Introduction
Noncoding RNAs (ncRNAs) refers to RNAs that do not
have a protein-coding function after gene transcription, and
include microRNAs (miRNAs/miRs), long non-coding RNAs (lncRNAs) and
circular RNAs (circRNAs). With the development of molecular
biological techniques, the status of ncRNA has changed from
‘transcription junk’ to an important entity that mediates the
regulation of cellular functions, serving a considerable role in
disease development, especially cancer (1). miRNAs are highly conserved endogenous
small RNAs, which primarily bind to the 3′-untranslated region (3′
UTR) of target mRNA to downregulate gene expression (1,2). Some
miRNAs are involved in the pathogenesis of various malignant tumors
(3). A number of these
cancer-related miRNAs possess oncogenic properties, such as miR-155
(4), and some, miR-488 for instance,
exert cancer-suppressive effects (5). It is worth noting that some miRNAs can
simultaneously exhibit cancer-promoting and -suppressing effects.
For example, miR-155 was found to be downregulated in ovarian and
gastric cancer (GC), suggesting that it acts as an oncogenic
molecule, but is upregulated in breast and pancreatic cancers
(4). In addition, in colon cancer,
miR-155 showed contradictory roles in the same cancer type, and its
expression patterns, also found for miR-490-3p, suggest that
miR-490-3p acts as a tumor regulator with complex mechanisms
(6–8). In addition, studies have shown that
miRNA expression patterns are related to cancer types and clinical
parameters, making miRNA analysis an effective tool for cancer
diagnosis and prognosis (3). The
emergence of miRNA mimics and miRNA-targeted molecules (antimiRs),
as well as research on miRNAs in drug resistance, also indicate
miRNAs as novel targets for cancer therapy (9,10).
Numerous studies have reported the association
between miRNAs and oncogenic effects. However, miR-490 and its two
mature products (miR-490-3p and miR-490-5p) have a unique appeal.
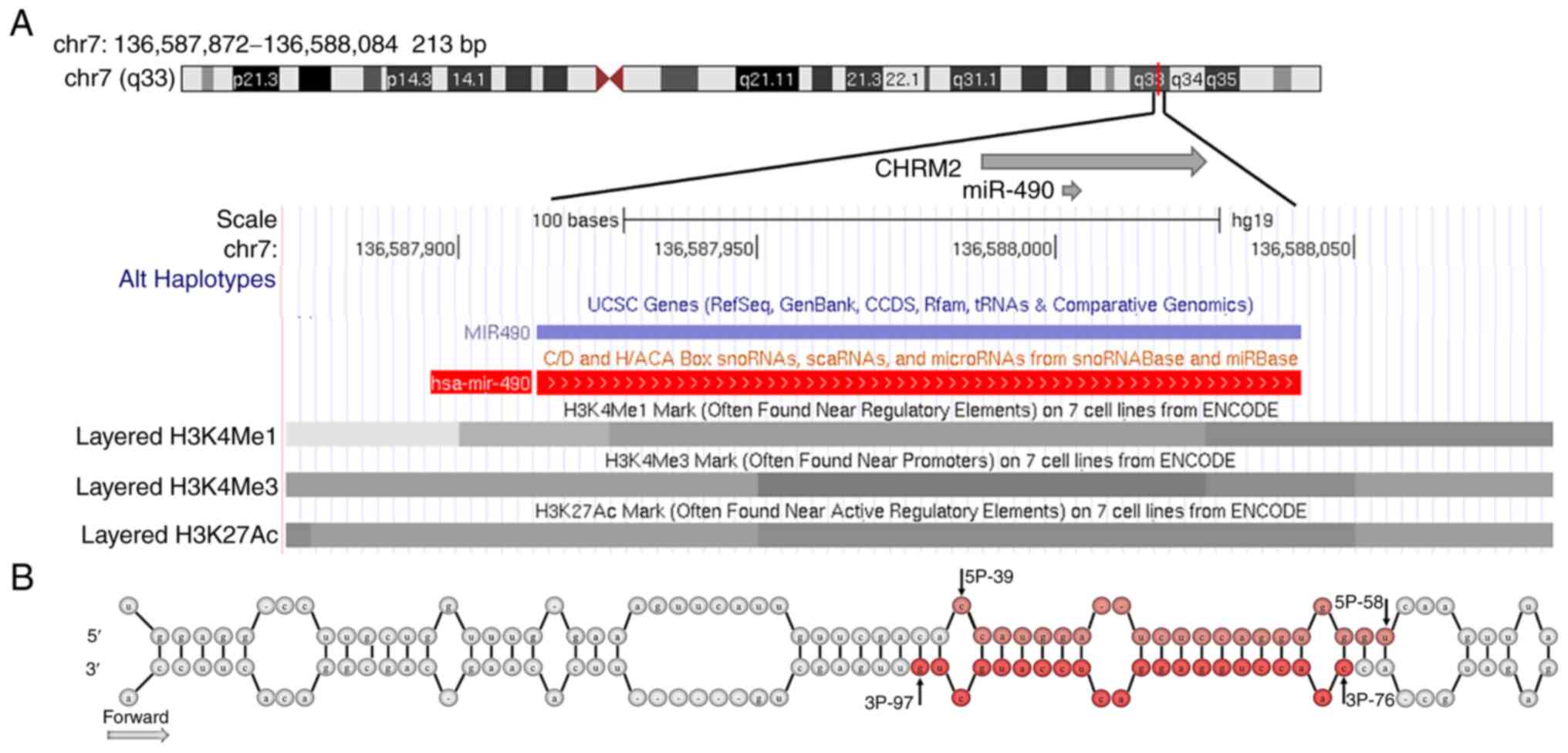
miR-490 is located in the cholinergic receptor muscarinic 2 (CHRM2)
gene in the 7q33 region. CHRM2 encodes a muscarinic acetylcholine
receptor, a G protein-coupled receptor that responds to
acetylcholine and plays an important signaling role in the nervous
system, inhibiting glioblastoma proliferation through the
Notch-1/EGFR signaling pathway (11). As such, activation of CHRM2 is a
viable strategy for the treatment of glioblastoma (12). miR-490 is an miRNA transcribed from
the intron of CHRM2 (13). miR-490
is transcribed in the same direction as the intron, suggesting a
co-transcriptional relationship, with the precursor miRNA being
excised from the intron. The miR-490 precursor comprises a
stem-loop structure composed of 128 bases (Fig. 1), which matures in the cytoplasm
(3), and generates two mature
products, miR-490-3p (22 bp) and miR-490-5p (20 bp). In
glioblastoma, CHRM2 affects the level of mature miR-490 due to low
expression of epigenetic modifications, and may be responsible for
miRNA dysregulation (13). Growing
research on miR-490-3p and miR-490-5p has been reported not only in
neurological tumors, such as glioblastoma, but also in a wide range
of cancer types and pathogenic networks, such as respiratory,
gastrointestinal and hematological tumors, and sarcomas. miR-490-5p
was initially reported in deeply-sequenced bladder cancer (BC)
tissues, and was the most significantly decreased miRNA (14), followed closely by miR-490-3p, which
was found to be expressed at low levels in high-throughput
sequenced colorectal adenocarcinoma (15). Thereafter, an in-depth study of
miR-490-3p in hepatocellular carcinoma (HCC) revealed that
miR-490-3p could act as an oncogene, which differs from the general
oncogenic effect of miRNAs (8), and
this is what prompted the investigation of these two miRNAs in the
present review. As a closely related entity, miR-490-5p may also be
a factor that plays a two-sided role in cancer. The current study
indicates that miR-490-5p only plays the role of an oncogene, while
miR-490-3p is indicated as a double-edged sword with regard to
cancer. Due to the difference in sequence, and the expression and
functionality of ‘3p’ and ‘5p’ miRNAs, it is possible that
miR-490-5p also serves both beneficial and detrimental roles in
cancer (16).
The low expression levels of miR-490-3p and
miR-490-5p in most cancer types suggests that they are associated
with the inhibition of tumorigenesis (6,17). This
was exemplified by Wang et al (6), who initially identified a substantial
downregulation in miR-490-3p levels in paired and unpaired HCC
tissues analyzed from a cohort of The Cancer Genome Atlas.
Secondly, in 50 paired HCC tissues, fluorescence in situ
hybridization revealed that miR-490-3p expression was downregulated
in HCC tissues compared with adjacent non-tumor tissues, primarily
in the cytoplasm, which may be associated with the maturation of
intron miRNA cleavage in the cytoplasm (3). Subsequent reverse
transcription-quantitative PCR analysis revealed that miR-490-3p
expression levels were lower in HepG2 hepatocellular carcinoma
cells than in normal liver tissue. Furthermore, cell function
assays (MTT analysis, agar assays and Transwell analysis) showed
that HepG2 cells transfected with miR-490-3p mimics exhibited
decreased viability, as well as decreased proliferative capacity,
anchorage-dependent growth and invasive potential. These results
suggest that miR-490-3p is dysregulated in tumor expression and
influences tumor cell behavior. Moreover, Kaplan-Meier analysis
revealed that low miR-490-3p expression levels were detrimental to
patient survival, and univariate and multifactorial Cox regression
analyses indicated that miR-490-3p expression was an independent
prognostic factor for poor survival and tumor recurrence in
patients with HCC (6). However,
miR-490-3p has also been found to be highly expressed in HCC
(8), lung cancer (18), thyroid carcinoma (19), multiple myeloma (MM) (20) and skin squamous cell carcinoma (SSCC)
(21). Therefore, it would be
interesting and meaningful to study the underlying mechanisms of
miR-490-3p and miR-490-5p in cancer. The current article reviews
the different roles of these two miRNAs in cancer formation,
competing endogenous RNA (ceRNA) regulatory networks,
chemoresistance, and patient diagnosis and prognosis (Table I). The review also discusses the
potential diagnostic, prognostic and therapeutic value of
miR-490-3p and miR-490-5p in cancer.
 | Table I.Clinical value and comparison of the
roles of miR-490-3p and miR-490-5p in cancer development. |
Table I.
