Introduction
Hepatocellular carcinoma (HCC) is one of the most
prevalent types of malignancy worldwide, with a rich blood supply
and high levels of metastasis and recurrence (1,2). The
overall 5-year survival rate for HCC is ~30% (3). HCC has been found to invade blood
vessels, resulting in intra- and extrahepatic metastases (4). At present, hepatectomy and liver
transplantation are key treatments with curative potential in
patients with HCC (5). However, the
overall survival (OS) rate of patients with HCC remains
unsatisfactory due to the high incidence of tumor recurrence and
metastasis (6). Thus, there is a
requirement to identify novel biomarkers for the progression of HCC
to enhance the OS rate of patients.
Sphingosine-1-phosphate (S1P) is a vital metabolite
that serves a key role in intra- and intercellular signal
transduction (7). S1P regulates
multiple cellular functions, such as cell proliferation, migration,
adhesion and inflammation, via high-affinity G protein-coupled S1P
receptors (S1PRs) (8), which affect
cellular activities by inhibiting cell apoptosis and promoting cell
proliferation (9). Furthermore,
activation of S1PR1 is involved in the regulation of numerous cell
behaviors associated with aggressiveness and cancer progression,
including tumor growth, invasion and migration (10,11).
Previous studies have also found that S1P and its receptors are
implicated in the progression of HCC (12,13).
The function of the renin-angiotensin system (RAS)
in tumor invasion or metastasis has previously been reported
(14). High protein and mRNA
expression levels of RAS components in human pancreatic cancer
tissues have been associated with tumor grade and clinical
prognosis (15). Angiotensin II (Ang
II), an important peptide of RAS, exerts its effects by activating
Ang II receptor type 1 (AT1R) and AT2R. AT1R has been found to be
involved in the majority of essential physiological actions, such
as blood pressure and sodium balance, and pathophysiological
actions, such as inflammation and angiogenesis, of Ang II (16,17). Ang
II and AT1R have essential roles in tumor survival, angiogenesis
and proliferation in all types of cancer (18), and have also been associated with the
progression and metastasis of HCC (19–21).
Ang II induces proliferation of smooth muscle cells
by the S1P signaling pathway (16).
Ang II is also able to augment S1PR1 protein and mRNA expression
levels and migration of rat aortic smooth muscle cells, which can
be inhibited by treatment with antagonists of AT1R and S1PR1
(22). However, the association and
roles of Ang II/AT1R and S1P/S1PR1 in HCC have remained largely
elusive. Therefore, the aim of the present study was to investigate
the association between AT1R and S1PR1 mRNA and protein expression
levels and the clinicopathological characteristics of patients with
HCC, as well as the progression of HCC. Additionally, the utility
of these potential biomarkers in the prediction of prognosis in
affected patients was assessed.
Materials and methods
Patients and tissue samples
A total of 75 patients with HCC (51 males and 24
females) who underwent resection of HCC at the Second Affiliated
Hospital of Xi'an Jiaotong University (Xi'an, China) between
January 2013 and January 2017 were included in the present
retrospective study. Data was also collected from the medical files
of the patients. Liver cancer and adjacent normal tissues (≥5 cm
from the tumor) were collected from the 75 patients and frozen at
−180°C for further analysis. Serum samples (n=75) were collected
from 4 ml peripheral blood and stored at 4°C in a 5-ml tube. After
centrifugation at 3,000 × g for 10 min at 4°C, the obtained serum
(1 ml) was stored at −80°C. Serum samples from the control group
were collected from 66 healthy subjects without any disease,
matched according to sex and age. The patients did not receive any
chemotherapy, radiotherapy or immunotherapy prior to surgery. The
liver cancer and adjacent normal tissues were collected for
immunohistochemical analysis. According to the Ethics Committee of
the Second Affiliated Hospital of Xi'an Jiaotong University (Xi'an,
China), all participants provided written informed consent.
Clinical data (including age, sex, stage, tumor size, intrahepatic
metastasis, portal vein invasion and Edmondson grade) were
collected from the medical records of each patient (Table I). All the specimens were graded
using the Edmonson method (23).
According to the differentiation degree of tumor cells, HCC tissues
were categorized into grades I–III. Grade I was defined as
low-grade HCC, grade II as medium-grade HCC and grade III as
high-grade HCC. Furthermore, the TNM stage was defined according to
the American Joint Committee on Cancer TNM Staging for Liver Tumors
as follows (24): TX, primary tumor
cannot be assessed; T0, no evidence of primary tumor; T1, solitary
tumor without vascular invasion; T2, solitary tumor with vascular
invasion or multiple tumors <5 cm in size; T3a, multiple tumors
>5 cm in size; T3b, single tumor or multiple tumors of any size,
involving a major branch of the portal vein or hepatic vein; and
T4, tumor(s) with direct invasion of adjacent organs, other than
the gallbladder, or with perforation of visceral peritoneum.
 | Table I.Association between AT1R and S1PR1
expression levels and pathological characteristics of patients with
hepatocellular carcinoma. |
Table I.
Association between AT1R and S1PR1
expression levels and pathological characteristics of patients with
hepatocellular carcinoma.
