Introduction
In 2018, prostate cancer was the second most common
malignancy and the fifth leading cause of cancer-associated
mortality in men worldwide (1). The
incidence differs by >25-fold among regions, with the highest
incidence being in Australia/New Zealand and the lowest in
South-Central Asia (1).
According to the World Health Organization (WHO),
acinar adenocarcinoma is an invasive carcinoma that consists of
neoplastic prostatic epithelial cells with secretory
differentiation arranged in a variety of histomorphological
patterns, including glands, cords, single cells and sheets, and
basal cells are typically absent (2). Antibodies against high molecular weight
cytokeratin (HMW-CK) and p63 may identify basal cells, and
α-methylacyl-CoA racemase (AMACR) is a positive marker for acinar
adenocarcinoma (2–4). In high-grade acinar adenocarcinoma, it
is crucial to use antibodies against cytokeratin 7 (CK7), CK20 and
prostate-specific antigen (PSA) to identify whether the tumor
derives from the colon, rectum, urinary bladder or a metastatic
disease (5,6). Gleason grading system is used for the
prognosis of prostate acinar adenocarcinoma. This standard method
is used worldwide to grade prostate cancer, and is based on the
degree of architectural differentiation (4,7,8). For therapeutic purposes, a new
prognostic grading group has been developed and is applied in
conjunction with the 2014 WHO/International Society of Urologic
Pathologists (9–11). The Gleason grading system should not
be applied in case of the therapy effect (2).
Androgen deprivation therapy (ADT) is a treatment
that alters the benign and cancerous prostatic epithelium by
inducing apoptosis, which is characterized by the fragmentation of
tumor DNA (12). The histological
features of the epithelium from the glandular component of
prostatic carcinoma following ADT include the loss of glandular
architecture, nuclear and nucleolar shrinkage, nuclear
hyperchromasia and pyknosis and mucinous degeneration (13). In addition, acinar atrophy, reduced
ratio of acini to stroma, enlargement and clearing of cytoplasm,
prominent clear cell change, basal cell hyperplasia and increase in
squamous metaplasia are also observed, following ADT. The
histological changes in the prostatic stroma following ADT include
an edema at early stages, fibrosis at late stages and patchy
condensation of the stroma resulting in focal hypercellularity and
focal chronic lympho-histiocytic inflammation (13,14).
Ultrastructural changes of prostate cancer cells
compared with normal or hypertrophic prostate cells are well known
and have been reported by numerous researchers (15–18). It
has been demonstrated that cancer prostate secretory cells present
with hyperchromatosis of the nucleus, hypertrophy of the nucleus
and nucleolus, an increased number of pleiomorphic mitochondria,
various configurations and positions of Golgi complexes and
increased amount of lipid droplets of different morphology
(15). In prostate cancer cells, an
increase in the number of mitochondria of different morphology has
been reported in patients with a high degree of prostate malignancy
according to the Gleason score (18).
Apoptosis, or programmed in situ cell death,
is a physiological homeostatic mechanism involving cell death that
naturally occurs during normal tissue turnover (19,20).
Apoptosis dysregulation serves a crucial role in numerous
pathological processes, including inflammation, hyperplasia, cancer
and responses to therapy (21).
Molecular imaging of apoptotic cells could therefore be useful for
early detection of anticancer therapy effects (22–24).
Apoptosis in acinar adenocarcinoma was described in small foci of
prostate cancer cells (2,3). Previous studies on apoptosis in
prostatic carcinoma reported a positive association between the
amount of apoptotic bodies and Gleason grade (25–29).
The present study aimed to characterize the complex
structural processes that occur in the malignant prostate through
histological, immunohistochemical (IHC) and ultrastructural
analyses using light, fluorescence and transmission electron
microscopy, in order to determine the importance of assessing
individual histological structures of the prostate in the clinical
course of the disease. To do so, prostate acinar adenocarcinoma
tissues from newly-diagnosed naïve patients with metastatic
disease, patients treated with ADT alone, or with ADT and
abiraterone acetate (Abi) were collected.
Materials and methods
Ethical approval
The Ethics Commission of the Faculty Hospital Nitra
(Nitra, Slovak Republic) approved the present study. All patients
provided written informed consent.
Patients and samples
Prostate tissue samples were collected between
November 2016 and June 2017 during transurethral resection from 22
patients with bladder outlet obstruction and histologically
confirmed benign prostatic hyperplasia (BPH) and acinar
adenocarcinoma prostate cancer at the Faculty Hospital Nitra
(Nitra, Slovak Republic). At the time of surgery, the mean age of
the patients was 70.7 years (age range, 58–85 years). Based on the
Tumor-Node-Metastasis (7th edition) clinical stage (30) and the type of treatment, samples were
divided into four groups as follows: i) Group 1, samples from
patients with BPH (adenocarcinoma-negative); ii) group 2, samples
from patients with metastatic hormone naïve acinar prostatic
adenocarcinoma (mHNPC); iii) group 3, samples from patients with
metastatic acinar prostatic adenocarcinoma (mPC) and receiving ADT;
and iv) group 4, samples from patients with metastatic
castration-resistant acinar prostatic adenocarcinoma (mCRPC) and
receiving ADT and Abi therapy (Table
I). The patient clinicopathological characteristics and the
number of patients in each group are presented in Table I. Patients in group 3 were treated
with leuprorelin acetate (5 mg subcutaneously every 3 months) ADT
drug for an average duration of 36 months (duration range, 6–80
months). Patients in group 4 were treated with leuprorelin acetate
(5 mg subcutaneously every 3 months) ADT drug for an average
duration of 50 months (duration range, 47–60 months). In groups 3
and 4 there was no statistically significant differences in the
length of ADT treatment (P=0.40). Patients from group 4 also
received abiraterone acetate, a standard treatment for mCRPC,
orally at a dose of 1,000 mg/day fasting for an average treatment
duration of 15 months (duration range, 3–27 months). At the time of
tissue collection, patients in groups 3 and 4 had castrated
testosterone levels (<50 ng/dl) (31) due to being treated with ADT.
Additionally, patients in group 3 did not have clinical or
biochemical signs of cancer progression, and patients in group 4
with CRPC continuing with ADT and Abi therapy, as is standard
treatment (31), did not have signs
of clinical progression.
 | Table I.Clinicopathological characteristics
of patients according to TNM classification. |
Table I.
