Introduction
The hypercoagulability state is a common condition
in patients with cancer, contributing to tumor-related morbidity
and mortality (1), or even revealing
silent malignancies (2). A close
association between cancer and thrombophilic states exists, both
physio-pathologically and clinically (1,3–5). Indeed, it is known that an imbalance
between coagulation and fibrinolytic system happens in malignancy,
because the cancer cells are able to produce and release
procoagulant factors, such as tissue factors, cancer procoagulants,
fibrinolityc protein (urokinase and tissue plasminogen activator),
citokines (including tumor necrosis factor-α and interleurk-1β) and
antiangiogenetic molecules (1,3–5). Moreover, cancer cells interact with
host vascular and blood cells, including endothelial cells,
leukocytes and platelets, and these mechanisms lead to signalling
cascades resulting in coagulation activation and thrombi formation
(1).
Thromboembolic events can affect the venous or/and
the arterious circulation (3).
Venous thromboembolism is found in 10–20% of patients with cancer
and the incidence is 4–7 times higher compared with patients
without cancer. Deep-vein-thrombosis (DVT) is the most common
manifestation (6). The incidence of
arterial thromboembolism ranges from 1 to 4.7% and non-bacterial
thrombotic endocarditis (NBTE) is a rare manifestation (7). The incidence of NBTE is unknown and
often is a post-mortem finding, occurring in 1.6% in the adult
autopsies (6). Several factors are
associated with increased risk of arterial and venous
thromboembolic events, including cancer-associated factors,
patients-associated factors and treatment-associated factors
(3,7). Among cancer-associated factors, lung,
gastric and pancreatic cancer, especially in advanced stages, are
associated with a higher risk of thromboembolic events (3,8–10). Patient-associated factors
contributing to higher risk include older and female patients with
low performance status and history of viral or bacterial infection,
chronic kidney disease, pulmonary disease and obesity (3,7).