|
|
| AT1R
expression |
| S1PR1
expression |
|
|---|
|
|
|
|
|
|
|
|---|
| Characteristic | Patients, n | Low, n (%) | High, n (%) | P-value | Low, n (%) | High, n (%) | P-value |
|---|
| Sex |
|
|
| 0.134 |
|
| >0.999 |
|
Male | 51 | 19 (37.3) | 32 (62.7) |
| 23 (45.1) | 28 (54.9) |
|
|
Female | 24 | 14 (58.3) | 10 (41.7) |
| 11 (45.8) | 13 (54.2) |
|
| Age, years |
|
|
| 0.337 |
|
| 0.097 |
|
≥50 | 49 | 23 (46.9) | 26 (53.1) |
| 20 (40.8) | 29 (59.2) |
|
|
<50 | 26 | 9 (34.6) | 17 (65.4) |
| 16 (61.5) | 10 (38.5) |
|
| Tumor size, cm |
|
|
| 0.602 |
|
| 0.589 |
| ≥4 | 55 | 25 (45.5) | 30 (54.5) |
| 17 (30.9) | 38 (69.1) |
|
|
<4 | 20 | 11 (55.0) | 9 (45.0) |
| 6 (30.0) | 14 (70.0) |
|
| Hepatitis B surface
antigen |
|
|
| >0.999 |
|
| 0.759 |
|
Positive | 61 | 28 (45.9) | 33 (54.1) |
| 22 (36.1) | 39 (63.9) |
|
|
Negative | 14 | 6 (42.9) | 8 (57.1) |
| 4 (28.6) | 10 (71.4) |
|
| Serum
α-fetoprotein, ng/ml |
|
|
| 0.450 |
|
| >0.999 |
|
≥400 | 53 | 23 (43.4) | 30 (56.6) |
| 20 (37.7) | 33 (62.3) |
|
|
<400 | 22 | 12 (54.5) | 10 (45.5) |
| 8 (36.4) | 14 (63.6) |
|
| Intrahepatic
metastasis |
|
|
| 0.017a |
|
| 0.008b |
|
Yes | 48 | 16 (33.3) | 32 (66.7) |
| 13 (27.1) | 35 (72.9) |
|
| No | 27 | 17 (63.0) | 10 (37.0) |
| 16 (59.3) | 11 (40.7) |
|
| Portal vein
invasion |
|
|
| 0.033a |
|
| 0.015a |
|
Yes | 46 | 15 (32.6) | 31 (67.4) |
| 12 (26.1) | 34 (73.9) |
|
| No | 29 | 17 (58.6) | 12 (41.4) |
| 16 (55.2) | 13 (44.8) |
|
| Edmondson
grade |
|
|
| 0.017a |
|
| 0.008b |
| I | 13 | 8 (61.5) | 5 (38.5) |
| 9 (69.2) | 4 (30.8) |
|
| II | 40 | 15 (37.5) | 25 (62.5) |
| 13 (32.5) | 27 (67.5) |
|
|
III | 22 | 12 (54.5) | 10 (45.5) |
| 15 (68.2) | 7 (31.8) |
|
| TNM stage |
|
|
| 0.004b |
|
| 0.015a |
|
I–II | 48 | 18 (37.5) | 30 (62.5) |
| 23 (47.9) | 25 (52.1) |
|
|
III–IV | 27 | 20 (74.1) | 7 (25.9) |
| 21 (77.8) | 6 (22.2) |
|
The patients were followed up from January 2016 to
January 2020, initially every 2 months and at least every 3–6
months following surgery. Furthermore, the liver function and serum
α-fetoprotein (AFP) levels were examined, and abdominal
ultrasonography was performed. If recurrence was suspected, CT or
MRI scan was performed immediately. OS and recurrence-free survival
(RFS) times were defined as the interval between surgery and death
or recurrence, respectively. If recurrence was not diagnosed, the
patient was examined at the date of death or at the last follow-up
evaluation. All of the aforementioned investigations were performed
as previously described (25,26).
ELISA
Serum samples were collected from the peripheral
blood samples, centrifuged at 4,000 × g for 10 min at −4°C and then
stored at −80°C for further analysis. According to the
manufacturer's instructions, Ang II (cat. no. ADI-900-204; Enzo
Life Sciences, Inc.) and S1P levels (cat. no. abx585002; Abbexa
Ltd.) were assessed using their respective ELISA kits. Each
analysis was performed in triplicate.
Western blot analysis
As previously described (27), total protein was extracted using RIPA
lysis buffer (cat. no. PP1901; BioTeke Corporation), and protein
concentration was determined using a BCA protein detection kit
(cat. no. P0010; Beyotime Institute of Biotechnology). Protein
samples (25 µg/lane) were separated via 10% SDS-PAGE and
transferred onto PVDF membranes (Bio-Rad Laboratories, Inc.), then
blocked with 5% skimmed milk in TBS buffer containing 0.1% Tween-20
at 25°C for 2 h, and incubated with specific antibodies against
AT1R (1:500; cat. no. ab124734), S1PR1 (1:500; cat. no. ab77076)
and β-actin (1:1,000; cat. no. ab200658) at −4°C overnight (all
Abcam). β-actin was used as the internal control. Subsequently,
membranes were incubated with an HRP-conjugated goat anti-rabbit
IgG H&L secondary antibody (1:5,000; cat. no. ab7090; Abcam) at
37°C for 1 h. Enhanced chemiluminescence reagents (Pierce; Thermo
Fisher Scientific, Inc.) were used to visualize the blots, and the
optical densities of the bands were scanned and quantified using
Syngene Gene Tools (Syngene Europe; model G box chem hr16; serial
no. SYDR4/2327). A total of three independent experiments was
performed.
Reverse transcription-quantitative
(RT-q)PCR
mRNA levels were detected as previously described
(27). Total RNA was extracted using
the TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). cDNA was synthesized from total RNA (1 µg) using
a PrimeScript™ RT master mix kit (Takara Bio, Inc.) according to
the manufacturer's instructions. qPCR was performed using a SYBR
Premix Ex Taq™ II Perfect Real-Time kit (Takara Bio, Inc.) on an
ABI qPCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. Each sample was
run in triplicate. Primers for AT1R, S1PR1 and β-actin were
designed using the Beacon designer 4.0 software (version 4.0;
Premier Biosoft International). The primer sequences were as
follows: AT1R forward, 5′-AGACTGGCCTTCTCTGGA-3′ and reverse,
5′-CACCGAGGAATACGCTTT-3′; S1PR1 forward, 5′-CACGCTTTCTGTGGCTTGGA-3′
and reverse, 5′-CGACGATGGCGCTCCAACA-3′; and β-actin forward,
5′-TGGCACCCAGCACAATGAA-3′ and reverse,
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′. The RT-PCR products were observed
using electrophoresis and 2% agarose gels with ethidium bromide. A
melting point dissociation curve was used to indicate that only a
single product was shown. For qPCR, relative mRNA levels were
analyzed using the 2−ΔΔCt method (28). Data were standardized to β-actin mRNA
levels. A total of three independent experiments were
performed.
Immunohistochemistry
Liver cancer tissue samples were collected from 75
patients with HCC following hepatic resection at the Second
Affiliated Hospital of Xi'an Jiaotong University between January
2013 and January 2017. During the same period, adjacent normal
liver tissue samples were also obtained. All specimens (adjacent
normal and liver cancer tissues) were investigated using
immunohistochemistry. Tissue samples were fixed in 10% formalin at
room temperature for 48 h and embedded in paraffin. Next, 5-µm
sections were deparaffinized for antigen retrieval in citric acid
buffer (pH 6.0; 95°C for 20 min). Sections were washed 3 times with
PBS for 3 min each, 3 times with xylene for 5 min each, 2 times
with 100% ethanol for 10 min each, 2 times with 95% ethanol for 10
min each and finally 2 times with double-distilled water for 5 min
each. Slides were treated with 3% hydrogen peroxide at room
temperature for 10 min to block endogenous peroxidase activity.
After blocking in 1% BSA (Sigma-Aldrich; Merck KGaA) at room
temperature for 20 min, the slides were incubated with rabbit
anti-human AT1R (1:50; cat. no. ab124734; Abcam) and S1PR1 (1:100;
cat. no. ab77076; Abcam) antibodies overnight at 4°C. Subsequently,
the slides were incubated with 2 µg/ml biotinylated anti-rabbit IgG
secondary antibody (XianFeng Bioengineering Institute) at room
temperature for 40 min. Next, the sections were stained using a
Standard Ultra-Sensitive ABC Peroxidase Staining kit (Thermo Fisher
Scientific, Inc.), incubating the tissue sections with the ABC
Reagent (Reagent A, 2 ml; Reagent B, 2 ml) at room temperature for
30 min. Bright-field images (magnification, ×200 and ×400) were
captured using an Axio Scan Z1 light microscope (Zeiss GmbH).