Clinicopathological characteristics
of patients according to TNM classification.
| Groups | T3-4, n | N+, n | M+, n | PSA level, ng/ml
(range) |
|---|
| BPH (n=5) | N/A | N/A | N/A | 9.30
(0.66–17.65) |
| mHNPC (n=6) | 6 | 2 | 6 | 88.00
(45.00–154.00) |
| mPC + ADT
(n=6) | 6 | 2 | 6 | 8.55
(1.19–22.40) |
| mCRPC + ADT + Abi
(n=5) | 5 | 3 | 5 | 91.98
(45.90–131.00) |
Histological and IHC analyses
The histological diagnosis was determined following
conventional biopsy. Fresh tissue samples following collection were
fixed in 10% neutrally-buffered formaldehyde solution for 24 h at
room temperature, dehydrated in an ascending ethanol series (80, 90
and 100%) at room temperature, and embedded in paraffin. Paraffin
sections of 5-µm thickness were prepared using a microtome.
Sections for histological analysis were stained with Mayer's
hematoxylin and eosin in Dako CoverStainer (Dako; Agilent
Technologies, Inc.) at room temperature for 20 min. Sections for
IHC analysis were deparaffinized and rehydrated in the automatic
pre-treatment module PT Link (Dako; Agilent Technologies, Inc.) at
97°C for 40 min. Sections were inserted into DakoAutostainer 48
Link, and the required protocols were selected according to the
manufacturer's instructions. Sections were incubated with primary
atibodies against HMW-CK (cat. no. IR051), AMACR (cat. no. IR060),
PSA (cat. no. IR514), CK7 (cat. no. IR619) and CK20 (cat. no.
IR777) at room temperature for 20 min, and subsequently with
EnVision™ Flex/HRP secondary antibody (cat. no. K8000) at room
temperature for 20 min (all Dako; Agilent Technologies, Inc.). All
primary and secondary antibodies were ready to use (undiluted). The
visualization step was performed with EnVision™ Flex/DAB and
Chromogen (cat. no. K8000) for 10 min, subsequently washed with
water for 5 min. Sections were stained with Mayer's hematoxylin for
5 min at room temperature. Finally, sections were rehydrated in a
descending ethanol series (100, 90 and 80%), cleared with 99%
xylene and mounted with DAKO Toluene-free mounting medium (Dako;
Agilent Technologies, Inc.). Sections were observed using a Nikon
Eclipse Ci-L light microscope (magnification, ×200; Nikon
Corporation), and images were digitally recorded using an Imaging
Source camera (The Imaging Source Europe GmbH). Samples from group
2 (patients with mHNPC) were evaluated using the Gleason grading of
prostatic carcinoma (9).
Electron microscopic analysis
Fresh prostatic tissue samples immediately after
collection were fixed in an aldehyde mixture containing 2.5%
glutaraldehyde and 2% paraformaldehyde in 0.1 M sodium cacodylate
buffer at pH 7.2–7.4 at 4°C for 1 h and subsequently post-fixed in
1% osmium tetroxide dissolved in 0.1 M sodium cacodylate for 1 h at
room temperature. Following dehydration in an ascending acetone
series (30, 50, 70, 80, 90, 95 and 100%), samples were embedded
into Poly/Bed Embedding Media (Polysciences, Inc.). Ultrathin
sections of 70-nm thickness were cut using a Leica EM UC6 ultra
microtome (Leica Microsystems GmbH) and contrasted with 10% uranyl
acetate solution dissolved in absolute methanol and lead citrate
according to methods by Reynolds (32), in order to visualize cellular
organelles. The contrasted sections were imaged using a JEM100 CXII
transmission electron microscope (JEOL, Ltd.) at an accelerating
voltage of 80 kV and a magnification range between ×1,900 and
×7,200.
Fluorescent determination of apoptosis
using terminal deoxynucleotidyl-transferase-mediated dUTP nick end
labelling (TUNEL) assay
Paraffin-embedded prostatic tissue sections (5 µm)
previously fixed in 10% neutrally-buffered formalin were analyzed
using TUNEL assay, using In Situ Cell Death Detection kit,
Fluorescein (Roche Diagnostics GmbH) according to the
manufacturer's instructions. The samples were deparaffinized using
pure xylene (≥99%), rehydrated in a descending ethanol series (100,
90 and 80%) and permeabilized using proteinase K (Roche Diagnostics
GmbH). Sections were labelled with TdT-reagent for 60 min in a wet
chamber at 37°C in a thermostat. Sections were covered with a
Vectashield anti-fade medium (Vector Laboratories, Inc.) and
mounted into a sandwich between a microslide and a coverslip.
Presence of apoptotic cells was analyzed under a Leica fluorescence
microscope (Leica Microsystems GmbH) equipped with a digital camera
DFC-480 with a total magnification of ×220. For the positive
control, sections were treated with 1,500 U/ml recombinant DNase I
(Roche Diagnostics GmbH) prior to TdT labelling and incubated in a
humidified atmosphere for 60 min at 37°C in the dark. For the
negative control, sections were incubated only with a fluorescein
isothiocyanate-labelling solution, according to the manufacturer's
protocol (In Situ Cell Death Detection kit; Roche
Diagnostics GmbH), in the absence of TdT-reagent for 60 min at 37°C
in the dark (data not shown).
Following TUNEL assay, apoptotic cells and fragments
exhibited green fluorescence on a dark background. The distribution
of apoptosis in the examined samples was different in all 4 groups.
In each sample, in areas where apoptosis occurred, images were
taken from 10 fields of view at ×200 magnification (ocular, ×10;
objective, ×20) under a fluorescence microscope. The number of
apoptotic cells was manually counted and statistically
analyzed.
Statistical analysis
ANOVA followed by least significant difference post
hoc test was used to analyze the difference in the number of
apoptotic cells between the 4 groups. Data were analyzed using SPSS
Statistics software (version 20; IBM Corp.). The level of α
significance was determined at 0.05, and P<0.05 was considered
to indicate a statistically significant difference. One-way ANOVA
followed by Tukey's post hoc test was used for comparisons among
multiple groups. The differences in the number of apoptotic cells
between all groups were evaluated. The calculated values were
plotted in a dot graph.