Meanwhile, treatment factors associated with increased risk of
thrombi formation include chemotherapy and antiangiogenetic drugs,
hormonal therapy, prolonged hospitalization or central venous
catheters (7).
The treatment depends on the severity of thrombotic
event; a massive pulmonary embolism could be fatal and requires
early hospitalization, whereas mild to moderate cases are treated
with anticoagulant therapy, namely unfranctioned heparins
(fondaparinux) or low molecular weight heparin (7). Recently, the Hokusai VTE Cancer Trial
demonstrated that non-inferiority of Edoxaban, an oral factor Xa
inhibitor, for at least 6 months and up to 12 months compared to
subcutaneous deltaparin in cancer patients with recurrent venous
thromboembolism (11). Thus,
Edoxaban could be a valid alternative to unfranctioned heparins
(fondaparinux) or low molecular weight heparin.
The present study reports a case of a patient
presenting with a newly diagnosed locally advanced non-small cell
lung cancer (NSCLC) who developed widespread thromboembolisms that
prevented any chance of local or systemic therapy. An underlying
NBTE, previously misdiagnosed as a patent foramen ovale (PFO), was
deemed responsible for the systemic hypercoagulability state.
Case report
Onset of embolic events
At December 12, 2018, a 63-year-old male with a
history of smoking (20 packs/year) and lacking significant
comorbidities developed DVT in the left leg and started rivaroxaban
anticoagulant therapy at a dose of 10 mg/daily. On 24th December
2018, the patient was hospitalized in an Italian communitry
hospital due to the abrupt onset of dyspnea. A thoracic CT scan
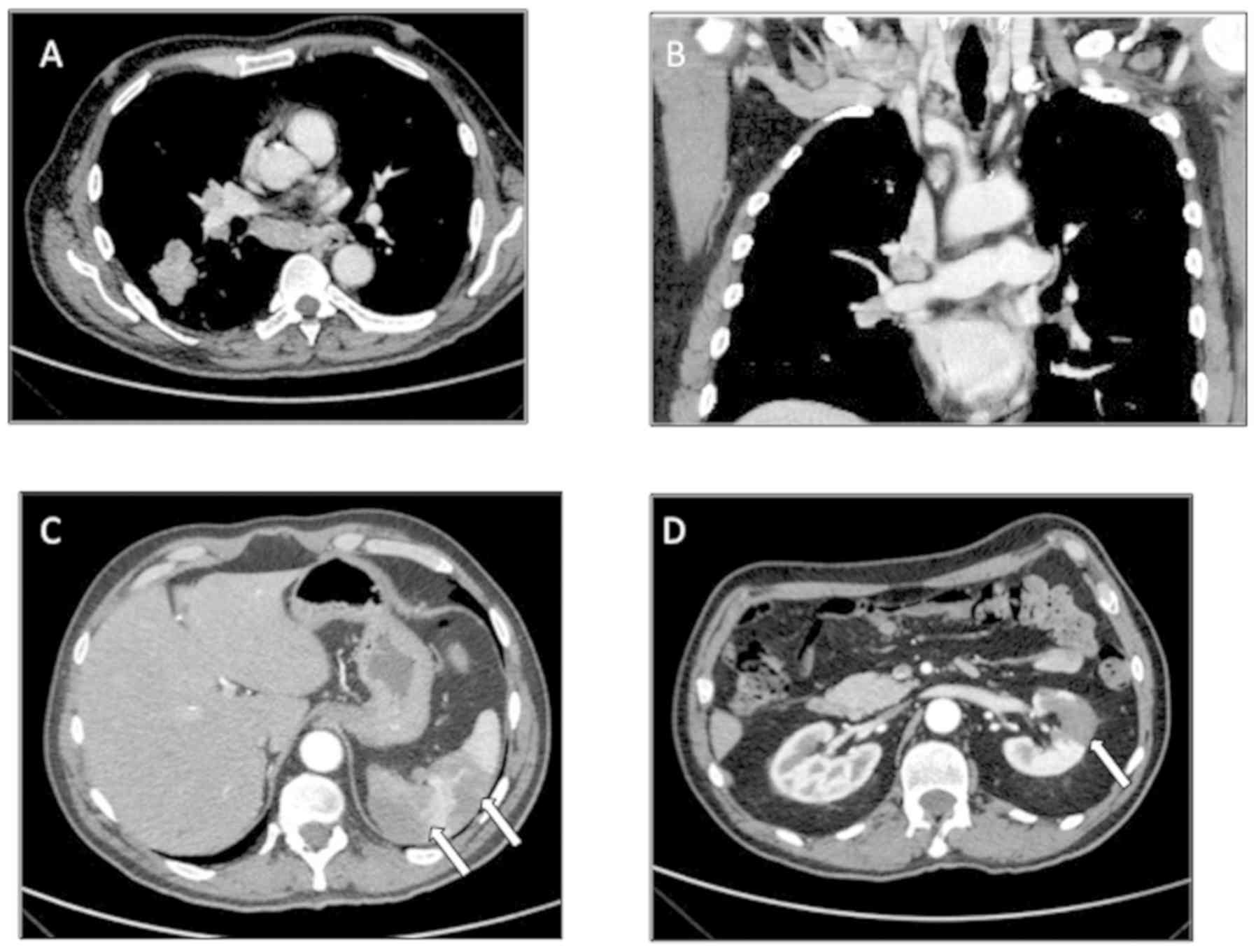
confirmed pumlonary thromboembolism (PTE), documented as a mass in
the lower lobe of the right lung, with ipsilateral ilo-mediastinal
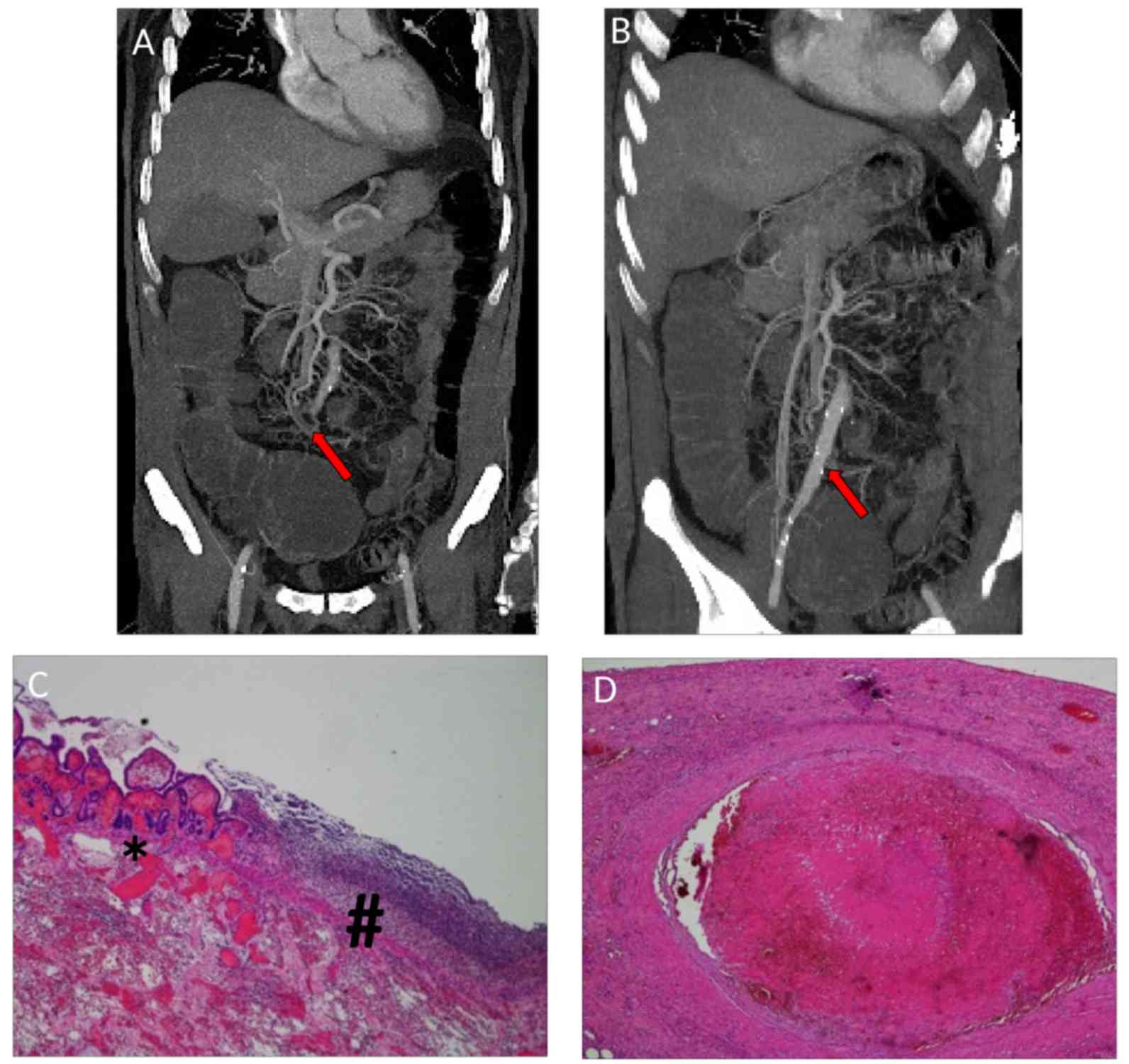
and infra-clavear pathological lymph nodes (Fig. 1A and B), evocative of lung cancer
with lymph nodal spreading. In addition, an abdominal CT scan
demonstrated signs of splenic and renal infarctions (Fig. 1C and D).