Immunostaining results were independently observed and interpreted
by two pathologists blinded to the clinical data. Weak and strong
staining were distinguished via the color of brown-yellow granules.
When the brown-yellow granules were dark in the HCC tissues, this
was considered as strong staining (high expression), while when the
brown-yellow granules were weak in the HCC tissues, this was
considered as weak staining (low expression).
Hepatitis B surface antigen (HBsAg)
quantification
Quantification of HBsAg was performed using an
automated chemiluminescent microparticle immunoassay using the
ARCHITECT i2000 system (Abbott Pharmaceutical Co., Ltd.). All
procedures were performed according to the manufacturer's protocol
(29). The sensitivity and the
specificity of this assay are 100 and 99.76%, respectively,
according to the manufacturer's protocol.
Statistical analysis
Data are expressed as the mean ± standard deviation.
A Fisher's exact test was performed to determine whether AT1R and
S1PR1 expression levels in HCC were associated with
clinicopathological characteristics. A two-tailed paired or
unpaired Student's t-test was used to determine significant
differences between groups. The Kaplan-Meier method was used for
survival analysis, and differences in survival were investigated
using the log-rank test. These statistical analyses were performed
using SPSS software (version 19.0; IBM Corp.). Correlation between
Ang II and S1P, and between AT1R and S1PR1 in patients with HCC
were analyzed using Pearson's correlation coefficient (GraphPad
Prism 8; GraphPad Software, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
AT1R and S1PR1 expression levels in
HCC tissue are associated with clinicopathological
characteristics
The clinical characteristics and laboratory
parameters of patients with HCC are summarized in Table I. The results indicated that AT1R and
S1PR1 expression levels were not significantly associated with
patient sex, age, levels of HBsAg or serum AFP, or tumor size.
However, AT1R and S1PR1 expression levels in HCC were associated
with intrahepatic metastasis (P=0.017 and P=0.008, respectively),
portal vein invasion (P=0.033 and P=0.015, respectively), Edmondson
grade (P=0.017 and P=0.008, respectively) and TNM stage (P=0.004
and P=0.015, respectively).
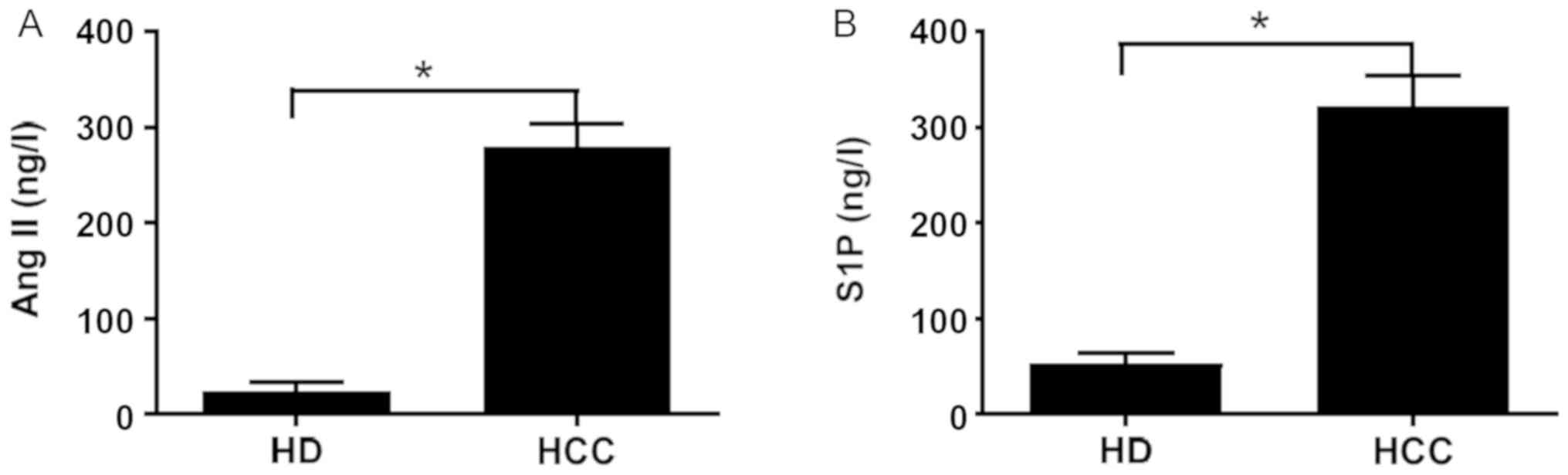
Ang II and S1P levels are elevated in
the serum of patients with HCC
The Ang II and S1P levels in the serum collected
from the 66 healthy donors and 75 patients with HCC were
determined, and the results revealed that both the serum levels
were significantly higher in patients with HCC compared with those
in healthy donors (Fig. 1).