Results
Histology and ultrastructure of BPH
samples
Histologically, BPH tissue from group 1 was formed
by dilated hyperplastic glands with two layers, epithelial and
myoepithelial. This was verified in one case (sample 5) following
IHC examination with HMW-CK and AMACR. The basal cell layer was
positive for the HMW-CK antibody, whereas the epithelial layer was
negative for the AMACR antibody. In two samples (no. 13 and 14), in
addition to gland hyperplasia, chronic active prostatitis with
periglandular chronic inflammatory infiltrate and presence of
neutrophil leukocytes in the lumen of the glands was detected (data
not shown).
Ultrastructurally, in the case of BPH (group 1),
well-developed microvilli were visible on the luminal surface of
the cells. The epithelial secretory cells were cylindrical in shape
and were attached to the basal membrane along with the small basal
cells (data not shown). The cytoplasm of the secretory cells
contained supranuclearly positioned secretion granules, oval
mitochondria, prominent and numerous vesicles of granular
endoplasmic reticulum, which were often expanded in the
supranuclear area and surface parts. Heterochromatic nuclei had an
oval shape and the nucleoli were of a reticular form. Some nuclei
were hyperchromatic and had numerous invaginations and signs of
degeneration. The incidence of compact nucleoli, swollen
mitochondria and lysosomes was low (Fig.
1A). The stroma was sporadically occupied with macrophages
(Fig. 1B).
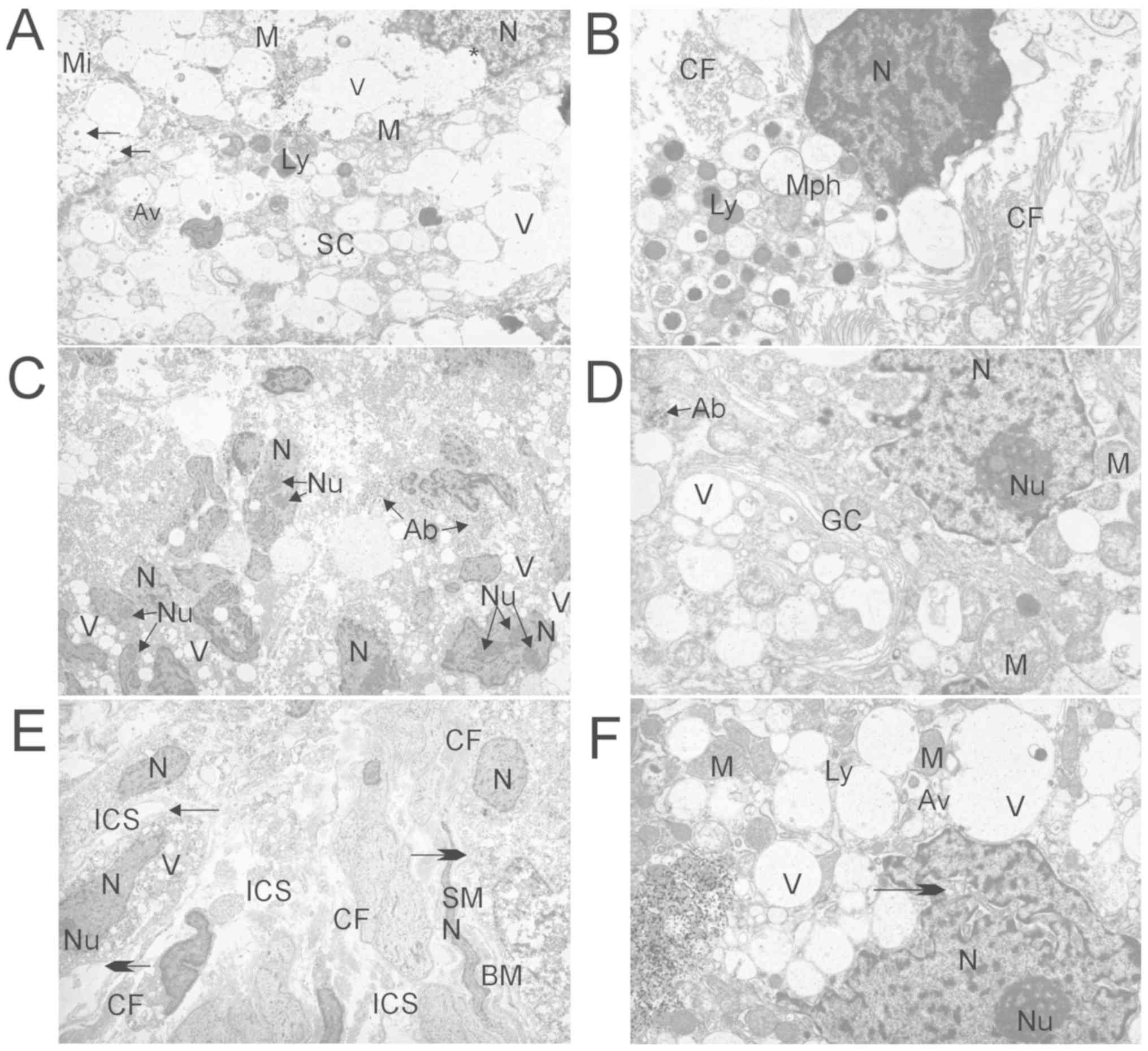 | Figure 1.Ultrastructural analysis of prostate
tumor tissue by EM. (A) EM analysis of benign prostatic hyperplasia
tissue (group 1) showing the supranuclear region of SC with Mi
oriented into the lumen of acinus, M, large V of the endoplasmic
reticulum, Ly, N with invagination (asterisk) and secretory
granules (arrows). Primary magnification, ×4,800. (B) EM analysis
of benign prostatic hyperplasia tissue (group 1) showing Mph in the
stroma of the prostate tissue with dense N, numerous Ly and CF
around. Primary magnification, ×7,200. (C) EM analysis of
metastatic hormone naïve prostate cancer tissue (group 2) showing
disintegrated acini with hyperchromatic N and prominent Nu, assumed
Ab near the N, and V of endoplasmic reticulum. Primary
magnification, ×1,900. (D) EM analysis of metastatic acinar
adenocarcinoma tissue following ADT (group 3) showing
hyperchromatic N, Nu, abnormal and swollen M with disrupted
cristae, cisternae of GC, V of the endoplasmic reticulum and
probably Ab. Primary magnification, ×7,200. (E) EM analysis of
metastatic castration-resistant prostate cancer tissue following
ADT and Abi treatment (group 4) showing solitary malignant cells
with small N and hypertrophic Nu. V of the endoplasmic reticulum
are less present compared with group 1 (Fig. 1A), and secretory granules are not
visible. Apical-basal cell polarity was lost, intercellular joints
were disrupted (small arrow), ICSs were dilated and the BM was
degraded (big arrows). These secretory cells appeared as
mesenchymal-like cells. There are also abundant CF and SM cells
with N. Primary magnification, ×1,900. (F) EM analysis of
