Introduction
The tumour microenvironment has received growing
interest owing to its role in metabolic dysregulation and
tumorigenesis. Recent studies have associated dysregulation of
extra cellular matrix (ECM) proteins, such as fibrillin-1, with
tumorigenesis. The structural glycoprotein, fibrillin-1, is one of
two cleavage products encoded by the FBN1 gene (1). FBN1 encodes a 66 exon proprotein
known as profibrillin-1, that is proteolytically cleaved within the
65th exon at the consensus sequence
X-Arg-X-Lys/Arg-Arg-X by the enzyme furin (1,2).
Cleavage produces the 320 kDa glycoprotein fibrillin-1 and the
recently discovered 30 kDa glucogenic hormone, asprosin (3).
Asprosin was recently identified by Romere et
al (3) through an investigation
of Neonatal Progeroid Syndrome (NPS); a disorder characterised by
reduced insulin despite maintenance of euglycemia, extreme leanness
and partial lipodystrophy (4). The
pathogenesis of NPS is attributed to premature ablation of
profibrillin-1 as a result of a truncation mutation within the
FBN1 gene (3). Investigation
of NPS pathophysiology led to the classification of asprosin - the
c-terminal cleavage product of profibrillin 1 - as a novel
orexigenic and glucogenic hormone, involved in the regulation of
glucose homeostasis (3).
Elevated circulating levels of asprosin are present
in patients with metabolic syndrome manifestations, such as insulin
resistance and type 2 diabetes mellitus (T2DM), and are associated
with obesity (5–7). Adipose tissue is the primary source of
asprosin secretion, with recent data showing that patients with
cancer-related anorexia exhibit significantly lower asprosin plasma
levels compared to control counterparts (8,9). There
is increasing evidence associating the expression of asprosin with
metabolic disorders and complications during pregnancy, such as
gestational diabetes, and preeclampsia, as well as intra-uterine
growth restriction (10).
Additionally, elevated circulating asprosin levels have been noted
in women with polycystic ovarian syndrome (PCOS), although further
research is required to clarify the relevant role of obesity in
this population (11).
Olfactory Receptor 743, an orphan G protein-coupled
receptor (GPCR), was recently identified as one of the possible
receptors of asprosin in mice, whilst the human ortholog, olfactory
receptor 4M1 (OR4M1), is considered to be the primary asprosin
receptor in humans (12).
Detection of peripherally expressed olfactory
receptors (ORs) is now well-documented; despite initial beliefs for
localised expression of these receptors solely within the olfactory
epithelium of the nasal cavity (12). Existing data suggest that expression
of OLFR734 (and its orthologue OR4M1) may involve the testis,
whilst emerging evidence further indicates that expression may also
extend to other reproductive tissues, such as the ovaries, with
further implications for fertility in mammals (13,14).
Recent data present expression of this receptor in the ovaries of
murine and bovine samples, supporting an auto/paracrine circuit
between asprosin and OR4M1 which may be implicated in female
fertility, as well as healthy ovarian follicular function (14). However, expression of OR4M1 is yet to
be explored in human tissues past the testis, with the exception of
peripheral blood mononuclear cell expression in cases of traumatic
brain injury (15).
Ovarian cancer is one of the most lethal
gynaecological malignancies, affecting over 295,000 women worldwide
(16). Dysregulation of FBN1
[which is expressed within the theca interna and stroma of healthy
ovarian tissue (17)], in ovarian
cancer, through Aurora A and BRCA 2 signalling, is associated with
invasion and metastasis of tumour cells (18). Moreover, FBN1 is linked with
worse overall survival, as well as advanced stage of disease in
high grade serous ovarian cancer (19). However, studies have yet to
investigate the expression of asprosin in reproductive tissues in
both healthy women and those with ovarian cancer.
The regulation of glucose metabolism in ovarian
cancer has been studied extensively, however, certain mechanisms
are not fully elucidated. For example, hyperglycaemia drives
ovarian tumour growth independently of insulin status (20). Of note, this heightened state of
glucose metabolism is thought to accelerate tumour growth through
increased aerobic glycolysis in what is known as the ‘Warburg
effect’, and leads to a worse prognosis in cancer, including
ovarian cancer (21). Increased
expression of the glucose transporter GLUT-1 in ovarian cancer is
also linked to a decrease in overall survival, suggesting that
glucose abundance is a rate limiting factor of glucose metabolism
(22). In this context,
investigating the expression of both FBN1 and the novel
glucogenic hormone asprosin in human ovarian tissues will enhance
our understanding of the underlying molecular mechanisms implicated
in ovarian cancer, as well as the regulation of its tumour
microenvironment (20).
In this study -apart from the in silico FBN1
pan-cancer expression- we provide novel evidence of the protein
expression of asprosin in ovarian cancer patients and healthy
controls. We also demonstrate -to the best of our knowledge- for
the first time expression of the olfactory receptor OR4M1 in the
same tissues, raising the prospect of an auto/paracrine regulation
at the ovarian level. Finally, we mapped the cellular distribution
of asprosin in human ovarian cell lines, as well as the expression
of the cognate receptor OR4M1.
Materials and methods
Bioinformatics analysis
A Pancancer set of TCGA data was downloaded through
cBioPortal (www.cbioportal.org) and Shiny Methylation Analysis
Tool (SMART) (www.bioinfo-zs.com/smartapp/). Expression was
validated through GEPIA (gepia.cancer-pku.cn/) and GTeX (gtexportal.org/home/).
Survival plots were obtained using Kaplan-Meier Plotter [www.kmplot.com; (23)].
TCGA data sets are described under abbreviations.
Cell culture
SKOV-3 (ECAAC 91091004), PEO1 (ECAAC 10032308), PEO4
(ECAAC 10032309) and MDAH-2774 (ATCC CRL-10303) ovarian cancer
cells were cultured using aseptic technique and incubated at 37°C
in humidified conditions at 5% CO2. Cells were regularly
sub-cultured at 80% confluency in T75 filter head flasks (Thermo
Fisher Scientific, Inc.). SKOV-3 and MDAH-2774 were cultured in
Dulbecco's modified Eagle's medium (DMEM) (Thermo Fisher
Scientific, Inc.). PEO1 and PEO4 were cultured in Roswell Park
Memorial Institute (RPMI) (Thermo Fisher Scientific, Inc.). Media
were supplemented with 10% foetal bovine serum (FBS) and 1%
penicillin-streptomycin (Thermo Fisher Scientific, Inc.). Normal
ovarian epithelial cells, HOSEpiC (cat. no. 7310) were cultured in
Poly-L-Lysine coated flasks (5 µg/ml) according to the protocol
provided by the supplier (ScienCell), with Ovarian Epithelial Cell
Medium (OEpICM) supplemented with 1% Ovarian Cell Growth Supplement
and 1% penicillin-streptomycin (ScienCell) and 10% FBS (Thermo
Fisher Scientific, Inc.). For disassociation of adherent cells,
TrypLE express (Thermo Fisher Scientific, Inc.) was used. Cell
count and viability were detected manually using a Neubauer
Counting chamber with trypan blue (Invitrogen; Thermo Fisher
Scientific, Inc.) exclusion method. SKOV-3 were derived from a
human epithelial ovarian cancer patient and are haplo-diploid
adherent cells that carry a P53 mutation. PEO1 are derived from
human ovarian adenocarcinoma. PEO4 were derived from the same
patient as PEO1 although were harvested following treatment with
platinum-based chemotherapeutics and are cisplatin resistant.
MDAH-2774 were derived from a patient with ovarian endometroid
adenocarcinoma. The primary cell line, Human Ovarian Surface
Epithelial cells (HOSEpiC), referred to as ovarian surface
epithelial cells (OSE), were obtained commercially at passage 1 and
are classified as normal ovarian epithelial cells.
Clinical ovarian samples
Clinical ovarian cancer samples (n=12, Table I) and samples from healthy volunteers
(n=6, Table II) were obtained from
patients at the First Department of Obstetrics and Gynaecology,
‘Papageorgiou’ General Hospital, Medical School, Aristotle
University, Thessaloniki, Greece. Specimens from the 12 (average,
61.8 years; range, 48–75) patients with ovarian cancer were taken
during laparotomy for debunking surgery. Furthermore, in 6
reproductive-age women (average, 41.7 years; range, 39–45) without
any ovarian pathology who had completed their reproductive cycle
and underwent laparoscopic myomectomy for leiomyomas during the
follicular phase of the cycle, an ovarian sample was taken.
Institutional ethical approval was provided, and informed consent
was obtained from each patient before the collection of samples
(Reference: 14/11/STF/06).
 | Table I.Clinical details of patients with
ovarian cancer. |
Table I.
Clinical details of patients with
ovarian cancer.
| Patient | Histology | Grade | Stage | Age, years |
|---|
| 1 | Serous | 3 | IIIC | 64 |
| 2 | Serous | 3 | IIIC | 48 |
| 3 | Serous | 3 | IIIC | 61 |
| 4 | Serous | 2 | IIIC | 54 |
| 5 | Serous | 3 | IIIC | 69 |
| 6 | Serous | 3 | IV | 65 |
| 7 | Serous | 3 | IIIC | 75 |
| 8 | Serous | 3 | IIIC | 65 |
| 9 | Serous | 3 | IIIC | 56 |
| 10 | Serous | 3 | IIIC | 64 |
| 11 | Serous | 3 | IIIC | 64 |
| 12 | Serous | 2 | IIIC | 56 |
 | Table II.Clinical details of the control
group. |
Table II.
Clinical details of the control
group.
| Patient | Number of
fibroids/patient | Mean diameter of
fibroids/patient, cm | Age, years |
|---|
| 1 | 1 |
8.9 | 39 |
| 2 | 4 |
3.2 | 42 |
| 3 | 2 |
6.5 | 40 |
| 4 | 6 |
3.7 | 43 |
| 5 | 2 |
6.0 | 45 |
| 6 | 1 | 10.0 | 41 |
Immunofluorescence
Cells were washed in phosphate buffered saline (PBS)
solution (Thermo Fisher Scientific, Inc.), fixed with ice cold
methanol, and washed three times with PBS. Samples were blocked
with 5% bovine serum albumin (BSA) buffer (Thermo Fisher
Scientific, Inc.), covered with parafilm, and left to incubate for
40 min at 37°C. Asprosin (BioLegend) and OR4M1 (Novus Biologicals)
primary antibodies (1:200/1:100 in 5% BSA) were added before
incubation at 37°C for 1 h (asprosin) or room temperature overnight
(OR4M1). The coverslips were washed three times with PBS before the
addition of secondary Alexa Flour 488 antibody (Merck Millipore) at
a concentration of 1:200. The samples were covered with parafilm
and placed in a humidified chamber for 30 min at room temperature,
before being washed three times with PBS. Coverslips were
transferred to a glass slide and sealed with a drop of Molecular
ProbesProLong Diamond Antifade Mountant with DAPI (Thermo Fisher
Scientific, Inc.) and clear nail varnish. The slides were then
analysed, and images captured using a DM4000 microscope (Leica)
lens at ×100 magnification.
