Introduction
More than 250,000 new cases of renal cell carcinoma
(RCC) are diagnosed annually and 116,000 patients die from the
disease worldwide. (1). A total of
25–30% of newly diagnosed renal RCC cases present with metastases
(2), and 20–40% of patients with
locally limited RCC undergo relapse during the follow-up period,
even if the tumors are surgically resected (3). Moreover, with regards to metastatic
RCC, 30% of patients have bone metastasis (4).
Bone metastasis from RCC is mainly osteolytic,
decreasing bone integrity and causing skeletal-related events
(SREs) (5), including pathologic
fractures and the need for radiotherapy for bone pain, as well as
surgical interventions to treat or prevent an impending fracture,
spinal cord and nerve root compressions, and hypercalcemia
(5). It has been reported that
>70% of patients with RCC with bone metastases experience ≥1 SRE
during their clinical course (5),
and subsequently experience a decrease in their quality of life
(QOL) (6).
The size of bone metastatic lesions is a risk factor
for pathological fractures (7), and
an enlarged bone metastasis in the vertebral body may induce spinal
cord and nerve root compression (8).
Bulky bone metastasis can cause SREs, and thus treatment to
decrease or inhibit the growth of bone metastases is often required
(9). Although radiotherapy for RCC
bone metastases can achieve a certain degree of local control
(10), there is not sufficient
evidence regarding the effects of systemic therapy to improve bone
metastasis.
Targeted therapy and immune checkpoint inhibitors
have been reported to prolong either the progression-free or
overall survival time of patients with metastatic RCC based on the
results of clinical trials. However, fewer patients with bone
metastases are included in these trials compared with patients with
lung or lymph node metastases (11–14), as
bone lesions are considered difficult to evaluate by the Response
Evaluation Criteria in Solid Tumors (RECIST) criteria (15), and the frequency of bone metastases
is lower compared with that of lung or lymph node metastases in RCC
(4). Therefore, the effect of
targeted therapy and immune checkpoint inhibitors on bone lesions
remains unknown, and which agents should be administered to
patients with bone dominant metastatic RCC is yet to be fully
elucidated.
Several methods for evaluating the treatment
efficacy for bone lesions have been suggested (16,17). For
example, Hamaoka et al (17)
proposed the MD Anderson bone response criteria, in which not only
a size reduction, but also osteoblastic changes are considered
evidence of treatment efficacy for bone lesions. These criteria
were originally developed for bone metastases from breast cancer,
but have been adopted during a clinical trial evaluating the
efficacy of radium-223 dichloride for RCC bone metastasis (18).
The present study aimed to evaluate the treatment
efficacy of targeted therapy and immune checkpoint inhibitors using
the MD Anderson bone response criteria, to examine whether systemic
therapy itself can shrink bone metastases of RCC.
Patients and methods
Enrollment of patients
The present study retrospectively reviewed 44
patients (32 men and 12 women) with RCC with bone metastases, who
were treated with systemic therapy at the National Hospital
Organization Kyushu Cancer Center and Graduate School of Medicine
and Pharmaceutical Sciences for Research University of Toyama
between October 2008 and March 2020. The median age at diagnosis of
bone metastasis was 66 years (age range, 39–83 years). Key
inclusion criteria were: Measurable bone metastasis on CT
(diameter, >1.0 cm); and systemic therapy after a diagnosis of
bone metastasis. Key exclusion criteria were: Bone metastasis that
could not be detected on CT; and the absence of systemic therapy
after a diagnosis of bone metastasis. The present study was
approved by the Institutional Review Board of the National Hospital
Organization Kyushu Cancer Center.
Evaluation of patient data
Patients were divided into two groups: Those who
underwent systemic therapy with concomitant or sequential
radiotherapy for bone lesions (n=29); and those who underwent
systemic therapy without radiotherapy for bone lesions (n=15). The
radiographical efficacy of systemic therapy was evaluated according
to the MD Anderson bone response criteria, which was previously
reported (Data S1) (17). The time to progression of bone
metastases and bone-specific overall response rate were calculated
and compared between groups. The starting point for the analysis
was set as the date of the initiation of systemic treatment after
the diagnosis of bone metastasis from RCC.
