Introduction
Colorectal cancer (CRC) is the most common malignant
tumour. It is estimated that CRC accounts for ~10% of all new
cancer cases and cancer-associated mortalities worldwide each year
(1). Due to its insidious onset,
20–22% of patients with CRC present with metastatic disease at
initial diagnosis, and 50–60% eventually develop metastasis. The
5-year overall survival rate for metastatic CRC is <14%
(2). In-depth exploration of the
mechanism of CRC occurrence and progression is critical for
improving existing detection methods and overcoming current
treatment limitations.
Interferon-induced protein 16 (IFI16) is a member of
the interferon-induced HIN200 gene family. IFI16 was first
identified in haematopoietic immune cells and was later found in
fibroblasts and epithelial cells derived from various human
tissues, such as lymph node, spleen, trachea and skin (3,4). Most
HIN200-family proteins contain a homotypic protein-protein
interaction PYRIN domain (PYD) region in the N-terminus and this
domain can bind to the PYD protein and induce cell apoptosis
(5). HIN200-family proteins also
share a partially conserved repeat of 200 amino acid residues in
the C-terminus by which the protein binds to foreign or damaged
double-stranded DNA (dsDNA) to activate the innate immune response
and inhibit cells growth (6,7). In addition, IFI16 protein has emerged
as an important stimulator of IFN-β expression in myeloid and
non-myeloid cells (8). IFI16 serves
a key role in connecting innate immunity and adaptive immunity
(7). Hence, IFI16 is an innate
immune sensor of foreign or damaged DNA and serves an important
role in innate immune responses, cell differentiation and
proliferation (9). Abnormal IFI16
expression is closely associated with immune system diseases and
the occurrence of various malignant tumours, such as systemic lupus
erythematosus and breast cancer (9,10).
IFI16 is upregulated during the onset of cellular
senescence in a variety of human cells, such as human fibroblasts
and bone and cartilage tumor cells (9–11). IFI16
can interact with p53, p-Rb, breast cancer gene 1 (BRCA1) protein,
among others, to active p53 target genes, such as the
cyclin-dependent kinase inhibitor p21WAF1/CIP1, the
inhibitor of cyclin-dependent kinase 4a (p16INK4a), Bax and Human
double minute 2 (Hdm2), and inhibit cell growth (10–12).
Accordingly, loss of IFI16 expression is associated with
immortalization of cells and the development of certain human
cancer types, such as breast cancer and prostatic cancer (13–15).
However, a few studies have indicated that the involvement of IFI16
in human cancer development varies depending on the cell source and
cell content. Cai et al (16)
demonstrated that IFI16 promoted cervical cancer progression by
upregulating programmed death-ligand 1 (PD-L1) in the
immunomicroenvironment through the stimulator of interferon
genes-TANK-binding kinase 1-NF-κB (STING-TBK1-NF-κB) pathway. In
familial inherited Wilms tumorigenesis, the WT1 gene participates
in tumorigenesis by regulating the spatial ectopic nature of IFI16
and thus combines with IFI16 protein to support cell survival
(17). The same phenomenon was
observed in liver cancer progression (18–20).
However, whether IFI16 is involved in the CRC development remains
unclear.
In our previous study (21), high-throughput gene expression
profiling was applied to assess gene expression characteristics
throughout the CRC development process, and the results
demonstrated that IFI16 was abnormally highly expressed during the
CRC process, which is consistent with the results reported by Yang
et al (22). Based on our
previous work and review of the current literature, we predicted
that IFI16 may serve an important role in CRC occurrence and
progression. In the present study, IFI16 expression and its
correlation with proliferation and immune signature markers was
investigated in CRC tissues and adjacent normal tissues. The
findings of the present study suggested that IFI16 takes part in
CRC occurrence via regulation of CRC cell proliferation.
Materials and methods
Tissue specimens
The human CRC tissue microarray (TMA) was purchased
from Shanghai Xinchao Biological Technology Co. Ltd. and contained
77 CRC tissues and adjacent normal tissues obtained by resection
between January 2012 and December 2013 from the First People's
Hospital of Yunnan Province (Kuming, China). However, 3 adjacent
normal tissues were lost due to repeated cutting. The inclusion
criteria used were as follows: i) All samples were selected from
patients with newly diagnosed CRC who had resection of colorectal
tumours without radiation therapy or chemotherapy; and ii) the
pathological information of the patients with CRC were complete.
The exclusion criteria were as follows: i) Patients aged <18
years; ii) patients with chronic diarrhea; and iii) patients who
had chemotherapy, radiation therapy or immunotherapy or viral
infection, such as Polyomaviruses (23). Among the 77 CRC patients, the median
age was 64 years (age range, 42–85 years), and the average age was
64.34±12.33 years. The 77 enrolled patients provided written
informed consent and the Ethics Committee of The First People's
Hospital of Yunnan Province (Kunming, China) ratified the study
(approval no. KHLL-2021-101). TMAs containing the tissue cores were
then cut into 4-µm sections for immunohistochemistry (IHC)
staining. The detailed clinical characteristics of the patients
enrolled in this study are summarized in Table I.
 | Table I.Clinical characteristics of patients
with CRC (n=77). |
Table I.
Clinical characteristics of patients
with CRC (n=77).
| Clinical
characteristic | n (%) |
|---|
| Sex |
|
|
Male | 42 (54.5) |
|
Female | 35 (45.5) |
| Age, years |
|
|
>55 | 42 (54.5) |
|
≤55 | 35 (45.5) |
|
Unknown | − |
| TNM stage |
|
|
I+II | 44 (57.1) |
|
III+IV | 33 (42.9) |
|
Differentiation |
|
|
Poorly | 11 (14.3) |
|
Well | 66 (85.7) |
| Lymph node
metastasis |
|
| No | 45 (58.4) |
|
Yes | 32 (41.6) |
| Primary tumor
sidedness |
|
|
Right-sided | 37 (48.1) |
|
Left-sided | 34 (44.2) |
|
Unknown | 6
(7.8) |
Antibodies
In the present study the primary antibodies used
were as follows: Rabbit anti-human phosphorylated (p)-ERK
monoclonal antibody (1:500; cat. no. cst4370; Cell Signaling
Technology Inc.); rabbit anti-human Ki-67 monoclonal antibody
(1:500; cat. cst12202; Cell Signaling Technology Inc.); a rabbit
anti-human IFI16 monoclonal antibody (immunohistochemistry,
1:1,000; western blotting, 1:1,000, cat. no. cst14970; Cell
Signaling Technology Inc.); rabbit anti-human CD8 monoclonal
primary antibody (1:50; cat. no. PA067; Suzhou Baidao Medical
Technology Co., Ltd.) rabbit anti-human PD-L1 monoclonal antibody
(cat. no. GT2280; Gene Tech Biotechnology, Co., Ltd.), rabbit
anti-β-actin polyclonal antibody (1:3,000; cat. no. 14395-1-AP;
Proteintech Group, Inc.). Human tonsil, which was purchased from
Shanghai Xinchao Biological Technology Co. Ltd. was stained with
Ki-67 antibody as a positive control according to the
recommendations from the International Ad Hoc Expert Committee
(24). Staining of human tonsil
slides with isotype control antibody (1:500; Rabbit (DA1E) mAb IgG
XP@ isotype control; cat. no. cst3900; Cell Signaling
Technology Inc.) was performed as negative controls. Human lung
tissues were stained with IFI16 and p-ERK1/2 antibodies as positive
controls. Staining of human lung slides with isotype control
antibody (cat. no. cst3900) was performed as negative controls.