A total of 5 days later, the patient developed right
cerebellar signs associated with confusion, including nystagmus,
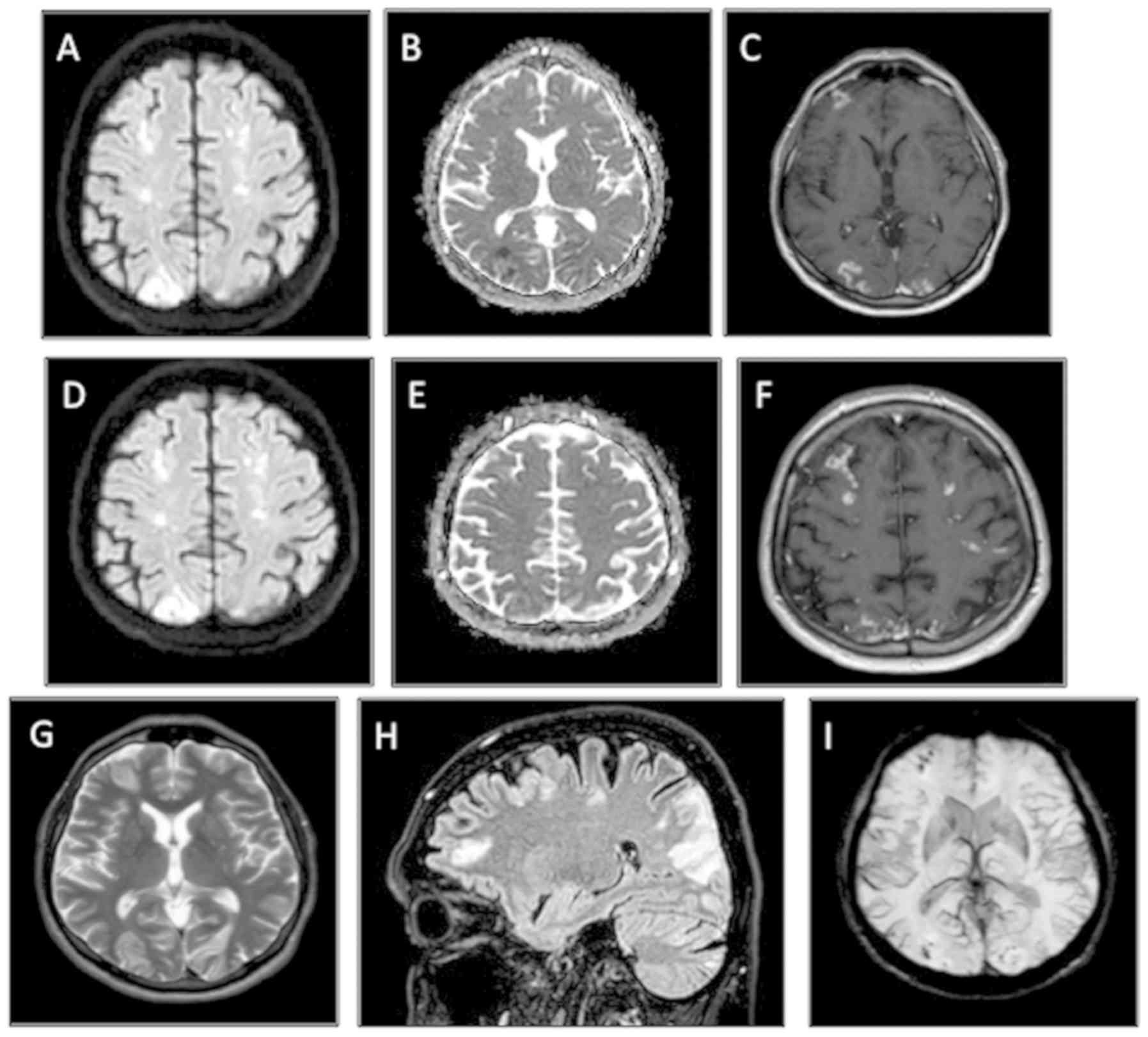
ataxia, dysmetria and adiadochokinesia. An MRI of the brain
revealed multiple diffuse bi-hemispheric cerebral and cerebellar
areas, both in the grey matter (especially of the occipital lobes)
and in the deep cerebral white matter, hyperintense in
fluid-attenuated inversion recovery/fast spin echo sequences when
using enhanced contrast (Fig. 2A-I).
These lesions were characterized by diffusion restriction and
demonstrated moderate patchy and gyriform enhancement. Given the
recent medical history of the patient, differential diagnoses were
hypothesized, including ischemic lesions on an embolic basis and
miliary brain metastases, although this was less likely the
radiology picture. Transthoracic echocardiogram (TTE) did not show
any valvular lesion or other anatomical defects. Because of the
critical patient condition, a transesophageal echocardiogram (TEE)
was not performed. In the following days, the patient experienced
spontaneous rapid improvement of the neurological state with
complete resolution of confusion and cerebellar symptoms.
In view of the neuro-radiological imaging and the
positive evolution of neurological condition, a cardio embolic
origin of the brain lesions was suspected. A TEE was carried out
and revealed a PFO that was assumed to be the cause of the
paradoxical arterial embolisms. No surgical correction of PFO was
envisaged by heart-surgeon, so, at January 10, 2019, the patient
was discharged and prescribed subcutaneous low-molecular-weight
heparin (LMWH) at dose of 6,000 IU twice daily.
