Introduction
Despite the low incidence rate, laryngeal carcinoma,
a common respiratory tract tumor (1), poses a threat to life safety and
quality of life of patients if not treated in time (2). According to statistics, the incidence
of laryngeal cancer is ~6.0% worldwide (3). The main treatments for laryngeal
carcinoma include surgery, chemoradiotherapy and other
comprehensive treatment measures (4); however, the remission rate is
relatively low, with a 5-year overall survival rate of <67%
(5). Moreover, patients with
laryngeal carcinoma often have no specific clinical manifestations
at the early stages (6). Therefore,
early detection and timely treatment are the keys to reducing
morbidity and mortality (7).
Autofluorescence endoscopy has been reported to have
high sensitivity and accuracy for the early diagnosis of laryngeal
carcinoma (8). Topuz et al
(9) have reported that circulating
calprotectin was abnormally expressed in patients with laryngeal
carcinoma, suggesting that calprotectin can be used as a biomarker
and be useful for the early diagnosis of the disease. Screening of
molecular biology-related indicators is the most promising
direction to improving the diagnosis of laryngeal carcinoma.
MicroRNAs (miRs), a class of short non-coding RNAs with a length of
~22 nucleotides, inhibit the translation and transcription of
target genes by binding to the 3′untranslated region of their
downstream target miRs, thus changing the expression levels of
target genes (10). miRs play a
regulatory role in various diseases, such as tumors and
cardiovascular diseases (11–13). In
a previous study, microarray and quantitative polymerase chain
reaction (PCR) were employed to examine different miR expression
profiles between cancerous and normal adjacent tissues, and it was
shown that miR-203 was downregulated in laryngeal carcinoma
(14). Saito et al (15) examined the miR expression profiles of
723 patients with laryngeal carcinoma by microarray, and the
results revealed that miR-133b was significantly downregulated in
laryngeal carcinoma. However, there are still few studies on the
clinical value of miR-203 and miR-133b in laryngeal cancer. It is
speculated that miR-203 and miR-133b may become effective tools for
screening and predicting laryngeal cancer.
In the present study, the expression levels of
miR-203 and miR-133b were measured in patients with laryngeal
carcinoma to explore whether miR-203 and miR-133b can be used as
indicators for the clinical diagnosis of laryngeal carcinoma and to
provide reference for clinical diagnosis.
Subjects and methods
General data
A total of 154 patients with laryngeal carcinoma
admitted to Yidu Central Hospital of Weifang (Weifang, China) from
February 2016 to October 2018 were assigned as the research group.
There were 98 males and 56 females, 30–64 years of age in the
research group. In addition, 100 healthy individuals receiving
physical examinations during the same period were assigned as the
control group, including 65 males and 35 females, 30–65 years of
age. The study was approved by the Ethics Committee of Yidu Central
Hospital of Weifang. Signed written informed consents were obtained
from the patients and/or guardians.
Inclusion and exclusion criteria
Inclusion criteria: Patients diagnosed with
laryngeal carcinoma based on the diagnostic criteria of laryngeal
carcinoma; patients receiving treatment in Yidu Central Hospital of
Weifang; patients 18–70 years of age; with elementary school
education and above; who cooperated with the research; with no
other organ serious disease; patients who they or their immediate
family members provided a signed informed consent form; patients
who had complete medical records. Exclusion criteria: Patients who
died during treatment; patients complicated with diseases of the
respiratory or blood system, or other infectious diseases; pregnant
or lactating women; patients who had recently received
immunosuppressants and hormone drugs.