Immunohistochemistry and image
analysis
Formalin-fixed paraffin-embedded (FFPE) tissue was
cut into 4-µm-thick sections and subjected to immunohistochemical
analysis. Sections were dewaxed in xylene 3 times for 3 min each
time at room temperature and rehydrated through a series of graded
alcohols in distilled water (100, 95 and 70% ethanol and in
distilled water finally). Heat-mediated antigen retrieval was
performed in citrate buffer (pH 6.0) using microwave treatment.
Then, the slides were washed with TBST (0.05% Tween-20) buffer and
the endogenous peroxidase activities were diminished with 0.3%
H2O2 for 10 min at room temperature.
Subsequently, blocking of the slices using 10% goat plasma (cat.
no. C0265; Beyotime Institute of Biotechnology) was conducted.
Rabbit anti-human CD8 monoclonal primary antibody and rabbit
anti-human PD-L1 monoclonal primary antibody were added and
incubated with the slides at 4°C overnight in a humidified chamber.
In all experiments, an isotype control antibody was used as a
negative control and no staining was obtained. Detection was
performed using the REAL EnVision Detection System (DAB; cat. no.
K500711; Dako; Agilent Technologies, Inc.) (25). In brief, the slides which were
incubated with primary antibody were wahsed in TBST 3 times for 3
min each time at room temperature. Then incubated the tissues with
REAL EnVision Detection System for 1 h and washed the slides again.
Following stained the tissues with DAB for 15 min. Sections were
counterstained with haematoxylin and eosin (H&E) at room
temperature for 5 mins and image acquisition was performed using a
light microscope (Nikon Corporation) (magnification, ×100). After
the immunostained slides had been reviewed by ≥2 independent
pathologists from Shanghai Xinchao Biological Technology Co. Ltd.
and consensus was achieved, the specific staining of defined
positive and negative cells of CD8 or PD-L1, such as position,
localization and cell types was matched with H&E staining. The
percentage of cells with CD8 and PD-L1 expression was recorded as
an average fraction of 100 neoplastic cells in every 3 fields, and
the intensity of CD8 and PD-L1 staining was graded on a scale of 0
to 3+ (0, absent staining; 1+, weak staining; 2+, moderate
staining; and 3+, strong staining). SPSS v.22.0 software (IBM
Corp.) was used for statistical analysis.
Multiplexed immunofluorescence
(MIF)
FFPE TMAs were deparaffinized, rehydrated and
subjected to antigen retrieval and diminishment of endogenous
peroxidase activities as aforementioned. The primary antibody for
p-ERK1/2 was incubated with the TMA for 30 min in a humidified
chamber at room temperature and detection was performed using a
Poal™ Polymer HRP Ms+Rb kit and Opal PPD520 TSA Plus (1:50; cat.
no. PPA200, PerkinElmer, Inc.) and were used according to the
manufacturer's instructions. Subsequently, the slide was again
placed in citrate buffer (pH 6.0) for microwave treatment for 15
min. The slide was then incubated with primary antibody targeting
IFI16 for 30 min at room temperature. This was followed by
detection using Opal PPD570 TSA Plus (1:50). The slide was placed
in citrate buffer (pH 6.0) for microwave treatment again. Then, the
slide was incubated with primary antibody against Ki-67 for 30 min
at room temperature. Ki-67 was visualized using Opal PPD650 TSA
Plus (1:50). The slide was then placed in citrate buffer (pH 6.0)
and heated via microwave treatment. The nuclei were subsequently
visualized with DAPI (Thermo Scientific Inc.) at room temperature
for 5 min. The slices were visualized under a PerkinElmer Vectra
Polaris™ fluorescent microscope (Neo Genomics Laboratories)
(magnifications, ×100 and ×400).
Fluorescence signal quantification and
image analysis
To obtain multispectral images, the stained slides
were scanned using the Vectra System (PerkinElmer, Inc.), which
captures the fluorescence spectra at 20-nm wavelength intervals
from 420 to 720 nm with an identical exposure time; the scans were
combined to build a single-stack image. Images of unstained
sections or single-stained sections were used to deduct the
autofluorescence and fluorescein fluorescence observed in tissues.
The extracted images were further used to establish a spectral
library required for multispectral unmixing using InForm Tissue
Finder™ Advanced image analysis software (PerkinElmer). The InForm
Tissue Finder™ Advanced image analysis software automatically
distinguished, the mucosal basal layer cells and the malignant
cells from the normal cells based on the atypia of tumour tissue
structure and the atypia of tumour cells. The atypia of tumour
tissue structure is characterized by a disordered arrangement,
direction, cell layers and cell rank order under a low power light
microscope (magnification, ×100) (26). The atypia of tumour cells usually
have features with difference in size, irregular in shape,
increasing the karyoplasmic ration and atypical pathologic mitoses
under a high-magnification light microscope (magnification, ×400)
(26).
Using this spectral library, reconstructed images of
sections with the autofluorescence removed were obtained. Each cell
was identified by detecting the nuclear spectral element (DAPI). To
define IFI16+ and Ki-67+ tumours, specimens
displaying unequivocal nucleus staining were classified as
positive. p-ERK1/2 expression that demonstrated a membranous,
cytoplasmic or nuclear staining pattern was classified as positive.
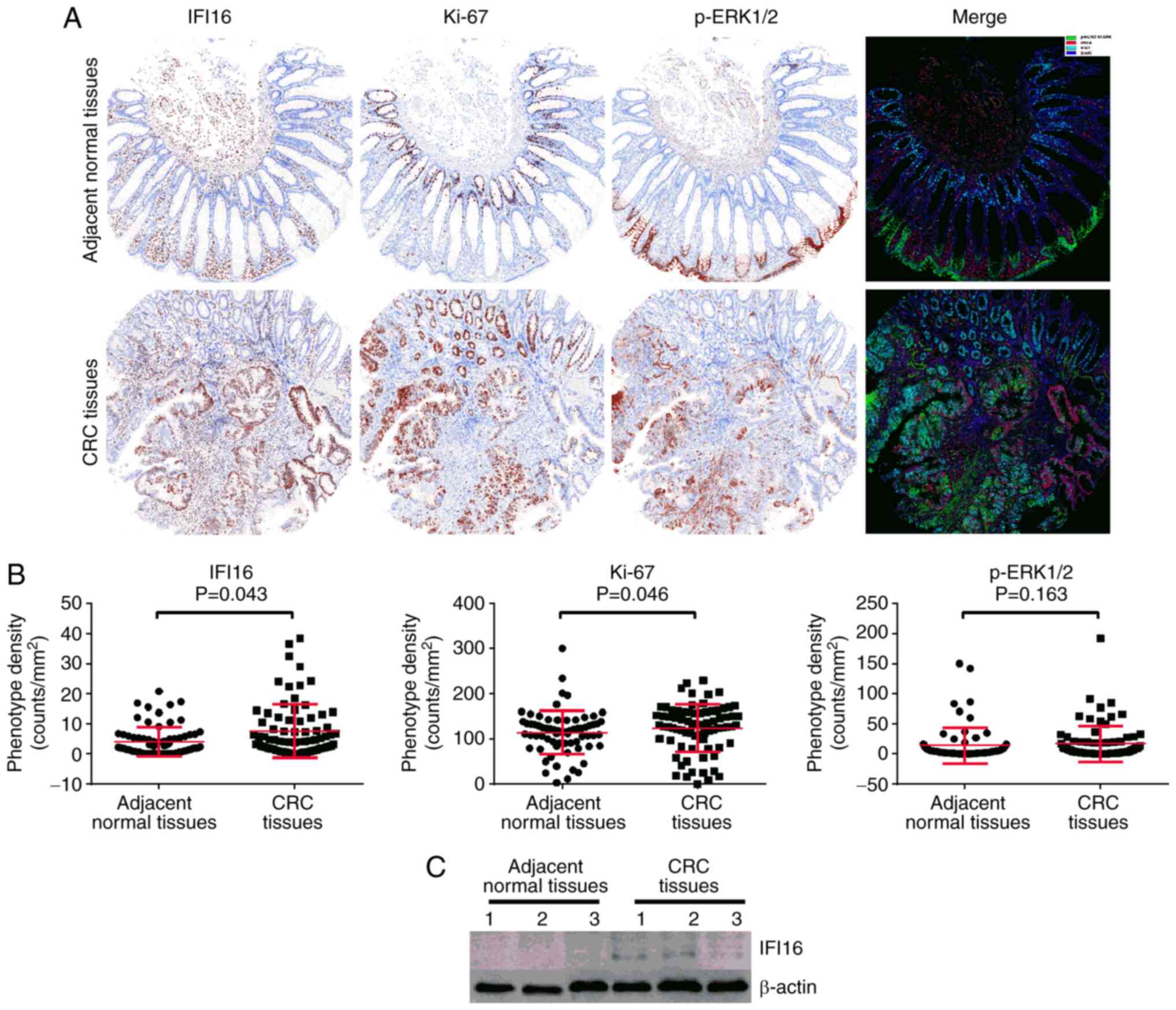
IFI16, Ki-67 and p-ERK1/2 are reported as normalized fluorescence
intensity in tumour and peritumoural tissue segments (Figs. 1 and 2). The proportion of IFI16, Ki-67 and
p-ERK1/2 positive tumour cells (TCs) was evaluated as the
percentage of total TCs (Fig. 3).