metastatic castration-resistant prostate cancer tissue following
ADT and Abi treatment (group 4) showing N with deep invagination
(arrow), compact Nu, electron-dense M, large V of the endoplasmic
reticulum, Ly and Av. Primary magnification, ×7,200. Abi,
abiraterone acetate; ADT, androgen-deprivation therapy; EM,
electron microscopy; M, mitochondria; N, nucleus/nuclei; Nu,
nucleoli; Mi, microvilli; V, vesicles; Ly, lysosomes; Mph,
macrophages; CF, collagen fibrils; Ab, apoptotic body; GC, Golgi
complex; ICS, intercellular space; BM, basal membrane; SM, smooth
muscle; AV, autophagic vacuoles; SC, secretory cell. |
Histology and ultrastructure of
samples from patients with metastatic acinar adenocarcinoma
following ADT or not
Samples from patients with acinar adenocarcinoma and
who did not receive treatment (group 2) presented with small glands
in a random arrangement, hyperchromatic nuclei and prominent
nucleoli, amphophilic cytoplasm without basal cell layer, according
to IHC staining for AMACR-positivity and HMW-CK negativity (data
not shown). The Gleason score was determined according to this
architectural pattern (3+3, 3+4, 5+5). In the samples from group 3
(patients who received ADT), groups of individually arranged cells
with round-shaped nuclei and light cytoplasm were observed as a
treatment effect (data not shown). Since the Gleason score is not
recommended to be applied in patients subjected to ADT (2,33), the
Gleason score was not used as a criteria in the present study.
The ultrastructural images of samples from the
groups 2 and 3 were similar and were characterized by the presence
of secretory cells without basal cells. In both groups,
disintegrated acinus and hyperchromatic nuclei were identified; the
nuclei presented with an oval shape with numerous invaginations and
signs of degeneration. In addition, the hypertrophic nuclei were of
compact form and high in number. The granular endoplasmic reticulum
and Golgi complex presented with similar structures in the two
groups. In group 2, some apoptotic bodies were identified (Fig. 1C). Furthermore, the number of swollen
mitochondria with altered cristae morphology in secretory cells of
samples from the group 3 was high (Fig.
1D). Impaired integrity of the basal membrane was observed in
samples from the 4 groups.
Histology and ultrastructure of
samples from patients with mCRPC who received ADT and Abi
treatment
Histologically mCRPC (group 4) was characterized by
solid and solid-alveolar arranged foci (33) of malignant cells with hyperchromatic
nuclei and prominent nucleoli, small to moderate amount of
amphophilic cytoplasm, a shift of the nucleus/cytoplasm ratio and
numerous mitoses, including atypical mitoses (Fig. 2A). Locally, foci of glandular
formation were noted. IHC staining of malignant cells revealed
positivity for PSA and AMACR (Fig. 2B
and C, respectively). IHC staining of the myoepithelial layer
with HMW-CK and staining for CK7 and CK20 was negative (data not
shown). A portion of the malignant cells were arranged in clusters,
rows, or as single cells (34), and
presented with a lightly stained eosinophilic cytoplasm and small
hyperchromatic nuclei as a sign of the therapeutic effect (Fig. 2D). As aforementioned, the Gleason