Clinical value and comparison of the
roles of miR-490-3p and miR-490-5p in cancer development.
| Variable | miR-490-3p | (Refs.) | miR-490-5p | (Refs.) |
|---|
| Carcinostatic | Gliomaa, ESCCa, HCCa, Osa, AML, GC, CCA, CRC, lung
cancer, BCa, OC, Eca, PC | (23,25,28,31,32,35,38,41,43,44,52,59,60) | Gliomaa, ESCCa, HCCa, RCC, BC, pharyngolaryngeal
cancer, neuroblastoma | (54,57,61,62,64–66) |
| Carcinogenesis | HCC, Lung cancer,
Thyroid carcinoma, SSCC, MM | (8,18–21) | N/A | – |
| Target genes | CCND1a, ABCC2, AKIRIN2, ATG7, AURKA,
CDK1, ERGIC3, FRAT1, HDAC2, HK2, HMGA2, hnRNPA1, MAPK1, MMP2, MMP9,
PCBP1, POU3F2, PPM1F, RAB14, RHOA, RSF1, SMARCD1, SP1, TGFα,
TGFβR1, TGIF2, TNKS2, TWIST1, VDAC1, VIM | (6,8,13,20–24,26–32,34,35,38–42,45,46,52,59,85,100,101) | CCND1a, BUB1, E2F2, ECT2, ROBO1, EGFR,
PIK3CA, MYEOV, SOX2, c-FOS, MAP3K9 | (17,50,51,53,54,57,61,62,65,66) |
| Signaling
pathways | MAPKa, TGF-βa, HIF-1A, Wnt, EGFR, AMPK, AKT | (13,22–24,28,40–42,44) | MAPKa, TGF-βa, PI3K-AKT | (54,64,66) |
| ceRNA network | Hsa_circ_SLC3A2,
Hsa_circ_0006948, Hsa_circ_101237, PPM1F, HMGA2, LncRNA BCYRN1,
lncRNA CCAT1, lncRNA DLEU1, lncRNA LINC00173, lncRNA LINC00483,
lncRNA RP11-81H3.2, lncRNA SNHG6, lncRNA SNHG15, lncRNA SNHG16,
lncRNA TP73-AS1, lncRNA TONSL-AS1, POU3F2, MAPK1, TGFβR1, CDK1,
hnRNPA1, TNKS2, RSF1, HDAC2, HK2 | (6,25,26,29,30,33–35,38,44,46–48,55,56,85,86,102) | Hsa_circ_0103809,
Hsa_circ_0023642, SOX2, EGFR, lncRNA LINC02532, LncRNA XLOC_001659,
PIK3CA | (17,50,57,87) |
| Diagnosis | In tissue and
plasma | (6,20) | In tissue | (93) |
|
Chemoresistance | Increase paclitaxel
resistance and decrease CDDP resistance | (36,94) | N/A | – |
| Prognosis | GCa, HCCa, Glioma, CRC, Osa | (6,13,22,90,91) | GCa, HCCa | (51,87) |
Aberrant expression of miR-490-3p and
miR-490-5p in cancer
Current research shows that the expression levels of
miR-490-3p and miR-490-5p are low in the majority of tumors. Tumor
types with low expression levels of miR-490-3p include colorectal
cancer (CRC) (22–24), GC (25–27),
cholangiocarcinoma (CCA) (28), lung
cancer (29–31), prostate cancer (32), ovarian cancer (OC) (33–37),
endometrial cancer (Eca) (38–40),
breast cancer (BCa) (41,42), osteosarcoma (Osa) (43) and acute myeloid leukemia (AML)
(44). Tumor types with low
expression levels of both miR-490-3p and miR-490-5p include HCC
(6,45–55),
esophageal squamous cell carcinoma (56–59) and
glioma (13,60,61). In
addition, tumor types with low miR-490-5p expression also include
BC (17,62,63),
renal cell carcinoma (64),
neuroblastoma (65) and
pharyngolaryngeal cancer (66). The
aforementioned results have been confirmed in corresponding tumor
tissues or tumor cells, and subsequent cell function experiments
further supported the tumor suppressor effects of high levels of
miR-490-3p and miR-490-5p, including inhibition of proliferation,
epithelial-mesenchymal transition (EMT), invasiveness and
metastasis, as well as apoptosis promotion (Fig. 2). The effects of miR-490-3p and
miR-490-5p on cancer cell behavior are achieved through 27 and 11
target genes, respectively (Table
II). Among them, only CCND1 is a common target gene (Table I).
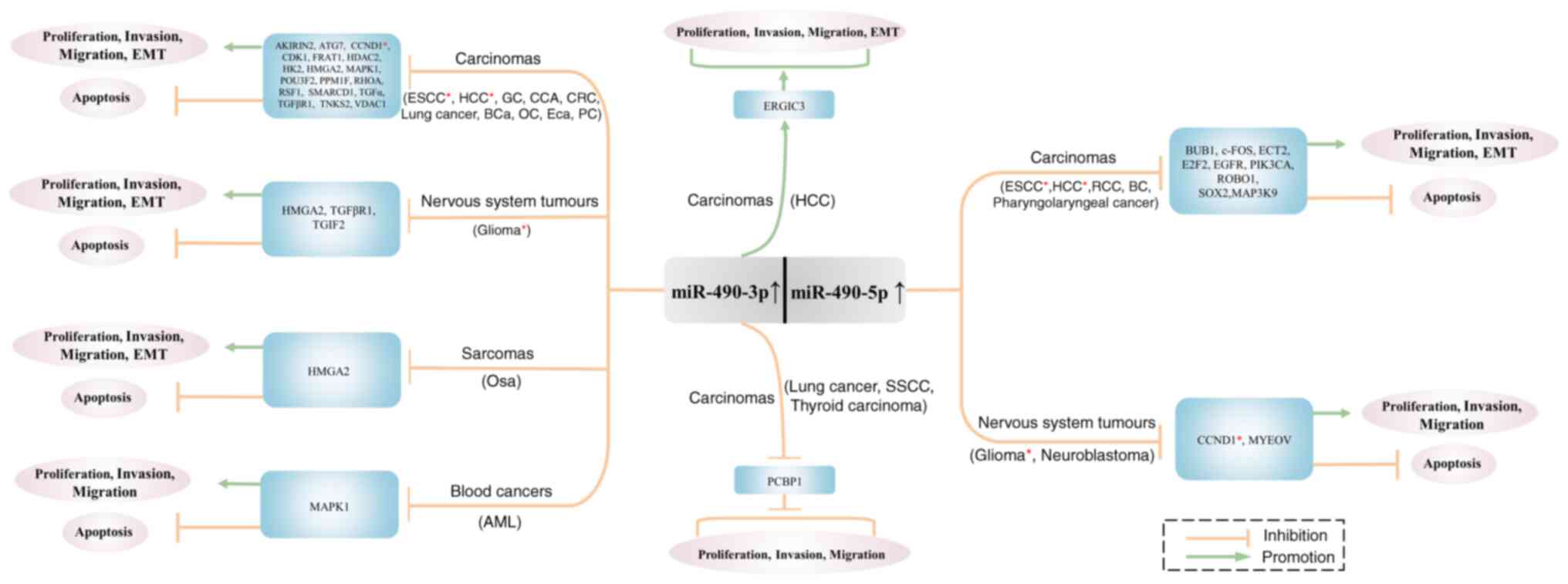 | Figure 2.Cell behavioral functions of
miR-490-3p and miR-490-5p target genes. miR-490-3p and miR-490-5p
have their own target genes. Expression of miR-490-3p and
miR-490-5p can inhibit cancer cell proliferation, migration,
invasiveness and EMT by inhibiting target genes and promoting
apoptosis. Among them, CCND1 is a common target gene. Additionally,
miR-490-3p promotes cellular proliferation, invasiveness, migration
and EMT by promoting the expression of ERGIC3. miR-490-3p promotes
cellular proliferation, migration and invasiveness by increasing
the expression of PCBP1. *Common target gene of miR-490-3p and
miR-490-5p. miR, microRNA; EMT, epithelial-mesenchymal transition;
CCND1, cyclin D1; ERGIC3, endoplasmic reticulum-Golgi intermediate
compartment protein 3; PCBP1, poly(RC) binding protein 1; ESCC,
esophageal squamous cell carcinoma; HCC, hepatocellular carcinoma;
GC, gastric cancer; CCA, cholangiocarcinoma; CRC, colorectal
cancer; BCa, breast cancer; OC, ovarian cancer; Eca, endometrial
carcinoma; PC, prostate cancer; Osa, osteosarcoma; AML, acute
myeloid leukemia; SSCC, skin squamous cell carcinoma; RCC, renal
cell carcinoma; BC, bladder cancer. |
 | Table II.Key roles of miR-490-3p and
miR-490-5p in cancer. |
Table II.
Key roles of miR-490-3p and
miR-490-5p in cancer.