The normalized fluorescence intensity was automatically calculated
and reported by the InForm image analysis software. In brief, a
threshold of positive fluorescence signal was set before the
calculation was performed. Then, the fluorescence intensity was
divided into 3 levels: An intensity of 1 was defined as a
cell-positive signal strength between 1 and 2 times the threshold;
an intensity of 2 was defined as a cell-positive signal between 2
and 3 times the threshold; and an intensity of 3 was defined as a
cell-positive signal that was more than 3 times the threshold.
Following this, the data were automatically calculated and reported
by the following formula: Normalized fluorescence intensity
(Hscore) = [(fluorescence intensity of 1+ cell positive rate) × 1+
(fluorescence intensity of 2+ cell positive rate) × 2+
(fluorescence intensity of 3+ cell positive rate) × 3] ×100.
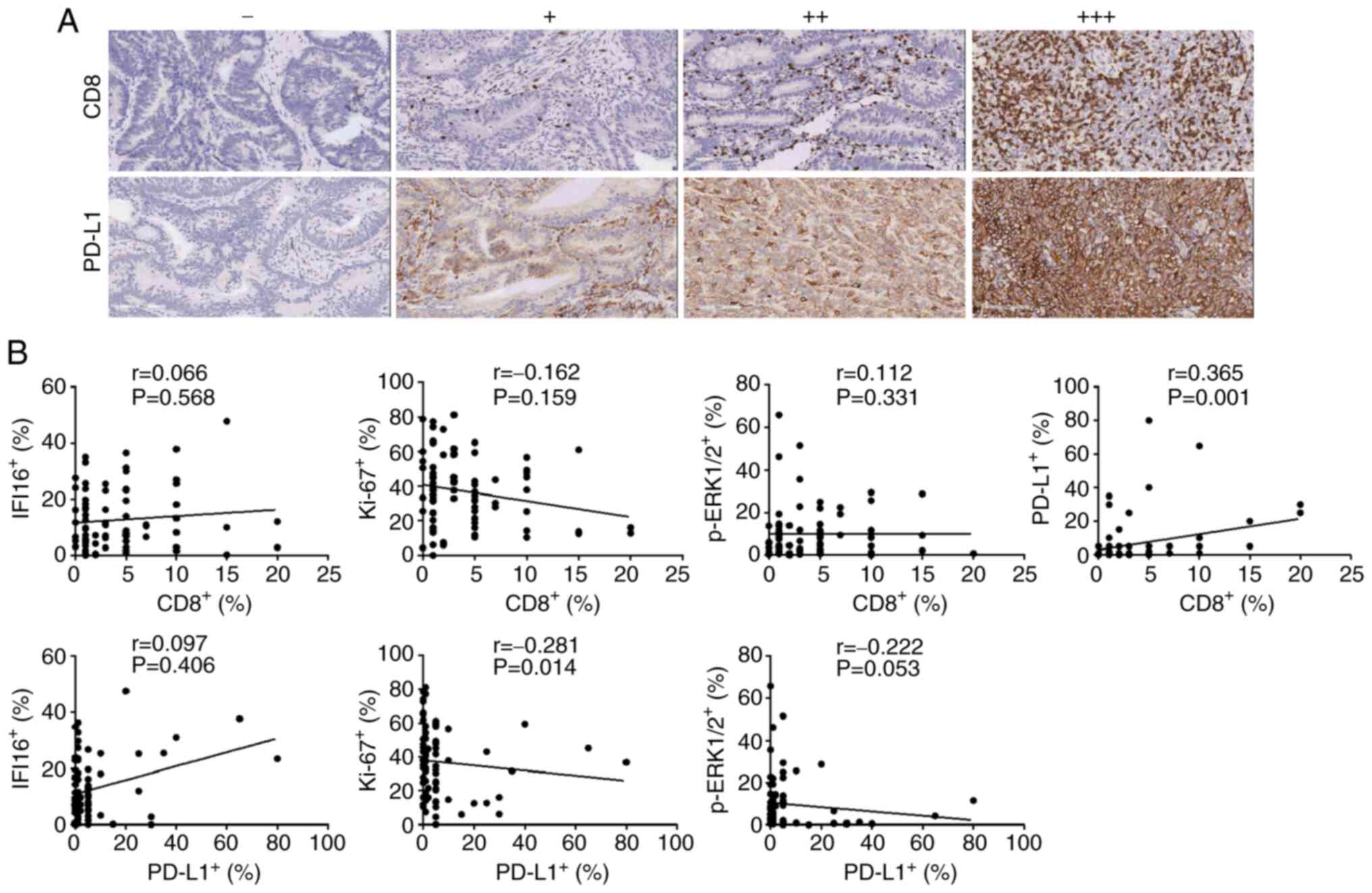 | Figure 3.Imaging of CD8+ TILs and
PD-L1 in CRC tissues and assessment of their correlation with
IFI16, Ki-67 and p-ERK1/2. (A) A negative to strong expression
level trend of CD8+ TILs and PD-L1 was displayed in the
immunohistochemical image (magnification, ×100). (B) Correlation
between CD8+ TILs and PD-L1 with IFI16, Ki-67 and
p-ERK1/2 protein expression. For CD8+ TILs, a
significant positive association with PD-L1 was observed in CRC
tissues. However, no significant association was observed with
IFI16, Ki-67 or p-ERK1/2 protein. For PD-L1, although no
significant association with IFI16 protein was observed, a
significant correlation with Ki-67 and a marginal association
between PD-L1 and p-ERK1/2 were observed. Correlations among IFI16,
Ki-67, p-ERK1/2 with CD8 and PD-L1 were analyzed with Spearman's
rank correlation. TILs, tumour infiltrating lymphocytes; PD-L1,
programmed death-ligand 1; CRC, colorectal cancer; IFI16,
interferon-induced protein 16; p, phosphorylated. |
Western blotting
Tissue lysates derived from CRC cancer tissues and
paired paracancerous tissues of 3 patients with CRC were prepared
using radioimunoprecipitation assay (RIPA) buffer (cat. no. P0013C;
Beyotime Institute of Biotechnology) in the presence of protease
inhibitor Phenylmethanesulfonyl fluoride (PFMS; cat. no. 36978;
Thermo Fisher Scientific Inc.) and inhibitor cocktail (cat. no.