Immunohistochemistry of tissue
microarray
Paraffin-embedded ovarian tissue microarray slides
were purchased from US Biomax Inc. (cat. no. BC11115c). All tissue
samples were collected under Health Insurance Portability and
Accountability Act (HIPAA) approved protocols, following the
appropriate ethical standards with the donors being fully informed
and with their consent. Slides comprised of 100 biopsy cores of
ovarian tissue: malignant and adjacent (Table SI). Slides were deparaffinised and
rehydrated, followed by antigen retrieval using sodium citrate
solution (10 mM Sodium citrate in dH2O, 0.05% Tween-20,
pH 6.0). They were then washed in 0.025% Triton-X in PBS (Thermo
Fisher Scientific, Inc.) before a 15-min incubation in 3%
H2O2 followed by additional washes in 0.025%
Triton-X in PBS. The slides were blocked with 5% BSA in PBS,
followed by incubation with Asprosin/OR4M1 Antibody (1:200/1:100)
overnight in a humidity chamber at 4°C.
Slides were then washed three times in 0.025%
Triton-X in PBS and incubated with a secondary antibody in 1%
rabbit serum (ZytoChem Plus HRP-DAB Kit, Zytomed Systems GmbH) for
1 h. The slides were then washed with 0.025% Triton-X in PBS to
ensure the removal of unbound secondary antibody. Then
streptavidin-HRP conjugate was added to the bound secondary
antibody and the slide incubated for a further 30 min within the
humidity chamber. Slides were washed with PBS before the addition
of DAB stain. These were then counterstained with haematoxylin and
washed with 0.1% sodium bicarbonate. Finally, slides were
dehydrated before the addition of DPX and coverslips, then left to
dry overnight. Immunoreactivity was analysed using a light
microscope (Zeiss GmbH). Results were calculated by two independent
reviewers using a percentage score of positive tumour cells, as
described previously (24).
RNA isolation, cDNA synthesis and
reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from cell lysates using the
RNeasy Mini Kit (Qiagen, Inc.), before being reverse transcribed
using a cDNA reverse transcription Kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.). Sample purity was assessed using
Nano-Drop 2000C (Thermo Fisher Scientific, Inc.) and relative gene
expression measured using SYBR Green PCR Master Mix (Bio-Rad) and
qPCR with a Bio-Rad CFX96 system according to the following
conditions (Table III).
 | Table III.Bio-Rad thermal cycling protocol for
use with iTaq™ Universal SYBR′ Green Supermix (Bio-Rad
Laboratories, Inc.). |
Table III.
Bio-Rad thermal cycling protocol for
use with iTaq™ Universal SYBR′ Green Supermix (Bio-Rad
Laboratories, Inc.).
| Step | Temperature,
°C | Time, sec | Cycle |
|---|
| Activation | 95 | 30 | 1 |
| Denaturation | 95 | 5 | 38 |
| Amplification | 60 | 30 |
| Melt curve
analysis | 60 | Increments of
5 | Infinite |
FBN1 primers were designed according to the
Harvard Primer bank, whereas OR4M1 were generated according
to a 2013 study (15). Additional
primers include the housekeeping gene YWHAZ (Table IV). RQ values were calculated as
previously described (24),
according to the comparative 2−ΔΔCq analysis method
(25).
 | Table IV.List of primers utilized in the
present study. |
Table IV.
List of primers utilized in the
present study.
| Gene | Primer sequences
(5′-3′) |
|---|
| YWHAZ | Forward:
AGACGGAAGGTGCTGAGAAA |
|
| Reverse:
GAAGCATTGGGGATCAAGAA |
| FBN1 | Forward:
TTTAGCGTCCTACACGAGCC |
|
| Reverse:
CCATCCAGGGCAACAGTAAGC |
| OR4M1 | Forward:
TCTGTTAATGTCCTATGCCTTCC |
|
| Reverse:
AATGTGGGAATAGCAGGTGG |
Statistical analysis
Statistical analyses were performed using GraphPad
prism9® software (GraphPad Software, Inc.). Error bars
in graphs are presented as standard error of the mean (SEM). Mann
Whitney U test and a one-way ANOVA (Analysis of variance) with
Tukey's multiple comparison post hoc statistical tests were applied
to the observed measurements from the data. Variances in survival
were generated using Kaplan-Meier curves with log-rank test. Beta
values were calculated using the SMART methylation tool (SMART).
The method for differential analysis conducted by GEPIA is listed
as a one-way ANOVA, where disease state (Tumour or Normal) is used
as a variable for calculating differential expression: Gene
expression against disease state. The expression data are first
log2(TPM+1) transformed for differential analysis and the log2FC is
defined as median (Tumour) - median (Normal). Genes with higher
|log2FC| values and lower q values than pre-set thresholds are
considered differentially expressed genes. More information can be
accessed at http://gepia.cancer-pku.cn/help.html. Unless stated
otherwise, significance was set at P-value <0.05.
Results
Expression of FBN1 in normal
tissues
Initial analyses of FBN1 expression were
conducted using publicly available data from The Genotype Tissue
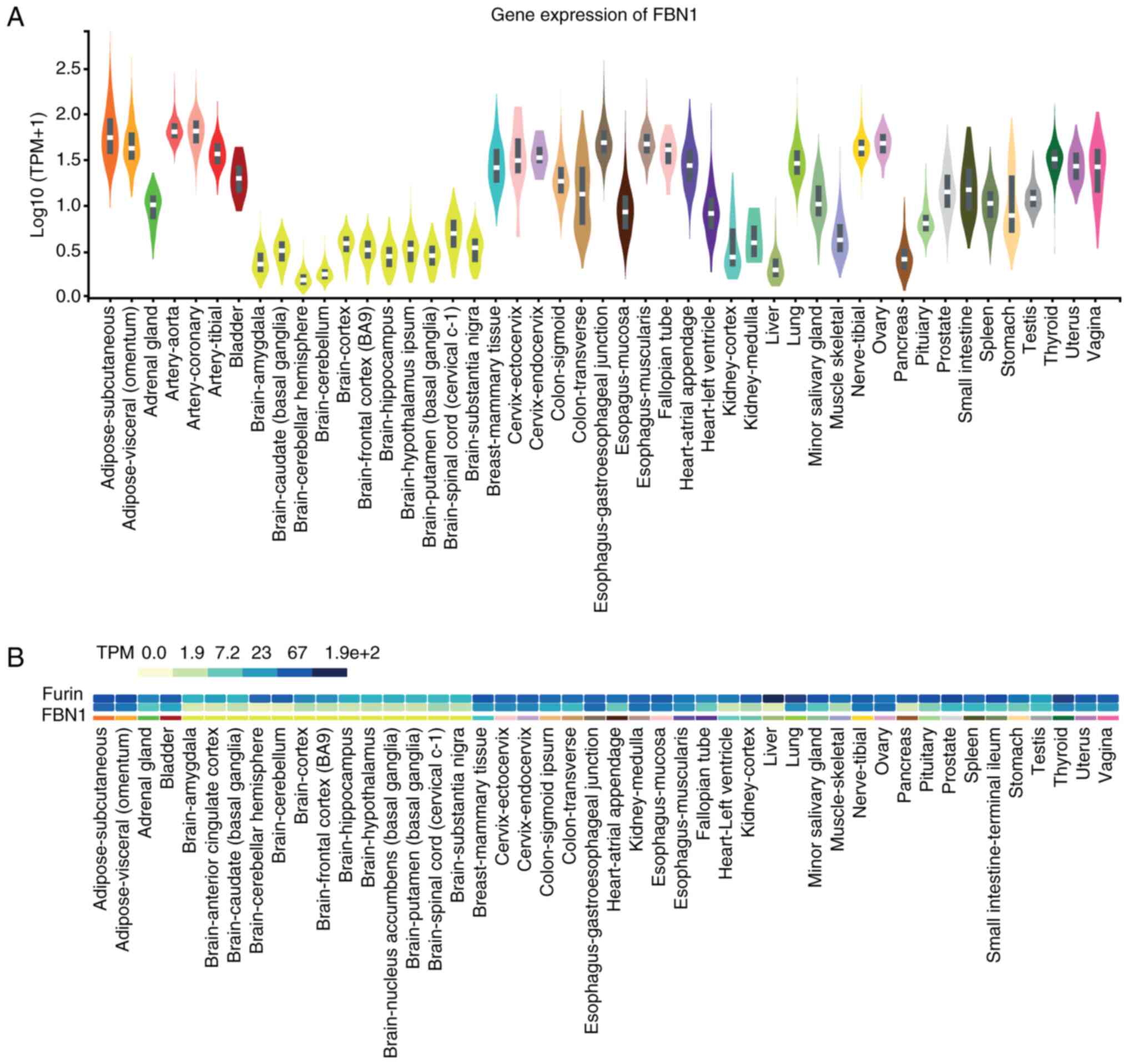
Expression (GTEX) project (Fig. 1).
Fibroblasts, arteries, adipose tissue (subcutaneous and visceral)
and the ovaries are amongst the tissues that express relatively
high levels of FBN1, as do the studied female reproductive
tissues. Brain and whole blood express the lowest FBN1
levels, along with the liver and pancreas (Fig. 1A). In the same dataset, we further
analysed the co-expression of FBN1 with the proteolytic
enzyme furin, which may provide an oversight of potential
furin-mediated cleavage release of asprosin in these tissues
(Fig. 1B). Furin is shown to
exhibit ubiquitous expression throughout the human body with high
levels detected across all tissues, including those with high
FBN1 expression (e.g., normal human reproductive tissues,
such as testis, vagina, uterus and ovaries).