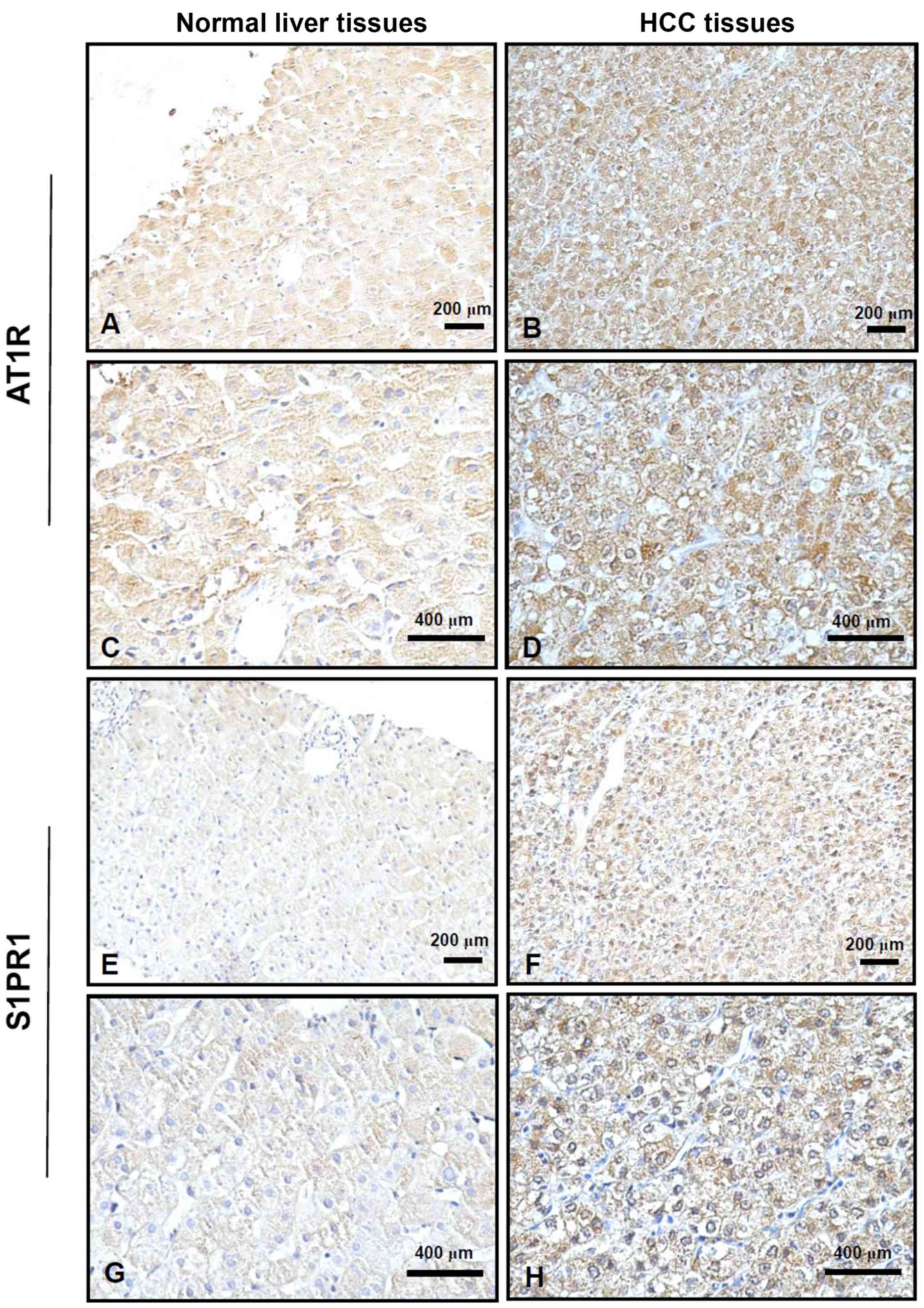
AT1R and S1PR1 are upregulated in HCC
tissue
Next, AT1R and S1PR1 protein expression levels were
investigated in HCC tissues from 75 patients using
immunohistochemistry. Positive staining for AT1R and S1PR1 was
displayed as brown-yellow granules, which were primarily located in
the cell membrane or cytoplasm. The cells with positive staining
exhibited a clear tissue cell structure, where uniform brown-yellow
granules were present in the cell membrane or cytoplasm and
staining was markedly higher compared with that in the background.
In human normal liver tissue, AT1R and S1PR1 were weakly expressed,
whereas they were strongly expressed in human HCC tissue (Fig. 2).
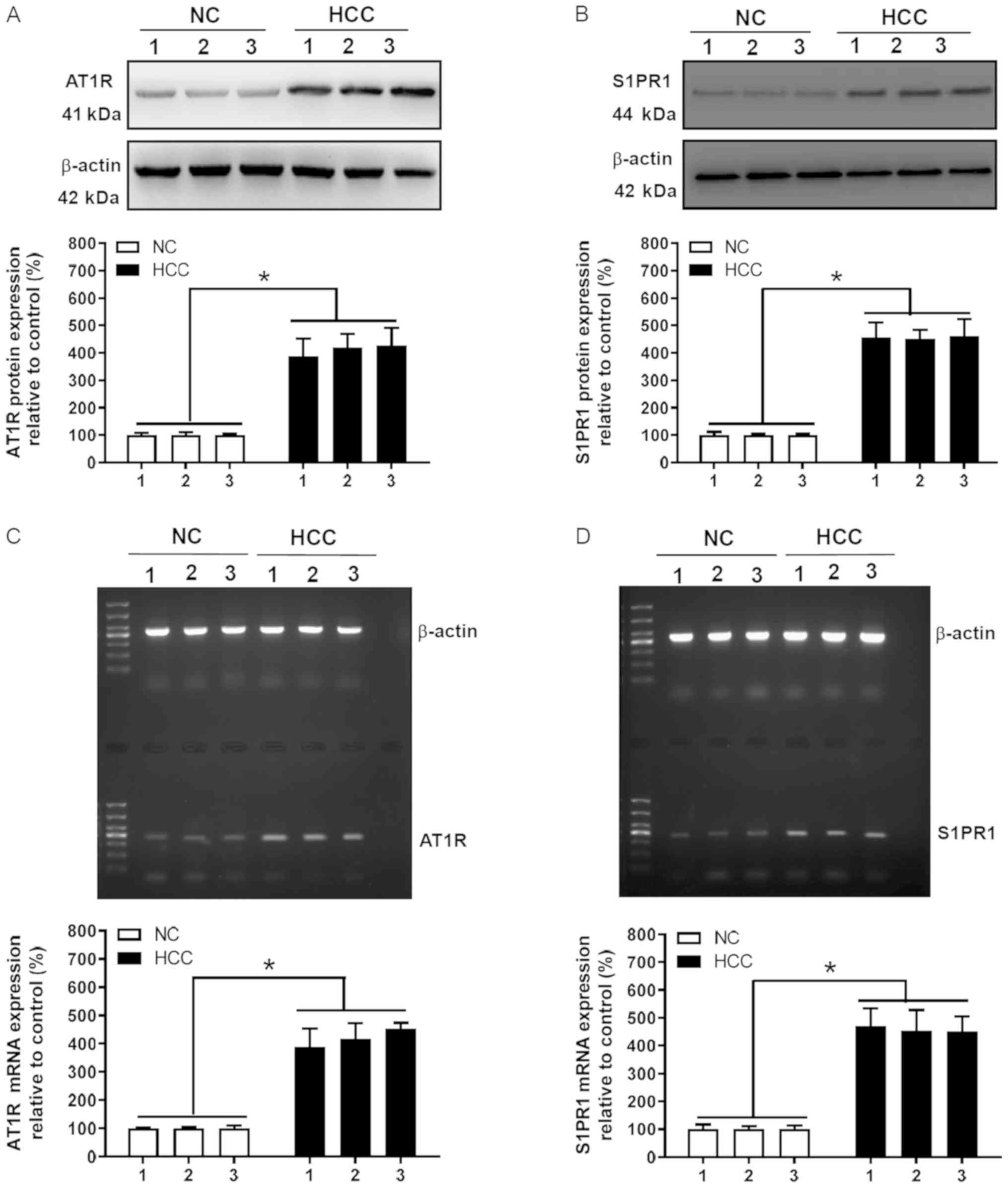
Protein and mRNA expression levels of
AT1R and S1PR1 are elevated in HCC tissues
To further assess AT1R and S1PR1 protein and mRNA
expression levels in HCC, liver cancer and adjacent normal tissue
obtained from 3 representative samples (according to IHC staining)
among the 75 HCC samples were subjected to western blot and RT-qPCR
analyses. AT1R and S1PR1 protein expression levels in HCC tissue
were significantly higher compared with those in normal liver
tissue (P<0.05; Fig. 3A and B).