score was not determined.
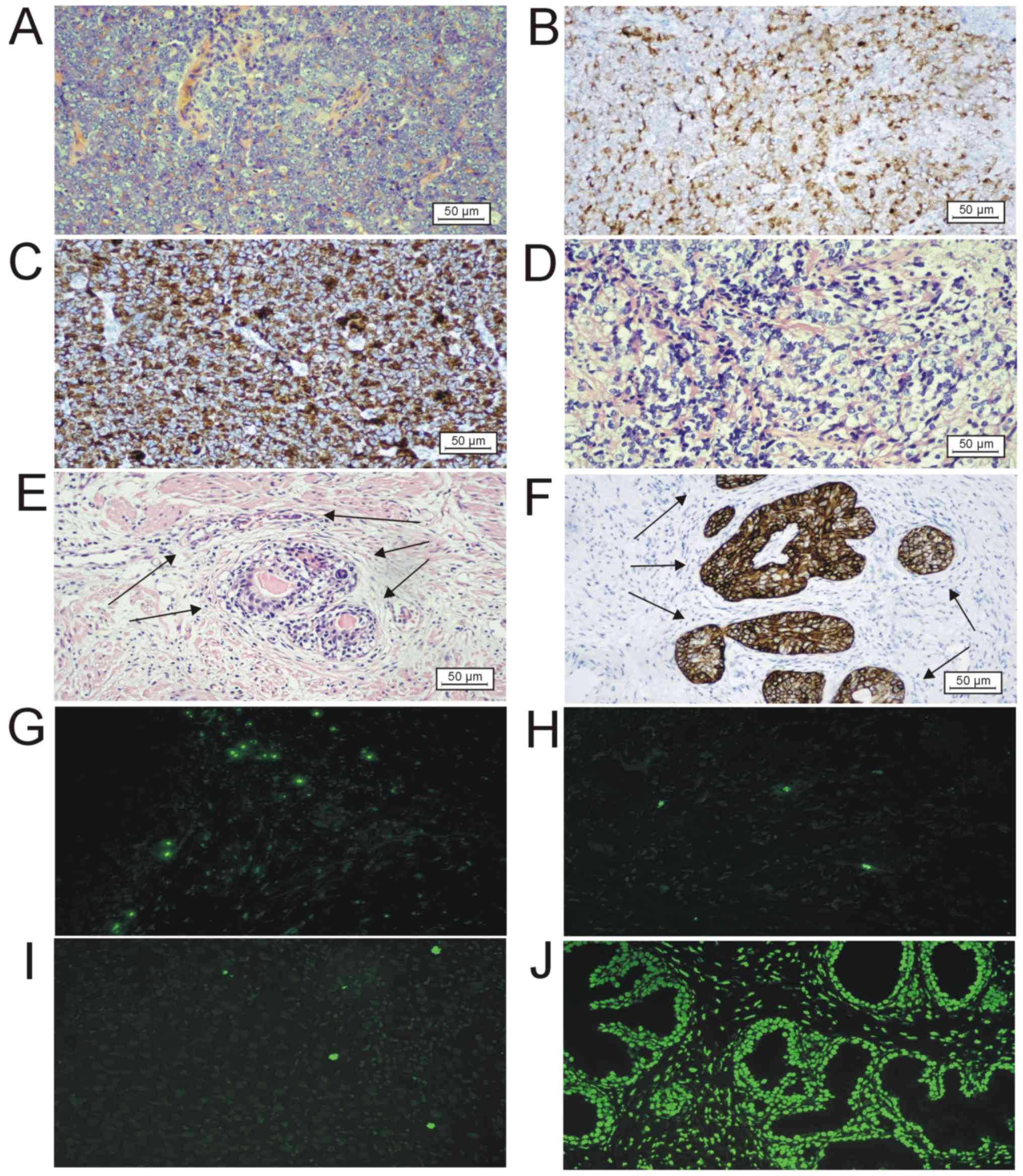 | Figure 2.Histological, immunohistochemical and
apoptotic images of prostatic tumor tissues. (A) Solid vital tumor
following ADT in group 4 (HE staining). Scale bar, 50 µm. (B)
Prostate-specific antigen-positive staining using IHC in group 4.
Scale bar, 50 µm. (C) α-Methylacyl-CoA racemase-positive staining
using IHC in group 4. Scale bar, 50 µm. (D) Histological image of
HE staining of the ADT therapeutic effect in group 4, showing
nuclear hyperchromasia and pyknosis, nuclear shrinkage and clear
cytoplasm. Scale bar, 50 µm. (E) Histological image of HE staining
of benign glands with basal cell hyperplasia (arrows) and
enlargement and clearing of the cytoplasm (group 4). Scale bar, 50
µm. (F) High molecular weight cytokeratin positive staining using
IHC demonstrating basal cell hyperplasia (arrows) in benign glands
(group 4). Scale bar, 50 µm. (G) TUNEL assay on sample no. 19
(group 2) of metastatic hormone naïve prostate cancer showing 10
apoptotic cells in one field of view in the malignant glandular
epithelium. Magnification, ×200. (H) TUNEL assay on sample no. 25
(group 3) of metastatic acinar adenocarcinoma with androgen
deprivation therapy, showing 4 apoptotic cells in one field of view
in a solidly arranged glandular epithelium. Magnification, ×200.
(I) TUNEL assay on sample no. 27 (group 4) of metastatis
castration-resistant prostate cancer following ADT and abiraterone
acetate treatment showing 3 apoptotic cells in one field of view in
malignant glandular epithelium. Magnification, ×200. (J) Positive
control for TUNEL assay (treated with recombinant DNase I).
Magnification, ×200. HE, hematoxylin-eosin; IHC,
immunohistochemistry; ADT, androgen-deprivation therapy; TUNEL,
terminal deoxynucleotidyl-transferase-mediated dUTP nick end
labelling. |
Benign glands with basal cell hyperplasia were also
identified in group 4, as confirmed by the IHC positivity for
HMW-CK (Fig. 2E). Basal cells in the
benign glands manifested enlargement and clearing of the cytoplasm
following treatment compared with the untreated BPH (Fig. 2E and F).
Ultrastructurally, mCRPC, in comparison with naïve
prostate cancer (group 2), was characterized by the presence of a
large amount of connective tissue, smooth muscle bundles and
solitary, malignant cells arranged into small groups located in the
prostate stroma with prominent fibrils of collagen (Fig. 1E). The nuclei of these malignant
cells were small, oval in shape and their membrane formed numerous
invaginations on the inside. Chromatin in the nucleus was evenly
distributed and formed hyperchromatic clusters. The nuclei
contained numerous, large, predominantly compact nucleoli. The
cytoplasm contained a great number of electron-dense mitochondria
with altered cristae, lysosomes and autophagic vacuoles (Fig. 1F).
Determination of cell apoptosis
Apoptosis is the most common form of death in
eukaryotic cells. In each group, apoptosis was observed in the
glands but rarely in the stroma (Fig.
2G-I; data not shown for group 1). Apoptotic fragments also
occurred in the lumen of the gland.