4693132001; Roche Diagnostics GmbH). Protein concentrations of the
extract tissue lysates were quantified using a bicinchoninic acid
(BCA) protein assay (cat. no. P0010; Beyotime Institute of
Biotechnology). A total of 10 µg protein/lane was run on a 10%
polyacrlamide gel under denaturing conditions. Proteins were
transferred onto a PVDF membrane and blocked for 2 h at room
temperature in 0.1% TBST with 5% skimmed milk. Subsequently the
membranes were blotted with IFI16 antibodies and rabbit
anti-β-actin polyclonal antibody at 4°C overnight. The membrane was
washed with TBST 3 times for 5 min each time and incubated with the
horseradish peroxidise (HRP)-conjugated Affinipure Goat Anti-Rabbit
IgG (H+L) secondary antibody at room temperature for 1 h (1:3,000;
cat. no. SA00001-2, Proteintech Group Inc.). The detection was
performed using Pierce™ ECL western blotting substrate (cat. no.
32106. Thermo Fisher Scientific Inc.).
Statistical analysis
SPSS v.22.0 software (IBM Corp.) was used for
statistical analysis. All experiments were performed in triplicate
and data are presented as the mean ± standard deviation (SD).
Differences in the means of continuous variables were compared
using Wilcoxon-signed rank tests. Unpaired Student's t-tests were
conducted to evaluate the association between protein expression
and clinical features. Co-expression analysis of the IFI16, Ki-67,
p-ERK1/2, CD8 and PD-L1 genes with clinical features was analyzed
with Spearman's rank correlation. P<0.05 was considered to
indicate a statistically significant difference.
Results
Differential expression of IFI16,
Ki-67 and p-ERK1/2 in CRC and adjacent tissues
To assess the expression level of IFI16 and
proliferation markers in CRC tissues and adjacent normal tissues, a
multiplex immunofluorescence panel of antibodies against IFI16,
Ki-67 and phosphorylated (p)-ERK1/2 was performed.
Immunofluorescence assay results demonstrated that in CRC and
adjacent normal tissues, IFI16 and Ki-67 were localized in the
nucleus of TCs, while p-ERK1/2 was located in both the cytoplasm
and the cell membrane and sometimes to the nucleus (Fig. 1A). IFI16 was highly expressed in
stromal cells in both cancer and adjacent normal tissues. Although
weak IFI16 expression was observed in the normal intestinal mucosal
epithelial cells adjacent to cancer tissues, weak to strong IFI16
expression was observed in different CRC tissues (Fig. 1). The mean fluorescence intensity
level of IFI16 protein in CRC tissues was significantly higher
compared with that in normal mucosal epithelial cells adjacent to
the tumour (7.48±8.84 vs. 4.38±4.93, respectively; P=0.043;
Fig. 1B). The result was further
confirmed using western blotting, although heterogeneous expression
was observed as patient 3 showed very faint expression compared
with the other 2 patients (Fig. 1C).
Ki-67 protein was highly expressed in cancer cells in the CRC
tissues, but was mainly expressed in the mucosal basal layer cells
in the adjacent normal tissues (Fig.
1C). The mean fluorescence intensity level of Ki-67 protein in
cancer cells was notably higher compared with in adjacent mucosal
epithelial cells (123.35±52.42 vs. 114.04±48.68, respectively;
P=0.046; Fig. 1B). p-ERK1/2 protein
could be detected in both cancer cells and stromal cells in CRC
tissues and was also detected in cells of adjacent normal tissues
(Fig. 1A). In addition, no
significant difference in the p-ERK1/2 expression was observed
between CRC cells and adjacent mucosal epithelial cells
(17.29±29.70 vs. 14.09±29.70, respectively; P=0.163; Fig. 1B). Taken together, these results
suggested that IFI16, Ki-67 were relatively higher expressed in CRC
tissues compared with normal tissues.
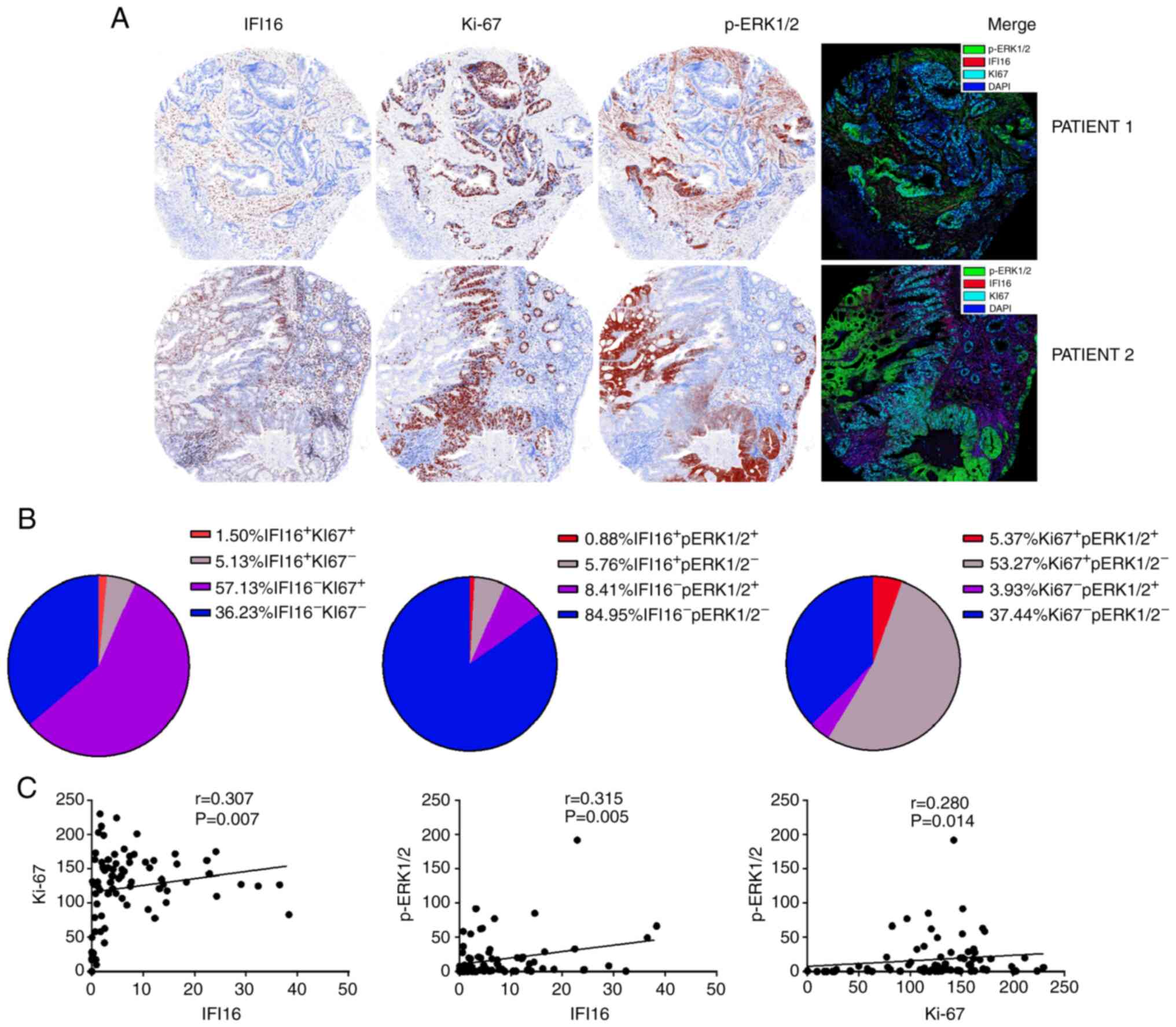
Co-localization and association of
IFI16 with Ki-67 and p-ERK protein in CRC tissues
InForm Tissue Finder™ Advanced image analysis
software was used to analysis the co-localization of the target
proteins. Although, a high IFI16 level was observed in the CRC
tissues of certain patients and the IFI16 expression level was
significantly positively correlated with that of Ki-67 and p-ERK
(IFI16 vs. Ki-67: r=0.307, P=0.007; IFI16 vs. p-ERK1/2: r=0.315,
P=0.005), IFI16 was not usually co-localized with Ki-67 or p-ERK1/2
within cells (Fig. 2A and C). The
proportion of double-positive cell subsets, such as
IFI16+/Ki-67+ cells (1.50%) or
IFI16+/p-ERK1/2+ cells (0.88%) was much lower