Radiotherapy for bone metastasis was performed using
a multidisciplinary conference consisting of medical oncologists,
orthopedic surgeons, radiation oncologists and urologists (9). Bone metastases, which can cause
pathological fracture and cord compression (19), were an indication for radiotherapy to
prevent SRE, even when patients did not have ostealgia. Patients
with ostealgia were suggested for radiotherapy to relieve pain. The
dose of palliative radiotherapy was 30–40 Gy. In the present study,
only one patient with solitary bone metastasis, without other organ
metastasis, underwent curative radiotherapy at a dose of 50 Gy. The
other patients underwent palliative radiotherapy.
Statistical analysis
Background characteristics of the groups were
compared using the χ2 test or Fisher's exact test, and
unpaired Student's t-test for continuous variables. The
Kaplan-Meier method was used to estimate the time to the
progression of bone metastases with systemic therapy. A log-rank
test was used to compare the time to progression between the
groups. A Cox proportional hazards model was used to evaluate the
predictors of the time to progression in univariable and
multivariable analyses. P<0.05 was considered to indicate a
statistically significant difference. All analyses were performed
using the JMP® Pro software package (version 14.2.0; SAS
Institute, Inc.).
Results
Patient characteristics
The characteristics of the 44 patients are presented
in Table I. In total, 29 patients
underwent radiotherapy for bone lesions, and 15 did not undergo
radiotherapy. More patients received bone-modifying agents (BMA) in
the group with radiotherapy compared with the group without
radiotherapy (P=0.021). The median follow-up period was 13.2 months
(range, 0.7–86.0 months), and the median radiation dose delivered
to the bone lesions was 30 Gy (range, 30–50 Gy). A total of 22
(50%) patients had bone metastases when they were diagnosed with
RCC, and 25 (57%) had multiple bone metastases when they were
diagnosed with bone metastasis. Regarding systemic therapy after
the diagnosis of bone metastasis, targeted therapy and immune
checkpoint inhibitors were administered to 36 (82%) and 8 (18%)
patients, respectively.
 | Table I.Characteristics of the patients (n=44)