In addition, RT-qPCR was performed to quantify AT1R and S1PR1 mRNA
expression levels in HCC tissues. AT1R and S1PR1 mRNA expression
levels in HCC tissue were significantly higher compared with those
in normal liver tissue (P<0.05; Fig.
3C and D), which was consistent with western blot analysis
results. In summary, western blot, RT-qPCR and immunohistochemical
analyses demonstrated that AT1R and S1PR1 expression was increased
in HCC tissue.
Significant positive correlation
between Ang II and S1P and between AT1R and S1PR1 in patients with
HCC
The serum levels of Ang II and S1P in 66 healthy
donors and 75 patients with HCC were determined using ELISA. A
positive correlation was identified between Ang II and S1P in
patients with HCC (P=0.002), but not in healthy donors (P=0.228;
Fig. 4A and B). Furthermore, the
correlation between AT1R and S1PR1 protein expression levels in the
75 HCC and normal adjacent tissues was also investigated and there
was no correlation between AT1R and S1PR1 levels in the normal
liver samples (P=0.081); however, there was a correlation in HCC
tissue samples (P=0.001; Fig. 4C and
D).
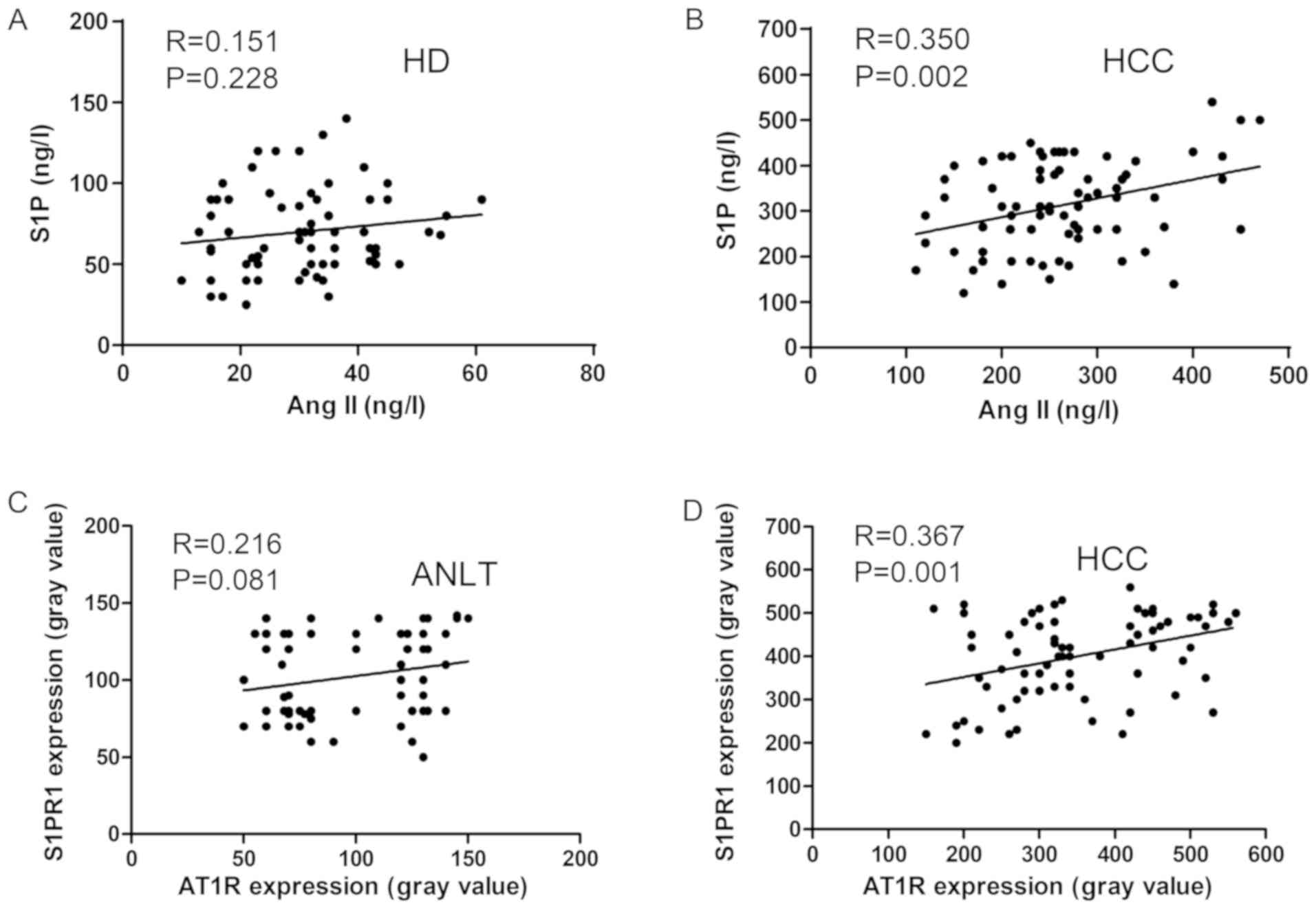 | Figure 4.Correlation between Ang II and S1P,
and AT1R and S1PR1 in HD (n=66) and patients with HCC (n=75). A
correlation between Ang II and S1P levels was not observed in (A)
HD, but was present in (B) patients with HCC. A correlation between
AT1R and S1PR1 protein expression levels was not observed in (C)
ANLT, but was present in (D) HCC tissues. Ang II and S1P levels
were detected using ELISA. AT1R and S1PR1 protein expression levels
were determined using western blot analysis. Ang II, angiotensin
II; S1P, sphingosine-1-phosphate; AT1R, angiotensin II receptor
type 1; S1PR1, sphingosine-1-phosphate receptor 1; HD, healthy
donors; HCC, hepatocellular carcinoma; ANLT, adjacent normal liver
tissues. |
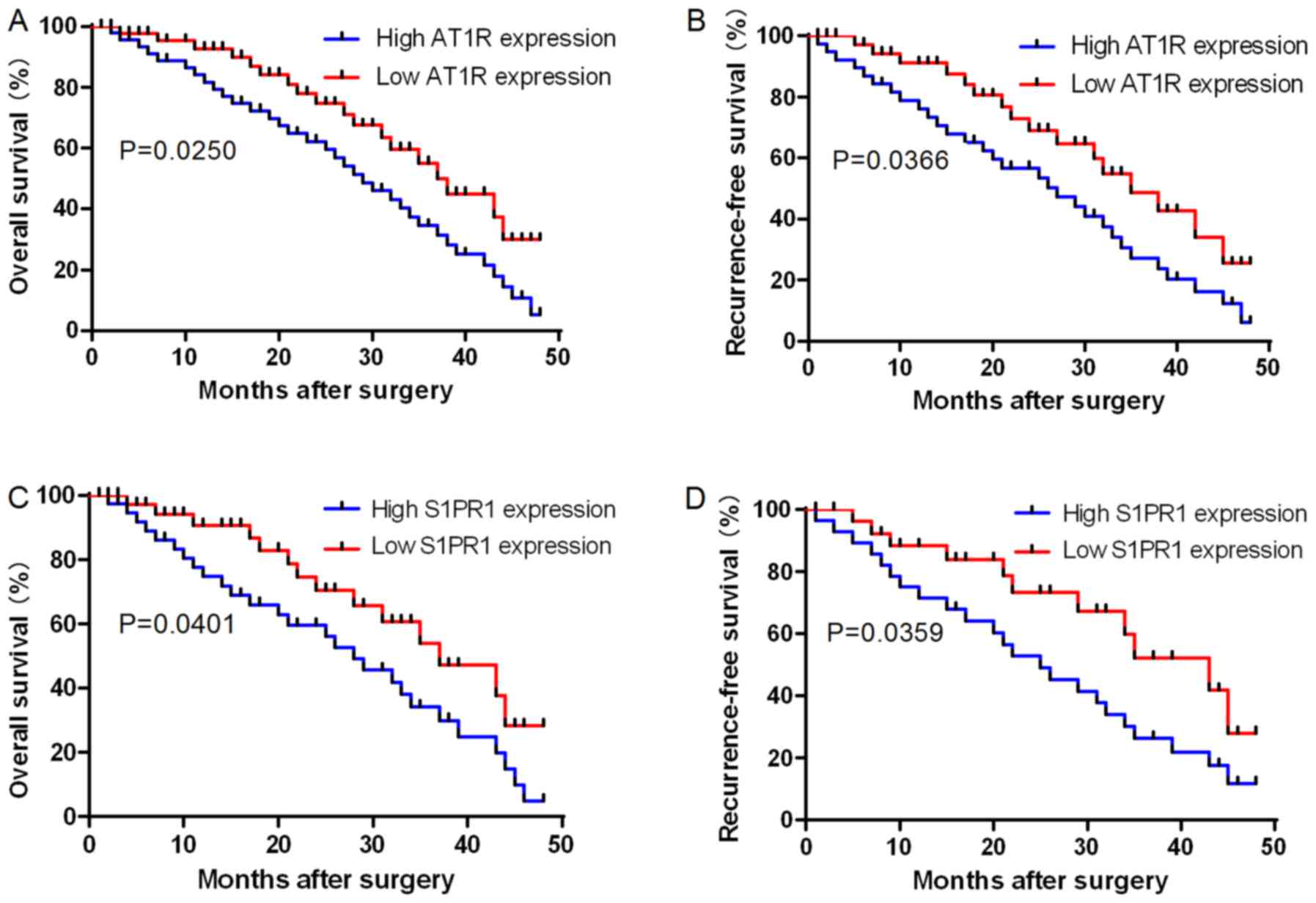
AT1R and S1PR1 protein levels are
associated with prognosis of patients with HCC
Kaplan-Meier survival curves were used to determine
the effects of AT1R and S1PR1 protein expression levels via
immunohistochemical analyses on the prognosis of patients with HCC.
The results revealed that OS and RFS rates were significantly lower
in patients with high AT1R expression levels compared with those in
patients with low AT1R levels (P=0.0250 and P=0.0366, respectively;
Fig. 5A and B). The results also
revealed that OS and RFS rates were significantly lower in patients
with high S1PR1 expression levels compared with those in patients
with low S1PR1 levels (P=0.0401 and P=0.0359, respectively;
Fig. 5C and D). The present results
indicated that the 50-month survival rate of patients with HCC with
high AT1R and S1PR1 expression was low. These results demonstrated
that AT1R and S1PR1 upregulation was significantly associated with
patient death and HCC recurrence.
Discussion
Evidence indicates that S1P and its receptors are
associated with the pathophysiology of cancer and have a key role
in cancer development (30).
Elevated serum levels of S1P in patients with different types of