compared with IFI16 single-positive cells (6.68%) (Fig. 2B). However, the proportion of cells
with mutually exclusive expression, such as
IFI16+/Ki-67− cells (5.13%) and
IFI16−/Ki-67+ cells (57.13%), was much higher
compared with that of the other 2 subsets
(IFI16−/Ki-67−, 36.23%;
IFI16+/Ki-67+, 1.50%; Fig. 2B). In contrast to IFI16 and Ki-67
expression, the proportions of IFI16+/p-ERK−
cell (5.76%) and IFI16−/p-ERK+ cell (8.41%)
subsets were much lower compared with that of the
IFI16−/p-ERK1/2− cell (84.95%) subset
(Fig. 2B). In addition, the
expression level of p-ERK1/2 was negligibly positively correlated
with that of Ki-67 (r=0.280; P=0.014; Fig. 2C and the proportion of
p-ERK1/2+/Ki-67+ double-positive cells was
only 5.37% (Fig. 2B). In addition,
the proportions of cells with double-positive
p-ERK1/2+/Ki-67+(5.37%), mutually exclusive
expression, such as p-ERK1/2−/Ki-67+ cells
(53.27%) and p-ERK1/2+/Ki-67− cells (3.93%),
and double-negative cell subsets (37.44%) displayed the same trend
with IFI16 as with Ki-67 (Fig. 2B).
The aforementioned results indicated that IFI16 protein in CRC
tissues showed mutually exclusive expression with Ki-67 and
p-ERK1/2.
CD8 and PD-L1 expression in CRC
tissues and its correlation with that of IFI16, Ki-67 and p-ERK1/2
expression
To explore the association of IFI16 with immune
micorenvironment of CRC cell, iimmunohistochemistry was used to
test the expression of CD8 and PD-L1. It was demonstrated that in
77 CRC tissues, the mean CD8 and PD-L1 expression rates were
4.26±4.523 and 6.86±13.849%, respectively, and a significant
positive correlation was observed between CD8 and PD-L1 (r=0.365;
P=0.001; Fig. 3B). No significant
association was observed between IFI16 and CD8 or PD-L1 (IFI16 vs.
CD8: r=0.066, P=0.568; IFI16 vs. PD-L1: r=0.097, P=0.406; Fig. 3B). Although neither Ki-67 nor
p-ERK1/2 expression demonstrated a significant association with
CD8, a significant negative association between Ki-67 and PD-L1 and
a marginally significant association between p-ERK1/2 and PD-L1
were observed (Ki-67 vs. CD8, r=0.162, P=0.159; Ki-67 vs. PD-L1,
r=−0.281, P=0.014; p-ERK1/2 vs. PD-L1, r=−0.222, P=0.053; Fig. 3B). Collectively, these results
suggested that IFI16 expression was not influenced by
CD8+ T cells and PD-L1 expression in CRC tissues.
Association between IFI16, Ki-67,
p-ERK1/2, CD8 and PD-L1 and clinical features
To further investigate clinical value of IFI16, SPSS
software was used to analysis the IFI16 expression level with
clinical parameters. Although IFI16 expression was significantly
negatively associated with patient age (age >55 vs. ≤55,
5.46±6.60 vs. 11.69±10.55, P=0.003; Table II), no significant association was
observed between IFI16 expression and tumor location, sex,
pathological grade, lymph node metastasis or tumour-node-metastasis
(TNM) stage (27) (P>0.05;
Table II). The cell
proliferation-associated nuclear antigen Ki-67 (28) was significantly positively associated
with lymph node metastasis (no vs. yes, 66.93±43.53 vs.
91.04±50.96; P=0.029; Table II) and
TNM stage (I+II vs. III+IV, 65.72±43.31 vs. 91.88±50.39; P=0.017;
Table II). The p-ERK1/2 expression
level was much higher in male patients compared with in female
patients (male vs. female, 26.03±36.73 vs. 6.80±11.56; P=0.004),
but there was no significant association of p-ERK1/2 expression
with TNM stage, lymph node metastasis, age, pathological grade and
tumor location (P>0.05; Table
II). CD8+ tumour-infiltrating lymphocytes (TILs)
were significantly negatively associated with lymph node metastasis
(5.18±4.88 vs. 2.97±3.66; P=0.034), TNM staging (5.25±4.91 vs.
2.94±3.61; P=0.026; Table II) and
positively associated with PD-L1 expression (r=0.365; P<0.001;
Fig. 3B). Although PD-L1 expression
was negatively associated with pathological grade (poorly vs. well
differentiated, 20.70±4.76 vs. 4.76±9.99; P<0.001; Table II), no significant association was
observed between PD-L1 and TNM stage, lymph node metastasis, age,
sex and tumor location (P>0.05; Table II). Taken together, these results
suggested that IFI16 demonstrated no significant association with
clinical outcomes.
 | Table II.Statistical analysis of the
clinicopathological characteristics and the expression levels of
candidate proteins in the cancer tissue of CRC patients (n=77). |
Table II.
Statistical analysis of the
clinicopathological characteristics and the expression levels of
candidate proteins in the cancer tissue of CRC patients (n=77).