with radiotherapy for bone metastasis (n=29) or without
radiotherapy (n=15). |
Table I.
Characteristics of the patients (n=44)
with radiotherapy for bone metastasis (n=29) or without
radiotherapy (n=15).
| Variable | All patients | Radiotherapy for bone
metastasis | No radiotherapy for
bone metastasis | P-value |
|---|
| Age at diagnosis of
bone metastasis, years | 66 (39–83) | 65 (39–83) | 63 (43–76) | 0.911 |
| Sex |
|
|
|
|
| Male | 32 (73) | 22 (76) | 10 (66) | 1.000 |
|
Female | 12 (27) | 7
(24) | 5
(34) |
|
| IMDC risk
stratification |
|
|
|
|
|
Favorable | 11 (25) | 7
(24) | 4
(27) | 0.854 |
|
Intermediate | 23 (52) | 16 (55) | 7
(46) |
|
| Poor | 10 (23) | 6
(21) | 4
(27) |
|
| Histology |
|
|
|
|
| Clear
cell | 37 (84) | 24 (83) | 13 (86) | 1.000 |
| Non-clear
cell | 7
(16) | 5
(17) | 2
(14) |
|
| Nephrectomy |
|
|
|
|
| Yes | 36 (82) | 25 (86) | 11 (73) | 0.414 |
| No | 8
(18) | 4
(14) | 4
(27) |
|
| Metastasis at the
diagnosis of RCC |
|
|
|
|
| Bone
metastasis with or without extraosseous metastasis | 22 (50) | 15 (52) | 7
(46) | 0.935 |
|
Extraosseous metastasis
only | 6
(14) | 4
(14) | 2
(14) |
|
| No
metastasis | 16 (36) | 10 (34) | 6
(40) |
|
| Multiplicity of bone
metastasis |
|
|
|
|
|
Solitary | 19 (43) | 12 (41) | 7
(46) | 0.737 |
|
Multiple | 25 (57) | 17 (59) | 8
(54) |
|
| Bone-modifying
agents |
|
|
|
|
|
Yes | 17 (39) | 15 (52) | 2
(14) | 0.021 |
| No | 27 (61) | 14 (48) | 13 (86) |
|
| Systemic therapy
after diagnosis of bone metastasis |
|
|
|
|
|
Sunitinib | 24 (56) | 14 (48) | 10 (66) | 0.110 |
|
Axitinib | 3
(7) | 1
(4) | 2
(14) |
|
|
Pazopanib | 3
(7) | 3
(10) | 0 |
|
|
Sorafenib | 2
(4) | 2
(7) | 0 |
|
|
Temsirolimus | 2
(4) | 2
(7) | 0 |
|
|
Everolimus | 2
(4) | 0 | 2
(14) |
|
|
Nivolumab | 4
(9) | 3
(10) | 1
(6) |
|
|
Nivolumab, Ipilimumab | 4
(9) | 4
(14) | 0 |
|
Treatment efficacy
The overall response rate of systemic therapy with
radiotherapy was 44% in the present study; in total, 13 patients
demonstrated a partial response, including 12 patients and one
patient administered targeted therapy and immune checkpoint
inhibitors, respectively. Only one patient (6%) had a partial
response among those who did not undergo radiotherapy (Table II).
 | Table II.Overall response rate of systemic
therapy with (n=29) or without radiotherapy (n=15). |