Pancancer mapping of FBN1
We expanded our observations by assessing the
expression of FBN1 across 33 different cancer types using
TCGA datasets through GEPIA. As presented in Fig. 2, significant differential regulation
of FBN1 is noted for the following cancer types: bladder urothelial
carcinoma (BLCA), cervical squamous cell carcinoma and endocervical
adenocarcinoma (CESC), cholangiocarcinoma (CHOL), lymphoid neoplasm
diffuse large B-cell lymphoma (DLBC), head neck and squamous cell
carcinoma (HNSC), lung adenocarcinoma (LUAD), lung squamous cell
carcinoma (LUSC), ovarian serous cystadenocarcinoma (OV),
pancreatic adenocarcinoma (PAAD), stomach adenocarcinoma (STAD),
thyroid carcinoma (THCA), thymoma (THYM), uterine corpus
endometrial carcinoma (UCEC), and uterine carcinosarcoma (UCS). Of
the presented cancers, the female reproductive tissues: uterine,
cervical, and ovarian exhibit lower FBN1 expression compared
to corresponding normal tissues.
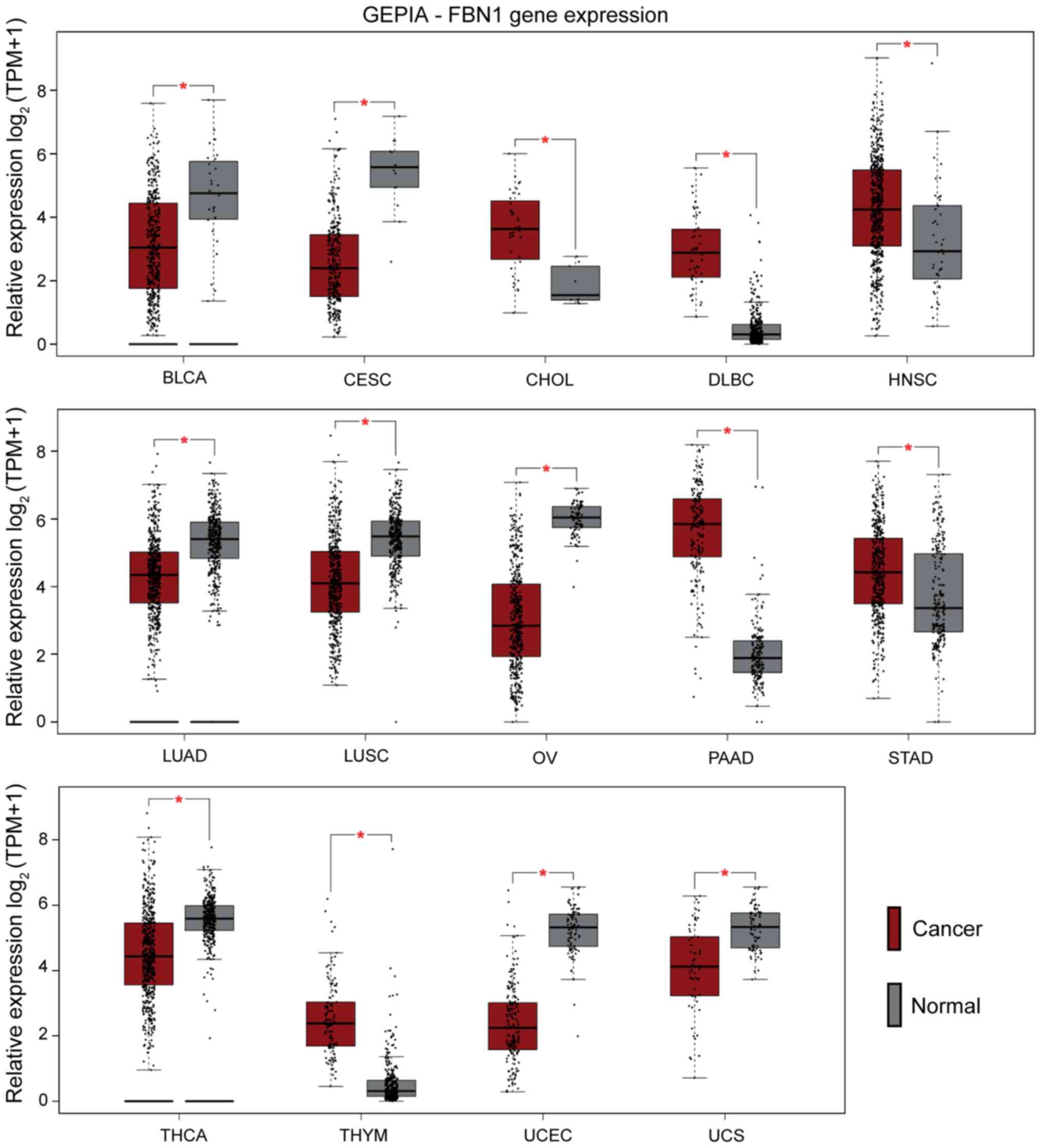 | Figure 2.Pancancer profiling of FBN1
expression. Cancer types with significant differences compared with
normal tissues (*P<0.01) are presented in the graphs [cancer
(red) and normal (grey)]. Cancers with lower expression levels of
FBN1 compared with normal samples included UCS, UCEC, THCA, OV,
LUSC, LUAD, CESC and BLCA. Those with higher FBN1 expression levels
included THYM, STAD, PAAD, HNSC, DLBC and CHOL. BLCA, bladder
urothelial carcinoma; CESC, cervical squamous cell carcinoma and
endocervical adenocarcinoma; CHOL, cholangiocarcinoma; DLBC,
lymphoid neoplasm diffuse large b-cell lymphoma; FBN1, fibrillin-1;
GEPIA, Gene Expression Profiling Interactive Analysis; HNSC, head
and neck squamous cell carcinoma; LUAD, lung adenocarcinoma; LUSC,
lung squamous cell carcinoma; OV, ovarian serous
cystadenocarcinoma; PAAD, pancreatic adenocarcinoma; STAD, stomach
adenocarcinoma; THCA, thyroid carcinoma; THYM, thymoma; TPM,
transcripts per million; UCEC, uterine corpus endometrial
carcinoma; UCS, uterine carcinosarcoma. |
Given that the methylation status of FBN1 is
of known biomarker potential (26),
the FBN1 methylation status for the above cancers was assessed in
the same dataset using SMART (Fig.
S1). FBN1 methylation in colon adenocarcinoma (COAD) is
significantly higher than healthy colon. Similar results are noted
for breast (BRCA) and uterine endometrial carcinoma (UCEC). The
methylation status of FBN1 within the ovarian cancer data set
appears to be highly variable compared to other cancers, as
indicated by the beta value of ~0.5; however, there is a lack of
comparable normal data for ovarian cancer from TCGA.
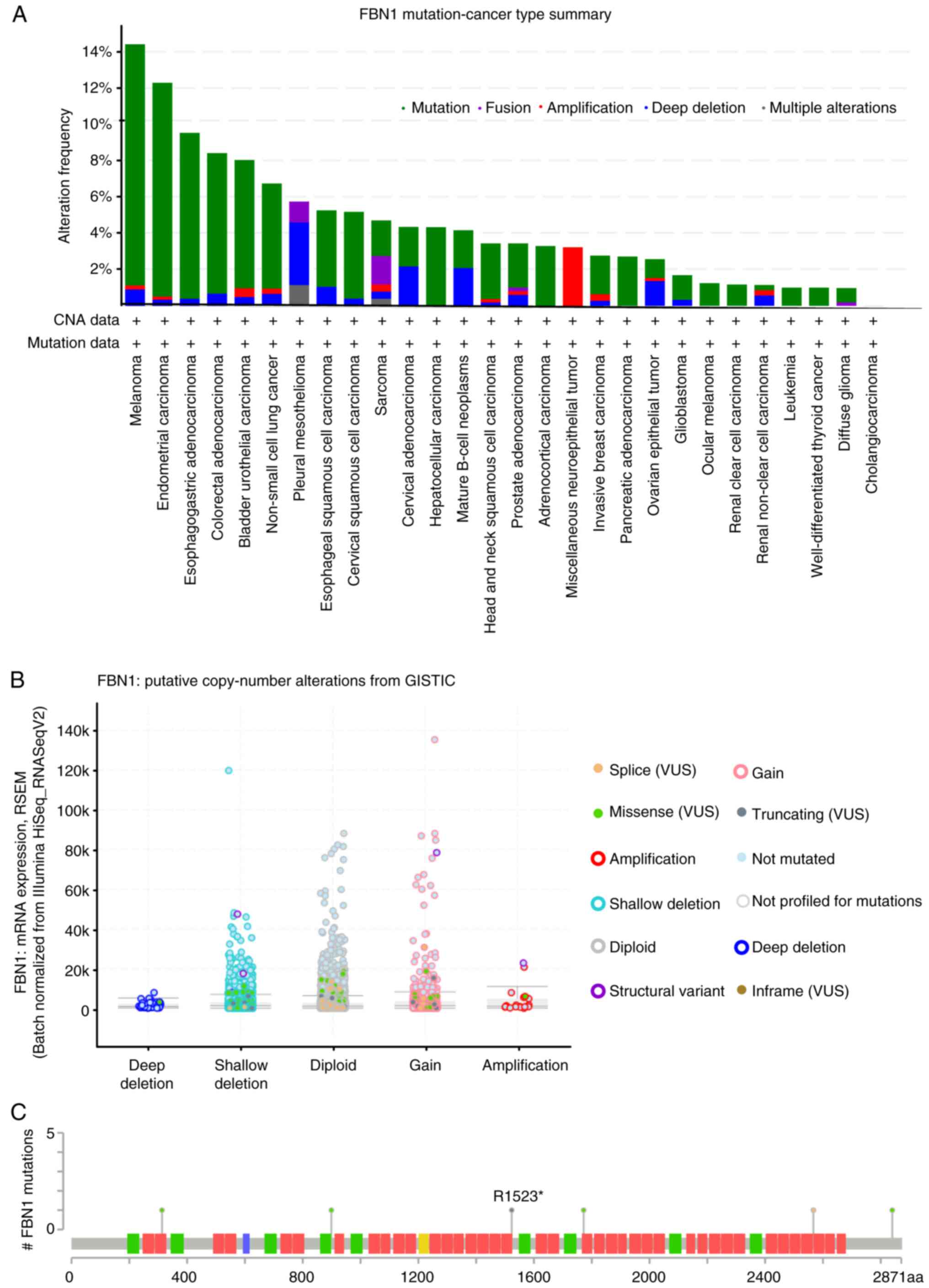
Additional insight was sought through the analysis
of FBN1 using cBioPortal. Mutations of FBN1 within the
pancancer cohort of TCGA cancers appear to be most frequent in
melanoma, uterine, stomach and colorectal cancer (Fig. 3A). A relatively lower frequency of
alterations were detected in ovarian cancer samples compared to the
other types of cancer, however, the high percentage of deep
deletion within the cases presented must be noted.