| Cancer type | Target gene | miR | Expression
level | Regulatory
mechanism | Effect in
vitro | (Refs.) |
|---|
| CRC | FRAT1, VDAC1,
TGFβR1, MMP2, MMP9 | miR-490-3p | Low | Inhibit FRAT1/Wnt
/β-catenin signaling pathway | Proliferation↓,
apoptosis↑, EMT↓ | (22–24) |
|
|
|
|
| Inhibit VDAC1/AMPK/
mTOR signaling pathway | Proliferation↓,
migration and invasion↓, apoptosis↑ |
|
|
|
|
|
| Inhibit
TGFβR1/SMAD2/4/TGF-β signaling pathway | Migration and
invasion↓ |
|
| GC | SMARCD1, MAPK1,
hnRNPA1 | miR-490-3p | Low | LncRNA
CCAT1/miR-490-3p/ hnRNPA1 axis LncRNA LINC00483/miR-490-3p/MAPK1
axis | Migration↓
Proliferation↓, migration and invasion↓, apoptosis↑ | (25–27) |
| HCC | PPM1F, ATG7, HDAC2,
POU3F2, CDK1, TNKS2, AURKA | miR-490-3p | Low | Hsa_circ_SLC3A2
/miR-490-3p/PPM1F axis | Proliferation↓,
invasion↓ | (6,45–48,51,52,55) |
|
|
|
|
| LncRNA
BCYRN1/miR-490-3p/POU3F2 axis | Proliferation↓,
migration and invasion↓ |
|
|
|
|
|
| LncRNA
CCAT1/miR-490-3p/CDK1 axis | Proliferation↓,
invasion↓ |
|
|
|
|
|
| LncRNA
RP11-81H3.2/miR-490-3p/TNKS2 axis | Proliferation↓,
migration and invasion↓ |
|
|
|
|
|
| LncRNA
SNHG15/miR-490-3p/HDAC2 axis | Proliferation↓,
migration and invasion↓ |
|
| HCC | ECT2, E2F2, ROBO1,
SOX2, BUB1 | miR-490-5p | Low |
Hsa_circ_0103809/miR-490-5p/SOX2 axis | Proliferation↓,
migration↓, apoptosis↑ | (49–51,53,54) |
|
|
|
|
| Inhibit BUB1/TGF-β
signaling pathway | Proliferation↓,
migration and invasion↓, apoptosis↑ |
|
| CCA | AKIRIN2 | miR-490-3p | Low | Inhibit
AKIRIN/IL-6/gp130/STAT3/VEGFA signaling pathway | Proliferation↓,
migration and invasion↓, EMT↓ | (28) |
| ESCC | HMGA2, MAPK1 | miR-490-3p | Low |
Hsa_circ_0006948/miR-490-3p/HMGA2
axis | Proliferation↓,
migration and invasion↓, EMT↓ | (56,58,59) |
| ESCC | PIK3CA | miR-490-5p | Low | LncRNA
XLOC_001659/miR-490-5p/PIK3CA axis | Proliferation↓,
invasion↓ | (57) |
| Lung cancer | CCND1, MAPK1,
RSF1 | miR-490-3p | Low |
Hsa_circ_101237/miR-490-3p/MAPK1 axis | Proliferation↓,
migration and invasion↓ | (29–31) |
|
|
|
|
| LncRNA
SNHG6/miR-490-3p/RSF1 axis | Proliferation↓,
apoptosis↑ |
|
| Pharyngolaryngeal
cancer | MAP3K9 | miR-490-5p | Low | Inhibit
MAP3K9/MAPKK/MAPK signaling pathway | Proliferation↓,
migration and invasion↓, EMT↓ | (66) |
| PC | HDAC2 | miR-490-3p | Low | – | Proliferation↓,
migration and invasion↓, apoptosis↑ | (32) |
| BC | EGFR, c-FOS | miR-490-5p | Low |
Hsa_circ_0023642/miR-490-5p/EGFR axis | Invasion↓ | (17,62,63) |
| RCC | PIK3CA | miR-490-5p | Low | Inhibit
PIK3CA/PI3K- Akt signaling pathway | Proliferation↓,
migration and invasion↓ | (64) |
| Glioma | HMGA2, TGFβR1,
TGIF2 | miR-490-3p | Low | Inhibit TGIF2/TGF-β
signaling pathway | Proliferation↓,
EMT↓ | (13,60) |
| Glioma | CCND1 | miR-490-5p | Low | – | Proliferation↓ | (61) |
| Neuroblastoma | MYEOV | miR-490-5p | Low | – | Proliferation↓,
migration and invasion↓, apoptosis↑ | (65) |
| OC | ABCC2, TGFβR1,
CDK1 | miR-490-3p | Low | LncRNA CCAT1
/miR-490-3p/TGFβR1 axis | Migration and
invasion↓, EMT↓ | (33–37) |
|
|
|
|
| LncRNA
DLEU1/miR-490-3p/CDK1 axis | Proliferation↓,
migration and invasion↓, apoptosis↑ |
|
|
|
|
|
| LncRNA
TONSL-AS1/miR-490-3p/CDK1 | Proliferation↓ |
|
| Eca | SP1, HK2, TGFα | miR-490-3p | Low | axis LncRNA
SNHG16/miR-490-3p/HK2 | Proliferation↓ | (38–40) |
|
|
|
|
| axis Inhibit
TGFα/EGFR signaling pathway | Proliferation↓,
migration and invasion↓, apoptosis↑ |
|
| BCa | TNKS2, RHOA | miR-490-3p | Low | Inhibit
RHOA/P70S6K/P85S6K signaling pathway | Proliferation↓,
apoptosis↑ | (41,42) |
|
|
|
|
| Inhibit
TNKS2/β-catenin/AXIN/ Wnt signaling pathway | Proliferation↓,
migration and invasion↓ |
|
| Osa | HMGA2 | miR-490-3p | Low | – | Proliferation↓,
apoptosis↑ | (43) |
| AML | MAPK1 | miR-490-3p | Low | Inhibit lncRNA
CCAT1/miR-490-3p/MAPK1/c-Myc positive feedback loop in MAPK
signaling pathway | Proliferation↓,
migration and invasion↓, apoptosis↑ | (44) |
| HCC | ERGIC3 | miR-490-3p | High | – | Proliferation↑,
migration and invasion↑, EMT↑ | (8) |
| Lung cancer | PCBP1 | miR-490-3p | High | – | Proliferation↑,
migration and invasion↑, EMT↑ | (18) |
| Thyroid | PCBP1 | miR-490-3p | High | – | Neoplasia↑ | (19) |
| carcinoma SSCC | PCBP1 | miR-490-3p | High | – | Maintenance of
cancer stem cells↑ | (21) |
In addition, unlike miR-490-5p, miR-490-3p is highly
expressed in HCC (8), lung cancer
(18), thyroid carcinoma (19), SSCC (21) and MM (20). Zhang et al (8), reported the upregulation of miR-490-3p
in 20 cases of HCC tissues and liver cancer cell lines (HepG2,
SK-Hep-1 and PLC/PRF/5), which is in contrast to the low expression
of miR-490-3p in other HCC tissues and HepG2 liver cancer cells
(47,52). Xia et al (21) observed high expression of miR-490-3p
in the SSCC cell line CD34 + COLO-16. In addition, Li et al
(18) and Zhang et al
(19) observed high expression of
miR-490-3p in lung cancer and thyroid cancer, respectively. In
plasma specimens, Jiang et al (20) reported the upregulation of miR-490-3p
in 35 patients with MM. However, the sample for these research
results was from Chinese hospitals with limited sample size.
Therefore, future verification of these findings in larger sample
cohorts from other regions is necessary.
In cancers with high expression levels of
miR-490-3p, the target sites of miR-490-3p are concentrated within
the ERGIC and golgi 3 (ERGIC3) and poly (rC) binding protein 1
(PCBP1) genes. As an oncogene, abnormal activation of ERGIC3 can
promote the occurrence of cancer (67), and silencing ERGIC3 can inhibit the
proliferation of lung adenocarcinoma cells (68). In HCC, the high expression levels of
miR-490-3p did not predictably silence the gene expression of
ERGIC3, but abnormally, significantly promoted ERGIC3 expression,
thereby promoting cellular proliferation, migration, invasiveness
and EMT (8). In cancer, the
promotion of ERGIC3 expression by miR-490-3p is likely to be
associated with argonaute RISC catalytic component 2 (Ago2), a key
component of the RNA-induced silencing complex (8). The Ago2 protein has been shown to
promote both protein translation and translation inhibition,
depending on the differential effect of the cell cycle and cellular
stress (69). However, the
underlying mechanism of Ago2 on miR-490-3p in HCC is yet to be
elucidated. PCBP1 is a tumor suppressor gene, which can promote the
immune response to tumors, the deletion of which leads to a
reduction in T cell-mediated antitumor immune responses and
promotes tumor metastasis and progression (70). In SSCC (21), lung cancer (18) and thyroid cancer (19), PCBP1 is post-transcriptionally
inhibited by the high expression levels of miR-490-3p, which
enhances tumor immune escape and promotes cancer proliferation,
migration and invasiveness, ultimately promoting the occurrence of
cancer. Zhang et al (71)
reported high expression levels of miR-490-3p in thyroid cancer.
Their study revealed that even with low expression of miR-490-3p,
the high expression of E3 ligase ubiquitin ligation factor E4 also
promoted PCBP1 degradation after translation. This suggests the
existence of other post-transcriptional modifications in
conjunction with miR-490-3p in carcinogenesis. Therefore, the tumor
suppressor and oncogenic mechanism of miR-490-3p is achieved by the
regulation of oncogene and tumor suppressor gene expression.
Signaling pathways associated with
miR-490-3p and miR-490-5p
Identification of the signaling pathways associated
with miR-490-3p and miR-490-5p are important for understanding
their complex regulatory mechanisms in cancer. The target genes of
miR-490-3p and miR-490-5p are involved in 7 and 3 signaling
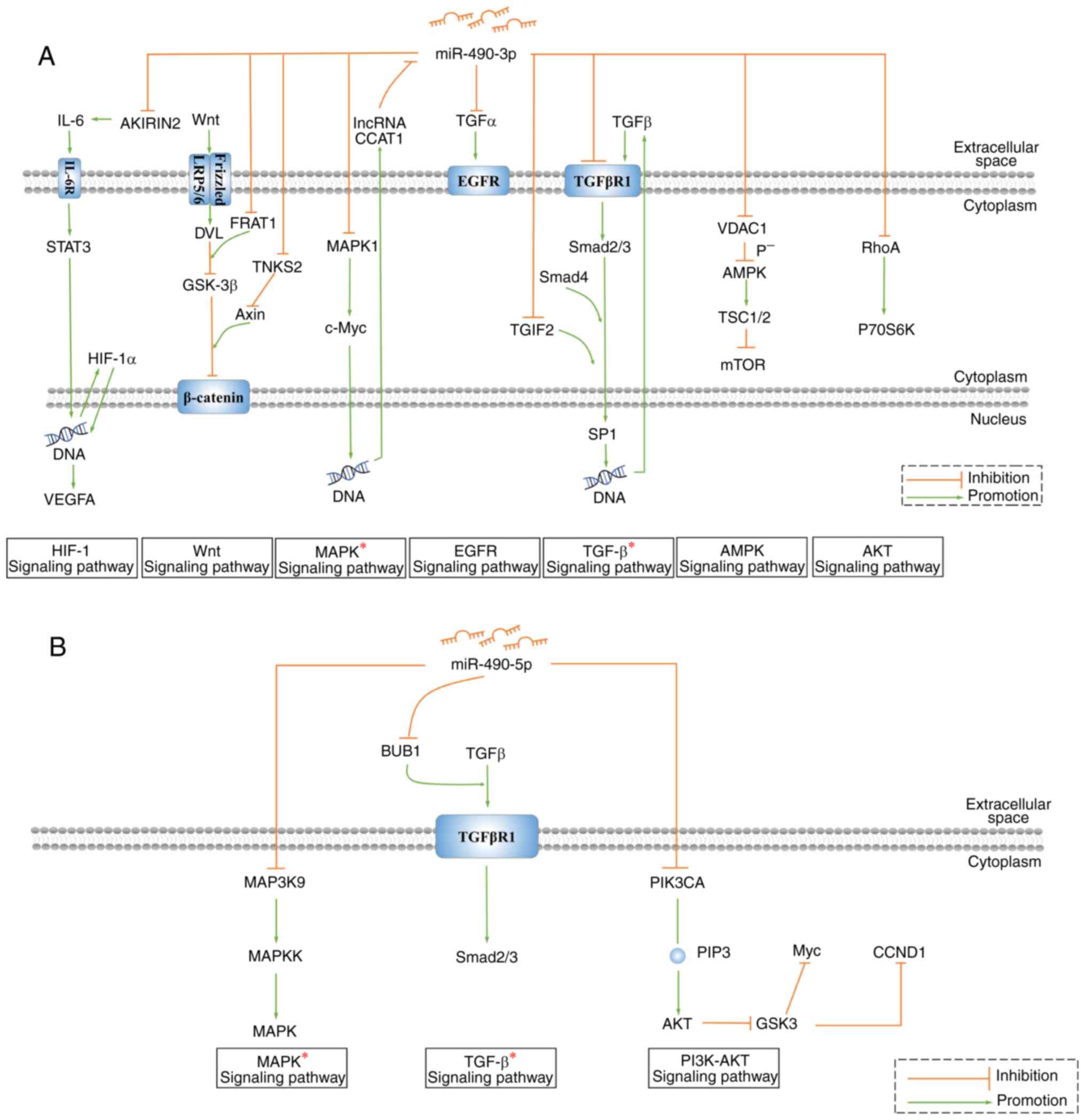
pathways respectively, (Fig. 3).