Table II.
Overall response rate of systemic
therapy with (n=29) or without radiotherapy (n=15).
| Best response, n
(%) | Systemic therapy
with radiotherapy | Systemic therapy
without radiotherapy |
|---|
| Partial
response | 13 (44) | 1
(6) |
| Stable disease | 4
(14) | 0 |
| Progressive
disease | 11 (38) | 11 (74) |
| Non-evaluable | 1
(4) | 3
(20) |
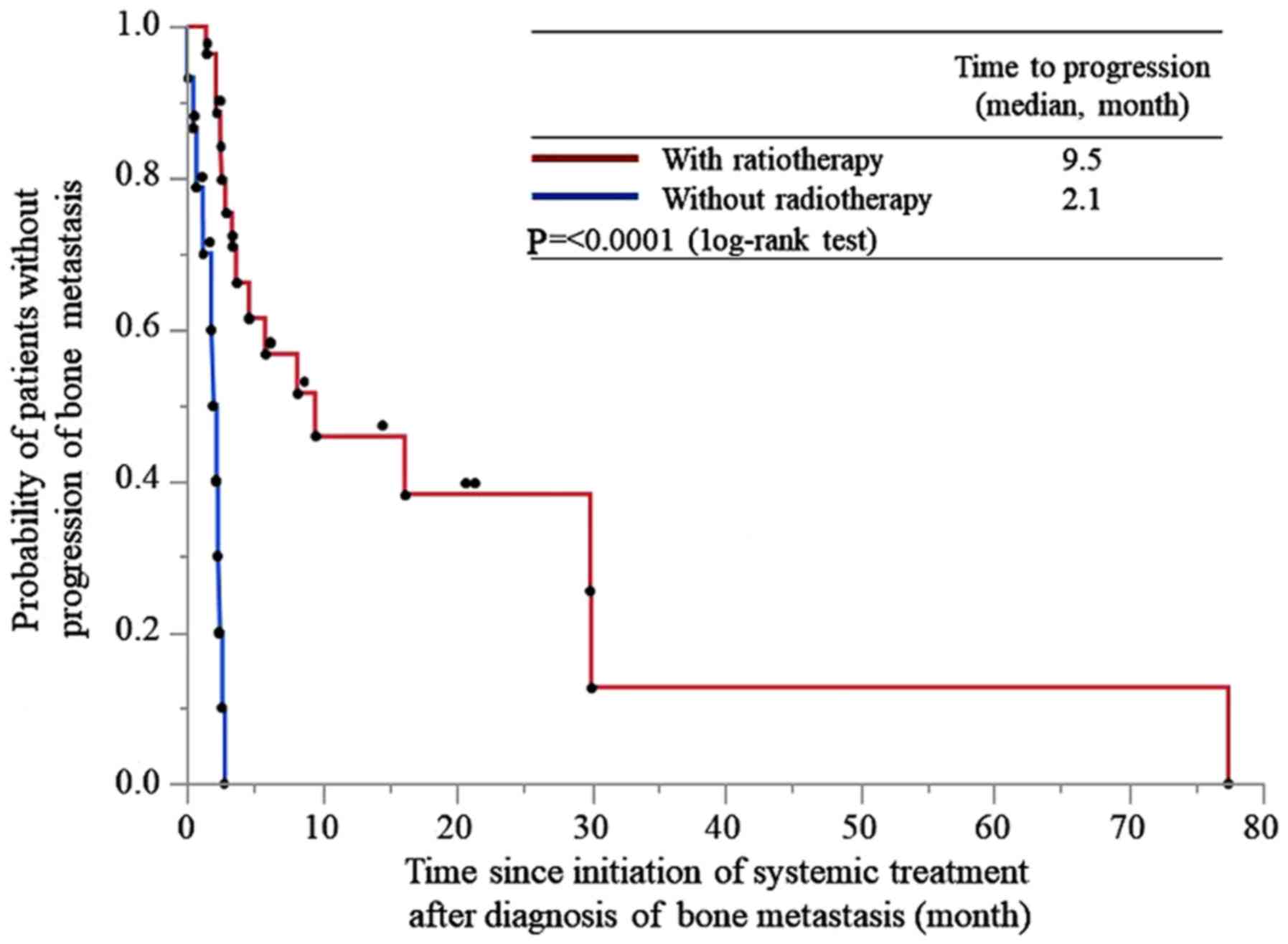
The time to progression of bone metastasis was 9.5
and 2.1 months in patients treated with and without radiotherapy,
respectively (P<0.0001; Fig.
1).
Cox proportional hazards model
A Cox proportional hazards model identified that a
systemic therapy regimen, BMA and International Metastatic RCC
Database Consortium risk stratification were not associated with
the time to progression. However, systemic therapy with
radiotherapy was a predictive factor of the time to progression of
bone metastasis (hazard ratio, 16.60; 95% CI, 4.53–60.90;
P<0.0001; Table III).
 | Table III.Univariate and multivariate analyses
to evaluate the predictors of the time to progression of bone
metastasis. |
Table III.
Univariate and multivariate analyses
to evaluate the predictors of the time to progression of bone
metastasis.
|
|
| Univariate
analysis |
| Multivariate
analysis |
|
|---|
|
|
|
|
|
|
|
|---|
| Variable | n | HR (95% CI) | P-value | HR (95%CI) | P-value |
|---|
| IMDC risk
stratification |
|
|
|
|
|
|
Favorable | 11 | 1 (reference) |
| 1 (reference) |
|
|
Intermediate | 23 | 1.88
(0.66–5.33) | 0.234 | 1.79
(0.55–5.90) | 0.336 |
|
Poor | 10 | 1.47
(0.45–4.82) | 0.527 | 1.24
(0.36–4.21) | 0.735 |
| Bone-modifying
agents |
|
|
|
|
|
|
Yes | 17 | 1 (reference) |
| 1 (reference) |
|
| No | 27 | 3.07
(1.28–7.38) | 0.012 | 1.45
(0.53–4.00) | 0.473 |
| Systemic
therapy |
|
|
|
|
|
| With
radiotherapy | 29 | 1 (reference) |
| 1 (reference) |
|
| Without
radiotherapy | 15 | 12.62
(4.17–38.25) | <0.0001 | 16.60
(4.53–60.90) | <0.0001 |
| Systemic therapy
regimen after diagnosis of bone metastasis |
|
|
|
|
|
|
Targeted therapy | 36 | 1 (reference) |
| 1 (reference) |
|
| Immune
checkpoint inhibitor | 8 | 2.17
(0.84–5.59) | 0.108 | 2.88
(0.88–9.43) | 0.080 |
Skeletal-related events
The details of the initial SREs experienced by
patients are presented in Table IV.