Gain of function, shallow deletion and diploid
appear to show the highest frequency of copy number variation
within the samples (Fig. 3B). Six
mutations on the FBN1 gene were identified in cases of
serous ovarian cancer (Fig. 3C).
Nonsense and splice-site mutations (black and orange lollipops)
give rise to a truncated FBN1-encoded protein, whereas the four
missense mutations (green lollipops) cause an amino acid
substitution. Of note, one mutation has been identified in the
asprosin coding region leading to a lysine to arginine (K2840R)
substitution.
Expression of FBN1, asprosin and OR4M1
in ovarian cancer
We have validated the in-silico data from TCGA and
GTEX (Fig. 4A), using a smaller
cohort of patients with ovarian cancer (n=12; stage III and IV).
Our data corroborates the previous findings, as it demonstrates
that the mRNA expression of FBN1 was significantly lower in
patients with ovarian cancer compared to healthy volunteers (n=6;
Fig. 4B). In addition, OR4M1
expression was significantly up regulated in the same ovarian
cancer samples (n=12) compared to the controls (n=6; Fig. 4C). We then measured expression of
FBN1 and OR4M1 in five ovarian cell lines: one normal ovarian
epithelial cell line (HOSEpiC), and four ovarian cancer cell lines
namely, SKOV-3, PEO1, PEO4 and MDAH-2774. FBN1 was significantly
over-expressed in HOSEpiC cells compared to all studied ovarian
cancer cell lines (Fig. 4D), whereas
no apparent change in the expression of OR4M1 was noted across all
five cell lines (Fig. 4E).
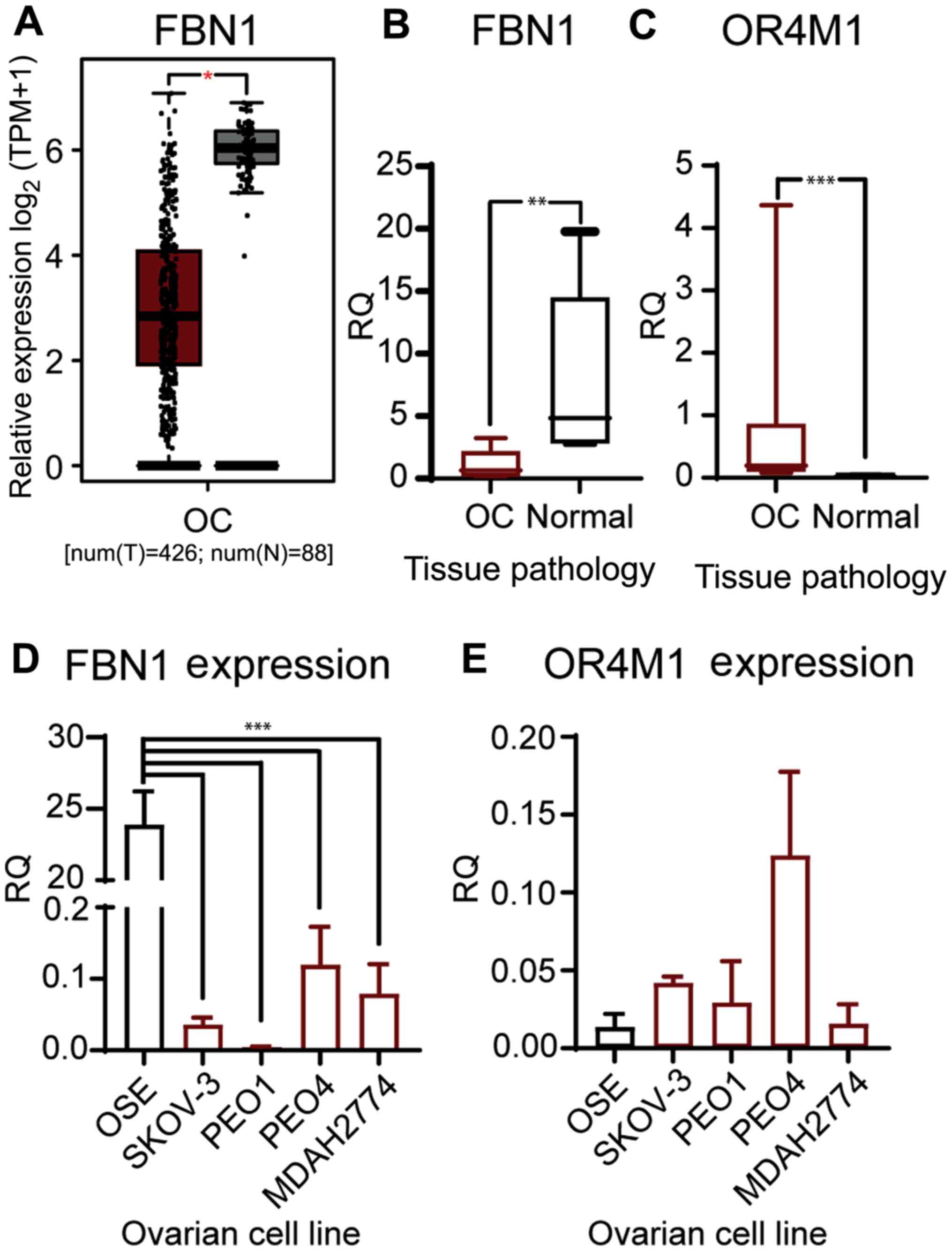 | Figure 4.Gene expression of FBN1 and OR4M1 at
the ovarian level. (A) Expression data of FBN1 in OC from GEPIA for
use as comparison. *P<0.05. Relative expression levels of FBN1
and OR4M1 in OC (red) and normal ovarian tissues (grey) were
determined using reverse transcription-quantitative PCR. (B)
Significantly lower expression levels of FBN1 in OC samples (OC,
n=12; stage III and IV) compared with FBN1 expression in normal
ovarian tissue samples from healthy volunteers (n=6). **P<0.001
(samples obtained for the present study; different from the GEPIA
cohort in A). (C) Significantly higher expression levels of OR4M1
in OC samples (OC, n=12; stage III and IV) compared with OR4M1
expression in normal ovarian tissue samples from healthy volunteers
(n=6). ***P<0.0001. (D) Higher relative expression levels of
FBN1 in normal ovarian surface epithelial cells (OSE), and lower
expression levels in the studied human ovarian cancer cell lines
(SKOV-3, PEO1, PEO4 and MDAH-2774). ***P<0.0001. (E) Lower
relative expression levels of OR4M1 in normal ovarian epithelial
cells, as well as in the PEO1 and MDAH-2774 human ovarian cancer
cell lines, compared with the relatively higher OR4M1 expression
noted in SKOV-3 and PEO4 cells. RQ indicates relative change in
fold expression to the calibrator gene YWHAZ. FBN1, fibrillin-1;
GEPIA, Gene Expression Profiling Interactive Analysis; OC, ovarian
cancer; OR4M1, olfactory receptor 4M1; OSE, HOSEpiC cells; TPM,
transcripts per million; num(T), number of patients for tumour
group; num(N), number of patients for normal group; RQ, relative
quantity. |
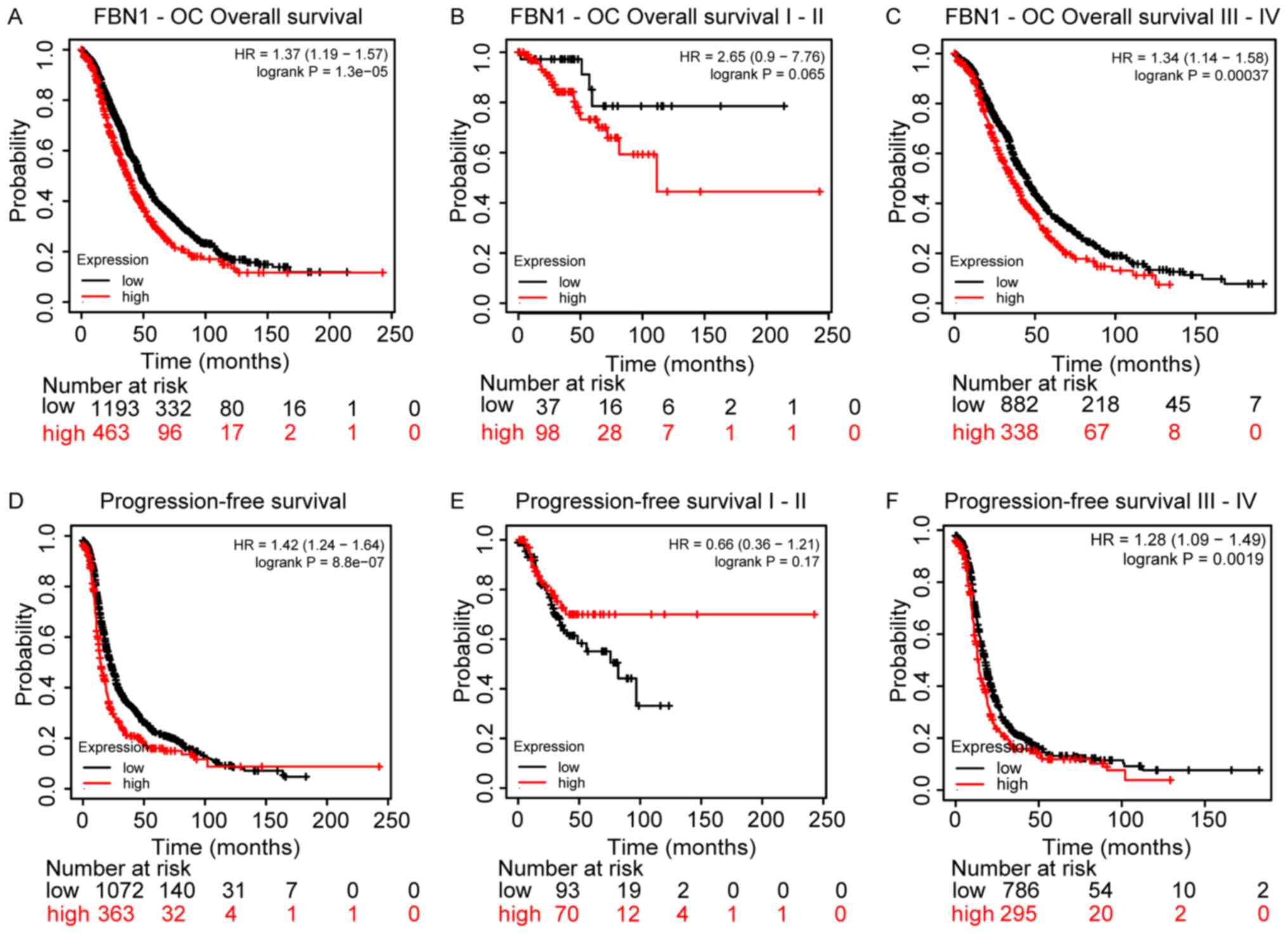
Since FBN1 is differentially regulated in ovarian
cancer, its prognostic value was also assessed using Kaplan-Meier
plots for overall survival (OS) and progression free survival
(PFS), Fig 5. Higher FBN1
expression was associated with poor OS and PFS, Fig. 5A and D, respectively. This predictive
power of FBN1 appears to be significant for patients with late
stage ovarian cancer (i.e., III and IV), rather than early stage
(i.e., I and II), Fig. 5B and C and E
and F for OS and PFS, respectively.