cancer, including HCC, have been associated with a poor clinical
prognosis (12,13). S1P is a metabolite that serves a
vital role in intra- and intercellular signal transduction
(7). Previous studies have found
that S1P and its receptors are involved in the development of HCC
(12,13). Consistent with the results from a
previous study, the present study indicated that the serum S1P
levels were significantly higher in patients with HCC compared with
those in healthy donors (13). The
present study inferred that the increased serum S1P levels may be
due to changes in sphingolipid metabolism in liver cancer cells.
Ang II, a central component of RAS, induces angiogenesis,
proliferation and inflammation in HCC tissues via its primary
receptor, AT1R (31). Furthermore,
Ang II and AT1R are reported to be associated with tumor grade and
clinical outcome in HCC (32). In
addition its roles in cardiovascular disease and renal injury, Ang
II has been associated with cell proliferation and inflammation,
and may offer a potential target for the prevention and treatment
of HCC (19). Consistent with
previous studies (32,33), the present study demonstrated that
serum Ang II levels were significantly higher in patients with HCC
compared with those in healthy donors. A previous study found that
inflammation is associated with liver cancer (34). The present study speculated that Ang
II stimulates angiogenesis, inflammation and proliferation in HCC
tissue, which may increase the levels of active peptide Ang II. In
the present study, Ang II and AT1R levels in the serum and liver
tissue of patients with HCC, respectively, were significantly
elevated. The present results suggested that Ang II and S1P levels
may be associated with cell growth, migration and invasion, and
with the prognosis of patients with HCC (Table I). However, it is not clear whether
the increase of Ang II and S1P levels in the serum was consistent
with that in HCC tissues.
Immunohistochemical analysis revealed that AT1R and
S1PR1 were strongly expressed in human HCC tissue. In addition,
AT1R and S1PR1 expression levels in patient tissue were assessed at
the mRNA and protein levels using RT-qPCR and western blot
analysis, respectively, which demonstrated that AT1R and S1PR1 mRNA
and protein expression levels were increased in HCC tissue compared
with those in normal adjacent liver tissue. Xu et al
(33) found that Ang II protein
expression levels were notably higher in human liver cancer tissue
compared with those in normal adjacent tissues. Further
investigations are required to assess protein and mRNA expression
levels of Ang II and S1P in liver cancer tissue, as this was not
performed in the present study. Notably, upregulation of AT1R and
S1PR1 was associated with well-known cancer progression-associated
clinicopathological parameters, including intrahepatic metastasis,
portal vein invasion, histological differentiation and TNM stage in
the present study, which indicates that upregulation of AT1R and
S1PR1 may be a significant contributing factor in the progression
of HCC.