| Clinical
characteristics | n (%) | IFI16 (mean ±
SD) | P-value | Ki-67 (mean ±
SD) | P-value | p-ERK1/2 (mean ±
SD) | P-value | CD8+
(mean ± SD) (%) | P-value | PD-L1+
(mean ± SD) (%) | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
|
|
|
|
|
>55 | 56 (73.7) | 5.46±6.60 |
| 119.23±54.46 |
| 15.76±30.84 |
| 4.18±4.72 |
| 5.95±12.58 |
|
|
≤55 | 20 (26.3) | 11.69±10.55 | 0.003a | 134.72±47.09 | 0.262 | 19.95±26.42 | 0.590 | 4.45±4.15 | 0.821 | 9.84±17.38 | 0.295 |
| Sex |
|
|
|
|
|
|
|
|
|
|
|
|
Male | 42 (54.5) | 8.79±9.80 |
| 128.30±46.95 |
| 26.03±36.73 |
| 3.71±3.83 |
| 6.14±12.34 |
|
|
Female | 35 (45.5) | 5.92±7.37 | 0.157 | 117.41±58.45 | 0.368 | 6.80±11.56 | 0.004a | 4.91±5.22 | 0.249 | 7.74±15.67 | 0.621 |
| Pathological
grade |
|
|
|
|
|
|
|
|
|
|
|
|
Poor | 11 (14.3) | 8.37±13.19 |
| 91.19±57.16 |
| 7.70±14.38 |
| 6.36±6.38 |
| 20.70±4.76 |
|
|
Well | 66 (85.7) | 7.33±8.03 | 0.721 | 128.71±50.05 | 0.027a | 18.89±31.29 | 0.250 | 3.91±4.10 | 0.096 | 4.76±9.99 |
<0.01a |
| LN metastasis |
|
|
|
|
|
|
|
|
|
|
|
| No | 45 (58.4) | 13.89±12.46 |
| 66.93±43.53 |
| 20.56±20.58 |
| 5.18±4.88 |
| 6.93±14.13 |
|
|
Yes | 32 (41.6) | 14.99±13.79 | 0.717 | 91.04±50.96 | 0.029a | 15.51±31.04 | 0.393 | 2.97±3.66 | 0.034a | 6.75±13.68 | 0.955 |
| TNM stage |
|
|
|
|
|
|
|
|
|
|
|
|
I+II | 44 (57.1) | 14.04±12.57 |
| 65.72±43.31 |
| 20.52±20.82 |
| 5.25±4.91 |
| 6.98±14.30 |
|
|
III+IV | 33 (42.9) | 14.78±13.63 | 0.806 | 91.88±50.39 | 0.017a | 15.71±30.57 | 0.895 | 2.94±3.61 | 0.026a | 6.70±13.46 | 0.931 |
| Tumor location |
|
|
|
|
|
|
|
|
|
|
|
| Left
colon | 34 (47.9) | 6.12±7.83 |
| 115.09±45.07 |
| 18.71±25.68 |
| 4.56±3.82 |
| 6.47±14.76 |
|
| Right
colon | 37 (52.1) | 9.02±10.07 | 0.184 | 131.31±56.29 | 0.187 | 15.47±33.47 | 0.652 | 4.22±5.21 | 0.755 | 8.00±14.13 | 0.659 |
Discussion
As a natural immune recognition receptor for foreign
DNA and damaged DNA, IFI16 activates the ATK/AMPK/p53 or
ATK/IKKB/NF-κB signalling pathway and promotes cell cycle arrest or
the expression of the inflammatory cytokines, such as IL-1, IL-18
and IFN-β (6,7). The release of IFN-β serves an important
role in the body's natural immune response and in age-related
cellular senescence (6,7). Increased expression of IFI16 in a
variety of cell types, such as human fibroblasts and bone and
cartilage tumor cells promotes cell senescence (10–12).
Although senescent cell are resistant to oncogenic challenge and do
not proliferate, they exhibit senescence-associated secretory
phenotype (SASP) (29–33). SASP is associated with secretion of
proinflammatory cytokines. Senescent cells or SASP release multiple
cytokines that may support the proliferation of non-senescent
neighbouring cell via paracrine mechanisms. Accumulation of
senescent cell or SASP in tissues or organs is thought to
contribute to organismal ageing and inflammation-associated human
diseases, including the development of certain cancer types, such
as colon cancer and breast cancer (34,35).
Increasing data have demonstrated that abnormal
expression of IFI16 is closely associated with cancer development
(6). Due to the different tissue
sources and cell contents, IFI16 can serve a dual role as a tumour
suppressor or a cancer promoter (12–21). The
present study demonstrated that IFI16 expression level was very low
in normal mucosal epithelial cells. However, barely detectable to
strong expression was observed in CRC cells in different patients
in the present study. The IFI16 expression level in CRC tissues was
higher compared with in normal mucosal epithelial tissues. In
addition, in the present study, a positive correlation in
expression was found between IFI16 and the cell
proliferation-associated nuclear antigen Ki-67. The finding of the
present study suggest that IFI16 may act as an oncogene to promote
CRC occurrence. However, IFI16 and Ki-67 were usually not
co-localized within cells and 60% of analysed cells in the present
study demonstrated mutually exclusive expression patterns. The
IFI16−/Ki-67+ phenotype was significantly
associated with TNM stage The results of the present study
suggested that IFI16 may serve a role in inhibiting CRC cell
proliferation. A potential explanation for these contradictory
phenomena may be that CRC can be divided into 2 categories based on
genomic instability: DNA repair deficiency/microsatellite
instability (dMMR/MSI) and DNA repair mechanism
integrity/microsatellite stability (pMMR/MSS) (36). The two tumour types show very
different genetic profiles (35).
dMMR/MSI tumours typically have increased tumour mutational burden,
with a 100- to 1,000-fold increased mutation rate compared with
pMMR/MSS tumours (37). This high
mutational rate leads to increased levels of tumour-associated
antigen and immune cell infiltration (37,38). We
hypothesized that patients with CRC in the present study with the
dMMR/MSI subtype have an even higher IFI16 expression compared with
patients with CRC with pMMR/MSS tumours as a much higher gene
mutational rate occurs in dMMR/MSI tumours. Hence, it was
speculated that the differential IFI16 expression in patients with
CRC in the present study may be associated with the different
MMR/MSI tumours present. However, increased IFI16 expression
inhibits cell growth and accelerates senescence (6). To maintain tumour cells growth, the
senescent cells may produce SASP to stimulate the survival and
proliferation of surrounding cells (33,35). At
present, there are 3 main methods for detecting MMR/MSI status,
including immunohistochemistry, polymerase chain reaction and
second-generation sequencing (39).
However, due to the scarcity of direct experimental evidence, as
the TMA tissue used in the present study did not have high enough
integrity to detect the MMR/MSI status, the association between
IFI16, MMR/MSI status and SASP in CRC occurrence remains
speculative and is a limitation of the present study.
In addition, the present study found that the IFI16
expression level was negatively associated with patient age.
Compared with patients older >55 years, higher IFI16 levels were
observed in patients younger than 55 years old. Studies by
Raffaella et al and Fujiuchi et al (13,14)
demonstrated taht the IFI16 expression was significantly increased
in old normal cells and senescent cells, and the expression was
significantly downregulated with cell immortalization and malignant
transformation. However, whether IFI16 expression level in
malignant cells is associated with age has not yet been reported
(6). In addition, sex hormones and
environmental factors also influence IFI16 expression, such as
prostaglandin and cervical cancer development (6,16).
Hence, further studies with larger sample sizes are required to
verify the findings of the present study.
The present study also analysed the correlation
between IFI16 and p-ERK1/2 protein. p-ERK1/2 is the phosphorylation
product of ERK1/2, a downstream protein in the MAPK signalling
pathway (40). The MAPK signalling
pathway serves an important role in regulating cell proliferation,
differentiation, migration, growth, survival and apoptosis, among
other processes (41).