Immunohistochemical analysis of a tissue microarray
containing 90 ovarian cancer cores and 10 normal adjacent tissue
(NAT) cores, each representing a different clinical case, was used
to measure the protein expression of asprosin and OR4M1 (Figs. 6 and 7). Asprosin was aberrantly expressed across
all different histological subtypes (Fig. 6A), with no stage-specific variation
when samples were grouped to early (I and II) and late (III and IV)
ovarian cancer stages (Fig. 6B).
Examination of OR4M1 protein expression revealed similar
non-specific expression across different histological subtypes
(Fig. 7A). However, higher
expression was detected in early (I and II) compared to late (III
and IV) ovarian cancer stages (Fig.
7B).
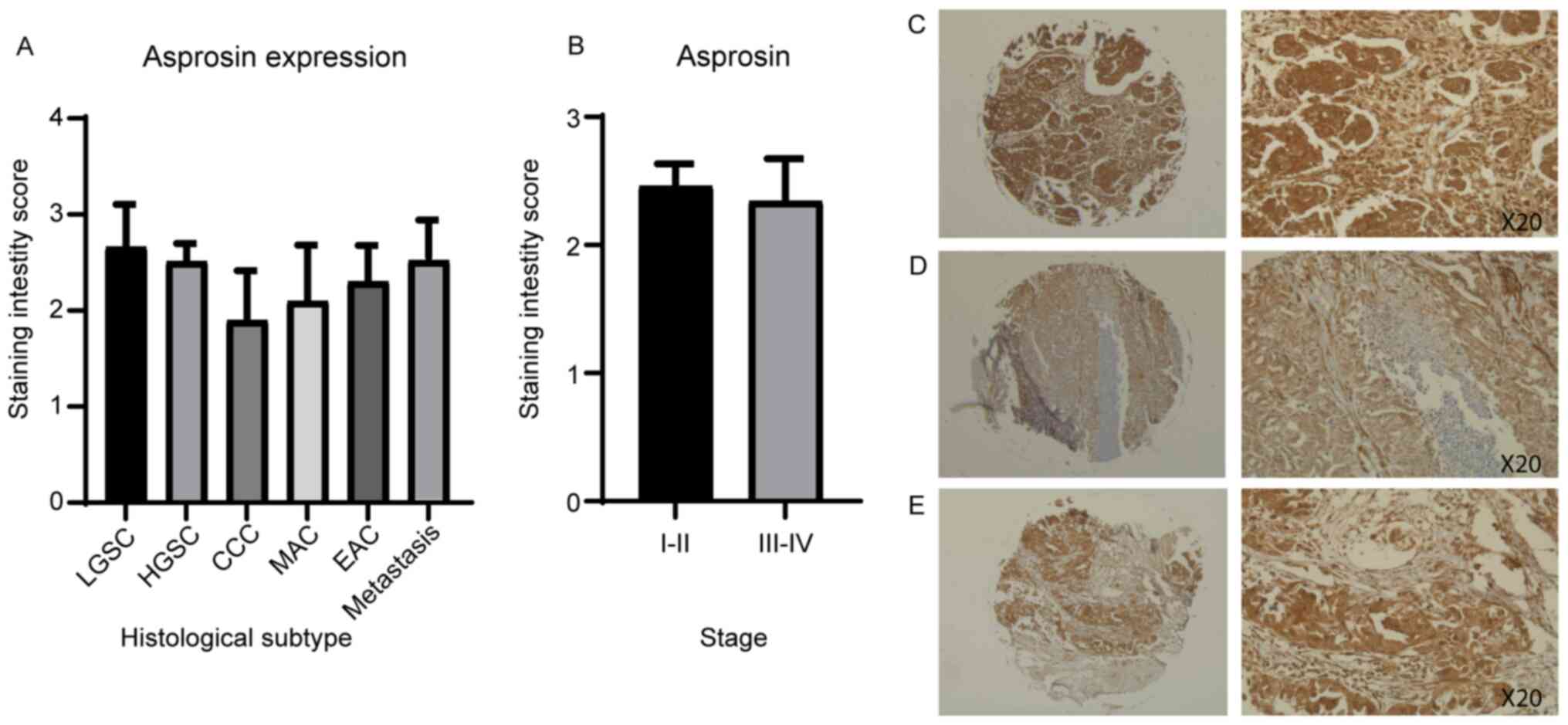 | Figure 6.Ovarian tissue microarray, including
90 ovarian cancer cores, stained with asprosin antibody (1:200).
Corresponding values for scoring: 0, <10%; 1, 10–25%; 2, 26–50%;
3, 51–75%; and 4, >76% of cells stained. (A) Asprosin staining
by histological subtype/grade: LGSC, HGSC, MAC, EAC and CCC. (B)
Asprosin staining of early (I and II) and late (III and IV) ovarian
cancer stages, revealing no significant difference. (C) HGSC, stage
II at ×5 (left) and ×20 (right) magnification. (D) HGSC, stage I at
×5 (left) and ×20 (right) magnification. (E) HGSC, stage III at ×5
(left) and ×20 (right) magnification. CCC, clear cell carcinoma;
EAC, endometroid adenocarcinoma; HGSC, high grade serous carcinoma;
LGSC, low grade serous carcinoma; MAC, mucinous adenocarcinoma. |
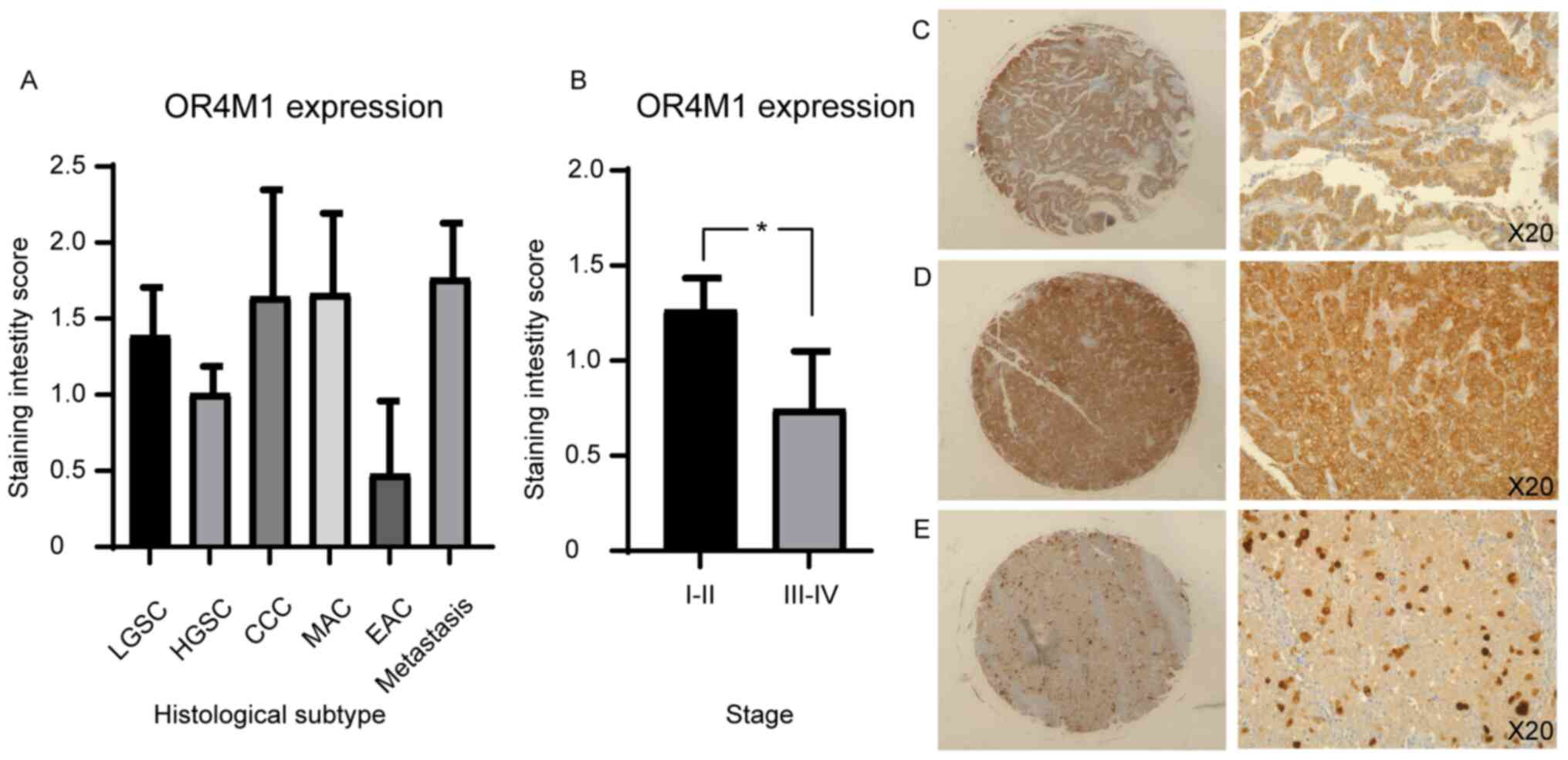 | Figure 7.Immunohistochemical staining of an
ovarian tissue microarray, including 90 ovarian cancer cores, with
OR4M1 antibody (1:100). (A) OR4M1 staining by histological
subtype/grade: LGSC, HGSC, MAC, EAC and CCC. (B) Higher OR4M1
staining in early (I and II) compared with late (III and IV)
ovarian cancer stages. *P=0.04. (C) HGSC, stage II at ×5 (left) and
×20 (right) magnification. (D) HGSC, stage I at ×5 (left) and ×20
(right) magnification. (E) HGSC, stage III at ×5 (left) and ×20
(right) magnification. CCC, clear cell carcinoma; EAC, endometroid
adenocarcinoma; HGSC, high grade serous carcinoma; LGSC, low grade
serous carcinoma; MAC, mucinous adenocarcinoma; NAT, normal
adjacent tissue; OR4M1, olfactory receptor 4M1. |
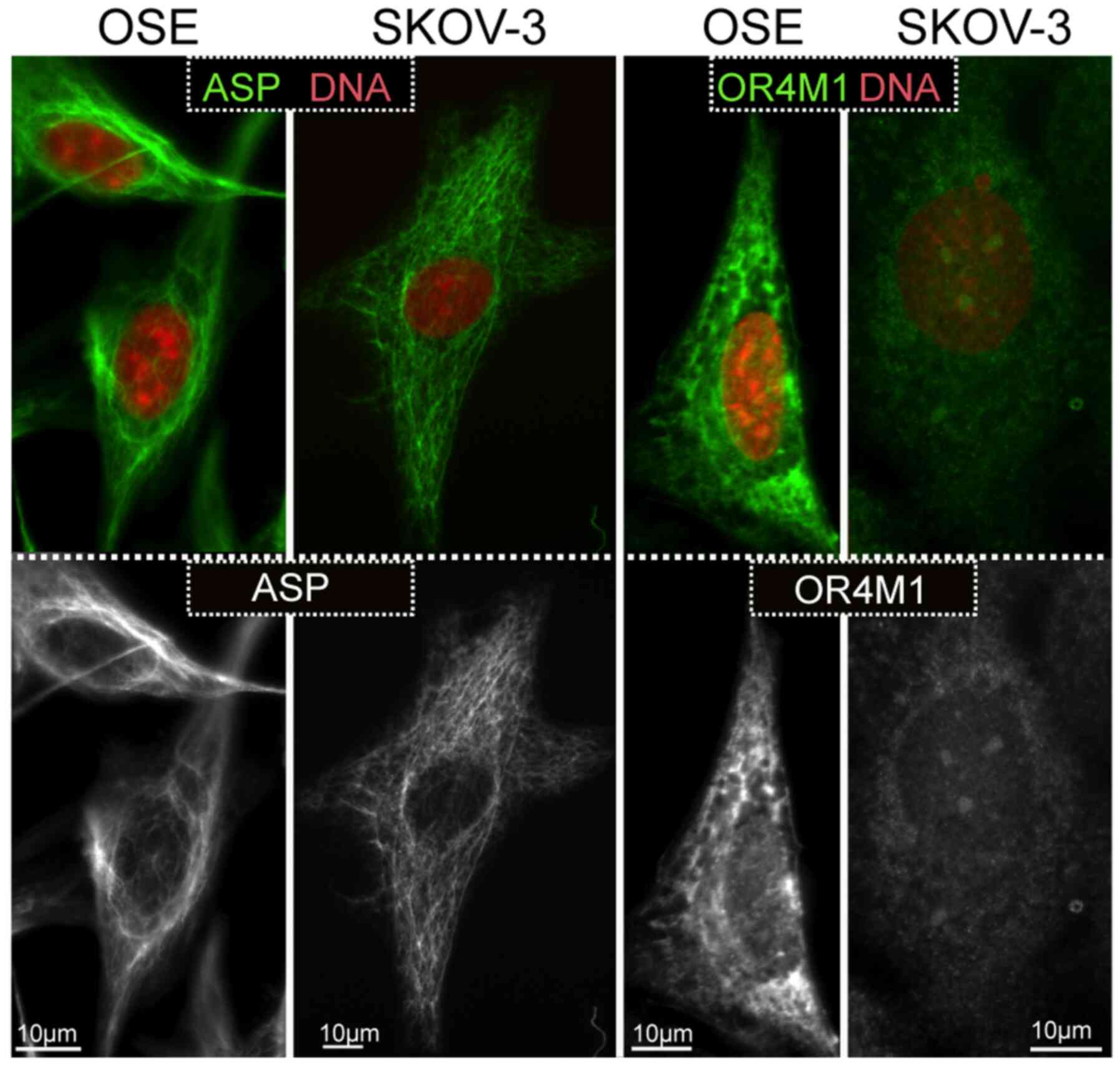
Observations on the expression of asprosin and OR4M1
were expanded using the SKOV-3 ovarian cancer cell line, as well as
the normal human ovarian epithelial cell line, HOSEpiC (OSE).
Similarly, to the tissue sections, asprosin exhibited a cytoplasmic
distribution (associated with structures resembling microtubules or
cytoskeleton), whereas OR4M1 appears to be expressed on the plasma
membrane and cytoplasm in accordance with the expected distribution
of a GPCR (Fig. 8).
Discussion
This study presents novel data regarding the
expression of FBN1 (the gene encoding profibrillin-1),
asprosin (the novel orexigenic/glucogenic hormone which is cleaved
from profibrillin-1), and OR4M1 (the human cognate receptor of
asprosin) in cancer, focusing on ovarian cancer. Using an in-silico
approach, we demonstrate that FBN1 expression is ubiquitous in
normal tissues, with high levels seen in fibrous tissues (e.g. in
fibroblast cells) and arteries, in addition to female reproductive
tissues, such as the uterus and ovaries. Being the main source for
the production of circulating asprosin (9), adipose tissue also exhibited high FBN1
expression. To date, asprosin production is thought to be specific
to adipose tissue. However, the noted co-expression of FBN1 with
the proteolytic enzyme furin in human tissues is indicative of
potential production and release of asprosin from other peripheral
tissues, such as the ovaries.
Although multiple studies have shown FBN1 mutations
as the cause of Marfan syndrome (MFS), which is further associated