HCC is one of the major causes of cancer-associated
morbidity and mortality. HCC is the sixth most common cancer
worldwide, being the fifth in men and the eighth in women, and
accounting for ~5.7% of all new cancer cases (35). Annually, around 1% of all deaths in
the world are associated with HCC (35). Despite the development of novel
diagnostic biomarkers, the OS rate of patients with HCC remains
unsatisfactory. Thus, in the present study, the potential
association between Ang II/S1P and AT1R/S1PR1 in HCC was
investigated, and the roles of AT1R and S1PR1 protein expression
levels in the progression and prognosis of HCC were examined. The
results indicated that a higher serum Ang II level was correlated
with a higher serum S1P level, and that higher AT1R expression
levels were correlated with higher expression levels of S1PR1 in
HCC tissue. Therefore, a positive correlation exists between Ang II
and S1P, as well as between AT1R and S1PR1 in HCC.
Consistent with previous studies (16,20), the
present study demonstrated that Ang II and AT1R were associated
with intrahepatic metastasis, portal vein invasion, tumor grade and
clinical outcome. S1P serves a key role in the tumor by regulating
cell survival, growth and invasion (36). In addition, S1P and S1PR1
overexpression in HCC have been associated with a poor prognosis
(37,38). The prognosis of patients with HCC
remains unfavorable due to high recurrence and metastasis rates,
and the 5-year OS rate of patients with HCC has been reported to be
16% (39,40). In the present study, the association
between AT1R and S1PR1 protein expression levels and OS and RFS in
patients with HCC was further investigated. AT1R and S1PR1 high
expression was significantly associated with patient mortality and
the recurrence of HCC. Notably, the 50-month survival rate of
patients with high AT1R and S1PR1 expression was low. Patients with
high AT1R and S1PR1 protein expression levels exhibited poor
outcomes with regard to OS and RFS compared with patients with low
AT1R and S1PR1 expression levels, suggesting that AT1R and S1PR1
may serve as valuable prognostic biomarkers for patients with
HCC.
In conclusion, the present study demonstrated that
Ang II and S1P levels were elevated in the serum of patients with
HCC, and that AT1R and S1PR1 mRNA and protein expression levels
were also upregulated in HCC tumor tissue. Furthermore, the results
demonstrated an association between AT1R and S1PR upregulation and
HCC progression and pathological characteristics, including
intrahepatic metastasis, portal vein invasion, TNM stage and
histological differentiation via Edmondson grade analysis. In
addition, a correlation between Ang II/S1P and AT1R/S1PR1 in HCC
was also identified. The present study indicated that Ang II/AT1R
and S1P/S1PR1 may be valuable indicators of a poor prognosis for
patients with HCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Basic Research Program of Shaanxi (grant no. 2020JZ-41),
the Key Research and Development Program of Shaanxi Province (grant
no. 2017SF-313), the Fundamental Research Funds for the Central
Universities of China (grant no. XJJ2017072) and the Personnel
Training Specialized Research Foundation of the Second Affiliated
Hospital of Xi'an Jiaotong University [grant no. RC(GG)201803].
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YYJ and ZW conceived the study. YYJ performed the
experiments and wrote the manuscript. YFJ, HC and WG analyzed data
and provided experimental technical support. LM and YFJ performed
the histopathological evaluation. ZW, ZY and BH interpreted results
from a clinical perspective and analyzed the data. ZW helped to
draft the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All of the experiments were approved by the Ethics
Committee of the Second Affiliated Hospital of Xi'an Jiaotong
University (approval no. 20160352; Xi'an, China). All patients
provided written informed consent in accordance with the
Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Ang II
|
angiotensin II
|
|
AT1R
|
Ang II receptor type 1
|
|
OS
|
overall survival
|
|
RAS
|
renin-angiotensin system
|
|
RFS
|
recurrence-free survival
|
References
|
1
|
Zhang X, Li J, Shen F and Lau WY:
Significance of presence of microvascular invasion in specimens
obtained after surgical treatment of hepatocellular carcinoma. J
Gastroenterol Hepatol. 33:347–354. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Serag HB: Hepatocellular carcinoma: An
epidemiologic view. J Clin Gastroenterol. 35 (5 Suppl 2):S72–S78.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dutta R and Mahato RI: Recent advances in
hepatocellular carcinoma therapy. Pharmacol Ther. 173:106–117.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bruix J, Reig M and Sherman M:
Evidence-based diagnosis, staging, and treatment of patients with
hepatocellular carcinoma. Gastroenterology. 150:835–853. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maluccio M and Covey A: Recent progress in
understanding, diagnosing, and treating hepatocellular carcinoma.
CA Cancer J Clin. 62:394–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dhir M, Melin AA, Douaiher J, Lin C, Zhen
WK, Hussain SM, Geschwind JF, Doyle MB, Abou-Alfa GK and Are C: A
Review and update of treatment options and controversies in the
management of hepatocellular carcinoma. Ann Surg. 263:1112–1125.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ogretmen B and Hannun YA: Biologically
active sphingolipids in cancer pathogenesis and treatment. Nat Rev
Cancer. 4:604–616. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Obinata H and Hla T: Sphingosine
1-phosphate and inflammation. Int Immunol. 31:617–625. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kawamori T, Kaneshiro T, Okumura M,
Maalouf S, Uflacker A, Bielawski J, Hannun YA and Obeid LM: Role
for sphingosine kinase 1 in colon carcinogenesis. FASEB J.
23:405–414. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Go H, Kim PJ, Jeon YK, Cho YM, Kim K, Park
BH and Ku JY: Sphingosine-1-phosphate receptor 1 (S1PR1) expression
in non-muscle invasive urothelial carcinoma: Association with poor
clinical outcome and potential therapeutic target. Eur J Cancer.
51:1937–1945. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vrzalikova K, Ibrahim M, Vockerodt M,
Perry T, Margielewska S, Lupino L, Nagy E, Soilleux E, Liebelt D,
Hollows R, et al: S1PR1 drives a feedforward signalling loop to
regulate BATF3 and the transcriptional programme of Hodgkin
lymphoma cells. Leukemia. 32:214–223. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang C, Mao J, Redfield S, Mo Y, Lage JM
and Zhou X: Systemic distribution, subcellular localization and
differential expression of sphingosine-1-phosphate receptors in
benign and malignant human tissues. Exp Mol Pathol. 97:259–265.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grammatikos G, Schoell N, Ferreirós N, Bon
D, Herrmann E, Farnik H, Köberle V, Piiper A, Zeuzem S,
Kronenberger B, et al: Serum sphingolipidomic analyses reveal an
upregulation of C16-ceramide and sphingosine-1-phosphate in
hepatocellular carcinoma. Oncotarget. 7:18095–18105. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoshiji H, Kuriyama S, Kawata M, Yoshii J,
Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H and Fukui H: The
angiotensin-I-converting enzyme inhibitor perindopril suppresses
tumor growth and angiogenesis: Possible role of the vascular
endothelial growth factor. Clin Cancer Res. 7:1073–1078.