Detection of serum miR-203 and
miR-133b
Fasting venous blood (5 ml) was collected from all
subjects in the morning, placed in a vacuum tube and subsequently
centrifuged at 1,050 × g at 4°C for 10 min. Total RNA was extracted
from serum using a TRIzol® extraction kit (Invitrogen;
Thermo Fisher Scientific, Inc.), and concentration and purity were
determined by a NanoDrop 2000 ultraviolet spectrophotometer
(KeyuXingye Science and Technology Development Co., Ltd.). Total
RNA was reversely transcribed into complementary DNA (cDNA) using a
reverse transcription kit (Invitrogen; Thermo Fisher Scientific,
Inc.), and the temperature protocol was 42°C for 60 min, 70°C for 5
min, storage at 4°C. The primers were designed and synthesized by
Shanghai GenePharma Co., Ltd. (Table
I). The reaction was carried out on an ABI PRISM 7500
fluorescence quantitative PCR instrument (Applied Biosystems;
Thermo Fisher Scientific, Inc.). PCR amplification conditions were
as follows: 90°C for 5 min, 90°C for 5 sec, 60°C for 30 sec, 72°C
for 5 sec, for a total of 40 cycles. Each sample was repeatedly
measured 3 times, with U6 as internal reference. The relative
expression levels of genes were quantified using the
2−ΔCq method (16).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Genes | Forward | Reverse |
|---|
| U6 |
5′-GCTTCGGCAGCACATATACTAAAT-3′ |
5′-CGCTTCACGAATTTGCGTGTCAT-3′ |
| miR-203 |
5′-GTATTCGCACTGGATACGACCGACC-3′ |
5′-TGCGCTAACAGTCTACAGCCA-3′ |
| miR-133b |
5′-GACGATCGATGCTAGCTACGTAGCT-3′ |
5′-CGAGCTAGCTAGCTAGCTAGTCCAG-3′ |
Statistical analysis
SPSS 24.0 software (Shanghai Yuchuang Network
Technology Co., Ltd.) was used for statistical analysis. GraphPad
Prism 8 software (Shenzhen Softhead Software Technology Co., Ltd.)
was used to generate the figures and double check the results. The
counting data were expressed as percentage (%) and χ2
test was used for their comparison between groups. The measurement
data were expressed as the mean ± standard deviation (SD) and
t-test was used for their comparison between groups. One-way ANOVA,
followed by LSD post hoc test, was used for the comparison of the
measurement data between multiple groups. Receiver operating
characteristic (ROC) curves were plotted to evaluate the diagnostic
values of miR-203 and miR-133b for laryngeal carcinoma. P<0.050
was considered to indicate a statistically significant