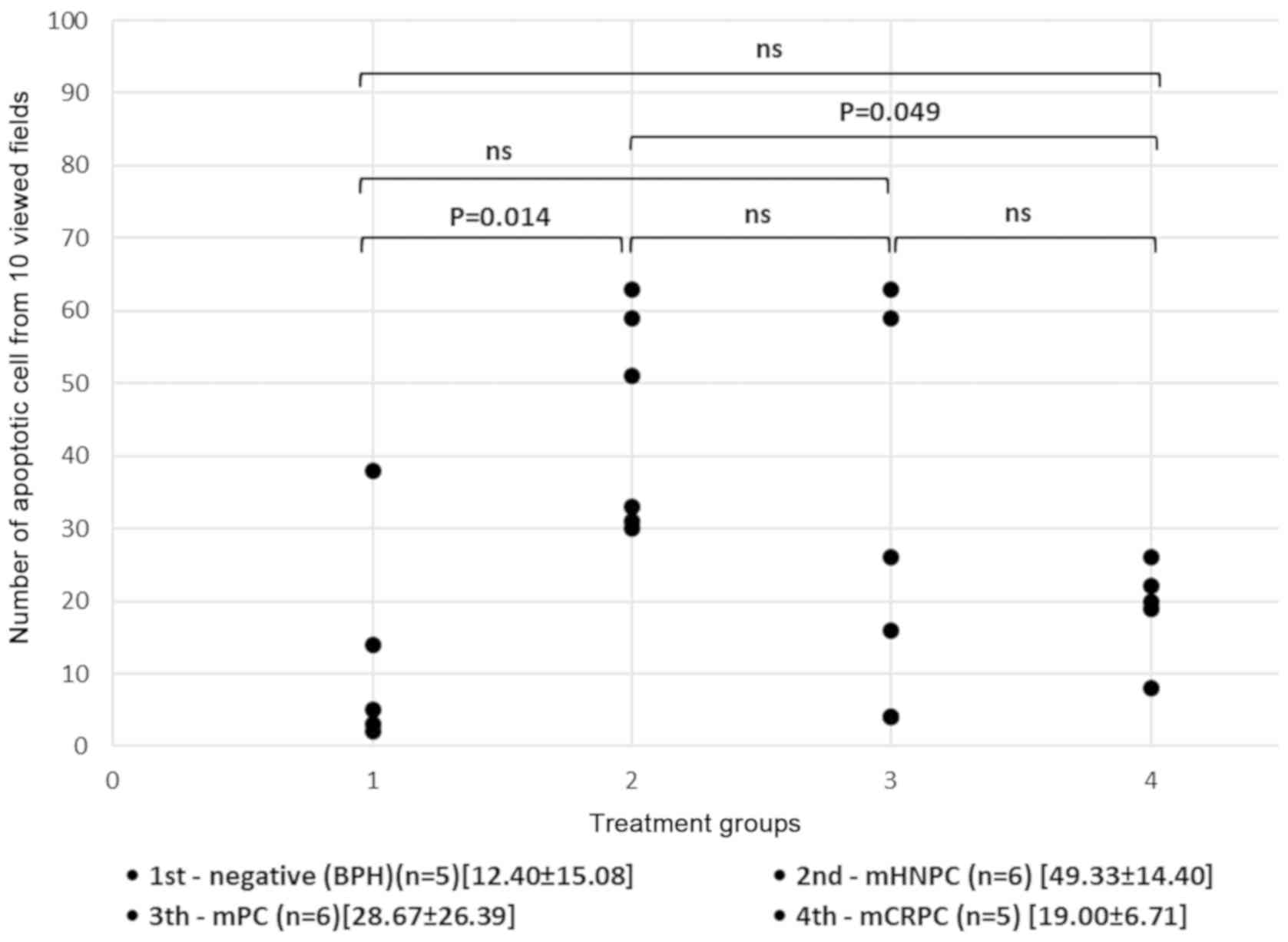
The results from the statistical analysis revealed
that the number of apoptotic cells in the assessed fields of view
was significantly increased in hormonally naïve, high-risk prostate
cancer tissues compared with benign tissue samples. The one-way
ANOVA test revealed significant differences among the groups
(P=0.014); the post-hoc Tukey's test confirmed the significant
difference in the apoptosis frequency was between group 1
(12.40±15.08) and group 2 (49.33±14.40) (P=0.014). Furthermore, the
number of apoptotic cells in samples from patients who received ADT
treatment (groups 3 and 4) was mainly decreased compared with
patients who did not receive any treatment (group 2). Although
there was no significant difference in the number of apoptotic
cells between groups 2 and 3 (P=0.215; group 3 mean ± SD,
28.67±26.39), the number of apoptotic cells in group 4 was
significantly decreased compared with that in group 2 (P=0.049;
group 4 mean ± SD, 19.00±6.71) (Fig.
3).
Discussion
The present study demonstrated that ADT and Abi
therapy (groups 3 and 4) had a therapeutic effect on the
histological structure of prostate cancer cells compared with group
2 (no treatment), which was consistent with other reports (13,14).
This effect was characterized by the loss of glandular
architecture, nuclear hyperchromasia and pyknosis, nuclear
shrinkage and a clear cytoplasm. In addition, malignant cells were
arranged in clusters, rows or remained individual. Furthermore,
benign prostate glands presented with basal cell hyperplasia,
enlargement and clearing of the cytoplasm following ADT in both
groups 3 and 4.
The changes in the ultrastructure of prostate cancer
secretory cells reported in the present study were consistent with
previous findings (15–18). The current study demonstrated the
high incidence of hyperchromatic nuclei and compact nucleoli in
prostate cancer secretory cells. Furthermore, pleiomorphic changes
in the mitochondria were observed in prostate cancer secretory
cells, including mitochondrial swelling and altered cristae
morphology, which were associated with clinical progression (groups
2–4). Similar to Kaighn et al (16), the present study reported a
disruption in the integrity of the desmosomes in the prostate
secretory cells from groups 3 and 4, and the presence of solitary
cells in the stroma. Occurrence of hyperchromatic nuclei and
compact nucleoli was high in cells from group 4 (mCRPC). In this
group, an increased incidence of electron-dense, dark mitochondria
with impaired cristae morphology were also identified. These
morphological changes were associated with clinical progression of
the disease.
Apoptosis involves numerous changes in cells,
leading to the death of functionally impaired cells (19,35). In
oncology, apoptosis is triggered by a variety of antitumor drugs,
radiation and hyperthermia, and the intrinsic propensity of tumor
cells to respond by apoptosis is modulated by expression of several
oncogenes (36). Apoptosis may
therefore be considered as a prognostic marker for cancer treatment
(37). Numerous markers are used for
the detection of apoptosis in tumor cells, including caspase-3,
Annexin V, poly ADP ribose polymerase, apoptotic peptidase
activating factor 1, Bax, Bid, Bcl-2, p53 tumor suppressor gene or
Fas receptor (21,38). The analysis of apoptotic markers was
not performed in the present study due to the small number of
tissue samples from patients. Subsequently, TUNEL assay, which is
based on DNA fragmentation, was used to assess apoptosis. In the
present study, the functional process of apoptosis was only
assessed as a final stage of cell death, which was a limitation.
However, apoptosis can also be identified morphologically. Further
investigation focusing on the functional and morphological
determination of apoptosis may therefore be beneficial.