Lung cancer diagnosis and staging
Cancer staging was completed using a PET scan, which
confirmed the locally advanced extension of the disease (IIIB stage
according to Tumor-Node-Metastasis staging system) (12) without distant metastases. An
endobronchial ultrasound (EBUS)-guided transbronchial needle
aspiration was performed, allowing the diagnosis of a large-cell
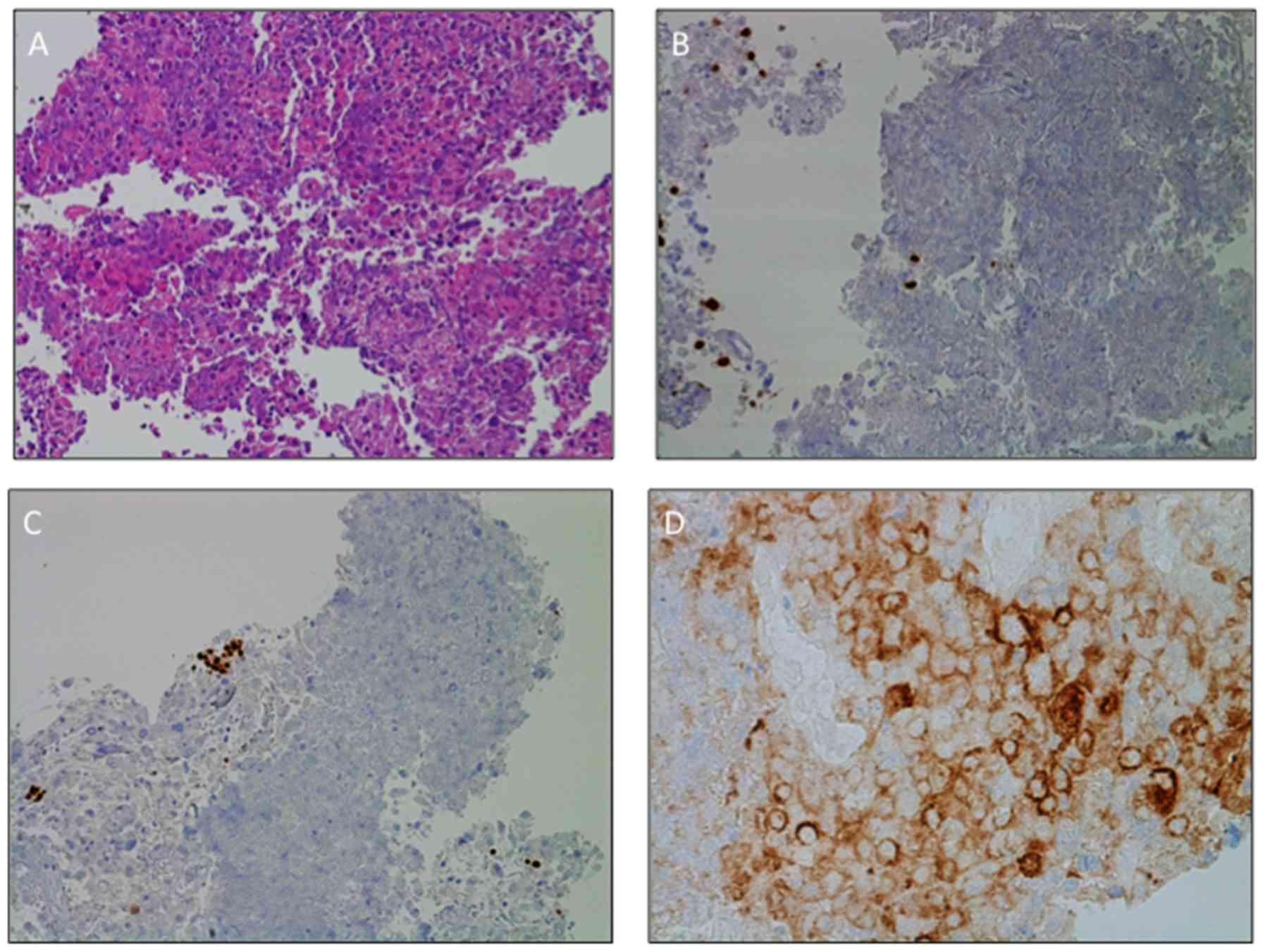
carcinoma of the lung (hematoxylin and eosin staining).
Immunohistochemistry (IHC) did not differentiate between squamous
or non-squamous type, was positive for cytokeratin pool and lacked
expression of thyroid transcription factor-1 (TTF-1), p40,
chromogranin A and synaptophysin (Fig.
3A-C) (13). The positivity for
cytokeratin CAM5.2 (data not shown) excluded the possibility of a
lymphoma. Neither EGFR mutations, researched with
Therascreen RGQ RT-PCR kit (Qiagen, Inc; qPCR) nor ALK gene
rearrangements (IHC, D5F3 clone) were detected. All the procedures
for the molecular analysis have been performed following the
specific manufacturer's instructions on the tumor material obtained
by the EBUS-guided needle aspiration (14). IHC demonstrated high positivity of
programmed death-ligand 1 (PD-L1), as PD-L1 tumor proportion score
was 55% (Fig. 3D). The patient was
therefore a candidate for immunotherapy with pembrolizumab, an
anti-programmed death-1 antibody (15).
Novel ischemic events leading to NBTE
detection
Only a few days before initiation of pembrolizumab
treatment, the onset of diplopia and gait impairment required a new
hospitalization (University Hospital of Parma, Italy). With the
limitation of a comparison between different imaging techniques,
the first CT scan already documented evolution of the lesion when
compared with the MRI performed 15 days before during the first
hospitalization (Fig. 4 vs. Fig. 2).