difference.
Results
General data comparison
There was no significant difference between the two
groups in terms of age, sex, body mass index (BMI), medical history
and marital status (P>0.050). However, the levels of
carcinoembryonic antigen (CEA), squamous cell carcinoma antigen
(SCC-Ag) and adenosine kinase (AK) were significantly different
between the two groups (P<0.050). Details are shown in Table II.
 | Table II.Comparison of patients' general data
[mean ± SD, n (%)]. |
Table II.
Comparison of patients' general data
[mean ± SD, n (%)].
| Characteristics | Research group
(n=154) | Control group
(n=100) | χ2 or
t | P-value |
|---|
| Age (years) | 48.25±5.39 | 48.71±6.67 | 0.604 | 0.546 |
| Sex |
|
| 0.049 | 0.824 |
| Male | 98 (63.64) | 65 (65.00) |
|
|
|
Female | 56 (36.36) | 35 (35.00) |
|
|
| BMI
(kg/m2) | 23.71±1.61 | 23.64±1.73 | 0.328 | 0.742 |
| Medical history |
|
|
|
|
|
Hypertension | 41 (26.62) | 24 (24.00) | 0.219 | 0.639 |
|
Diabetes | 31 (20.13) | 18 (18.00) | 0.176 | 0.674 |
| High
blood lipid | 27 (17.53) | 17 (17.00) | 0.012 | 0.912 |
| Marital status |
|
| 1.208 | 0.271 |
|
Married | 116 (75.32) | 81 (81.00) |
|
|
|
Unmarried | 38
(24.68) | 19 (19.00) |
|
|
| CEA (ng/ml) |
3.48±0.32 | 1.18±1.39 | 13.291 | <0.001 |
| SCC-Ag level |
9.13±5.18 | 0.78±0.34 | 16.090 | <0.001 |
| AK |
0.34±0.08 | 1.14±0.16 | 52.750 | <0.001 |
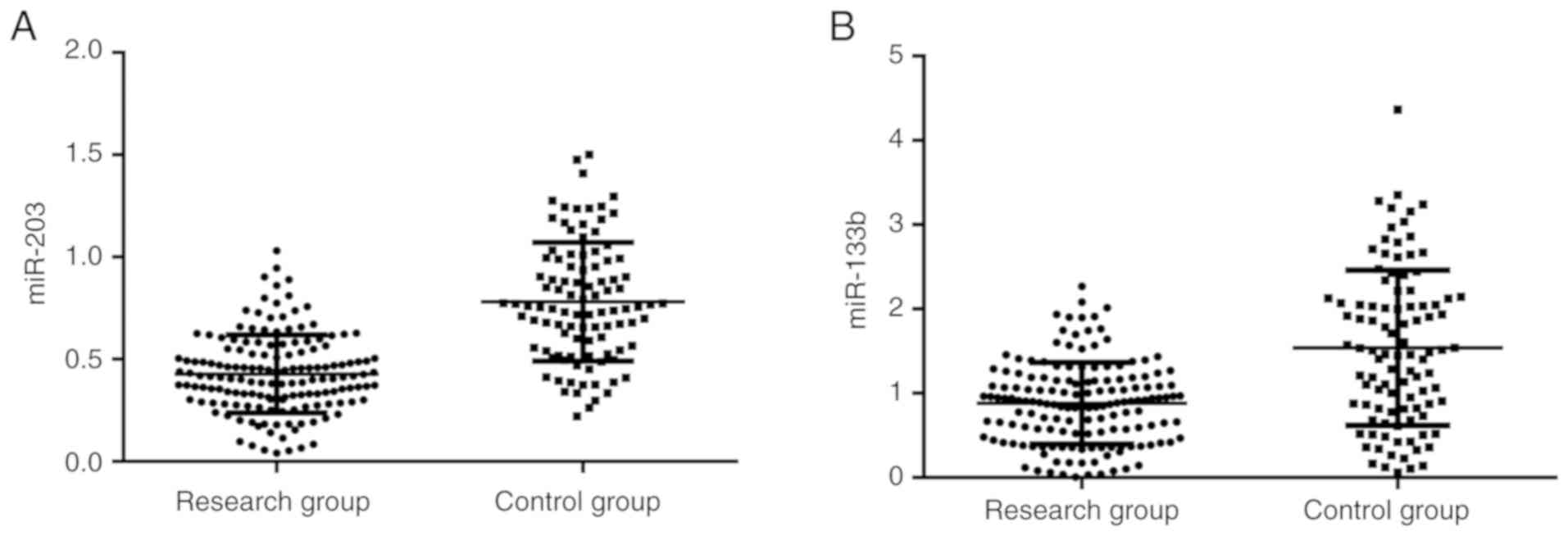
Serum miR-203 and miR-133b expression
levels in the two groups
The expression of miR-203 in the research group was
significantly lower than that in the control group (0.43±0.20 vs.
0.85±0.29; P<0.05) (Fig. 1A). In
addition, the expression of miR-133b in the research group was
significantly lower than that in the control group (0.84±0.51 vs.
1.38±1.07; P<0.05) (Fig. 1B).
Association of miR-203 and miR-133b
expression levels with clinicopathological characteristics
The analysis of clinicopathological data and miR-203
and miR-133b expression levels showed that miR-203 expression was
related to tumor-node-metastasis (TNM) stages and tumor types
(P<0.050; Table III). However,
the expression of miR-133b varied in patients with different
pathological stages, differentiation degrees and lymph node
metastasis (P<0.050; Table
IV).
 | Table III.Association of miR-203 expression
with clinicopathological characteristics [mean ± SD, n (%)]. |
Table III.