The present study demonstrated that the numbers of
apoptotic cells between patients with BPH (group 1) and patients
with mHNPC (group 2) and mCRPC (group 4) were statistically
different. The association between apoptosis, mHNPC and mCRPC,
despite the small sample size, has a certain scientific
significance. The decrease in apoptosis incidence in tissues from
patients following ADT (group 2 vs. groups 3 and 4) was consistent
with a previous study (39). The
differences observed between the groups were due to the third
group, which was non-homogenous (treatment duration of each patient
was different). Dysregulation of the apoptotic signaling pathway is
associated with the progression of androgen deprivation in CRPC,
which reflects the blockade of apoptosis following ADT (40). In the present study, patients treated
with ADT and presenting with clinical remission (group 3) showed a
high amount of variability in the incidence of apoptosis.
Development of CRPC is individual and depends on the patient. The
expression of numerous protein members of the Bcl-2 family,
including Bcl-2, Bcl-XL and Mci-1, is highlighted during
progression into a castration-resistant metastatic phenotype by
losing the ability for extracellular matrix proteins to bind to the
cell surface (40).
A previous study (41) reported the pathological role of the
Myb gene, which is a transcription factor that is
overexpressed in mCRPC (41). Myb
promotes cell cycle progression and stimulates cell survival in
androgen-supplemented and deprived conditions, respectively,
through induction of Bcl-xL and Bcl2 proteins and downregulation of
p27 and the pro-apoptotic protein Bax. Furthermore, Srivastava
et al (41) reported the
positive role of Myb in the enhanced motility and invasive capacity
and decreased homotypic interactions of prostate cancer cells.
Myb overexpression is also associated with actin
reorganization, which leads to the formation of filopodia-like
cellular protrusions that promote cell migration; furthermore,
Myb enhances the proliferation and androgen
deprivation-resistance of prostate cancer cells and confers to
these cells an aggressive phenotype by facilitating the
epithelial-to-mesenchymal transition (EMT) (42). Several studies have indicated a
direct link between EMT and the generation and development of a
tumor (43,44). Mesenchymal cells are relatively more
motile and exhibit less cell-to-cell communication compared with
cancer cells. Cancer cells gain mesenchymal features during their
progression through EMT (42–44). At
the ultrastructural level, the present study demonstrated that in
cells from group 4, the apical-basal cell polarity was lost,
intercellular joints were disrupted, the basal membrane was
degraded and cells seemed to acquire a mesenchymal-like phenotype
(42).
In conclusion, the aim of the present study was to
describe the biological changes that appear during the clinical
progression of metastatic prostate cancer. A combined approach
including apoptosis assay, IHC, fluorescent and transmission
electron microscopy was used. The results indicated that samples
from patients with mPC consisted of randomly arranged glands with
hyperchromatic nuclei and prominent nucleoli without basal cell
layer, which was also confirmed using IHC examination of
AMACR-positivity and HMW-CK-negativity and ultrastructural
analysis. Furthermore, a decrease in the number of apoptotic cells
was identified in end-stage prostate cancer (group 4). In addition,
ADT therapy caused changes in histological structure and
ultrastructure of prostate tissues. The results from the TUNEL
assay following treatment suggested that the progression of the
disease may be associated with apoptosis dysregulation.
Acknowledgements
The authors would like to thank Mrs. Daniela
Rusevova (Department of Pathology, Faculty Hospital Nitra, Nitra,
Slovak Republic) for the preparation of histological samples.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MK collected, analyzed and interpreted the patient
data, and performed statistical evaluation. MS, HG, AVM, LO and JP
performed the experiments. LB contributed to the analysis and the
interpretation of the data, and reviewed the clinical data. All
authors contributed in writing the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted according to the
Declaration of Helsinki and approved by the Ethics Commission of
the Faculty Hospital Nitra (approval no. 11.10.2018). All patients
provided written informed consent for the use of their tissue and
clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moch H, Humphrey PA, Ulbright TM and
Reuter VI: Tumours of the prostate. WHO Classification of Tumours
of the Urinary System and Male Genital Organs. 4th. Humphrey P:
IARC; Lyon: pp. 138–162. 2016
|
|
3
|
Cheng L and Bostwick DG: Urologic surgical
pathology. E-book. Expert consult title: Online and print. 2nd.
Elsevier Health Sciences; pp. 468–513. 2008
|
|
4
|
Epstein JI and Netto GJ: Grading of
Prostatic adenocarcinoma. Biopsy interpretation of the prostate.
4th. Pine J and McGough J: Wolters Kluwer and Lippincott Wiliams
& Wilkins; pp. 175–198. 2008
|
|
5
|
Bahrami A, Truong LD and Ro JY:
Undifferentiated tumor. Arch Pathol Lab Med. 132:326–348.
2008.PubMed/NCBI
|
|
6
|
Lin F and Liu H: Immunohistochemistry in
undifferentiated neoplasm/tumor of uncertain origin. Arch Pathol
Lab Med. 138:1583–1610. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gleason DF: Histologic grading of prostate
cancer: A perspective. Hum Pathol. 23:273–279. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kryvenko ON and Epstein JI: Prostate
cancer grading. Arch Pathol Lab Med. 140:1140–1152. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Epstein JI, Egewad L, Amin MB, Delahunt B,
Srigley JR and Humphrey PA; Grading Committee, : The 2014
International Society of Urological Pathology (ISUP) Consensus
conference on Gleason grading of prostatic carcinoma: Definition of
grading patterns and proposal for a new grading system. Am J Surg
Pathol. 40:244–252. 2016.PubMed/NCBI
|
|
10
|
Khochikar M: Newly proposed prognostic
grade group system for prostate cancer: Genesis, utility and its
implications in clinical practice. Curr Urol Rep. 17:802016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Offermann A, Hohensteiner S, Kuempers C,
Ribbat-Idel J, Schneider F, Becker F, Hupe MC, Duensing S,
Merseburge AS, Kirfel J, et al: Prognostic value of the new
prostate cancer international society of urological pathology grade
groups. Front Med (Lausanne). 4:1572017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reuter VE: Pathological changes in benign
and malignant prostatic tissue following androgen deprivation
therapy. Urology. 49 (Suppl 3A):S16–S22. 1997. View Article : Google Scholar
|
|
13
|
Montironi R and Schulman CC: Pathological
changes in prostate lesions after androgen manipulation. J Clin
Pathol. 51:5–12. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bostwick DG and Meiers I: Diagnosis of
prostatic carcinoma after therapy. Arch Pathol Lab Med.