Ras-Raf-Mek1/2-ERK1/2 is one of the important downstream cascades
of the MAPK signalling pathway (42). When the body's proinflammatory
cytokines bind to receptors on the cell surface, the MAPK
signalling pathway is activated to promote cell growth,
proliferation, differentiation or apoptosis, such as tumor necrosis
factor-α (TNFα) (37). Previous
studies have demonstrated that IFI16 is involved in Ras signalling
pathway activation (43–45). Kim et al (45) demonstrated that in the occurrence of
thyroid cancer, IFI16 acts as a downstream regulator in the
RAS/RAF/ERK signalling pathway to inhibit the continuous activation
of cells induced by the Ras oncogene. In 2010, Lengyel et al
(46) demonstrated that the p204
protein (a mouse family member homologous to the IFI16 protein) is
an important protein that regulates Ras and its downstream
signalling pathways. Hence, the correlation between IFI16 and
p-ERK1/2 was investigated in the present study. The present study
revealed that p-ERK1/2 was located mainly in the cytoplasm and cell
membrane rather than the nucleus. Although the IFI16 expression
level was significantly positively correlated with that of
p-ERK1/2, the co-localization rate of IFI16 and p-ERK1/2 in the
same cell was very low (0.88%), and ~84.95% of the analysed cells
were double-negative for IFI16 and p-ERK1/2. The IFI16 and p-ERK1/2
expression model was quite different from that of IFI16 and Ki-67
observed in the present study. Surprisingly, only 5.86% of the
analysed cells showed a p-ERK1/2+/Ki-67+
phenotype, and 57.2% of the analysed cells showed a mutually
exclusive expression phenotype, such as p-ERK1/2−/
Ki-67+ or p-ERK1/2+/Ki-67−. In
addition, patients with the p-ERK1/2−/Ki-67+
phenotype had more lymph node migration and a more advanced TNM
stage and the opposite was observed in patients with the
p-ERK1/2+/Ki-67− phenotype in the present
study. These results suggested that p-ERK1/2, similar to IFI16, may
serve a role in inhibiting CRC cell proliferation.
p-ERK1/2 is located in the cytoplasm and
subsequently translocates to the nucleus, where it activates
transcription factors, such as c-Jun and Fos (40). Nuclear translocation of ERK/MAPK is
required for mitogenesis (46).
Cytosolic retention of p-ERK1/2 can activate certain proapoptotic
proteins, such as cytosolic death-associated protein kinase 1
(47). The nucleocytoplasmic
distribution of ERK/MAPK is used for regulating ERK/MAPK signalling
(48). There are quite a few
proteins involved in the regulation of localizing ERK/MAPK
signalling, such as LYN proto-oncogene (Lyn), proliferation and
apoptosis adaptor protein 15 (PEA15) and human Sef gene (46–48). Our
present data indicated that p-ERK1/2 is expressed in both the
membrane and cytoplasm of CRC cells and in the nucleus. p-ERK1/2
expression was also positively correlated with Ki-67 protein
expression. These results indicated that the p-ERK1/2 expression
level was closely associated with the proliferation potential of
CRC cells, which is consistent with the aforementioned studies
(49–51). However, the results of the present
study demonstrated that p-ERK1/2 does not usually co-localize with
Ki-67 in CRC cells, which contradicted the aforementioned results.
The inconsistent results may be partially due to the inability to
divide the p-ERK1/2 protein into nuclear-located p-ERK1/2 and
non-nuclear located ERK1/2 and analyse the subsets separately in
the present study. Hence, future studies should clarify this issue
using in vitro cell culture experiments.
In addition, the present study demonstrated that the
p-ERK1/2 expression level in males was much higher compared with in
females. We hypothesised that this result may be associated with
the samples used in the present study. p-ERK1/2 expression is
affected by numerous factors, such as hypoxia and chronic
inflammation and thus, whether there is a direct correlation
between p-ERK1/2 and sex in CRC tissues must be further verified in
future studies.
IFI16 is an important protein that links innate
immunity and adaptive immunity (7).
Intracellular DNA activation of human monocyte-derived dendritic
cells (DCs) as well as primary DCs was dependent upon IFI16 protein
expression and IFN-β expression (52). More important, activated DCs induce
naive CD4+ T cells to promote Th1-type cytokine
production, such as IL-2, IFN-γ (53) and generate CD4+ and
CD8+ cytotoxic T cells (54). Qi et al (55) reported that IFI16 expression may be a
good prognostic biomarker and immunotherapeutic target in patients
with HCC. Hence, the present study detected and analysed the
relationship between IFI16 expression and PD-L1/CD8+
TILs in CRC tissues. However as the findings of the present study
demonstrated no significant association was observed between IFI16
and CD8+ TILs or PD-L1 expression. This result was
opposite of that by Cai et al (16), who demonstrated that IFI16 promotes
cervical cancer progression by upregulating PD-L1 expression. This
difference may be attributed to differences in the diseases or the
methods. In addition, the present study did not subtype the
patients with CRC based on molecular characteristics, such as
MMR/MSI phenotype, which may influence the expression model
obtained for IFI16, PD-L1 and CD8+ TIL.
CD8+ TIL infiltration was positively
associated with PD-L1 expression in the present study. Infiltration
of CD8+ TILs boosts PD-L1 expression in cancer cells to
facilitate their escape from attack by CD8+ TILs
(56–59). Therefore, the findings of the present
study also supported the notion that the expression level of PD-L1
in CRC tissues can be used as an effective predictor of the
response to the programmed death/PD-L1 immune checkpoint therapy.
CD8+ TIL infiltration in CRC tissues was related to a
low rate of lymph node metastasis and early TNM stage in the
present study, which further supports the notion that
CD8+ TIL abundance is a good predictor of clinical
outcome for patients with CRC (60,61). In
conclusion, to the best of our knowledge, the present study was the
first time multiplex immunofluorescence and IHC techniques were
used to explore the expression and potential mechanism of IFI16 in
CRC tissues. Although IFI16 expression was significantly increased
in CRC tissues compared with adjacent normal tissues and had a
positive association with Ki-67 and p-ERK1/2 expression, IFI16
usually showed mutually exclusive expression patterns with Ki-67
p-ERK1/2. The findings of the present study suggested that
increased IFI16 expression may serve a role in inhibiting CRC cell
proliferation and contribute to cell senescence. In addition, the
senescent cells likely stimulate the survival and proliferation of
surrounding cells through production of SASP. However, future
studies need to be conducted to support this hypothesis and uncover
the molecular mechanism underlying IFI16 in CRC occurrence.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Health Bureau
of Yunnan Province (grant nos. 2017NS226 and 2018NS0247); the
Yunnan Digestive Endoscopy Clinical Medical Center Foundation for
Health Commission of Yunnan Province (grant no. 2019LCZXKF-XH04);
the Yunnan Blood Disease Clinical Medical Center Foundation for
Health Commission of Yunnan Province (grant no. 2020LCZXKF-XY08);
and in part by the Science and Technology Department of Yunnan
Province (grant no. 2018DG010).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and XY designed the study and wrote the
manuscript. JZ and LZ performed the experiments and collected the
data. YZ and XY analysed and interpreted the data. YL and XY
confirmed the authenticity of all the raw data. All the authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
All participants provided written informed consent,
and the study was approved by the Ethics Committee of the First
People's Hospital of Yunnan Province (Kunming, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dekker E, Tanis PJ, Vleugels J, Kasi PM
and Wallace MB: Colorectal cancer. Lancet. 394:1467–1480. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Goding Sauer A,
Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA and Jemal
A: Colorectal cancer statistics, 2020. CA Cancer J Clin.
70:145–164. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dawson MJ and Trapani JA: IFI 16 gene
encodes a nuclear protein whose expression is induced by
interferons in human myeloid leukaemia cell lines. J Cell Biochem.
57:39–51. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wei W, Clarke CJ, Somers GR, Cresswell KS,
Loveland KA, Trapani JA and Johnstone RW: Expression of IFI16 in
epithelial cells and lymphoid tissues. Histochem Cell Biol.
119:45–54. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stehlik C: The PYRIN domain in signal
transduction. Curr Protein Pept Sci. 8:293–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choubey D and Panchanathan R: IFI16, an
amplifier of DNA-damage response: Role in cellular senescence and
aging-associated inflammatory diseases. Ageing Res Rev. 28:27–36.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin H and Cao X: Nuclear innate sensors
for nucleic acids in immunity and inflammation. Immunol Rev.