Association of miR-203 expression
with clinicopathological characteristics [mean ± SD, n (%)].
|
Characteristics | Cases (n=154) | miR-203 relative
expression | t or F | P-value |
|---|
| Age, years |
|
| 0.300 | 0.764 |
|
≤50 | 74 (48.05) | 0.42±0.19 |
|
|
|
>50 | 80 (51.95) | 0.43±0.22 |
|
|
| Sex |
|
| 0.260 | 0.795 |
|
Male | 98 (63.64) | 0.42±0.24 |
|
|
|
Female | 56 (36.36) | 0.43±0.21 |
|
|
| Tumor type |
|
| 0.0065 | 0.006 |
|
Supraglottic carcinoma | 33 (21.43) | 0.41±0.17 |
|
|
| Glottic
carcinoma | 29 (18.83) | 0.40±0.23 |
|
|
|
Subglottic carcinoma | 38 (24.68) | 0.54±0.18 |
|
|
|
Transglottic carcinoma | 54 (35.06) | 0.41±0.21 |
|
|
| Degree of
differentiation |
|
| 0.552 | 0.583 |
| Highly
differentiated | 81 (52.60) | 0.44±0.23 |
|
|
|
Moderately and poorly
differentiated | 73 (47.40) | 0.42±0.22 |
|
|
| Lymph node
metastasis |
|
| 0.603 | 0.547 |
|
Yes | 68 (44.16) | 0.41±0.21 |
|
|
| No | 86 (55.84) | 0.43±0.20 |
|
|
| TNM stage |
|
| 3.123 | <0.02 |
|
I–II | 89 (57.79) | 0.47±0.22 |
|
|
|
III–IV | 65 (42.20) | 0.36±0.21 |
|
|
 | Table IV.Association of miR-133b expression
with clinicopathological characteristics [mean ± SD, n (%)]. |
Table IV.
Association of miR-133b expression
with clinicopathological characteristics [mean ± SD, n (%)].
|
Characteristics | Cases (n=154) | miR-203 relative
expression | t or F | P-value |
|---|
| Age, years |
|
| 0.419 | 0.675 |
|
≤50 | 74 (48.05) | 0.86±0.40 |
|
|
|
>50 | 80 (51.95) | 0.83±0.48 |
|
|
| Sex |
|
| 0.243 | 0.808 |
|
Male | 98 (63.64) | 0.86±0.48 |
|
|
|
Female | 56 (36.36) | 0.84±0.51 |
|
|
| Tumor type |
|
| 0.055 | 0.983 |
|
Supraglottic carcinoma | 33 (21.43) | 0.84±0.57 |
|
|
| Glottic
carcinoma | 29 (18.83) | 0.83±0.46 |
|
|
|
Subglottic carcinoma | 38 (24.68) | 0.81±0.51 |
|
|
|
Transglottic carcinoma | 54 (35.06) | 0.85±0.39 |
|
|
| Degree of
differentiation |
|
| 3.409 | <0.001 |
| Highly
differentiated | 81 (52.60) | 0.96±0.44 |
|
|
|
Moderately and poorly
differentiated | 73 (47.40) | 0.71±0.47 |
|
|
| Lymph node
metastasis |
|
| 3.623 | <0.001 |
|
Yes | 68 (44.16) | 0.68±0.47 |
|
|
| No | 86 (55.84) | 0.98±0.54 |
|
|
| TNM stage |
|
| 2.632 | 0.009 |
|
I–II | 89 (57.79) | 0.93±0.47 |
|
|
|
III–IV | 65 (42.20) | 0.73±0.46 |
|
|
Diagnostic efficacy of miR-203,
miR-133b and their combination in laryngeal carcinoma
ROC curve analysis showed that the area under curve
(AUC) of serum miR-203 was 0.788 [95% confidence interval (CI):
0.728–0.848, P<0.001] and when the cut-off value was 0.659 the
sensitivity and specificity were 60.00 and 90.26%, respectively.
The AUC of serum miR-133b was 0.712 (95% CI: 0.642–0.782,
P<0.001) and when the cut-off value was 1.398 the sensitivity
and specificity were 55.00 and 87.66%, respectively. In addition,
when the cut-off value was 0.416 the sensitivity and specificity of
the joint detection were 70.00 and 83.77%, respectively. Details
are shown in Fig. 2 and Table V.