AKIRIN2 is the direct target gene of miR-490-3p.
gp130 is the signal transduction subunit of IL-6, STAT3, VEGFA and
the IL-6 receptor in the hypoxia inducible factor 1 subunit α
(HIF-1Α) signaling pathway (28,72). In
CCA, the high expression of AKIRIN2 increases the expression of
gp130, indicating that miR-490-3p regulates the HIF-1A signaling
pathway to inhibit the proliferation, invasion and migration
abilities of CCA cells (28). FRAT
regulator of WNT signaling pathway 1 (FRAT1) and tankyrase 2
(TNKS2) are the other two target genes of miR-490-3p. FRAT1 is a
cofactor for DVL for the inhibition of GSK-3β in the Wnt signaling
pathway (73). TNKS2 induces Axin-1
degradation, thereby weakening the inhibition of β-catenin by
GSK-3β, and ultimately enhancing β-catenin activity (74). Therefore, in CRC and BCa, the low
expression levels of miR-490-3p can promote β-catenin accumulation
and Wnt signaling pathway activation, thereby enhancing the cancer
cell aggressiveness (23,42).
In AML, miR-490-3p is associated with the MAPK
signaling pathway. Inhibition of miR-490-3p by lncRNA-colon cancer
associated transcript 1 (CCAT1) causes the accumulation of MAPK1
and c-Myc in the MAPK signaling pathway. As a nucleoprotein, c-Myc
stimulates the expression of lncRNA CCAT1 to form a positive
feedback loop, and enhance cancer cell proliferation and
invasiveness (44).
In BCa, P70S6 kinase (P70S6K) is the downstream
effector of Transforming protein RhoA, a target gene of miR-490-3p
(41). Through the P70S6K/P85S6K
pathway, miR-490-3p may affect the proliferation and apoptosis of
BCa T47D and MCF-7 cells (41,75). In
Eca, the high expression of TGFα is the possible reason for the
activation of the EGFR signaling pathway. Upregulation of TGFα can
promote the expression of EGFR downstream proteins NF-kB, MMP-2,
cyclin D1 and survivin, and inhibit the production of pro-apoptotic
protein Bax (40). miR-490-3p is
involved in the development of Eca by inhibiting TGFα and the EGFR
pathway (40). Transfection of
miR-490-3p mimics reduced the level of voltage-dependent
anion-selective channel protein 1 and increased the level of
phosphorylated AMPK in CRC (22).
The activated AMPK pathway inhibits the mTOR signaling pathway
through tuberous sclerosis complex subunit 1 and 2 (76), indicating that miR-490-3p plays an
anticancer role through its regulation of the AMPK signaling
pathway (22). The target genes of
miR-490-3p comprise TGFβR1 and TGFB induced factor homeobox 2,
which regulate the TGF-β signaling pathway to promote cancer
(13,24,77,78).
The target genes of miR-490-5p are also involved in
the regulation of the TGF-β signaling pathway. BUB1 is an activator
of TGF-β. By inhibiting the expression of BUB1, miR-490-5p inhibits
the TGF-β signaling pathway, suppresses the proliferation,
migration and invasiveness, and increases the apoptosis of HCC
cells (54). miR-490-5p is also
associated with the MAPK signaling pathway, and MAP3K9 is the
direct target of miR-490-5p. The phosphorylation activation of
MAP3K9 can sequentially activate MAPKK and MAPK, and promote
cellular proliferation, migration, invasiveness and EMT (66). Furthermore, miR-490-5p directly
targets PIK3CA to regulate the PI3K/Akt signaling pathway and
inhibit the carcinogenicity of renal cancer cells (64,79,80).
Although both miR-490-3p and miR-490-5p are involved
in regulating the TGF-β and MAPK signaling pathways, their
molecular mechanisms are different (13,44,54,66).
This indicates that miR-490-3p and miR-490-5p have different
mechanisms for inhibiting cancer development.
To the best of our knowledge, there are currently no
published studies on the oncogenic signaling pathway of miR-490-3p
(81). Notably, the antioncogene
PCBP1 was reported to be an immune checkpoint required for effector
T-cell function, as well as a key molecule in the conversion of the
TGF-β signaling pathway from a growth factor inhibitor to a tumor
growth promoter in advanced cancer stages (70). PCBP1 was also reported to be
associated with the HIF and Akt signaling pathways (82,83).
However, the role of miR-490-3p in the regulation of these pathways
is yet to be elucidated. By contrast, ERGIC3 has not been reported
in association with a corresponding signaling pathway; however, in
non-small cell lung cancer (NSCLC), ERGIC3 is negatively regulated
by miR-230a and no effect of miR-490-3p on ERGIC3 was observed
(84). Whether this is affected by
tissue specificity, or perhaps other influencing factors, may be
worthy of further investigation.
Primary ceRNA regulatory networks of
miR-490-3p and miR-490-5p
In cancer, the dysregulation of miR-490-3p and
miR-490-5p is significantly associated with ncRNAs, including
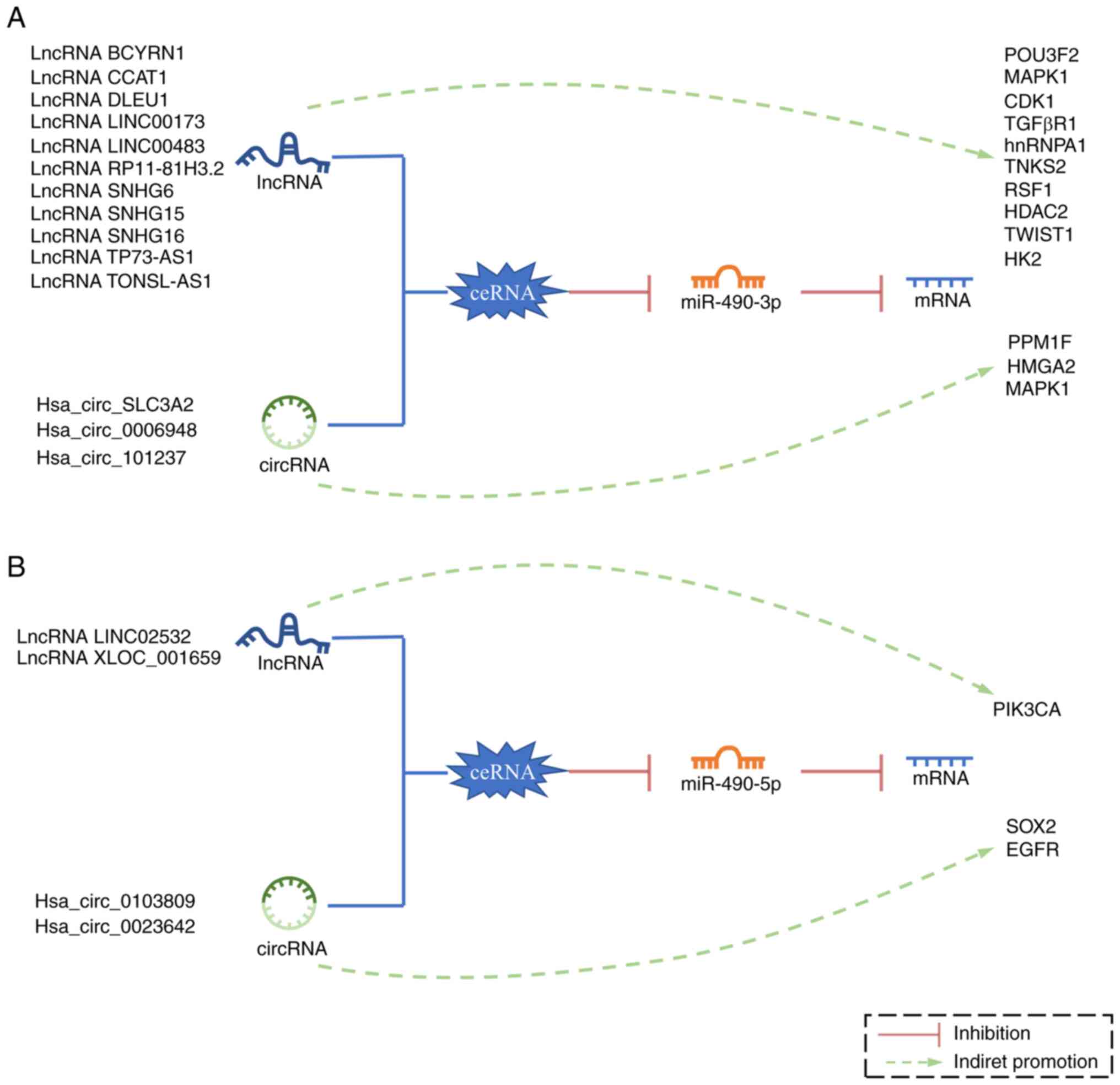
circRNA and lncRNA (Fig. 4). In the
HepG2 liver cancer cell line, circSLC3A2 can sponge miR-490-3p, and
thus promote the expression of protein phosphatase 1F, and
increases tumor proliferation and invasion (6). In the TE-1 and KYSE30 ESCC cell lines,
Hsa_circ_0006948 can reverse the inhibition of high mobility group
AT-hook 2 expression by miR-490-3p, thereby promoting cancer
(56). In NSCLC cell lines (A549 and
H1299), enhancement of the circRNA_101237/miRNA-490-3p/MAPK1
pathway was found to have a cancer-promoting effect (29).