One day later, the patient developed substernal
chest pain and dyspnea; however, body temperature, blood pressure
and heart rate were regular. Cardiac auscultation revealed a
diastolic murmur consistent with aortic regurgitation, without any
other notable symptoms at the physical examination. A 12-lead
electrocardiogram showed sinus tachycardia (90 beats/min) with ST
segment elevation in DII-DIII-aVF derivations. Laboratory tests
revealed elevated serum troponin I with a peak value of 22.91 ng/ml
(normal value <0.006 ng/ml) (16). TTE showed a reduced left ventricular
ejection fraction (LVEF) of 45%, a mobile mass on the anterior
leaflet of mitral valve and a moderate aortic insufficiency. The
following development of acute pulmonary edema with respiratory
failure required noninvasive ventilation. Coronary angiography
showed reduced flow (Thrombolysis in Myocardial Infarction score=2)
(17) of the recurrent branch of the
descending artery rapidly after injection of the contrast agent,
whose appearance was consistent with an embolus. No atherosclerotic
lesions were observed in the remaining vessels. Due to the
hemodynamic and respiratory instability, the patient was admitted
to the intensive care unit. Therapy with aspirin and β-blockers was
initiated while maintaining LMWH treatment. Suspicion of infective
endocarditis led to blood culture tests as well as empiric
intravenous antibiotic therapy with linezolid 600 mg twice daily,
daptomycin 6 mg/kg once daily and piperacillin/tazobactam 4.5 g
three times daily. Clinical examination at this timepoint revealed
petechiae on the skin of the lower limbs, which is highly
suggestive of peripheral embolization (18).
An abdominal CT scan was performed at the onset of
abdominal pain and loss of blood in the stool, revealing occlusion
of a distal branch of the superior mesenteric artery, accompanied
by the thinning of the corresponding ileal loop (Fig. 5A and B). The patient underwent
emergency segmental ileo-cecal resection, with the histological
diagnosis of bowel infarction (hematoxylin and eosin staining;
Fig. 5C and D) and remained
intubated thereafter.
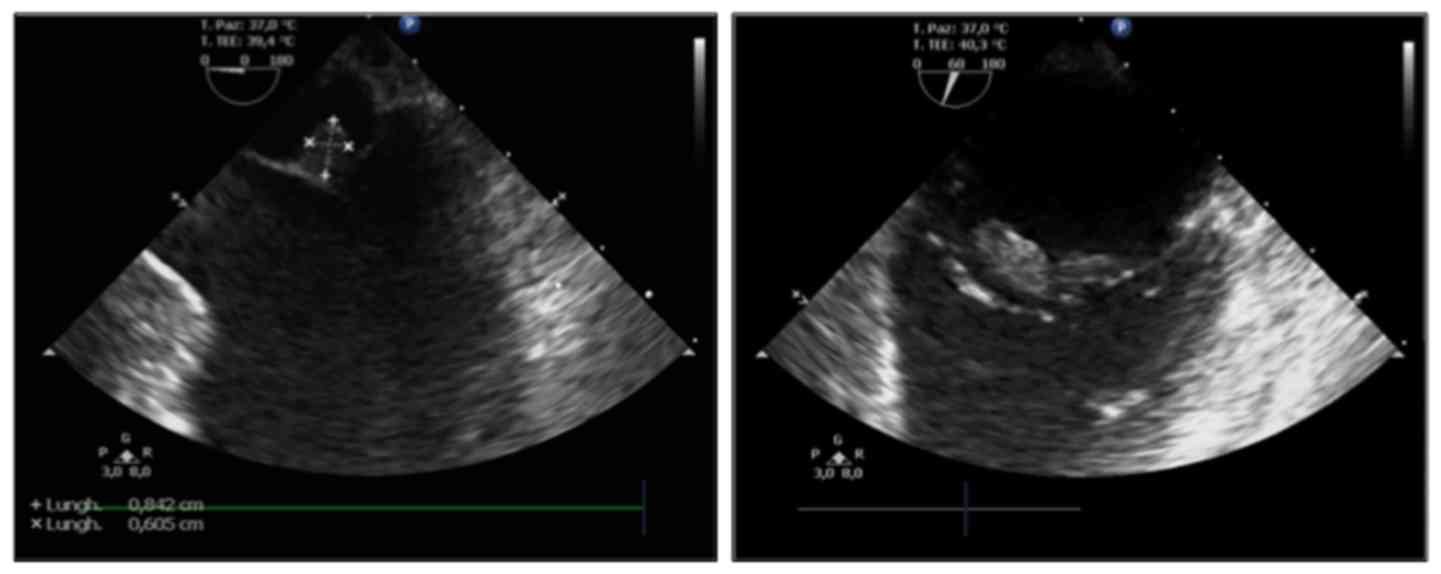
A TEE was performed following clinical stabilization
and revealed a mobile echogenic mass of 0.8×0.6 cm on the atrial
surface of the anterior leaflet of the mitral valve, moderate
aortic insufficiency and reduced LVEF (40%) (Fig. 6). No signs of PFO were detected.
The negativity of blood cultures and the normal
values for inflammatory markers (procalcitonin 0.26 ng/ml, range
0.00–0.5 ng/ml), together with the diffuse embolic manifestations
led to a final diagnosis of NBTE.