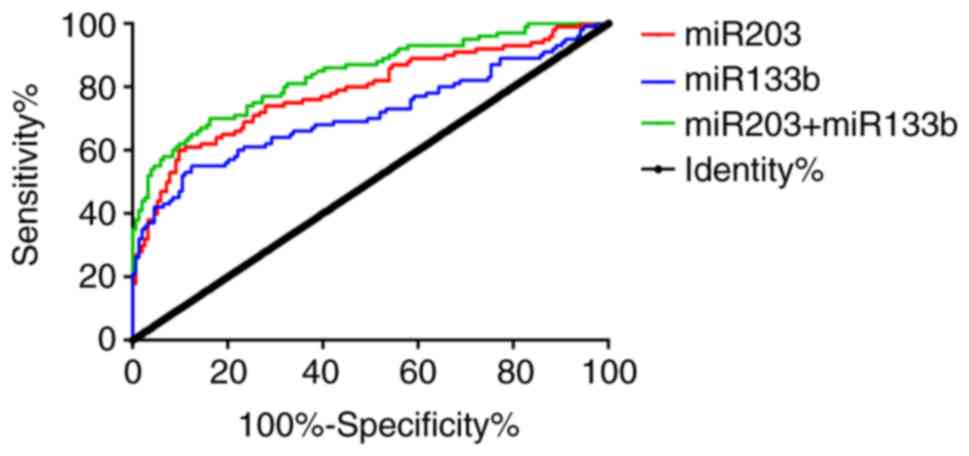 | Figure 2.ROC curves of serum miR-203 and
miR-133b in diagnosing laryngeal carcinoma. ROC curve analysis of
serum miR-203 revealed that, when the cut-off value was 0.659, the
sensitivity and specificity were 60.00 and 90.26%, respectively.
When the cut-off value was 1.398, the sensitivity and specificity
of serum miR-133b were 55.00 and 87.66%, respectively. In addition,
when the cut-off value was 0.416, the sensitivity and specificity
of the joint detection were 70.00 and 83.77%, respectively. miR,
microRNA; ROC, receiver operating characteristic; miR,
microRNA. |
 | Table V.ROC curve analysis. |
Table V.
ROC curve analysis.
| Index | miR-203 | miR-133b | Joint
detection |
|---|
| AUC | 0.788 | 0.712 | 0.838 |
| 95% CI | 0.728–0.848 | 0.642–0.782 | 0.787–0.890 |
| Cut-off | 0.659 | 1.398 | 0.416 |
| Sensitivity
(%) | 60.00 | 55.00 | 70.00 |
| Specificity
(%) | 90.26 | 87.66 | 83.77 |
| P-value | <0.001 | <0.001 | 0.001 |
Discussion
Although great progress has been achieved in the
treatment of laryngeal carcinoma with surgery, radiotherapy and
chemotherapy (17), the long-term
prognosis of patients is still unsatisfactory due to various
factors (18). Seeking tumor markers
with high sensitivity and accuracy has become a research hotspot
(19,20). In the present study, the expression
levels of miR-203 and miR-133b were detected to explore their
potential role as indicators for the diagnosis of laryngeal
carcinoma.
The results of the present study revealed that the
expression levels of serum miR-203 and miR-133b in the research
group were significantly lower than those in the control group, in
agreement with the relevant literature results (21,22),
suggesting that miR-203 and miR-133b may have key biological
functions in the occurrence and progression of laryngeal carcinoma.