In AML, there is a positive feedback loop associated
with lncRNA CCAT1/miR-490-3p/MAPK1/c-Myc. Within this, the
downregulation of miR-490-3p can lead to the upregulation of MAPK1
and c-Myc, which in turn increases the expression of CCAT1 and
accelerates tumorigenesis (44).
lncRNA CCAT1 is also involved in the development of GC, and the
lncRNA CCAT1/miR-490-3p/heterogeneous nuclear ribonucleoprotein A1
axis can promote the migration of GC cells (26). In addition, the lncRNA
CCAT1/miR-490-3p/CDK1 axis was also found in HCC (55). In OC cell lines (SKOV3 and CaOV3),
the lncRNA CCAT1/miR-490-3p/TGFβR1 axis can promote EMT (34).
In the H460 NSCLC cell line, lncRNA small nucleolar
RNA host gene (SNHG) 6 promotes the expression of oncogene
remodeling and spacing factor 1 (RSF1) through the miR-490-3p/RSF1
axis, and also indirectly promotes the expression of anti-apoptotic
protein Bcl-2, and inhibits the expression of apoptosis-related
proteins (cleaved caspase-3 and Bax) (30). In the MKN-45GC cell line, LINC00483
not only regulates the miR-490-3p/MAPK1 axis, but also indirectly
promotes the expression of anti-apoptotic proteins c-Myc and MMP9,
and inhibits the expression of pro-apoptotic protein Bax (25). Furthermore, In HCC cell lines (Huh-1
and Huh-7), lncRNA SNHG15 promotes HCC progression by regulating
the miR-490-3p/histone deacetylase 2 axis (48). In other liver cancer cell lines
(HepG2 and Huh7), the lncRNA RP11-81H3.2/miR-490-3p/TNKS2 pathway
was also identified to be active. miR-490-3p is not only the
downstream target of RP11-81H3.2, but is also responsible for the
degradation of RP11-81H3.2; the positive feedback loop between
RP11-81H3.2 and miR-490-3p can promote the proliferation, migration
and invasiveness of tumor cells (47). Additionally, there exists a
dysregulation of the lncRNA BCYRN1/miR-490-3p/POU3F2 axis in the
HepG2 cell line (46). In OC cell
lines (OVCAR3 and A2780), the inhibition of lncRNA deleted in
lymphocytic leukemia 1 on miR-490-3p increased the expression of
CDK1, CCND1 and SMARCD1 protein, and indirectly promoted the
expression of anti-apoptosis-related proteins MMP2, Bcl-xL and
P70S6K (35). In addition, there is
also a dysfunction of the lncRNA TONSL-AS1/miR-490-3p/CDK1 axis in
the OVCAR3 OC cell line (33). In
Eca, the TFAP2A/lncRNA SNHG16/miR-490-3p/HK2 axis can promote
cancer. The transcription factor TFAP2A can activate SNHG16
transcription, thereby reversing the inhibitory effect of
miR-490-3p on HK2, and ultimately promoting the proliferation and
glycolysis of Eca (38). In the
MDA-MB-231 triple-negative BCa (TNBC) cell line, the lncRNA
TP73-AS1/miR-490-3p/TWIST1 axis can promote the formation of
vasculogenic mimicry, thereby promoting tumor progression (85). In addition, the inhibitory effect of
lncRNA LINC00173 on miR-490-3p was identified in TNBC (86).
Members of the ceRNA regulatory network centered on
miR-490-5p are different from those of the miR-490-3p network. In
the BC cell lines (J82 and UMUC3), the
ERα/circ_0023642/miR-490-5p/EGFR pathway can inhibit the progress
of BC (17). In liver cancer cell
lines (HepG2 and Huh7), highly expressed Hsa_circ_0103809 promotes
the development of cancer through the Hsa_circ_0103809/miR-490-5p/
SRY-box transcription factor 2 (SOX2) signaling pathway (50). Additionally, in ESCC cell lines
(EC9706 and EC-1), the highly expressed lncRNA XLOC_001659
inhibited miR-490-5p and promoted the expression of PIK3CA, which
ultimately promoted the proliferation and invasiveness of ESCC
cells (57). Furthermore, in GC, the
highly expressed lncRNA LINC02532 can inhibit the expression of
miR-490-5p (87).
Clinical diagnostic and prognostic values of
miR-490-3p and miR-490-5p
Early diagnosis of cancer is a hot spot of clinical
concern, and early cancer discovery is conducive to improved
prognosis. As shown in Table III,
the abnormal expression of miR-490-3p in tumor tissues has been
applied to the diagnosis of a variety of tumors, including HCC
(6,88), rectal adenocarcinoma, ESCC, GC and
CRC (89). In addition, plasma
miR-490-3p may be a diagnostic biomarker for CRC (22) and MM (20).
 | Table III.Diagnostic and prognostic values of
miR-490-3p and miR-490-5p. |
Table III.
Diagnostic and prognostic values of
miR-490-3p and miR-490-5p.
| miR | Cancer type | Sample type | Expression
level | Target gene |
Diagnostic/prognostic value | (Refs.) |
|---|
| miR-490-3p | CRC | 457 patients from
TCGA | Low | – | AUC=0.797 | (89) |
| miR-490-3p | CRC | 55 plasma | Low | VDAC1 | Prognostic factor
of OS, AUC=0.66, sensitivity=65.45%, specificity=71.43% | (22) |
| miR-490-3p | ESCC | 90 patients from
TCGA | Low | – | AUC=0.826 | (89) |
| miR-490-3p | GC | 446 patients from
TCGA | Low | – | AUC=0.798 | (89) |
| miR-490-3p | Glioma | 58 patients | Low | TGIF2 | Prognostic factor
of OS | (13) |
| miR-490-3p | GC | 36 paired tissues
and 82 patients | Low | SMARCD1 | Prognostic factor
of OS, DFS and Survival rate | (27,91) |
| miR-490-3p | HCC | 114 patients | Low | PPM1F | Prognostic factor
of OS, relapse, AUC=0.63 | (6) |
| miR-490-3p | HCC | 41 patients and 375
patients | Low | POU3F2 | Prognostic factor
of Survival rate, AUC=0.695 | (46,88) |
| miR-490-3p | MM | 35 plasma | High | – | AUC=0.87,
sensitivity=60%, specificity=85% | (20) |
| miR-490-3p | Osa | 148 patients | Low | – | Prognostic factor
of OS and RFS | (90) |
| miR-490-3p | READ | 162 patients from
TCGA | Low | – | AUC=0.965 | (89) |
| miR-490-5p | CRC | 115 patients | Low | – | AUC=0.737,
sensitivity=70.79%, specificity=64.52% | (93) |
| miR-490-5p | HCC | 41 patients | Low | – | AUC=0.715 | (92) |
| miR-490-5p | HCC | 50 patients and 92
patients | Low | E2F2, ECT2 | Prognostic factor
of OS, DFS and survival rate | (49,51) |
Current research shows that miR-490-3p also plays an
important role in predicting the prognosis of cancer patients
(Table III). Liu et al
(22) reported that the low
expression levels of miR-490-3p in CRC tissues were not only
associated with poorer clinicopathological characteristics, but
also with shorter overall survival (OS) in patients with CRC. Wang
et al (6) found that the low
expression of miR-490-3p was associated with a lower OS and tumor
recurrence in patients with HCC. Li et al (88) showed that the low expression of
miR-490-3p represents the low OS rate of patients with HCC, and
Ding et al (46) found that
the low expression of miR-490-3p decreased the OS of patients with
HCC. In Osa, Tang et al (90)
found that low expression of miR-490-3p was associated with OS and
relapse-free survival. Qu et al (91) showed that in helicobacter
pylori-infected patients with GC, low expression of miR-490-3p was
associated with shorter OS and disease-free survival (DFS) times.
Shen et al (27) found that
low expression levels of miR-490-3p promotes the expression of
SMARCD1, and is associated with shorter OS times in patients with
GC. In addition, Vinchure et al (13) showed that higher expression levels of
miR-490-3p in patients with the mesenchymal subtype of glioblastoma
is associated with improved OS (13).
miR-490-5p also has potential diagnostic
capabilities in HCC (92) and CRC
(93). Fang et al (51) revealed that the expression level of
miR-490-5p is associated with the OS and DFS rate of patients with
HCC. Other studies have also found that low expression levels of
miR-490-5p are associated with lower OS (49,92) and
DFS (49) in patients with HCC. In
GC, high expression of LINC02532 can result in reduced expression
of miR-490-5p, which is associated with poor prognosis (87).
miR-490-3p-related anticancer drug
resistance
Tumor chemoresistance frequently results in
recurrence and poor prognosis. Tian et al (36) ascertained that miR-490-3p promotes
chemosensitivity to cisplatin (CDDP) by downregulating the target
gene ATP binding cassette subfamily C member 2 in OC cells,
suggesting that it may be a potential therapeutic target for the
treatment of patients with CDDP-resistant OC. In addition, Chen
et al (94) revealed that
miR-490-3p can upregulate the protein expression levels of P-gp and
GST-π, thereby enhancing the resistance of A2780 OC cells to
paclitaxel. At present, research on miR-490-3p and chemoresistance
is limited, and the underlying mechanism of its differential action
on the sensitivity of different chemotherapeutic agents towards
cancer may be associated with miR-490-3p targeting of different
downstream genes.
Discussion
The dysfunction mechanism of miR-490-3p and
miR-490-5p in cancer is worthy of attention. Hypermethylation of
the miR-490 promoter results in decreased expression of the
precursor miR-490 (27), which
influences the maturation of miR-490-3p and miR-490-5p. In
addition, the competitive inhibition of miR-490 by oncogenic ncRNAs
(such as lncRNAs and circRNAs) is one of the reasons for the low
expression of miR-490-3p and miR-490-5p (Fig. 4). However, numerous studies have
shown that miR-490-3p has a different mechanism of action than
miR-490-5p in tumorigenesis. Due to the difference in base sequence
between the two mature miRNAs (Fig.