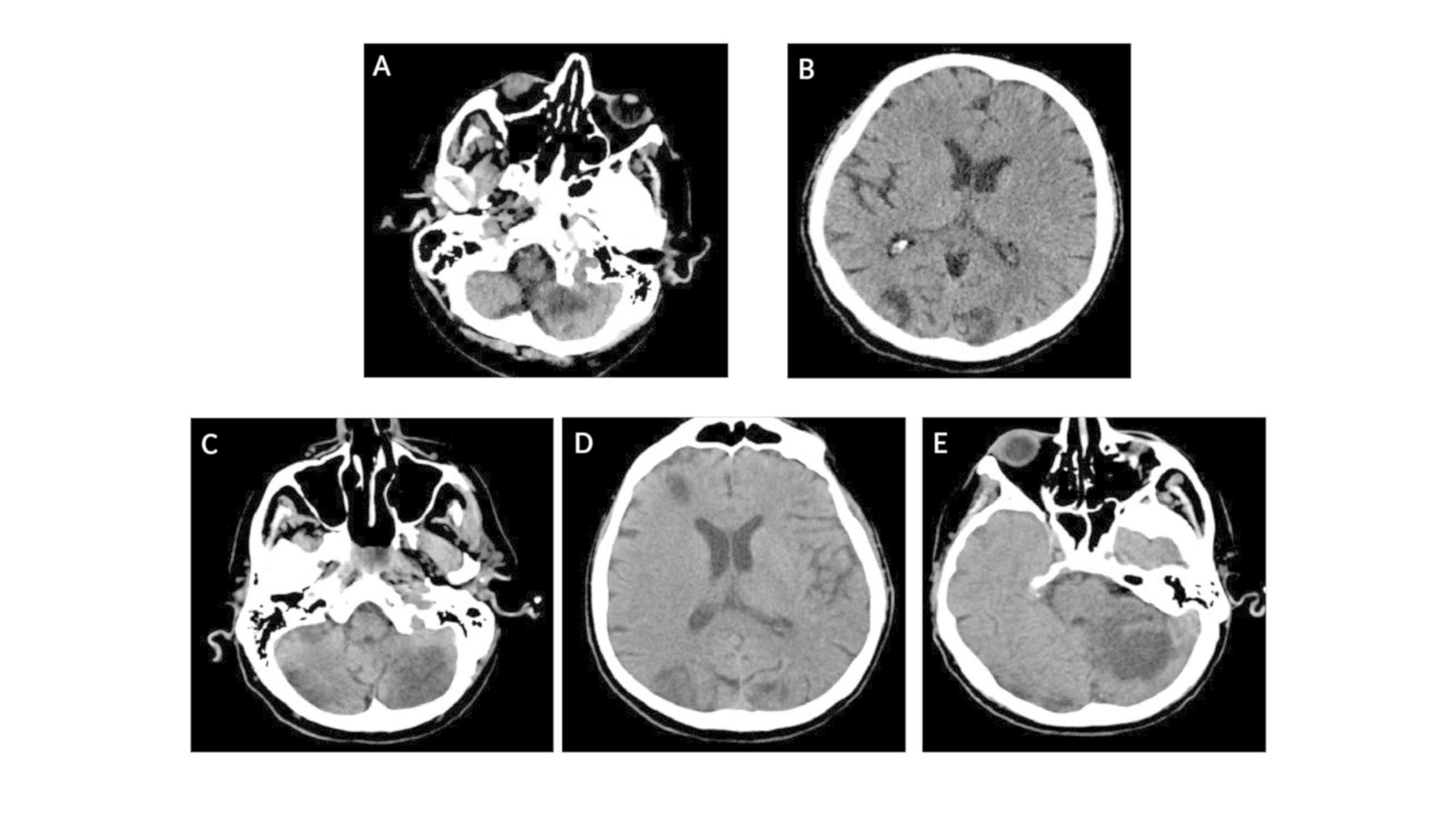
The patient then went into a coma, with an
additional brain CT scan documenting increased number and dimension
of the aforementioned described bi-hemispheric infarct lesions in
different phases of evolution, compatible with the progression of
the cerebral ischemic events and the onset of novel interested foci
(Fig. 4C-E).
Then, the patient developed multi-organ failure and
passed away, ~70 days after lung cancer diagnosis. Post-mortem
examination was not carried out.
Discussion
NBTE is a rare but serious manifestation of the
hypercoagulability state in patients with cancer, observed in
~1.25% of autoptic cases compared with 0.2% of autopsies performed
in patients without cancer (16).
Adenocarcinoma histology is frequently involved in the occurrence
of NBTE with pancreatic adenocarcinoma being the most common
(6,19).
The pathogenesis of NBTE endocarditis is not
completely understood. The local and systemic milieu of
pro-thrombotic factors secreted by tumors, such as tumor necrosis
factor, interleukin 1, tissue factor and cancer procoagulant, are
likely to be involved in the development and maintenance of cardiac
vegetations (1). Cardiac vegetations
are composed of aseptic thrombi, lacking inflammatory reaction and
growing on valvular surface. Aortic and mytralic valves are the
most commonly implicated, while the valves of the right side of the
heart are less frequently affected (20).
In patients with cancer, embolization occurs in
around 50% of NBTE cases (21).
Notably, NBTE leads to embolization events more frequently compared
with bacterial forms, given their mild cellular composition and the
weaker intercellular links (22).
The spleen, kidneys, brain, mesenteric and coronary arterial
circulation are the most commonly structures implicated in NBTE
(21,23,24).
The peculiarity of the present case was the
development of systemic embolisms in a short period of time, while
undergoing LMWH for a misdiagnosed PFO. Asymptomatic kidney and
splenic infarctions were the first to be detected concomitantly
with DVT, PTE and cerebral dissemination. The venous and pulmonary
circulation is less frequently involved in NBTE embolization but
are the most common manifestations of the hypercoagulability state
(6). Therefore, differential
pathogenetic mechanisms of NBTE on the basis of DVT and PTE cannot
exclude. After presenting with these thromboembolic manifestations,
the patient then developed cardiac, mesenteric and lower limb
ischemia. Notably, the last brain CT scan (Fig. 4) documented a variety of lesions
compatible with strokes occurring at different time points,
suggesting that there were continuous emboli shedding from the
valvular vegetations.
