-based delivery of fused
NKG2D-IL-21 gene suppresses colon cancer growth in mice. Int J
Nanomedicine. 12:3095–3107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ma W, Wang X, Wang C, Gong M and Ren P:
Up-regulation of P21-activated kinase 1 in osteoarthritis
chondrocytes is responsible for osteoarthritic cartilage
destruction. Biosci Rep. 40:BSR201910172020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ohori S, Mitsuhashi S, Ben-Haim R, Heyman
E, Sengoku T, Ogata K and Matsumoto N: A novel PAK1 variant
causative of neurodevelopmental disorder with postnatal
macrocephaly. J Hum Genet. 65:481–485. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Horn S, Au M, Basel-Salmon L,
Bayrak-Toydemir P, Chapin A, Cohen L, Elting MW, Graham JM,
Gonzaga-Jauregui C, Konen O, et al: De novo variants in PAK1 lead
to intellectual disability with macrocephaly and seizures. Brain.
142:3351–3359. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kernohan KD, McBride A, Hartley T, Rojas
SK; Care4Rare Canada Consortium, ; Dyment DA, Boycott KM and Dyack
S: p21 protein-activated kinase 1 is associated with severe
regressive autism, and epilepsy. Clin Genet. 96:449–455. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zynda ER, Maloy MH and Kandel ES: The role
of PAK1 in the sensitivity of kidney epithelial cells to
ischemia-like conditions. Cell Cycle. 18:596–604. 2019. View Article : Google Scholar : PubMed/NCBI
|