131:360–371. 2007.PubMed/NCBI
|
|
15
|
Mao P, Nakao K and Angrist A: Human
prostatic carcinoma: An electron microscope study. Cancer Res.
26:955–973. 1966.PubMed/NCBI
|
|
16
|
Kaighn ME, Narayan KS, Ohnuki Y, Lechner
JF and Jones LW: Establishment and characterization of a human
prostatic carcinoma cell line (PC-3). Invest Urol. 17:16–23.
1979.PubMed/NCBI
|
|
17
|
Hernandez-Verdun D: The nucleolus: A model
for the organisation of nuclear functions. Histochem. Cell Biol.
126:135–148. 2006.
|
|
18
|
Sun X, Liao NK and Yu JJ: Prognostic value
of a mitochondrial functional score in prostate cancer. J Int Med
Res. 40:371–376. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kerr JFR, Wyllie AH and Currie AR:
Apoptosis: A basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schmied M, Breitschopf H, Gold R, Zichler
R, Rothe G, Wekerle H and Lassmannet A: Apoptosis of T lymphocytes
in experimental autoimmune encephalomyelitis: Evidence for
programmed cell death as a mechanism to control inflammation in the
brain. Am J Pathol. 143:446–452. 1993.PubMed/NCBI
|
|
21
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carson DA and Ribeiro JM: Apoptosis and
disease. Lancet. 341:1251–1254. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gougeon ML and Montagnier L: Apoptosis in
AIDS. Science. 260:1269–1270. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rył A, Rotter I, Kram A, Teresiński L,
Słojewski M, Dołęgowska B, Lubkowska A, Piasecka M and Laszczyńska
M: Apoptosis and proliferation of the prostate cells in men with
benign prostatic hyperplasia and concomitant metabolic disorders.
Histol Histopathol. 33:389–397. 2017.PubMed/NCBI
|
|
25
|
Gaffney EF: The extent of apoptosis in
different types of high-grade prostatic carcinoma. Histopathology.
25:269–273. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aihara M, Truong LD, Dunn JK, Wheeler TM,
Scardino PT and Thompson TC: Frequency of apoptosis bodies
positively correlates with Gleason grade in prostate cancer. Hum
Pathol. 25:797–801. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aihara M, Scardino PT, Truong LD, Wheeler
TM, Goad JR, Yang G and Thompson TC: The frequency of apoptosis
correlates with the prognosis of Gleason grade 3 adenocarcinoma of
the prostate. Cancer. 75:522–529. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Staunton MJ and Gaffney EF: Tumor type is
a determinant of susceptibility to apoptosis. Am J Clin Pathol.
103:300–307. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Drachenberg CB, Ioffe OB and Papadimitriou
JC: Progressive increase of apoptosis in prostatic intraepithelial
neoplasia and carcinoma: Comparison between in situ end-labeling of
fragmented DNA and detection by routine hematoxylin-eosin staining.
Arch Pathol Lab Med. 121:54–58. 1997.PubMed/NCBI
|
|
30
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mottet N, Bellmunt J, Bolla M, Briers E,
Cumberbatch MG, De Santis M, Fossati N, Gross T, Henry AM, Joniau
S, et al: EAU-ESTRO-SIOG Guidelines on prostate cancer. Part 1:
Screening, diagnosis, and local treatment with curative intent. Eur
Urol. 71:618–629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Reynolds ES: The use of lead citrate at
high pH as an electron-opaque stain in electron microscopy. J Cell
Biol. 17:2081963. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Têtu B: Morphological changes induced by
androgen blockade in normal prostate and prostatic carcinoma. Best
Pract Res Clin Endocrinol Metab. 22:271–283. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Krakhmal NV, Zavyalova MV, Denisov EV,
Vtorushin SV and Perelmuter VM: Cancer invasion: Patterns and
mechanisms. Acta Naturae. 7:17–28. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sebastiano C, Vincenzo F, Tommaso C,
Giuseppe S, Marco R, Ivana C, Giorgio R, Massimo M and Giuseppe M:
Dietary patterns and prostatic diseases. Front Biosci (Elite Ed).
4:195–204. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stoff JA: Selected office based anticancer
treatment strategies. J Oncol. 2019:74625132019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hickmen JA: Apoptosis induced by
anti-cancer drugs. Cancer Metastasis Rev. 11:121–139. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
D'Arcy MS: Cell death: A review of the
major forms of apoptosis, necrosis and autophagy. Cell Biol Int.
43:582–592. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li M, Yang X, Wang H, Xu E and Xi Z:
Inhibition of androgen induces autophagy in benign prostate
epithelial cells. Int J Urol. 21:195–199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Krajewska M, Krajewski S, Epstein JI,
Shabaik A, Sauvageot J, Song K, Kitada S and Reed JC:
Immunohistochemical analysis of bcl-2, bax, bcl-X, and mcl-1
expression in prostate cancers. Am J Pathol. 148:1567–1576.
1996.PubMed/NCBI
|
|
41
|
Srivastava SK, Bhardwaj A, Singh S, Arora
S, McClellan S, Grizzle WE, Reed E and Singh AP: Myb overexpression
overrides androgen depletion-induced cell cycle arrest and
apoptosis in prostate cancer cells, and confers aggressive
malignant traits: Potential role in castration resistance.
Carcinogenesis. 33:1149–1157. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sun BO, Fang Y, Li Z, Chen Z and Xiang J:
Role of cellular cytoskeleton in epithelial-mesenchymal transition
process during cancer progression. Biomed Rep. 3:603–610. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shankar J, Messenberg A, Chan J, Underhill
TM, Foster LJ and Nabi IR: Pseudopodial actin dynamics control
epithelial-mesenchymal transition in metastatic cancer cells.
Cancer Res. 70:3780–3790. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jolly MK and Celià-Terrassa T: Dynamics of
phenotypic heterogeneity associated with emt and stemness during
cancer progression. J Clin Med. 8:15422019. View Article : Google Scholar
|