A total of 36/44 patients experienced SREs, with the most commonly
being the requirement for radiotherapy for bone pain. Moreover,
6/15 patients who underwent systemic therapy without radiotherapy,
received radiotherapy for bone metastases due to pain after the
failure of systemic therapy.
 | Table IV.Details of the initial
skeletal-related events experienced by patients (n=36) treated with
systemic therapy with (n=27) or without (n=9) radiotherapy. |
Table IV.
Details of the initial
skeletal-related events experienced by patients (n=36) treated with
systemic therapy with (n=27) or without (n=9) radiotherapy.
| Skeletal-related
events, n (%) | Total no. patients
Total no. patients | Systemic therapy
with radiotherapy | Systemic therapy
without radiotherapy |
|---|
| Need for
radiotherapy for bone pain | 30 (83) | 24 (89) | 6 (67) |
| Spinal cord and
nerve root compression | 3
(8) | 3
(11) | 0 |
| Hypercalcemia | 2
(6) | 0 | 2 (22) |
| Pathologic
fracture | 1
(3) | 0 | 1 (11) |
| Surgical
interventions | 0 | 0 | 0 |
Discussion
Bone metastasis is a poor prognostic factor for
metastatic RCC (20,21), and it can worsen despite the
administration of systemic chemotherapy, even when it is effective
for metastases in other organs. Enlarged bone lesions cause SRE,
decrease the QOL and result in a poor prognosis (5,6); thus,
it is important to control bone lesions in order to avoid these
outcomes. However, while improved efficacy of radiotherapy and
surgery for controlling bone lesions has been reported (10,22),
whether systemic therapy itself can control bone lesions remains
unknown.
Despite a number of reports having been published
discussing the prognosis of patients with RCC with bone metastases,
to the best of our knowledge, these have not mention the overall
response rate of targeted therapy or immune checkpoint inhibitors
for bone lesions (22,23). Bone lesions are difficult to measure
accurately or evaluate via the RECIST criteria. Therefore, the
present study adopted the MD Anderson bone response criteria, which
can be used to evaluate the treatment efficacy for bone lesions by
considering the size and sclerotic changes (17). While these criteria were originally
developed for evaluating bone metastases from breast cancer, they
have been used in translational research to evaluate the effect of
radium-223 dichloride combined with targeting therapy on bone
metastases of RCC (18).
Bone metastases in RCC are mainly osteolytic in
nature and decrease the bone integrity, induce bone pain and result
in significant morbidity for patients due to associated SREs
(24). Osteoclast activation due to
the presence of malignant cells leads to bone destruction (9), and a decline in malignant cells as a
result of treatment can decrease osteoclast activity and induce
sclerotic changes. It is therefore reasonable to regard sclerotic
changes as indicating treatment efficacy.
The present results suggested that targeted therapy
or immune checkpoint inhibitor without radiotherapy had only a
slight effect on bone metastasis control. With regards to targeted
therapy, a beneficial effect of cabozantinib, which is not
available in Japan, on bone metastasis has been reported in a
previous clinical trial, but the overall response rate was not
mentioned, and how bone lesions are altered by cabozantinib is yet
to be elucidated (25). By contrast,
the results of the present study demonstrated that systemic therapy
with sequential or concomitant radiotherapy exhibited a significant
impact on bone metastasis control and prolonged the time to
progression. This suggests a synergic effect of radiotherapy
combined with targeted therapy or immune checkpoint inhibitors
(26,27). Targeted therapy or immune checkpoint
inhibitors can enhance radiosensitization and improve local
control, but the optimal timing and schedule remain unknown
(26,27). Surgical resection is promising for
controlling bone lesions (9);
however, in a clinical setting, numerous cases are unresectable due
to a poor physical condition or unfavorable location of the bone
lesion, among other reasons (28).
For such cases, targeted therapy or immune checkpoint inhibitor
therapy alone is not adequate, and radiotherapy should be
considered to maintain the QOL.