TEE, the best diagnostic tool to identify bacterial
or non-bacterial endocarditis, has higher sensitivity compared with
TTE (25,26). In the present case, TTE did not show
abnormalities. Otherwise, the TEE documented a different cardiac
involvement in terms of a PFO as the origin of the embolic foci.
Both TTE and TEE, performed 40 days later, demonstrated
sub-centimetric vegetation on the mitral valve, which explained the
development of the cardiogenic thromboembolic state. Considering
this, the importance of performing TEE by experienced physicians to
rule out the possibility of endocarditis should be reiterated,
particular when accompanying symptoms, radiological features and
medical history (for example history of malignancy, as in the
present case, or recurrent thrombotic events) are suggestive of
NBTE.
The treatment of NBTE relies on therapy targeting
the underlying pathology, for example lung cancer in the present
case, but inflammatory chronic diseases (including systemic lupus
erythematosus or connective tissue disorders) can also be a cause
(27). NBTE also relies on
anticoagulant therapy (6). Surgical
intervention is not recommended for NBTE in patients affected by
advanced and non-curable cancer (28). Both unfractionated heparin and LMWH
are potentially effective in decreasing the risk of embolization
(6). On the other hand, vitamin K
antagonists, such as warfarin, fail to prevent the systemic emboli
in NBTE, potentially due to the not involvement of vitamin
K-dependent factors (factors II, VII, IX and X and protein C and
S), inducing the trombophilic state in NBTE (29). There is no definite evidence
demonstrating the efficacy of fondaparinux, a synthetic
pentassaccharide like heparin, for the treatment of NBTE. Instead,
direct oral anticoagulants, such as rivaroxaban and edoxaban, are
acceptable alternatives to LMWH but should be used carefully, due
to the considerable risk of bleeding events (30). However, recently a case of metastatic
pancreatic cancer underlying a NBTE was reported, highlighting the
clinical improvement and tumor control obtained with LMWH and
chemotherapy administration (31).
Unfortunately, in the present case, LMWH
administration for the suspected PFO was not sufficient to avoid
diffuse embolization, even at the curative dose. Although, the
patient was a candidate for Pembrolizumab therapy due to locally
advanced disease stage (IIIB) and high expression levels of PD-L1
(≥50%) (15), multi-organ failure
due the multiple foci embolization did not allow sufficient time
for specific anticancer therapy to be administered.
NBTE should be strongly suspected when a patient
with underlying malignancy experiences multiple arterial thrombotic
events. Diagnosis of NBTE should not be excluded if the TTE does
not demonstrate cardiac anomalies and a TEE should still be
performed, given the risk of false negatives (32). In hindsight, the precocious putative
detection of a NBTE in the present patient could have sped up the
diagnosis and improved patient care, for example prolonging
hospitalization with close clinical monitoring. Moreover, the
current availability of targeted and immunotherapy agents, such as
tyrosine kinase inhibitors and pembrolizumab, respectively, are
characterized by lacking significant pro-thrombotic effects
compared with traditional cytotoxic agents. So, these drugs in the
treatment of patients with NBTE and cancer are deemed safe
(33).
On the other hand, due to the strong association
between cancer and NBTE, an unknown neoplastic disease should be
considered for patients who develop systemic embolisms from sterile
cardiac vegetations. It should be mandatory to suspect NBTE in
these cases. NBTE quickly worsens and can be fatal if left
untreated and the early start of adequate anticoagulant and
specific anticancer therapy may improve survival and risk of
complications due to NBTE.
Acknowledgements
The authors would like to thank Dr Tala Tayoun
(National Institute of Health and Medical Research, University
Paris-Saclay) for her medical writing assistance.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
FB performed the brain radiological examinations.
LG, MM and VA performed the histological examinations of the lung
tissue and contributed the histopathological details written in the
manuscript. AR, AV and TM managed the cardiac complication of the
patient and were involved in the collection and analysis of
clinical data regarding the evolution of the cardiac disease. FP,
AB, FF and MT analyzed patient's data and wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Consent for publication was obtained verbally from
patient's next of kin.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Caine JG, Stonelake PS, Lip GYH and Kehoe
ST: The hypercoagulable state of malignancy: Pathogenesis and
current dabate. Neoplasia. 4:465–473. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carrier M, Lazo-Langner A, Shivakumar S,
Tagalakis V, Zarychanski R, Solymoss S, Routhier N, Douketis J,
Danovitch K, Lee AY, et al: Screening for occult cancer in
unprovoked venous thromboembolism. New Engl J Med. 373:697–704.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abdol Razak NB, Jones G, Bhandari M,
Berndt MC and Metharom P: Cancer-associated thrombosis: An overview
of mechanisms, risk factors, and treatment. Cancers (Basel).
10:3802018. View Article : Google Scholar
|
|
4
|
Aronson D and Brenner B: Arterial
thrombosis and cancer. Thromb Res. 164 (Suppl 1):S23–S28. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lip GYH, Chin BSP and Blann AD: Cancer and
the prothrombotic state. Lancet Oncol. 3:27–34. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
el-Shami K, Griffiths E and Streiff M:
Nonbacterial thrombotic endocarditis in cancer patients:
Pathogenesis, diagnosis, and treatment. Oncologist. 12:518–523.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mukai M and Oka T: Mechanism and
management of cancer-associated thrombosis. J Cardiol. 72:89–93.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Horsted F, West J and Grainge MJ: Risk of
venous thromboembolism in patients with cancer: A systematic review
and meta-analysis. PLoS Med. 9:e10012752012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haddad TC and Greeno EW:
Chemotherapy-induced thrombosis. Thrombo Res. 118:555–568. 2006.