1B), miR-490-3p not only suppresses oncogenes to exert
anticancer effects, but also suppresses the cancer suppressor gene
PCBP1 and promotes cancer development. In addition, miR-490-3p has
a role in promoting the expression of target genes, which may be
associated with the Ago2 protein (69) and the 5′-UTR of mRNAs that encode
ribosomal proteins (95). Therefore,
the molecular mechanism by which miR-490-3p inhibits or promotes
cancer warrants further investigation.
The ceRNA regulatory network is an important factor
in cancer regulation by miR-490-3p and miR-490-5p. Through an
endogenous competition mechanism, these ncRNAs downregulate the
expression of miR-490-3p and miR-490-5p, and relieve their
inhibitory effects on downstream target genes (Fig. 4). The existence of feedback loops
suggests that the aberrant expression of miR-490 is not only the
initiating factor for cancer, but also the effector of accelerated
cancer development (44). The ceRNA
network of miR-490-3p includes 11 lncRNAs, 3 circRNAs and 12
protein-coding genes. In the ceRNA network of miR-490-5p, there are
2 lncRNAs, 2 circRNAs and 3 protein-coding genes. The two ceRNA
networks also communicate with each other. For example, SOX2 in the
miR-490-5p regulatory network can activate lncRNA CCAT1 in the
miR-490-3p regulatory network, thereby promoting the progression of
squamous cell carcinoma (96). This
shows that the ceRNA regulatory network is more complex and
sophisticated than the current findings indicate.
Numerous studies have shown that miR-490-3p and
miR-490-5p have high specificity and sensitivity in the early
diagnosis of tumors (Table III).
The diagnostic capabilities of miR-490-3p can be applied to bodily
fluids such as tissue, blood and urine. miR-490-3p and miR-490-5p
are also independent prognostic factors for HCC and GC (51,91). At
the same time, the imbalance of miR-490-3p is also related to the
chemoresistance of cancer (36,94). In
addition, though it is not clear whether miR-490-3p or miR-490-5p
is involved, miR-490 exists in the exosomes of mast cells. This
suggests the possibility of using exosomes as miR-490 vectors to
transform the tumor microenvironment (97).
At present, it is necessary to further understand
the specific mechanisms of the ceRNA regulatory networks associated
with miR-490-3p and miR-490-5p. At the same time, the unique
cancer-promoting mechanism of miR-490-3p remains to be clarified.
Besides the involvement of Ago2, whether there are other
influencing factors that make miR-490-3p a unique cancer-promoting
factor remains to be studied. There are also conflicting results
regarding the role of miR-490-3p in OC drug resistance (36,94), and
further experimental verification is required. At present, there is
a lack of clinical studies using miR-490-3p and miR-490-5p as
therapeutic targets, and the side effects of related therapies are
not yet clear.
Previously, Vinchure and Kulshreshtha (81) reviewed miR-490 as a potential
biomarker and therapeutic target in cancer and other pathologies.
However, the differences between miR-490-3p and miR-490-5p in this
context were not well distinguished. According to current research,
it is necessary to treat the two mature products of miR-490
differently. Further research is required to focus on the ceRNA
networks centered on miR-490-3p and miR-490-5p, which will provide
a solid theoretical foundation for their applications in early
clinical diagnosis, tumor prognosis prediction and chemotherapeutic
resistance.
We hypothesize that further investigation is
required to thoroughly explore the molecular mechanisms of
miR-490-3p and miR-490-5p in tumors. In addition, there is still a
lack of research surrounding the effects of miR-490-3p and
miR-490-5p on chemoresistance. In the future, whether miR-490-3p
and miR-490-5p can be targeted as tumor treatment options will be
an interesting topic (9), as well as
their value in the treatment of non-cancer diseases (98).
Conclusions
miR-490-3p and miR-490-5p are derived from the same
precursor miRNA and both exhibit low expression levels in glioma,
ESCC and HCC. Both have a common target gene, CCND1, and both
function in the MAPK and TGF-β pathways to regulate cancer cell
characteristics, such as proliferation, migration and invasiveness.
However, the differences between miR-490-3p and miR-490-5p are of
interest. Firstly, miR-490-3p is involved in a wider range of
cancer types, with dysregulated expression in up to 13 cancers,
while miR-490-5p has been more widely studied in BC, RCC and
pharyngeal cancer. Secondly, miR-490-3p targets a higher number of
downstream genes. miR-490-5p targets 11 downstream genes, while
miR-490-3p targets 30. Furthermore, miR-490-3p is involved in more
signaling pathways, including those of HIF-1A, Wnt, EGFR, AMPK and
AKT; and compared with miR-490-5p, miR-490-3p has a more complex
ceRNA regulatory network. Notably, the current review suggests that
miR-490-3p has both oncogenic and pro-oncogenic effects, while
miR-490-5p has only oncogenic effects. It has been well documented
that tissue and plasma miR-490-3p have diagnostic capacities in
malignancies such as CRC, MM and HCC, and that miR-490-3p has a
higher value as an independent prognostic factor in CRC, glioma,
GC, HCC and Osa than miR-490-5p. In addition, focusing on
miR-490-3p may help to address the resistance of OC to
chemotherapeutic drugs such as cisplatin and paclitaxel.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
The article was conceived by YL and SD. YL, DT, HC,
YC and SC collected and analyzed the associated publications, and
drafted the manuscript. SD and YL critically revised the work and
gave the final approval of the submitted version. All authors have
read and approved the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Anastasiadou E, Jacob LS and Slack FJ:
Non-coding RNA networks in cancer. Nat Rev Cancer. 18:5–18. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu Y, Mao Q and Liang X: Targeting the
MicroRNA-490-3p-ATG4B-Autophagy axis relieves myocardial injury in
ischemia reperfusion. J Cardiovasc Transl Res. 14:173–183. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee YS and Dutta A: MicroRNAs in cancer.
Annu Rev Pathol. 4:199–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gulei D, Raduly L, Broseghini E, Ferracin
M and Berindan-Neagoe I: The extensive role of miR-155 in malignant
and non-malignant diseases. Mol Aspects Med. 70:33–56. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu Y, Yuan MH, Wu HT, Chen WJ, Zhang ML,
Ye QQ, Liu J and Zhang GJ: MicroRNA-488 inhibits proliferation and
motility of tumor cells via downregulating FSCN1, modulated by
Notch3 in breast carcinomas. Cell Death Dis. 11:9122020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang H, Chen W, Jin M, Hou L, Chen X,
Zhang R, Zhang J and Zhu J: CircSLC3A2 functions as an oncogenic
factor in hepatocellular carcinoma by sponging miR-490-3p and
regulating PPM1F expression. Mol Cancer. 17:1652018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Z, Ma T, Huang C, Hu T and Li J: The
pivotal role of microRNA-155 in the control of cancer. J Cell
Physiol. 229:545–550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang LY, Liu M, Li X and Tang H:
miR-490-3p modulates cell growth and epithelial to mesenchymal
transition of hepatocellular carcinoma cells by targeting
endoplasmic reticulum-Golgi intermediate compartment protein 3
(ERGIC3). J Biol Chem. 288:4035–4047. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsui M and Corey DR: Non-coding RNAs as
drug targets. Nat Rev Drug Discov. 16:167–179. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Di Bari M, Bevilacqua V, De Jaco A, Laneve
P, Piovesana R, Trobiani L, Talora C, Caffarelli E and Tata AM:
miR-34a-5p mediates cross-talk between M2 muscarinic receptors and
Notch-1/EGFR pathways in U87MG glioblastoma cells: Implication in
cell proliferation. Int J Mol Sci. 19:16312018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cristofaro I, Spinello Z, Matera C, Fiore
M, Conti L, De Amici M, Dallanoce C and Tata AM: Activation of M2
muscarinic acetylcholine receptors by a hybrid agonist enhances
cytotoxic effects in GB7 glioblastoma cancer stem cells. Neurochem
Int. 118:52–60. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vinchure OS, Sharma V, Tabasum S, Ghosh S,
Singh RP, Sarkar C and Kulshreshtha R: Polycomb complex mediated
epigenetic reprogramming alters TGF-β signaling via a novel
EZH2/miR-490/TGIF2 axis thereby inducing migration and EMT
potential in glioblastomas. Int J Cancer. 145:1254–1269. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han Y, Chen J, Zhao X, Liang C, Wang Y,
Sun L, Jiang Z, Zhang Z, Yang R, Chen J, et al: MicroRNA expression
signatures of bladder cancer revealed by deep sequencing. PLoS One.
6:e182862011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hamfjord J, Stangeland AM, Hughes T,
Skrede ML, Tveit KM, Ikdahl T and Kure EH: Differential expression
of miRNAs in colorectal cancer: Comparison of paired tumor tissue
and adjacent normal mucosa using high-throughput sequencing. PLoS
One. 7:e341502012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lv Y, Yang H, Ma X and Wu G:
Strand-specific miR-28-3p and miR-28-5p have differential effects
on nasopharyngeal cancer cells proliferation, apoptosis, migration
and invasion. Cancer Cell Int. 19:1872019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu L, Zhang M, Qi L, Zu X, Li Y, Liu L,
Chen M, Li Y, He W, Hu X, et al: ERα-mediated alterations in
circ_0023642 and miR-490-5p signaling suppress bladder cancer
invasion. Cell Death Dis. 10:6352019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li J, Feng Q, Wei X and Yu Y: MicroRNA-490
regulates lung cancer metastasis by targeting poly r(C)-binding
protein 1. Tumour Biol. 37:15221–15228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang M, Wang X, Tan J, Zhao M, Lian L and
Zhang W: Poly r(C) binding protein (PCBP) 1 is a negative regulator
of thyroid carcinoma. Am J Transl Res. 8:3567–3573. 2016.PubMed/NCBI
|
|
20
|
Jiang Y, Luan Y, Chang H and Chen G: The
diagnostic and prognostic value of plasma microRNA-125b-5p in
patients with multiple myeloma. Oncol Lett. 16:4001–4007.
2018.PubMed/NCBI
|
|
21
|
Xia S, Zhao Z, Xie F, He J and Li H: Poly
r(C) binding protein is post-transcriptionally repressed by
miR-490-3p to potentiate squamous cell carcinoma. Tumour Biol.