Several limitations associated with the present
study should be discussed. For example, this was a retrospective
study that contained a relatively small sample size and a
heterogeneous group of patients. However, the current data
reflected the clinical practice and demonstrated a radiographically
overall response rate specialized for bone lesions. To the best of
our knowledge, the present findings have not been previously
reported and may be useful for treating patients with RCC with bone
metastases.
In conclusion, almost none of the patients who
received systemic therapy without radiotherapy achieved a response
in bone metastasis from RCC. Systemic therapy with radiotherapy
exhibited a significant impact on bone metastasis control; however,
it is still not adequate. Bone metastasis from RCC remains
challenging to manage, despite multimodal therapy. Radium-223
dichloride, an α-emitting therapy that induces DNA double-strand
breaks leading to cellular death in areas with increased
osteoblastic activity, is reported to be effective when combined
with targeted therapy for controlling RCC bone metastasis (15). Moreover, novel approaches for
treating RCC bone metastasis are expected to be developed.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Inoue and Dr
Kumagai (National Hospital Organization Kyushu Cancer Center,
Fukuoka, Japan) for analyzing the samples.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
TN conceived and designed the analysis. All authors
acquired the data. TN and NF analyzed the data. TN and NF drafted
the manuscript. DT, KI, NN, HK and MN acquired the data, assisted
with statistical analysis, supervised the study and revised the
manuscript critically for important intellectual content. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of National Hospital Organization Kyushu Cancer Center
(Fukuoka, Japan; approval no. 2014–99) and the Graduate School of
Medicine and Pharmaceutical Sciences for Research University of
Toyama (Toyama, Japan; approval no. RCOI2019133). Written informed
consent was provided by all patients prior to the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ljungberg B, Campbell SC, Cho HY, Jacqmin
D, Lee JE, Weikert S and Kiemeney LA: The epidemiology of renal
cell carcinoma. Eur Urol. 60:615–621. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gupta K, Miller JD, Li JZ, Russell MW and
Charbonneau C: Epidemiologic and socioeconomic burden of metastatic
renal cell carcinoma (mRCC): A literature review. Cancer Treat Rev.
34:193–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Du YJ, Pahernik S, Hadaschik B, Teber D,
Duensing S, Jäger D, Hohenfellner M and Grüllich C: Survival and
prognostic factors of patients with renal cell cancer with bone
metastasis in the era of targeted therapy: A single-institution
analysis. Urol Oncol. 34:433.e1–e8. 2016. View Article : Google Scholar
|
|
4
|
Bianchi M, Sun M, Jeldres C, Shariat SF,
Trinh QD, Briganti A, Tian Z, Schmitges J, Graefen M, Perrotte P,
et al: Distribution of metastatic sites in renal cell carcinoma: A
population-based analysis. Ann Oncol. 23:973–980. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Santini D, Procopio G, Porta C, Ibrahim T,
Barni S, Mazzara C, Fontana A, Berruti A, Berardi R, Vincenzi B, et
al: Natural history of malignant bone disease in renal cancer:
Final results of an Italian bone metastasis survey. PLoS One.
8:e830262013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kinnane N: Burden of bone disease. Eur J
Oncol Nurs (11 Suppl 2). S28–S31. 2007. View Article : Google Scholar
|
|
7
|
Mirels H: Metastatic disease in long
bones: A proposed scoring system for diagnosing impending
pathologic fractures. 1989. Clin Orthop Relat Res. (415
Suppl):S4–S13. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ruppert LM: Malignant spinal cord
compression: Adapting conventional rehabilitation approaches. Phys
Med Rehabil Clin N Am. 28:101–114. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Umer M, Mohib Y, Atif M and Nazim M:
Skeletal metastasis in renal cell carcinoma: A review. Ann Med Surg
(Lond). 27:9–16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Taunk NK, Spratt DE, Bilsky M and Yamada
Y: Spine radiosurgery in the management of renal cell carcinoma
metastases. J Natl Compr Cancer Netw. 13:801–809. 2015. View Article : Google Scholar
|
|
11
|
Motzer RJ, Hutson TE, Cella D, Reeves J,
Hawkins R, Guo J, Nathan P, Staehler M, de Souza P, Merchan JR, et
al: Pazopanib versus sunitinib in metastatic renal-cell carcinoma.