2001.PubMed/NCBI
|
|
15
|
Nakai Y, Isayama H, Ijichi H, Sasaki T,
Sasahira N, Hirano K, Kogure H, Kawakubo K, Yagioka H, Yashima Y,
et al: Inhibition of renin-angiotensin system affects prognosis of
advanced pancreatic cancer receiving gemcitabine. Br J Cancer.
103:1644–1648. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chowdhury A, Sarkar J, Pramanik PK,
Chakraborti T and Chakraborti S: Cross talk between
MMP2-Spm-Cer-S1P and ERK1/2 in proliferation of pulmonary artery
smooth muscle cells under angiotensin II stimulation. Arch Biochem
Biophys. 603:91–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tirapelli CR, Bonaventura D, Tirapelli LF
and de Oliveira AM: Mechanisms underlying the vascular actions of
endothelin 1, angiotensin II and bradykinin in the rat carotid.
Pharmacology. 84:111–126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dolley-Hitze T, Verhoest G, Jouan F, Le
Pogamp P, Arlot-Bonnemains Y, Oger E, Belaud-Rotureau MA,
Rioux-Leclercq N and Vigneau C: Angiotensin-2 type 1 receptors
(AT1R) and cancers. Nephrol Ther. 9:85–91. 2013.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ji Y, Wang Z, Li Z, Zhang A, Jin Y, Chen H
and Le X: Angiotensin II enhances proliferation and inflammation
through AT1/PKC/NF-κB signaling pathway in hepatocellular carcinoma
cells. Cell Physiol Biochem. 39:13–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hsu YC, Ho HJ, Wu MS, Lin JT and Wu CY:
Postoperative peg-interferon plus ribavirin is associated with
reduced recurrence of hepatitis C virus-related hepatocellular
carcinoma. Hepatology. 58:150–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Facciorusso A, Del Prete V, Crucinio N,
Muscatiello N, Carr BI, Di Leo A and Barone M: Angiotensin receptor
blockers improve survival outcomes after radiofrequency ablation in
hepatocarcinoma patients. J Gastroenterol Hepatol. 30:1643–1650.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee DY, Won KJ, Lee KP, Jung SH, Baek S,
Chung HW, Choi WS, Lee HM, Lee BH, Jeon BH and Kim B: Angiotensin
II facilitates neointimal formation by increasing vascular smooth
muscle cell migration: Involvement of APE/Ref-1-mediated
overexpression of sphingosine-1-phosphate receptor 1. Toxicol Appl
Pharmacol. 347:45–53. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kanai T, Hirohashi S, Upton MP, Noguchi M,
Kishi K, Makuuchi M, Yamasaki S, Hasegawa H, Takayasu K, Moriyama
N, et al: Pathology of small hepatocellular carcinoma. A proposal
for a new gross classification. Cancer. 60:810–819. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Y, Liu P, Jiang Y, Dou X, Yan J, Ma
C, Fan Q, Wang W, Su F, Tang H and Su X: High expression of
neuropilin-1 associates with unfavorable clinicopathological
features in hepatocellular carcinoma. Pathol Oncol Res. 22:367–375.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhai X, Zhu H, Wang W, Zhang S, Zhang Y
and Mao G: Abnormal expression of EMT-related proteins, S100A4,
vimentin and E-cadherin, is correlated with clinicopathological
features and prognosis in HCC. Med Oncol. 31:9702014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ji Y, Wang Z, Chen H, Zhang L, Zhuo F and
Yang Q: Serum from chronic hepatitis B patients promotes growth and
proliferation via the IGF-II/IGF-IR/MEK/ERK signaling pathway in
hepatocellular carcinoma cells. Cell Physiol Biochem. 47:39–53.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abbott. Operations manual or user guide.
https://www.corelaboratory.abbott/registration-ous/login
|
|
30
|
Pyne NJ and Pyne S: Sphingosine
1-phosphate and cancer. Nat Rev Cancer. 10:489–503. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pinter M, Weinmann A, Wörns MA, Hucke F,
Bota S, Marquardt JU, Duda DG, Jain RK, Galle PR, Trauner M, et al:
Use of inhibitors of the renin-angiotensin system is associated
with longer survival in patients with hepatocellular carcinoma.
United European Gastroenterol J. 5:987–996. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ye G, Qin Y, Lu X, Xu X, Xu S, Wu C, Wang
X, Wang S and Pan D: The association of renin-angiotensin system
genes with the progression of hepatocellular carcinoma. Biochem
Biophys Res Commun. 459:18–23. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu ZW, Yan SX, Wu HX, Zhang Y and Wei W:
Angiotensin II and tumor necrosis factor-alpha stimulate the
growth, migration and invasion of BEL-7402 cells via
down-regulation of GRK2 expression. Dig Liver Dis. 51:263–274.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stoyanov E, Ludwig G, Mizrahi L, Olam D,
Schnitzer-Perlman T, Tasika E, Sass G, Tiegs G, Jiang Y, Nie T, et
al: Chronic liver inflammation modifies DNA methylation at the
precancerous stage of murine hepatocarcinogenesis. Oncotarget.
6:11047–11060. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kew MC: Epidemiology of chronic hepatitis
B virus infection, hepatocellular carcinoma, and hepatitis B
virus-induced hepatocellular carcinoma. Pathol Biol (Paris).
58:273–277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pyne NJ, Ohotski J, Bittman R and Pyne S:
The role of sphingosine 1-phosphate in inflammation and cancer. Adv
Biol Regul. 54:121–129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bao M, Chen Z, Xu Y, Zhao Y, Zha R, Huang
S, Liu L, Chen T, Li J, Tu H and He X: Sphingosine kinase 1
promotes tumour cell migration and invasion via the S1P/EDG1 axis
in hepatocellular carcinoma. Liver Int. 32:331–338. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Matsushima-Nishiwaki R, Yamada N, Fukuchi
K and Kozawa O: Sphingosine 1-phosphate (S1P) reduces hepatocyte
growth factor-induced migration of hepatocellular carcinoma cells
via S1P receptor 2. PLoS One. 13:e02090502018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fong ZV and Tanabe KK: The clinical
management of hepatocellular carcinoma in the United States,
Europe, and Asia: A comprehensive and evidence-based comparison and
review. Cancer. 120:2824–2838. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wong RJ, Devaki P, Nguyen L, Cheung R and
Nguyen MH: Ethnic disparities and liver transplantation rates in
hepatocellular carcinoma patients in the recent era: Results from
the Surveillance, Epidemiology, and End Results registry. Liver
Transpl. 20:528–535. 2014. View Article : Google Scholar : PubMed/NCBI
|