37:14773–14778. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu X, He B, Xu T, Pan Y, Hu X, Chen X and
Wang S: miR-490-3p functions as a tumor suppressor by inhibiting
oncogene VDAC1 expression in colorectal cancer. J Cancer.
9:1218–1230. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng K, Zhou X, Yu J, Li Q, Wang H, Li M,
Shao Z, Zhang F, Luo Y, Shen Z, et al: Epigenetic silencing of
miR-490-3p promotes development of an aggressive colorectal cancer
phenotype through activation of the Wnt/β-catenin signaling
pathway. Cancer Lett. 376:178–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu X, Chen R, Li Z, Huang N, Wu X, Li S,
Li Y and Wu S: MicroRNA-490-3p inhibits colorectal cancer
metastasis by targeting TGFβR1. BMC Cancer. 15:10232015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo M and Liang C: LncRNA LINC00483
promotes gastric cancer development through regulating MAPK1
expression by sponging miR-490-3p. Biol Res. 53:142020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou B, Wang Y, Jiang J, Jiang H, Song J,
Han T, Shi J and Qiao H: The long noncoding RNA colon
cancer-associated transcript-1/miR-490 axis regulates gastric
cancer cell migration by targeting hnRNPA1. IUBMB Life. 68:201–210.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shen J, Xiao Z, Wu WK, Wang MH, To KF,
Chen Y, Yang W, Li MS, Shin VY, Tong JH, et al: Epigenetic
silencing of miR-490-3p reactivates the chromatin remodeler SMARCD1
to promote Helicobacter pylori-induced gastric carcinogenesis.
Cancer Res. 75:754–765. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Leng K, Xu Y, Kang P, Qin W, Cai H, Wang
H, Ji D, Jiang X, Li J, Li Z, et al: Akirin2 is modulated by
miR-490-3p and facilitates angiogenesis in cholangiocarcinoma
through the IL-6/STAT3/VEGFA signaling pathway. Cell Death Dis.
10:2622019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang ZY, Gao XH, Ma MY, Zhao CL, Zhang YL
and Guo SS: CircRNA_101237 promotes NSCLC progression via the
miRNA-490-3p/MAPK1 axis. Sci Rep. 10:90242020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dong Z, Liu H and Zhao G: Long noncoding
RNA SNHG6 promotes proliferation and inhibits apoptosis in
Non-small cell lung cancer cells by regulating miR-490-3p/RSF1
Axis. Cancer Biother Radiopharm. 35:351–361. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gu H, Yang T, Fu S, Chen X, Guo L and Ni
Y: MicroRNA-490-3p inhibits proliferation of A549 lung cancer cells
by targeting CCND1. Biochem Biophys Res Commun. 444:104–108. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fan H and Zhang YS: MiR-490-3p modulates
the progression of prostate cancer through regulating histone
deacetylase 2. Eur Rev Med Pharmacol Sci. 23:539–546.
2019.PubMed/NCBI
|
|
33
|
Liu Y, Li L, Wang X, Wang P and Wang Z:
LncRNA TONSL-AS1 regulates miR-490-3p/CDK1 to affect ovarian
epithelial carcinoma cell proliferation. J Ovarian Res. 13:602020.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mu Y, Li N and Cui YL: The lncRNA CCAT1
upregulates TGFβR1 via sponging miR-490-3p to promote TGFβ1-induced
EMT of ovarian cancer cells. Cancer Cell Int. 18:1452018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang LL, Sun KX, Wu DD, Xiu YL, Chen X,
Chen S, Zong ZH, Sang XB, Liu Y and Zhao Y: DLEU1 contributes to
ovarian carcinoma tumourigenesis and development by interacting
with miR-490-3p and altering CDK1 expression. J Cell Mol Med.
21:3055–3065. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tian J, Xu YY, Li L and Hao Q: miR-490-3p
sensitizes ovarian cancer cells to cisplatin by directly targeting
ABCC2. Am J Transl Res. 9:1127–1138. 2017.PubMed/NCBI
|
|
37
|
Chen S, Chen X, Xiu YL, Sun KX and Zhao Y:
MicroRNA-490-3P targets CDK1 and inhibits ovarian epithelial
carcinoma tumorigenesis and progression. Cancer Lett. 362:122–130.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang G, Ma A, Jin Y, Pan G and Wang C:
LncRNA SNHG16 induced by TFAP2A modulates glycolysis and
proliferation of endometrial carcinoma through miR-490-3p/HK2 axis.
Am J Transl Res. 11:7137–7145. 2019.PubMed/NCBI
|
|
39
|
Shao W, Li Y, Chen F, Jia H, Jia J and Fu
Y: Long non-coding RNA DLEU1 contributes to the development of
endometrial cancer by sponging miR-490 to regulate SP1 expression.
Pharmazie. 73:379–385. 2018.PubMed/NCBI
|
|
40
|
Sun KX, Chen Y, Chen S, Liu BL, Feng MX,
Zong ZH and Zhao Y: The correlation between microRNA490-3p and TGFα
in endometrial carcinoma tumorigenesis and progression. Oncotarget.
7:9236–9249. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao L and Zheng XY: MicroRNA-490 inhibits
tumorigenesis and progression in breast cancer. Onco Targets Ther.
9:4505–4516. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jia Z, Liu Y, Gao Q, Han Y, Zhang G, Xu S,
Cheng K and Zou W: miR-490-3p inhibits the growth and invasiveness
in triple-negative breast cancer by repressing the expression of
TNKS2. Gene. 593:41–47. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu W, Xu G, Liu H and Li T:
MicroRNA-490-3p regulates cell proliferation and apoptosis by
targeting HMGA2 in osteosarcoma. FEBS Lett. 589((20 Pt B)):
3148–3153. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang C, Chen F, Fan Z, Yao C and Xiao L:
lncRNA CCAT1/miR-490-3p/MAPK1/c-Myc positive feedback loop drives
progression of acute myeloid leukaemia. J Biochem. 167:379–388.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang H, Bao J, Zhao S, Huo Z and Li B:
MicroRNA-490-3p suppresses hepatocellular carcinoma cell
proliferation and migration by targeting the aurora kinase A gene
(AURKA). Arch Med Sci. 16:395–406. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ding S, Jin Y, Hao Q, Kang Y and Ma R:
LncRNA BCYRN1/miR-490-3p/POU3F2, served as a ceRNA network, is
connected with worse survival rate of hepatocellular carcinoma
patients and promotes tumor cell growth and metastasis. Cancer Cell
Int. 20:62020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen W, Li K, Zhu K, Yan R, Cai QC, Li WH
and Dang CX: RP11-81H3.2 acts as an oncogene via microRNA-490-3p
inhibition and consequential Tankyrase 2 Up-Regulation in
hepatocellular carcinoma. Dig Dis Sci. 65:2949–2958. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dai W, Dai JL, Tang MH, Ye MS and Fang S:
lncRNA-SNHG15 accelerates the development of hepatocellular
carcinoma by targeting miR-490-3p/ histone deacetylase 2 axis.
World J Gastroenterol. 25:5789–5799. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yu Y, Cai O, Wu P and Tan S: miR-490-5p
inhibits the stemness of hepatocellular carcinoma cells by
targeting ECT2. J Cell Biochem. 120:967–976. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cai H, Hu B, Ji L, Ruan X and Zheng Z:
Hsa_circ_0103809 promotes cell proliferation and inhibits apoptosis
in hepatocellular carcinoma by targeting miR-490-5p/SOX2 signaling
pathway. Am J Transl Res. 10:1690–1702. 2018.PubMed/NCBI
|
|
51
|
Fang ZQ, Li MC, Zhang YQ and Liu XG:
miR-490-5p inhibits the metastasis of hepatocellular carcinoma by
down-regulating E2F2 and ECT2. J Cell Biochem. 119:8317–8324. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ou Y, He J and Liu Y: miR-490-3p inhibits
autophagy via targeting ATG7 in hepatocellular carcinoma. IUBMB
Life. 70:468–478. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen W, Ye L, Wen D and Chen F: miR-490-5p
inhibits hepatocellular carcinoma cell proliferation, migration and
invasion by directly regulating ROBO1. Pathol Oncol Res. 25:1–9.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xu B, Xu T, Liu H, Min Q, Wang S and Song
Q: miR-490-5p suppresses cell proliferation and invasion by
targeting BUB1 in hepatocellular carcinoma cells. Pharmacology.
100:269–282. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Dou C, Sun L, Jin X, Han M, Zhang B and Li
T: Long non-coding RNA colon cancer-associated transcript 1
functions as a competing endogenous RNA to regulate
cyclin-dependent kinase 1 expression by sponging miR-490-3p in
hepatocellular carcinoma progression. Tumour Biol.
39:10104283176975722017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Pan Z, Lin J, Wu D, He X, Wang W, Hu X,
Zhang L and Wang M: Hsa_circ_0006948 enhances cancer progression
and epithelial-mesenchymal transition through the miR-490-3p/HMGA2
axis in esophageal squamous cell carcinoma. Aging (Albany NY).
11:11937–11954. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li FZ and Zang WQ: Knockdown of
lncRNAXLOC_001659 inhibits proliferation and invasion of esophageal
squamous cell carcinoma cells. World J Gastroenterol. 25:6299–6310.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zabihula B, Yiliyasi M, Lu Y and Salai A:
MicroRNA-490-3p inhibits proliferation and stimulates apoptosis of
ESCC cells via MAPK1 downregulation. Oncol Lett. 18:3170–3176.
2019.PubMed/NCBI
|
|
59
|
Kang NN, Ge SL, Zhang RQ, Huang YL, Liu SD
and Wu KM: MiR-490-3p inhibited the proliferation and metastasis of
esophageal squamous cell carcinoma by targeting HMGA2. Eur Rev Med
Pharmacol Sci. 22:8298–8305. 2018.PubMed/NCBI
|
|
60
|
Zhang F, Wu A, Wang Y and Liu J:
miR-490-3p functions as a tumor suppressor in glioma by inhibiting
high-mobility group AT-hook 2 expression. Exp Ther Med. 18:664–670.
2019.PubMed/NCBI
|
|
61
|
Zhao L, Tang X, Luo R, Duan J, Wang Y and
Yang B: MicroRNA-490-5P Targets CCND1 to suppress cellular
proliferation in glioma cells and tissue through cell cycle arrest.