N Engl J Med. 369:722–731. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson D, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C,
Kim ST, et al: Sunitinib versus interferon alfa in metastatic
renal-cell carcinoma. N Engl J Med. 356:115–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: Nivolumab versus everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1803–1813. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Motzer RJ, Tannir NM, McDermott DF, Arén
Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P,
Porta C, George S, et al: Nivolumab plus ipilimumab versus
sunitinib in advanced renal-cell carcinoma. N Engl J Med.
378:1277–1290. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Woolf DK, Padhani AR and Makris A:
Assessing response to treatment of bone metastases from breast
cancer: What should be the standard of care? Ann Oncol.
26:1048–1057. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hamaoka T, Madewell JE, Podoloff DA,
Hortobagyi GN and Ueno NT: Bone imaging in metastatic breast
cancer. J Clin Oncol. 22:2942–2953. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McKay RR, Bossé D, Gray KP, Michaelson MD,
Krajewski K, Jacene HA, Walsh M, Bellmunt J, Pomerantz M, Harshman
LC and Choueiri TK: Radium-223 dichloride in combination with
vascular endothelial growth factor-targeting therapy in advanced
renal cell carcinoma with bone metastases. Clin Cancer Res.
24:4081–4088. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jehn CF, Diel IJ, Overkamp F, Kurth A,
Schaefer R, Miller K and Lüftner D: Management of metastatic bone
disease algorithms for diagnostics and treatment. Anticancer Res.
36:2631–2637. 2016.PubMed/NCBI
|
|
20
|
Motzer RJ, Escudier B, Bukowski R, Rini
BI, Hutson TE, Barrios CH, Lin X, Fly K, Matczak E and Gore ME:
Prognostic factors for survival in 1059 patients treated with
sunitinib for metastatic renal cell carcinoma. Br J Cancer.
108:2470–2477. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Motzer RJ, Escudier B, Oudard S, Hutson
TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA,
Hollaender N, et al: Phase 3 trial of everolimus for metastatic
renal cell carcinoma: Final results and analysis of prognostic
factors. Cancer. 116:4256–4265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kitamura H, Takahashi A, Takei F, Hotta H,
Miyano N, Shindo T, Igarashi M, Tachiki H, Kunishima Y, Muranaka T,
et al: Molecular-targeted therapy and surgery may prolong survival
of renal cell carcinoma patients with bone metastasis: A
multi-institutional retrospective study in Japan. Anticancer Res.
36:5531–5536. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ruatta F, Derosa L, Escudier B, Colomba E,
Guida A, Baciarello G, Loriot Y, Fizazi K and Albiges L: Prognosis
of renal cell carcinoma with bone metastases: Experience from a
large cancer centre. Eur J Cancer. 107:79–85. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zekri J, Ahmed N, Coleman RE and Hancock
BW: The skeletal metastatic complications of renal cell carcinoma.
Int J Oncol. 19:379–382. 2001.PubMed/NCBI
|
|
25
|
Choueiri TK, Escudier B, Powles T, Tannir
NM, Mainwaring PN, Rini BI, Hammers HJ, Donskov F, Roth BJ, Peltola
K, et al: Cabozantinib versus everolimus in advanced renal cell
carcinoma (METEOR): Final results from a randomised, open-label,
phase 3 trial. Lancet Oncol. 17:917–927. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morris ZS and Harari PM: Interaction of
radiation therapy with molecular targeted agents. J Clin Oncol.
32:2886–2893. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sharabi AB, Lim M, DeWeese TL and Drake
CG: Radiation and checkpoint blockade immunotherapy:
Radiosensitisation and potential mechanisms of synergy. Lancet
Oncol. 16:e498–e509. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tokuhashi Y, Matsuzaki H, Oda H, Oshima M
and Ryu J: A revised scoring system for preoperative evaluation of
metastatic spine tumor prognosis. Spine (Phila Pa 1976).
30:2186–2191. 2005. View Article : Google Scholar : PubMed/NCBI
|