Subsequently, the association of miR-203 and miR-133b expression
levels with the patients' clinicopathological data in the research
group was analyzed. The results showed that miR-203 expression was
related to TNM stage and tumor type, but not lymph node metastasis,
indicating that miR-203 might play an antitumor role in early
laryngeal carcinoma. In addition, the expression of miR-133b was
shown to vary significantly in patients with different pathological
stages, differentiation degrees and lymph node metastasis. Tian
et al (23) have reported
that miR-203 was downregulated in laryngeal squamous cell carcinoma
and was closely related to poor differentiation, advanced clinical
stage (III–IV), tumor stages (T3-4), lymph node metastasis and
5-year overall survival reduction. These results are contrary to
our findings, i.e., that miR-203 has no association with lymph node
metastasis in laryngeal carcinoma, suggesting that miR-203, like
many other miRs, might have a biphasic effect on human cancers and
act as an oncogene or tumor suppressor depending on the cellular
environment of the tumor. Finally, in the present study, ROC curve
analysis showed that the AUC of miR-203 and miR-133b was 0.788 and
0.712, respectively, indicating that both miRs are valuable in the
diagnosis of laryngeal carcinoma and could be useful screening and
prediction tools for this disease. There have been numerous studies
on the diagnostic value of single serum markers in laryngeal
carcinoma, pointing out that single marker detection tends to cause
missed diagnosis and misdiagnosis, as well as delay in treatment,
whereas the joint detection has a better diagnostic effect
(24–26). Therefore, the ROC curve of the joint
detection of miR-203 and miR-133b was also plotted in the present
study, and it was shown that the AUC and cut-off value were 0.838
and 0.416, respectively, whereas the sensitivity and specificity
for diagnosing laryngeal carcinoma were 70.00 and 83.77%
respectively, indicating better effectiveness compared with that of
the single detection. Thus, the joint detection of miR-203 and
miR-133b could be useful for the diagnosis of laryngeal carcinoma.
In the past, laryngeal cancer blood markers, such as CEA and
SCC-Ag, have been commonly used in clinical practice. Because of
the significant degree of response to tumor lesions and injuries,
these markers have in general extremely high sensitivity and low
specificity. In clinic, the detection of the aforementioned markers
can be used to determine whether the patient has a tumor; however
it is not ruled out that some highly specific diseases could also
cause the rise of CEA. Therefore, to determine the patient's
disease, follow-up inspections are still needed. Compared with
conventional laryngeal cancer tumor markers, the advantages of
miR-203 and miR-133b detection in peripheral blood are as follows:
i) The detection is convenient and the cycle is short. Only
peripheral blood is needed. ii) The test results are intuitive and
the results do not need to be interpreted compared with imaging
techniques. iii) The sample is easy to maintain, which can be
conducive to the long-term treatment of patients. iv) In addition
to the significant sensitivity and the excellent specificity, it
can effectively assist doctors to quickly and effectively judge
whether the patient has laryngeal cancer. Previous studies have
shown that traditional tumor diagnostic markers, such as CEA and
SCC-Ag, are highly sensitive to the occurrence of laryngeal cancer,
although the specificity is low (27,28).
Studies have also shown that CEA and SCC-Ag levels increase during
the occurrence of uremia and inflammatory reactions (29,30). The
combined detection of miR-203 and miR-133b has better specificity,
is more effective and accurate in identifying laryngeal cancer and
more conducive to early clinical screening, improving the early
detection rate of laryngeal cancer and prognosis.
Previous studies have reported that miR-203 and
miR-133b are effective in the prognosis of patients with
osteosarcoma and bladder cancer (31,32). In
the present study, due to the short experimental period, the
patients were not followed up and whether miR-203 and miR-133b have
an impact on the prognosis of patients remains unknown. In
addition, due to the lack of support from basic experiments, the
specific impact mechanism of miR-203 and miR-133b on laryngeal
cancer is not clear yet. The extended experimental time, the
expanded sample size and in vitro experiments will be
included in our future study to further explore the effects of
miR-203 and miR-133b on laryngeal cancer and provide reference for
clinical practice.
In conclusion, miR-203 and miR-133b were expressed
at low levels in patients with laryngeal carcinoma. The expression
of miR-203 was related to TNM stage and tumor type, whereas the
expression of miR-133b was related to TNM stage, differentiation
degree and lymph node metastasis. The joint detection of miR-203
and miR-133b is expected to be an excellent marker for the
diagnosis and treatment of laryngeal carcinoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
NZ conceived and designed the study, and drafted the
manuscript. HL acquired, analyzed and interpreted the experimental
data. AZ and MW performed serum miR-203 and miR-133b detection. NZ
wrote the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Yidu Central Hospital of Weifang (Weifang, China). Signed written
informed consents were obtained from the patients and/or
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wierzbicka M, Winiarski P and
Osuch-Wójcikiewicz E: The incidence of laryngeal cancer in Europe
with special regard to Poland in last 2 decades. Otolaryngol Pol.