View Article : Google Scholar
|
|
10
|
Connolly G and Francis CW:
Cancer-associated thrombosis. Hematology Am Soc Hematol Educ
Program. 2013:684–691. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raskob GE, van Es N, Verhamme P, Carrier
M, Di Nisio M, Garcia D, Grosso MA, Kakkar AK, Kovacs MJ, Mercuri
MF, et al: Edoxaban for treatment of cancer-associated venous
thromboembolism. N Engl J Med. 378:615–624. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Amin MB, Edge SB, Greene FL, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC Cancer Manual. 8th. Springer; New York,
NY: 2017
|
|
13
|
Coons AH, Creech HJ and Jones RN:
Immunological properties of an antibody containing a fluorescent
group. Exp Biol Med. 47:200–202. 1941. View Article : Google Scholar
|
|
14
|
Vallée A, Le Loupp A-G and Denis MG:
Efficiency of the Therascreen® RGQ PCR kit for the
detection of EGFR mutations in non-small cell lung carcinomas. Clin
Chim Acta. 429:8–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saenger AK, Beyrau R, Braun S, Cooray R,
Dolci A, Freidank H, Giannitsis E, Gustafson S, Handy B, Katus H,
et al: Multicenter analytical evaluation of a high-sensitivity
troponin T assay. Clin Chim Acta. 412:748–754. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mok Y, Ballew SH, Bash LD, Bhatt DL, Boden
WE, Bonaca MP, Carrero JJ, Coresh J, D'Agostino RB Sr, Elley CR, et
al: International validation of the thrombolysis in myocardial
infarction (TIMI) risk score for secondary prevention in post-MI
patients: A collaborative analysis of the chronic kidney disease
prognosis consortium and the risk validation scientific committee.
J Am Heart Assoc. 7:e0084262018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Layden J, Michaels J, Bermingham S and
Higgins B; Guideline Development Group, : Diagnosis and management
of lower limb peripheral arterial disease: Summary of NICE
guidance. BMJ. 345:e49472012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Quintela AG, Candela MJ, Vidal C, Román J
and Aramburo P: Non-bacterial thrombotic endocarditis in cancer
patients. Acta Cardiol. 46:1–9. 1991.PubMed/NCBI
|
|
20
|
Biller J, Challa VR, Toole JF and Howard
VJ: Nonbacterial thrombotic endocarditis. A neurologic perspective
of clinicopathologic correlations of 99 patients. Arch Neurol.
39:95–98. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barry WE and Scarpelli D: Nonbacterial
thrombotic endocarditis. A clinicopathologic study. Arch Intern
Med. 109:79–84. 1962. View Article : Google Scholar
|
|
22
|
Liu J and Frishman WH: Nonbacterial
thrombotic endocarditis: Pathogenesis, diagnosis and management.
Cardiol Rev. 24:244–247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kooiker JC, MacLean JM and Sumi SM:
Cerebral embolism, marantic endocarditis, and cancer. Arch Neurol.
33:260–264. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mazokopakis EE, Syros PK and Starakis IK:
Nonbacterial thrombotic endocarditis (marantic endocarditis) in
cancer patients. Cardiovasc Hematol Disord Drug Targets. 10:84–86.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Joffe II, Jacobs LE, Owen AN, Ioli A and
Kotler MN: Noninfective valvular masses: Review of the literature
with emphasis on imaging techniques and management. Am Heart J.
131:1175–1183. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dutta T, Karas MG, Segal AZ and Kizer JR:
Yield of transesophageal echocardiography for nonbacterial
thrombotic endocarditis and other cardiac sources of embolism in
cancer patients with cerebral ischemia. Am J Cardiol. 97:894–898.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abouarab AA, Elmously A, Leonard JR,
Arisha MJ, Gaudino M, Narula N and Salemi A: Nonbacterial trombotic
endocarditis presenting with leg pain and a left atrial mass
lesion. Cardiology. 139:208–211. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Asopa S, Patel A, Khan OA, Sharma R and
Ohri SK: Non-bacterial thrombotic endocarditis. Eur J Cardiothorac
Surg. 32:696–701. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee AY, Levine MN, Baker RI, Bowden C,
Kakkar AK, Prins M, Rickles FR, Julian JA, Haley S, Kovacs MJ, et
al: Low-molecular-weight heparin versus a coumarin for the
prevention of recurrent venous thromboembolism in patients with
cancer. N Eng J Med. 349:146–153. 2003. View Article : Google Scholar
|
|
30
|
Franco-Moreno A, Cabezòn-Gutierréz L,
Palka-Kotlowsa M, Villamayor-Delgado M and García-Navarro M:
Evaluation of direct oral anticoagulants for the treatment of
cancer-associated thrombosis: An update. J Thromb Thrombolysis.
47:409–419. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fournier JB and Testa EJ: Nonbacterial
thrombotic endocarditis. N Engl J Med. 380:e482019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee RJ, Bartzokis T, Yeoh TK, Grogin HR,
Choi D and Schnittger I: Enhanced detection of intracardiac source
of cerebral emboli by transesophageal echocardiography. Stroke.
22:734–739. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brosseau S, Gounant V, Naltet C,
Théou-Anton N, Cazes A, Smonig R, Neuville M, Khalil A, Mikail N,
Meignin V, et al: Lazarus syndrome with crizotinib in a non-small
cell lung cancer patient with ROS1 rearrangement and disseminated
intravascular coagulation. Clin Lung Cancer. 19:e57–e61. 2018.
View Article : Google Scholar : PubMed/NCBI
|