Curr Neurovasc Res. 15:246–255. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lan G, Yang L, Xie X, Peng L and Wang Y:
MicroRNA-490-5p is a novel tumor suppressor targeting c-FOS in
human bladder cancer. Arch Med Sci. 11:561–569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Li S, Xu X, Xu X, Hu Z, Wu J, Zhu Y, Chen
H, Mao Y, Lin Y, Luo J, et al: MicroRNA-490-5p inhibits
proliferation of bladder cancer by targeting c-Fos. Biochem Biophys
Res Commun. 441:976–981. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chen K, Zeng J, Tang K, Xiao H, Hu J,
Huang C, Yao W, Yu G, Xiao W, Guan W, et al: MiR-490-5p suppresses
tumour growth in renal cell carcinoma through targeting PIK3CA.
Biol Cell. 108:41–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wang J, Zhang X, Yao H, Le Y, Zhou W, Li
J, Lu L, Chen M and Li X: miR-490-5p functions as tumor suppressor
in childhood neuroblastoma by targeting MYEOV. Hum Cell.
33:261–271. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Abdeyrim A, Cheng X, Lian M and Tan Y:
miR4905p regulates the proliferation, migration, invasion and
epithelial-mesenchymal transition of pharyngolaryngeal cancer cells
by targeting mitogen-activated protein kinase kinasekinase 9. Int J
Mol Med. 44:240–252. 2019.PubMed/NCBI
|
|
67
|
Wu M, Tu T, Huang Y and Cao Y: Suppression
subtractive hybridization identified differentially expressed genes
in lung adenocarcinoma: ERGIC3 as a novel lung cancer-related gene.
BMC Cancer. 13:442013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhao Q, Wu M, Zheng X, Yang L, Zhang Z, Li
X and Chen J: ERGIC3 silencing additively enhances the growth
inhibition of BFA on lung adenocarcinoma cells. Curr Cancer Drug
Targets. 20:67–75. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Buchan JR and Parker R: Molecular biology.
The two faces of miRNA. Science. 318:1877–1878. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ansa-Addo EA, Huang HC, Riesenberg B,
Iamsawat S, Borucki D, Nelson MH, Nam JH, Chung D, Paulos CM, Liu
B, et al: RNA binding protein PCBP1 is an intracellular immune
checkpoint for shaping T cell responses in cancer immunity. Sci
Adv. 6:eaaz38652020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhang MP, Zhang WS, Tan J, Zhao MH, Lian
LJ and Cai J: Poly r(C) binding protein (PCBP) 1 expression is
regulated by the E3 ligase UBE4A in thyroid carcinoma. Biosci Rep.
37:BSR201701142017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Jones SA, Scheller J and Rose-John S:
Therapeutic strategies for the clinical blockade of IL-6/gp130
signaling. J Clin Invest. 121:3375–3383. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Hino S, Michiue T, Asashima M and Kikuchi
A: Casein kinase I epsilon enhances the binding of Dvl-1 to Frat-1
and is essential for Wnt-3a-induced accumulation of beta-catenin. J
Biol Chem. 278:14066–14073. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Mariotti L, Templeton CM, Ranes M,
Paracuellos P, Cronin N, Beuron F, Morris E and Guettler S:
Tankyrase requires SAM domain-dependent polymerization to support
Wnt-β-catenin signaling. Mol Cell. 63:498–513. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kozma SC and Thomas G: p70s6k/p85s6k:
Mechanism of activation and role in mitogenesis. Semin Cancer Biol.
5:255–260. 1994.PubMed/NCBI
|
|
76
|
Inoki K, Ouyang H, Zhu T, Lindvall C, Wang
Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, et al: TSC2
integrates Wnt and energy signals via a coordinated phosphorylation
by AMPK and GSK3 to regulate cell growth. Cell. 126:955–968. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhao M, Mishra L and Deng CX: The role of
TGF-β/SMAD4 signaling in cancer. Int J Biol Sci. 14:111–123. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Feng XH, Lin X and Derynck R: Smad2, Smad3
and Smad4 cooperate with Sp1 to induce p15(Ink4B) transcription in
response to TGF-beta. EMBO J. 19:5178–5193. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Duda P, Akula SM, Abrams SL, Steelman LS,
Martelli AM, Cocco L, Ratti S, Candido S, Libra M, Montalto G, et
al: Targeting GSK3 and associated signaling pathways involved in
cancer. Cells. 9:11102020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Shimura T: Acquired radioresistance of
cancer and the AKT/GSK3β/cyclin D1 overexpression cycle. J Radiat
Res. 52:539–544. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Vinchure OS and Kulshreshtha R: miR-490: A
potential biomarker and therapeutic target in cancer and other
diseases. J Cell Physiol. 236:3178–3193. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhang X, Di C, Chen Y, Wang J, Su R, Huang
G, Xu C, Chen X, Long F, Yang H and Zhang H: Multilevel regulation
and molecular mechanism of poly (rC)-binding protein 1 in cancer.
FASEB J. 34:15647–15658. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Tripathi V, Sixt KM, Gao S, Xu X, Huang J,
Weigert R, Zhou M and Zhang YE: Direct regulation of alternative
splicing by SMAD3 through PCBP1 Is essential to the tumor-promoting
role of TGF-β. Mol Cell. 64:549–564. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Lin QH, Zhang KD, Duan HX, Liu MX, Wei WL
and Cao Y: ERGIC3, which is regulated by miR-203a, is a potential
biomarker for non-small cell lung cancer. Cancer Sci.
106:1463–1473. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Tao W, Sun W, Zhu H and Zhang J: Knockdown
of long non-coding RNA TP73-AS1 suppresses triple negative breast
cancer cell vasculogenic mimicry by targeting miR-490-3p/TWIST1
axis. Biochem Biophys Res Commun. 504:629–634. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Fan H, Yuan J and Li X, Ma Y, Wang X, Xu B
and Li X: LncRNA LINC00173 enhances triple-negative breast cancer
progression by suppressing miR-490-3p expression. Biomed
Pharmacother. 125:1099872020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhang C, Ma MH, Liang Y, Wu KZ and Dai DQ:
Novel long non-coding RNA LINC02532 promotes gastric cancer cell
proliferation, migration, and invasion in vitro. World J
Gastrointest Oncol. 11:91–101. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Li MF, Zeng JJ, Pan AP, Lin YH, Lin HS,
Zhang RZ, Yang L, Zhang Y, Dang YW and Chen G: Investigation of
miR-490-3p expression in hepatocellular carcinoma based on reverse
transcription-polymerase chain reaction (RT-qPCR) and a
meta-analysis of 749 cases. Med Sci Monit. 24:4914–4925. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lai CH, Liang XZ, Liang XY, Ma SJ, Li JG,
Shi MF, Zhu X, Lan HH and Zeng JH: Study on miRNAs in Pan-cancer of
the digestive tract based on the Illumina HiSeq system data
sequencing. Biomed Res Int. 2019:80161202019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Tang B, Liu C, Zhang QM and Ni M:
Decreased expression of miR-490-3p in osteosarcoma and its clinical
significance. Eur Rev Med Pharmacol Sci. 21:246–251.
2017.PubMed/NCBI
|
|
91
|
Qu M, Li L and Zheng WC: Reduced
miR-490-3p expression is associated with poor prognosis of
Helicobacter pylori induced gastric cancer. Eur Rev Med
Pharmacol Sci. 21:3384–3388. 2017.PubMed/NCBI
|
|
92
|
Yang H, Zhang L, Wang XD, Huang ML, Lin P,
Pang YY, Feng ZB and Chen G: Potential targets and clinical value
of miR-490-5p in hepatocellular carcinoma: A study based on TCGA,
qRT-PCR and bioinformatics analyses. Int J Clin Exp Pathol.
11:1123–1134. 2018.PubMed/NCBI
|
|
93
|
Xu X, Wu X, Wu S, Jiang Q, Liu H, Chen R
and Sun Y: Study on miR-490-5p and miR-363 as novel biomarkers for
the diagnosis of colorectal cancer. Zhonghua Wei Chang Wai Ke Za
Zhi. 17:45–50. 2014.(In Chinese). PubMed/NCBI
|
|
94
|
Chen S, Chen X, Xiu YL, Sun KX, Zong ZH
and Zhao Y: MicroRNA 490-3P enhances the drug-resistance of human
ovarian cancer cells. J Ovarian Res. 7:842014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Orom UA, Nielsen FC and Lund AH:
MicroRNA-10a binds the 5′UTR of ribosomal protein mRNAs and
enhances their translation. Mol Cell. 30:460–471. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Jiang Y, Jiang YY, Xie JJ, Mayakonda A,
Hazawa M, Chen L, Xiao JF, Li CQ, Huang ML, Ding LW, et al:
Co-activation of super-enhancer-driven CCAT1 by TP63 and SOX2
promotes squamous cancer progression. Nat Commun. 9:36192018.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Xiong L, Zhen S, Yu Q and Gong Z: HCV-E2
inhibits hepatocellular carcinoma metastasis by stimulating mast
cells to secrete exosomal shuttle microRNAs. Oncol Lett.
14:2141–2146. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ding L, Ning J, Wang Q, Lu B and Ke H:
Sevoflurane improves nerve regeneration and repair of neurological
deficit in brain damage rats via microRNA-490-5p/CDK1 axis. Life
Sci. 271:1191112021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Kent WJ, Sugnet CW, Furey TS, Roskin KM,
Pringle TH, Zahler AM and Haussler D: The human genome browser at
UCSC. Genome Res. 12:996–1006. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Bai J, Yeh S, Qiu X, Hu L, Zeng J, Cai Y,
Zuo L, Li G, Yang G and Chang C: TR4 nuclear receptor promotes
clear cell renal cell carcinoma (ccRCC) vasculogenic mimicry (VM)
formation and metastasis via altering the miR490-3p/vimentin
signals. Oncogene. 37:5901–5912. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Wang B, Yin M, Cheng C, Jiang H, Jiang K,
Shen Z, Ye Y and Wang S: Decreased expression of miR4903p in
colorectal cancer predicts poor prognosis and promotes cell
proliferation and invasion by targeting RAB14. Int J Oncol.
53:1247–1256. 2018.PubMed/NCBI
|
|
102
|
Zhang C, Wang W, Lin J, Xiao J and Tian Y:
lncRNA CCAT1 promotes bladder cancer cell proliferation, migration
and invasion. Int Braz J Urol. 45:549–559. 2019. View Article : Google Scholar : PubMed/NCBI
|