with increased risk of tumourigenesis (27), very little is known about the role of
FBN1 mutations in cancer. Analysis of over one million cancer
cases, including stomach, liver, oesophagus, prostate,
gynaecological and other cancers, in a national cohort of patients
with MFS in Taiwan showed a higher risk of developing cancer in
these patients (27). Of note, the
data presented from cBioportal in our study, indicate that six FBN1
mutations were present in patients with ovarian cancer, with one of
the missense mutations located in the coding region for asprosin.
Future GWAS studies are required to explore the potential
involvement of these mutations in ovarian cancer.
The presented data from GEPIA in this study, show
differential FBN1 expression in 14 cancers, with higher expression
noted in cancers of the stomach (STAD) and pancreas (PAAD). The
latter is in line with previous research associating increased FBN1
expression in pancreatic islets with cellular progression from
hyperplastic to angiogenic to insulinoma (28). Lower FBN1 expression, however, was
noted in cancers that originate from fibrous tissues, including
gynaecological cancers, such as cervical (CESC), endometrial
(UCEC), uterine (UCS) and ovarian (OV) cancers. The downregulation
of FBN1 in this cohort of cancers may be suggestive of
tissue-specific expression compared to up-regulation in other
malignancies. Based on a previous study, FBN1 has a single CpG-rich
dominant promoter that is highly conserved in mammals (29). Interestingly, a study showed that
gene expression and activity of the promoter was significantly
higher in MG63 cells (a human osteosarcoma line) when compared to
MDA-MB-231 cells (a breast cancer cell line) (29). This agrees with previous observations
that variations in the activity of the promoter region can exert a
heritable transcriptional effect (30,31). As
such, this might also explain, at least in part, the varying
expression of FBN1 among different cancer types. Indeed,
transcription factor binding motifs identified in the promoter
region of FBN1, subserve tissue-specific functions (29). Of note, furin expression is slightly
elevated in ovarian cancer compared to controls (data not shown).
Therefore, in terms of the secretion of the cleaved peptide
asprosin, the dynamics may be different. Moreover, it is possible
that FBN1/asprosin may exert different effects in health and
disease. For example, in the normal ovary, it might affect
steroidogenesis and in the cancerous tissue may be implicated in
the Warburg effect. Especially the later, warrants further
investigation given that asprosin is a glucogenic peptide, that
stimulates the release of glucose from hepatic cells. It is well
known that in cancer cells, there is dramatic increase of the rate
of glucose uptake and subsequent lactate production (32). Recently, inhibition of Bcl2 in SKOV3
ovarian cancer cells, appeared to reverse the Warburg effect and
promoted oxidative stress-induced apoptosis in vitro
(33). Further studies are needed to
investigate the clinical application of asprosin as a potential
mediator of the Warburg effect in ovarian cancer.
Furthermore, changes in the methylation status of
FBN1 have shown biomarker value. For example, hypermethylation of
Synuclein Alpha (SNCA) and FBN1 in stool samples show excellent
sensitivity and specificity for colon cancer (34). Additional data has shown similar
potential for colorectal cancer (26). The data presented in our study
support these findings as methylation of FBN1 is significantly
higher in colon adenocarcinoma (COAD) compared to healthy colon.
Similar results are seen for breast (BRCA) and uterine endometrial
carcinoma (UCEC). Methylation of FBN1 in normal ovarian tissue
requires further investigation, since there is a lack of comparable
normal methylation data held through TCGA and SMART for ovarian
cancer.