70:16–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Souza DL, Pérez MM and Curado MP:
Predicted incidence of oral cavity, oropharyngeal, laryngeal, and
hypopharyngeal cancer in Spain and implications for cancer control.
Cancer Epidemiol. 35:510–514. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin CC, Fedewa SA, Prickett KK, Higgins KA
and Chen AY: Comparative effectiveness of surgical and nonsurgical
therapy for advanced laryngeal cancer. Cancer. 122:2845–2856. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aydil U, Akmansu M, Gumusay Ö, Bakkal FK,
Yazıcı Ö, Kızıl Y, Köybaşıoğlu A, Yıldız R, Büyükberber S and İnal
E: Comparison of three different concurrent chemoradiation regimens
for treatment of laryngeal cancer. Eur Arch Otorhinolaryngol.
273:2795–2803. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fararouei M, Daneshi N, Mohammadianpanah
M, Reza Tabatabaei H, Zare-Bandamiri M and Dianatinasab M: Factors
predicting survival in patients with early stage laryngeal cancer:
A cohort study between 2000 to 2015. J BUON. 22:996–1003.
2017.PubMed/NCBI
|
|
7
|
Sethi N, Rafferty A, Rawnsley T and Jose
J: Short, sharp shock public health campaign had limited impact on
raising awareness of laryngeal cancer. Eur Arch Otorhinolaryngol.
273:2747–2754. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fostiropoulos K, Arens C, Betz C and Kraft
M: Noninvasive imaging using autofluorescence endoscopy: Value for
the early detection of laryngeal cancer. HNO. 64:13–18. 2016.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Topuz MF, Binnetoglu A, Yumusakhuylu AC,
Sarı M, Baglam T and Gerin F: Circulating calprotectin as a
biomarker of laryngeal carcinoma. Eur Arch Otorhinolaryngol.
274:2499–2504. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ha M and Kim VN: Regulation of microRNA
biogenesis. Nat Rev Mol Cell Biol. 15:509–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arvidsson Y, Rehammar A, Bergström A,
Andersson E, Altiparmak G, Swärd C, Wängberg B, Kristiansson E and
Nilsson O: miRNA profiling of small intestinal neuroendocrine
tumors defines novel molecular subtypes and identifies miR-375 as a
biomarker of patient survival. Mod Pathol. 31:1302–1317. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nagy Z, Decmann Á, Perge P and Igaz P:
Pathogenic and diagnostic roles of microRNAs in adrenocortical
tumours. Orv Hetil. 159:245–251. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He L, Chen Y, Hao S and Qian J: Uncovering
novel landscape of cardiovascular diseases and therapeutic targets
for cardioprotection via long noncoding RNA-miRNA-mRNA axes.
Epigenomics. 10:661–671. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu ZM, Lin YF, Jiang L, Chen LS, Luo XN,
Song XH, Chen SH and Zhang SY: Micro-Ribonucleic acid expression
profiling and bioinformatic target gene analyses in laryngeal
carcinoma. Onco Targets Ther. 7:525–533. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saito K, Inagaki K, Kamimoto T, Ito Y,
Sugita T, Nakajo S, Hirasawa A, Iwamaru A, Ishikura T, Hanaoka H,
et al: MicroRNA-196a is a putative diagnostic biomarker and
therapeutic target for laryngeal cancer. PLoS One. 8:e714802013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei WI and Chan JY: Surgical treatment of
advanced staged hypopharyngeal cancer. Adv Otorhinolaryngol.
83:66–75. 2019.PubMed/NCBI
|
|
18
|
Hsueh C, Tao L, Zhang M, Cao W, Gong H,
Zhou J and Zhou L: The prognostic value of preoperative
neutrophils, platelets, lymphocytes, monocytes and calculated
ratios in patients with laryngeal squamous cell cancer. Oncotarget.