In silico data for ovarian cancer were
further validated using clinical ovarian tissue samples from
patients with stage III/IV ovarian cancer, as well as both cancer
and normal human ovarian epithelial cell lines Our present findings
show significantly downregulated FBN1 expression in the ovarian
cancer samples compared to those from healthy controls, in accord
with the data noted in GEPIA. Moreover, FBN1 expression was
detected in the human ovarian cancer cell line SKOV-3, the
high-grade serous PEO1 and PEO4 cell lines, and the human
endometroid ovarian cancer cell line MDAH-2774, as well as the
normal ovarian epithelial cells (OSE). As noted in the clinical
ovarian cancer samples, these human ovarian cancer cell lines
exhibit relatively lower FBN1 expression compared with the normal
ovarian tissue.
The differential - albeit not-significant -
expression of FBN1 in the BRCA2 mutant and silent (wild-type) PEO1
and PEO4 cell lines, respectively, is of interest given that the
tumour suppressor gene BRCA2 is an inhibitor of FBN1 (18). BRCA2 inhibition of FBN1 is associated
with the inhibition of matrix metalloproteases (MMPs), including
MMP2, MMP9 and MMP13, as well as the activation of cellular
adhesion molecules which protect against metastasis (18). However, it should be noted that
database analyses on survival times are often biased because of
limited clinical information. These should be validated ideally
with prospective cohorts employing sophisticated statistical
considerations. Due to the nature of this study, such advanced
statistical considerations were not feasible, thus this should be
acknowledged as a limitation of the present study. The prognostic
value of FBN1 in cancer is continually evolving (19,35,36). In
our study, high FBN1 expression predicts lower overall- and
progression-free survival of patients with late stages (i.e., III
and IV) of ovarian cancer, corroborating previous findings which
show promising prognostic potential of FBN1 as part of a panel of
genes (19,35). Similarly, elevated FBN1 expression in
both colon and bladder cancer are associated with worse overall
survival (19,37,38).
Interestingly, previous data suggest that glucose
metabolism including hyperglycaemia in ovarian cancer is associated
with tumour growth and progression as well as worse survival
outcome (20,39). As such, further research is required
to elucidate the potential role of the glucogenic hormone and
product of FBN1, asprosin, at the level of the ovaries. To that aim
and following studies on the expression in normal murine and bovine
ovaries (13,14), we provide novel data regarding the
expression of both asprosin and its cognate receptor, OR4M1, in
normal human ovaries and ovarian cancer.
Using immunohistochemical staining, we show aberrant
protein expression of asprosin in ovarian cancer samples and normal
adjacent tissue. In routine examination, normal adjacent tissue is
often taken from the vicinity (<2 cm) of malignant cells and is
frequently used as a control for cancer studies. Of note, recent
transcriptome profiling data comparing normal adjacent tissue
samples to healthy control tissue - which is removed from a
substantial distance away from the primary tumour or from an age
matched healthy control - suggest that there is premalignant
conditioning of normal adjacent tissue (40). In the present study no apparent
differences in asprosin protein expression were observed amongst
different histological subtypes or stages of ovarian cancer, with
staining representative of high asprosin expression in most cases
(cancer and normal adjacent tissue samples). Similar widespread
protein expression of asprosin was recently documented in malignant
mesothelioma (41).
In our cell lines, this cytoplasmic distribution
appeared associated with structures resembling microtubules or
cytoskeleton. As this is a secreted protein, one would expect to
observe a pattern that resembles the endoplasmic reticulum, or the
Golgi, or even a vesicular pattern. One possibility is that
asprosin production by furin-mediated cleavage, escapes the
conventional secretory route, and follows a non-conventional
secretory pathway that may not be dependent on vesicular
exocytosis. Future in vitro studies using specific markers
of cytoplasmic organelles should address this finding. One of the
limitations of this study is the inability to measure mRNA
expression for asprosin, as this is a cleaved peptide therefore
only protein and precursor FBN1 mRNA expression can be
measured. The fact that FBN1 colocalises with furin in the ovary,
favours local production of the cleaved product. Future studies
should include the measure of asprosin levels from conditioned
media of ovarian cell lines and/or ovarian explants to elucidate
the secretion rate of asprosin from this tissue.
Moreover, given that asprosin binds to a GPCR, it is
expected to have a discrepancy between mRNA and protein levels. It
is possible after prolonged exposure to the ligand (i.e. asprosin),
OR4M1 might undergo desensitisation, as a mechanism limiting GPCR
signalling and subsequent activation of adenylyl cyclase. In doing
so, OR4M1 can be detected in the cytoplasm (rather the cytoplasmic
membrane) as a process of internalisation, or in lesser amounts if
it undergoes lysosomal degradation rather than recycling (42). Future studies should concentrate on
activation of second messengers in vitro. It is known that
GPCRs are capable of activating multiple G proteins (43), therefore it is important to measure
release of cAMP, or IP3 and activation of PKA or PKC in
vitro. It has also been shown that asprosin is capable of
binding to Toll-like receptor 4 (TLR4) and activating JNK
mediated-pathway in pancreatic β-cells (44). Of note, the role of TLR4 in ovarian
cancer is well documented (45), and
future studies should also investigate the possibility of asprosin
binding to TLR4 as well in ovarian cells.
We acknowledge that there are certain additional
limitations in this study. The validation of the in silico
data is performed on a small number of clinical samples and
controls. Moreover, the samples of ovarian cancer where RT-qPCR was
performed were all stage III/IV, as such we do not have the data to
compare mRNA expression of FBN1 and OR4M1 in early stages of
ovarian cancer. In silico analysis (Ualcan; http://ualcan.path.uab.edu) indicated that FBN1 is
overexpressed across all stages with significantly increased
expression when comparing stages II vs. III and II vs IV (data not
shown). In addition, we demonstrate protein expression of asprosin
in clinical samples and cells, however the study does not examine
whether the ovaries are capable of secreting this peptide, since
this was beyond the scope of the present paper. Future experiments
using conditioned media from ovarian cell lines and/or ovarian
explants are planned which will enable us to answer this
question.
In conclusion, to the best of our knowledge, this is
the first study to demonstrate expression of asprosin and its
cognate receptor, OR4M1, in the human ovaries in health and cancer,
focusing specifically on ovarian cancer. The presence of the
recently identified orexigenic and glucogenic hormone asprosin (the
cleaved product of profibrillin-1) in the human ovaries suggests a
specific endocrine and/or auto/paracrine role for asprosin in human
female reproduction. Indeed, the novel findings of the present
study open two distinct lines of investigation: the potential role
and effects of asprosin in normal ovaries in terms of fertility and
steroidogenesis; as well as the potential involvement of asprosin
as a gluconeogenic peptide in cancer. The latter is of particular
importance given that hyperglycaemia is a contributing factor to
the onset and progression of epithelial ovarian cancer (20). However, it should be noted that the
exact role of asprosin and its receptor in the pathogenesis of
ovarian cancer and its precise clinical relevance remains to be
clarified. Further research is required to expand on the present
findings and elucidate the potential role of asprosin in health and
disease using in vitro and in vivo models, as well as
larger cohorts of patients undergoing treatment for ovarian
cancer.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by Cancer Treatment
& Research Trust and University Hospitals Coventry and
Warwickshire NHS Trust (grant no. 12899).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MH, HSR, IK, GP and EK conceived the study. RK, JJ,
PV and EK developed the methodology. RK, IK, JJ and EK performed
data analysis. RK, JJ and EK performed experiments. GP provided
resources. GP, MH, HSR, IK and EK collected and prepared clinical
samples. RK, EK and IK wrote the original draft. GP, PV, MH, HSR,
GP, IK and EK reviewed and edited the manuscript. MH, IK and EK
supervised the study. GP, HSR, IK and EK were involved in project
administration. GP, HSR and MH acquired funding. EK and IK confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The study was conducted according to the guidelines
of the Declaration of Helsinki and approved by the Institutional
Review Ethics Committee (School of Health Sciences and Social Care;
current College of Health, Medicine and Life Sciences) of Brunel
University London (Uxbridge, UK) and the Committee for Medical
Ethics and Deontology School of Medicine, University of
Thessaloniki (reference, 14/11/STF/06; Thessaloniki, Greece). All
patients provided oral informed consent for participation to this
research study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ACC
|
adrenocortical carcinoma
|
|
BLCA
|
bladder urothelial carcinoma
|
|
BRCA
|
breast invasive carcinoma
|
|
CESC
|
cervical squamous cell carcinoma and
endocervical adenocarcinoma
|
|
CHOL
|
cholangiocarcinoma
|
|
COAD
|
colon adenocarcinoma
|
|
DLBC
|
lymphoid neoplasm diffuse large B cell
lymphoma
|
|
ESCA
|
oesophageal carcinoma
|
|
GBM
|
glioblastoma multiforme
|
|
HNSC
|
head and neck squamous cell
carcinoma
|
|
KICH
|
kidney chromophobe
|
|
KIRC
|
kidney renal clear cell carcinoma
|
|
KIRP
|
kidney renal papillary cell
carcinoma
|
|
LAML
|
acute myeloid leukaemia
|
|
LGG
|
brain lower grade glioma
|
|
LIHC
|
liver hepatocellular carcinoma
|
|
LUAD
|
lung adenocarcinoma
|
|
LUSC
|
lung squamous cell carcinoma
|
|
MESO
|
mesothelioma
|
|
OV
|
ovarian serous cyst-adenocarcinoma
|
|
PAAD
|
pancreatic adenocarcinoma
|
|
PCPG
|
pheochromocytoma and
paraganglioma
|
|
PRAD
|
prostate adenocarcinoma
|
|
READ
|
rectum adenocarcinoma
|
|
SARC
|
sarcoma
|
|
SKCM
|
skin cutaneous melanoma
|
|
STAD
|
stomach adenocarcinoma
|
|
TGCT
|
testicular germ cell tumours
|
|
THCA
|
thyroid carcinoma
|
|
THYM
|
thymoma
|
|
UCEC
|
uterine corpus endometrial
carcinoma
|
|
UCS
|
uterine carcinosarcoma
|
|
UVM
|
uveal melanoma
|
References
|
1
|
Lee B, Godfrey M, Vitale E, Hori H, Mattei
MG, Sarfarazi M, Tsipouras P, Ramirez F and Hollister DW: Linkage
of Marfan syndrome and a phenotypically related disorder to two
different fibrillin genes. Nature. 352:330–334. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Klimstra WB, Heidner HW and Johnston RE:
The furin protease cleavage recognition sequence of Sindbis virus
PE2 can mediate virion attachment to cell surface heparan sulfate.
J Virol. 73:6299–6306. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Romere C, Duerrschmid C, Bournat J,
Constable P, Jain M, Xia F, Saha PK, Del Solar M, Zhu B, York B, et
al: Asprosin, a Fasting-Induced Glucogenic Protein Hormone. Cell.
165:566–579. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
O'Neill B, Simha V, Kotha V and Garg A:
Body fat distribution and metabolic variables in patients with
neonatal progeroid syndrome. Am J Med Genet A. 143A:1421–1430.
2007. View Article : Google Scholar
|
|
5
|
Wang Y, Qu H, Xiong X, Qiu Y, Liao Y, Chen
Y, Zheng Y and Zheng H: Plasma Asprosin Concentrations Are
Increased in Individuals with Glucose Dysregulation and Correlated
with Insulin Resistance and First-Phase Insulin Secretion.
Mediators Inflamm. 2018:94715832018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li X, Liao M, Shen R, Zhang L, Hu H, Wu J,
Wang X, Qu H, Guo S, Long M, et al: Plasma Asprosin Levels Are
Associated with Glucose Metabolism, Lipid, and Sex Hormone Profiles
in Females with Metabolic-Related Diseases. Mediators Inflamm.
2018:73752942018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang CY, Lin TA, Liu KH, Liao CH, Liu YY,
Wu VCC, Wen MS and Yeh TS: Serum asprosin levels and bariatric
surgery outcomes in obese adults. Int J Obes. 43:1019–1025. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Du C, Wang C, Guan X, Li J, Du X, Xu Z, Li
B, Liu Y, Fu F, Huo H, et al: Asprosin is associated with anorexia
and body fat mass in cancer patients. Support Care Cancer.
29:1369–1375. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Duerrschmid C, He Y, Wang C, Li C, Bournat
JC, Romere C, Saha PK, Lee ME, Phillips KJ, Jain M, et al: Asprosin
is a centrally acting orexigenic hormone. Nat Med. 23:1444–1453.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang X, Jiang H, Ma X and Wu H: Increased
serum level and impaired response to glucose fluctuation of
asprosin is associated with type 2 diabetes mellitus. J Diabetes
Investig. 11:349–355. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li E, Shan H, Chen L, Long A, Zhang Y, Liu
Y, Jia L, Wei F, Han J, Li T, et al: OLFR734 Mediates Glucose
Metabolism as a Receptor of Asprosin. Cell Metab. 30:319–328.e8.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kerslake R, Hall M, Randeva HS, Spandidos
DA, Chatha K, Kyrou I and Karteris E: Co expression of peripheral
olfactory receptors with SARS CoV 2 infection mediators: Potential
implications beyond loss of smell as a COVID 19 symptom. Int J Mol
Med. 46:949–956. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wei F, Long A and Wang Y: The
Asprosin-OLFR734 Hormonal Signaling Axis Modulates Male Fertility.
Cell Discov. 5:552019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maylem ERS, Spicer LJ, Batalha I and
Schutz LF: Discovery of a possible role of asprosin in ovarian
follicular function. J Mol Endocrinol. 66:35–44. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao W, Ho L, Varghese M, Yemul S,
Dams-O'Connor K, Gordon W, Knable L, Freire D, Haroutunian V and
Pasinetti GM: Decreased level of olfactory receptors in blood cells
following traumatic brain injury and potential association with
tauopathy. J Alzheimers Dis. 34:417–429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Prodoehl MJ, Hatzirodos N, Irving-Rodgers
HF, Zhao ZZ, Painter JN, Hickey TE, Gibson MA, Rainey WE, Carr BR,
Mason HD, et al: Genetic and gene expression analyses of the
polycystic ovary syndrome candidate gene fibrillin-3 and other
fibrillin family members in human ovaries. Mol Hum Reprod.
15:829–841. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Z, Liu Y, Lu L, Yang L, Yin S, Wang
Y, Qi Z, Meng J, Zang R and Yang G: Fibrillin-1, induced by
Aurora-A but inhibited by BRCA2, promotes ovarian cancer
metastasis. Oncotarget. 6:6670–6683. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Millstein J, Budden T, Goode EL, Anglesio
MS, Talhouk A, Intermaggio MP, Leong HS, Chen S, Elatre W, Gilks B,
et al AOCS Group, : Prognostic gene expression signature for
high-grade serous ovarian cancer. Ann Oncol. 31:1240–1250. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kellenberger LD and Petrik J:
Hyperglycemia promotes insulin-independent ovarian tumor growth.
Gynecol Oncol. 149:361–370. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hanahan D and Weinberg R A: Hallmarks of
Cancer: The next Generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cantuaria G, Fagotti A, Ferrandina G,
Magalhaes A, Nadji M, Angioli R, Penalver M, Mancuso S and Scambia
G: GLUT-1 expression in ovarian carcinoma: Association with
survival and response to chemotherapy. Cancer. 92:1144–1150. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gyorffy B, Lánczky A and Szállási Z:
Implementing an online tool for genome-wide validation of
survival-associated biomarkers in ovarian-cancer using microarray
data from 1287 patients. Endocr Relat Cancer. 19:197–208. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saravi S, Katsuta E, Jeyaneethi J, Amin
HA, Kaspar M, Takabe K, Pados G, Drenos F, Hall M and Karteris E:
H2A Histone Family Member X (H2AX) Is Upregulated in Ovarian Cancer
and Demonstrates Utility as a Prognostic Biomarker in Terms of
Overall Survival. J Clin Med. 9:28442020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo Q, Song Y, Zhang H, Wu X, Xia P and
Dang C: Detection of Hypermethylated Fibrillin-1 in the Stool
Samples of Colorectal Cancer Patients. Med Oncol. 30:6952013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hsu CW, Wang JC, Liao WI, Chien WC, Chung
CH, Tsao CH, Wu YF, Liao MT and Tsai SH: Association between
malignancies and Marfan syndrome: A population-based, nested
case-control study in Taiwan. BMJ Open. 7:e0172432017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Naba A, Clauser KR, Mani DR, Carr SA and
Hynes RO: Quantitative proteomic profiling of the extracellular
matrix of pancreatic islets during the angiogenic switch and
insulinoma progression. Sci Rep. 7:404952017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Summers KM, Bokil NJ, Baisden JM, West MJ,
Sweet MJ, Raggatt LJ and Hume DA: Experimental and bioinformatic
characterisation of the promoter region of the Marfan syndrome
gene, FBN1. Genomics. 94:233–240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
De Koning DJ and Haley CS: Genetical
Genomics in Humans and Model Organisms. Genetical genomics in
humans and model organisms. Trends Genet. 21:377–381. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hubner N, Wallace CA, Zimdahl H, Petretto
E, Schulz H, Maciver F, Mueller M, Hummel O, Monti J, Zidek V, et
al: Integrated transcriptional profiling and linkage analysis for
identification of genes underlying disease. Nat Genet. 37:243–253.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liberti MV and Locasale JW: The Warburg
Effect: How Does It Benefit Cancer Cells? Trends Biochem Sci.
41:211–218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dong D, Dong Y, Fu J, Lu S, Yuan C, Xia M
and Sun L: Bcl2 inhibitor ABT737 reverses the Warburg effect via
the Sirt3-HIF1α axis to promote oxidative stress-induced apoptosis
in ovarian cancer cells. Life Sci. 255:117846. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li WH, Zhang H, Guo Q, Wu XD, Xu ZS, Dang
CX, Xia P and Song YC: Detection of SNCA and FBN1 methylation in
the stool as a biomarker for colorectal cancer. Dis Markers.
2015:6575702015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen J, Cai Y, Xu R, Pan J, Zhou J and Mei
J, Zhou J and Mei J: Identification of four hub genes as promising
biomarkers to evaluate the prognosis of ovarian cancer in silico.
Cancer Cell Int. 20:2702020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mo X, Su Z, Yang B, Zeng Z, Lei S and Qiao
H: Identification of key genes involved in the development and
progression of early-onset colorectal cancer by co-expression
network analysis. Oncol Lett. 19:177–186. 2020.PubMed/NCBI
|
|
37
|
Shi S and Tian B: Identification of
biomarkers associated with progression and prognosis in bladder
cancer via co-expression analysis. Cancer Biomark. 24:183–193.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhai X, Xue Q, Liu Q, Guo Y and Chen Z:
Colon cancer recurrence associated genes revealed by WGCNA co
expression network analysis. Mol Med Rep. 16:6499–6505. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kellenberger LD, Bruin JE, Greenaway J,
Campbell NE, Moorehead RA, Holloway AC and Petrik J: The role of
dysregulated glucose metabolism in epithelial ovarian cancer. J
Oncol. 2010:5143102010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Aran D, Camarda R, Odegaard J, Paik H,
Oskotsky B, Krings G, Goga A, Sirota M and Butte AJ: Comprehensive
analysis of normal adjacent to tumor transcriptomes. Nat Commun.
8:10772017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kocaman N and Artaş G: Can novel
adipokines, asprosin and meteorin-like, be biomarkers for malignant
mesothelioma? Biotech Histochem. 95:171–175. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rajagopal S and Shenoy SK: GPCR
desensitization: Acute and prolonged phases. Cell Signal. 41:9–16.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Karteris E and Randeva HS: Orexin
Receptors and G-Protein Coupling: Evidence for Another
‘Promiscuous’ Seven Transmembrane Domain Receptor. J Pharmacol Sci.
93:126–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee T, Yun S, Jeong JH and Jung TW:
Asprosin impairs insulin secretion in response to glucose and
viability through TLR4/JNK-mediated inflammation. Mol Cell
Endocrinol. 486:96–104. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zandi Z, Kashani B, Poursani EM, Bashash
D, Kabuli M, Momeny M, Mousavi-Pak SH, Sheikhsaran F, Alimoghaddam
K, Mousavi SA, et al: TLR4 blockade using TAK-242 suppresses
ovarian and breast cancer cells invasion through the inhibition of
extracellular matrix degradation and epithelial-mesenchymal
transition. Eur J Pharmacol. 853:256–263. 2019. View Article : Google Scholar : PubMed/NCBI
|