8:60514–60527. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang YW, Luo J, Weng YI, Mutch DG,
Goodfellow PJ, Miller DS and Huang TH: Promoter hypermethylation of
CIDEA, HAAO and RXFP3 associated with microsatellite instability in
endometrial carcinomas. Gynecol Oncol. 117:239–247. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zimonjic DB and Popescu NC: Role of DLC1
tumor suppressor gene and MYC oncogene in pathogenesis of human
hepatocellular carcinoma: Potential prospects for combined targeted
therapeutics (review). Int J Oncol. 41:393–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yao XD, Li P and Wang JS: MicroRNA
differential expression spectrum and microRNA125a5p inhibition of
laryngeal cancer cell proliferation. Exp Ther Med. 14:1699–1705.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu B, Xiong X, Jia J and Zhang WF:
MicroRNAs: New actors in the oral cancer scene. Oral Oncol.
47:314–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tian L, Li M, Ge J, Guo Y, Sun Y, Liu M
and Xiao H: miR-203 is downregulated in laryngeal squamous cell
carcinoma and can suppress proliferation and induce apoptosis of
tumours. Tumour Biol. 35:5953–5963. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pakkanen PP, Aaltonen LM, Sorsa TA,
Tervahartiala TI, Hagström JK and Ilmarinen TT: Serum matrix
metalloproteinase 8 and tissue inhibitor of metalloproteinase 1:
Potential markers for malignant transformation of recurrent
respiratory papillomatosis and for prognosis of laryngeal cancer.
Head Neck. 41:309–314. 2018.PubMed/NCBI
|
|
25
|
Memar MY, Alizadeh N, Varshochi M and
Kafil HS: Immunologic biomarkers for diagnostic of early-onset
neonatal sepsis. J Matern Fetal Neonatal Med. 32:143–153. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao FX, Liu GH and Zhang J: Value of IL-6
and IL-8 in the diagnosis of neonatal sepsis. Zhongguo Dang Dai Er
Ke Za Zhi. 17:1311–1315. 2015.(In Chinese). PubMed/NCBI
|
|
27
|
Wang W, Xu X, Tian B, Wang Y, Du L, Sun T,
Shi Y, Zhao X and Jing J: The diagnostic value of serum tumor
markers CEA, CA19-9, CA125, CA15-3, and TPS in metastatic breast
cancer. Clin Chim Acta. 470:51–55. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu F, Li Y, Fan L, Ma J, Yu L, Yi H, Chen
X, Wei W, Wu P, Liang L, et al: Preoperative SCC-Ag and
thrombocytosis as predictive markers for pelvic lymphatic
metastasis of squamous cervical cancer in early FIGO stage. J
Cancer. 9:1660–1666. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kerr D, Laber D, Visweshwar N and Jaglal
M: Case report: CEA elevation can be a marker of increased
inflammation during treatment with oxaliplatin. Anticancer Res.
38:1711–1713. 2018.PubMed/NCBI
|
|
30
|
Kwack JY, Roh HJ, You SG, Cho HJ, Lee SH
and Kwon YS: Chemotherapy-induced hemolytic uremic syndrome in
locally advanced cervical cancer treated with combination
chemotherapy after laparoscopic radical hysterectomy: A case report
and review of the literature. Eur J Gynaecol Oncol. 38:956–959.
2017.
|
|
31
|
Chen X, Chen XG, Hu X, Song T, Ou X and
Zhang C, Zhang W and Zhang C: miR-34a and miR-203 inhibit survivin
expression to control cell proliferation and survival in human
osteosarcoma cells. J Cancer. 7:1057–1065. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen X, Wu B, Xu Z, Li S, Tan S, Liu X and
Wang K: Downregulation of miR-133b predict progression and poor
prognosis in patients with urothelial carcinoma of bladder. Cancer
Med. 5:1856–1862. 2016. View
Article : Google Scholar : PubMed/NCBI
|