297:162–173. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pancanathan R, Liu H, Leung YK, Ho SM and
Choubey D: Bisphenol A (BPA) stimulates the interferon signaling
and activates the inflammasome activity in meloid cells. Mol Cell
Endocrinol. 415:45–55. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cui J, Chen Y, Wang HY and Wang RF:
Mechanisms and pathways of innate immune activation and regulation
in health and cancer. Hum Vaccin Immunother. 10:3270–3285. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ouchi M and Ouchi T: Role of IFI16 in DNA
damage and checkpoint. Front Biosci. 13:236–239. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duan X, Ponomareva L, Veeranki S and
Choubey D: IFI16 induction by glucose restriction in human
fibroblasts contributes to autophagy through activation of the
ATM/AMPK/p53 pathway. PLoS One. 6:e195322011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Howell RD, Alfonso DT, Yu J, Kong
L, Wittig JC and Liu CJ: IFI16 inhibits tumorigenicity and cell
proliferation of bone and cartilage tumor cells. Front Biosci.
12:4855–4863. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Raffaella R, Gioia D, De Andrea M,
Cappello P, Giovarelli M, Marconi P, Manservigi R, Gariglio M and
Landolfo S: The interferon-inducible IFI16 gene inhibits tube
morphogenesis and proliferation of primary, but not HPV16 E6/E7
immortalized human endothelial cells. Exp Cell Res. 293:331–345.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fujiuchi N, Aglipay JA, Ohtsuka T, Maehara
N, Sahin F, Su GH, Lee SW and Ouchi T: Requirement of IFI16 for the
maximal activation of p53 induced by ionizing radiation. J Biol
Chem. 279:20339–20344. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alimirah F, Chen J, Davis FJ and Choubey
D: IFI16 in human prostate cancer. Mol Cancer Res. 5:251–259. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cai H, Yan L, Liu N, Xu M and Cai H: IFI16
promotes cervical cancer progression by upregulating PD-L1 in
immunomicroenvironment through STING-TBK1-NF-κB pathway. Biomed
Pharmacother. 123:1097902020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim MK, Mason JM, Li CM, Berkofsky-Fessler
W, Jiang L, Choubey D, Grundy PE, Tycko B and Licht JD: A
pathologic link between Wilms tumor suppressor gene, WT1, and
IFI16. Neoplasia. 10:69–78. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu F, Hao X, Zhao H, Ge C, Yao M, Yang S
and Li J: Delta-like 1 contributes to cell growth by increasing the
interferon-inducible protein 16 expression in hepatocellular
carcinoma. Liver Int. 30:703–714. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi XL, Yang J, Mao N, Wu JH, Ren LF, Yang
Y, Yin XL, Wei L, Li MY and Wang BN: Nutlin-3-induced
redistribution of chromatin-bound IFI16 in human hepatocellular
carcinoma cells in vitro is associated with p53 activation. Acta
Pharmacol Sin. 36:252–258. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi X, Liu J, Liu Q and Li M: IFI16
mis-localization can be a contributing factor to hepatocellular
carcinoma progression. Med Hypotheses. 82:398–400. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang H, Guo Q, Zhang C, Zhu J, Yang H, Zou
YL, Yan Y, Hong D, Shou T and Yan XM: Identification of an
intermediate signature that marks the initial phases of the
colorectal adenoma-carcinoma transition. Int J Mol Med. 26:631–641.
2010.PubMed/NCBI
|
|
22
|
Yang CA, Huang HY, Chang YS, Lin CL, Lai
IL and Chang JG: DNA-Sensing and nuclease gene expressions as
markers for colorectal cancer progression. Oncology. 92:115–124.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Turkington CJR, Varadan AC, Grenier SF and
Grasis JA: The viral Janus: Viruses as aetiological agents and
treatment options in colorectal cancer. Front Cell Infect
Microbiol. 10:6015732020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Torlakovic EE, Nielsen S, Francis G,
Garratt J, Gilks B, Goldsmith JD, Hornick JL, Hyjek E, Ibrahim M,
Miller K, et al: Standardization of positive controls in diagnostic
immunohistochemistry: Recommendations from the international Ad Hoc
Expert Committee. Appl Immunohistochem Mol Morphol. 23:1–18. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Skaland I, Nordhus M, Gudlaugsson E, Klos
J, Kjellevold KH, Janssen EA and Baak JP: Evaluation of 5 different
labeled polymer immunohistochemical detection systems. Appl
Immunohistochem Mol Morphol. 18:90–96. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Muto T, Bussey HJ and Morson BC: The
evolution of cancer of the colon and rectum. Cancer. 36:2251–2270.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Greene FL: Current TNM staging of
colorectal cancer. Lancet Oncol. 8:572–573. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li LT, Jiang G, Chen Q and Zheng JN: Ki67
is a promising molecular target in the diagnosis of cancer
(review). Mol Med Rep. 11:1566–1572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Piccaluga PP, Agostinelli C, Righi S,
Ciccone M, Re MC, Musumeci G, Diani E, Signoretto C, Bon L, Piccin
O, et al: IFI16 reduced expression is correlated with unfavorable
outcome in chronic lymphocytic leukemia. APMIS. 125:511–522. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Man SM, Karki R and Kanneganti T:
DNA-sensing inflammasomes: Regulation of bacterial host defense and
the gut microbiota. Pathog Dis. 74:ftw282016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Veeranki S and Choubey D: Systemic lupus
erythematosus and increased risk to develop B cell malignancies:
Role of the p200-family proteins. Immunol Lett. 133:1–5. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Salama R, Sadaie M, Hoare M and Narita M:
Cellular senescence and its effector programs. Genes Dev.
28:99–144. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sharpless NE and Sherr CJ: Forging a
signature of in vivo senescence. Nat Rev Cancer. 15:397–408. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guo Y, Ayers JL, Carter KT, Wang T, Maden
SK, Edmond D, Newcomb PP, Li C, Ulrich C, Yu M and Grady WM:
Senescence-associated tissue microenvironment promotes colon cancer
formation through the secretory factor GDF15. Aging Cell.
18:e130132019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Demaria M, Q'Leary MN, Chang J, Shao L,
Liu S, Alimirah F, Koening K, Le C, Mitin N, Deal AM, et al:
Cellular senescence promotes adverse effects of chemotherapy and
cancer relapse. Dancer Dissov. 7:165–176. 2017.PubMed/NCBI
|
|
36
|
Lai E, Liscia N, Donisi C, Mariani S, Tolu
S, Pretta A, Persano M, Pinna G, Balconi F, Pireddu A, et al:
Molecular-Biology-Driven treatment for metastatic colorectal
cancer. Cancers (Basel). 12:12142020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Le DT, Uram JN, Wang H, Bartlett BR,
Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et
al: PD-1 blockade in tumors with mismatch-repair deficiency. N Engl
J Med. 372:2509–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lizardo DY, Kuang C, Hao S, Yu J, Huang Y
and Zhang L: Immunotherapy efficacy on mismatch repair-deficient
colorectal cancer: From bench to bedside. Biochim Biophys Acta Rev
Cancer. 1874:1884472020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Evrard C, Tachon G, Randrian V,
Karayan-Tapon L and Tougeron D: Microsatellite instability:
Diagnosis, heterogeneity, discordance, and clinical impact in
colorectal cancer. Cancers (Basel). 11:15672019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Keshet Y and Seger R: The MAP kinase
signaling cascades: A system of hundreds of components regulates a
diverse array of physiological functions. Mothods Mol Biol.
661:3–38. 2010.PubMed/NCBI
|
|
41
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ye Q, Cai W, Zheng Y, Evers BM and She QB:
ERK and AKT signaling cooperate to translationally regulate
survivin expression for metastatic progression of colorectal
cancer. Oncogene. 33:1828–1839. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ding B and Lengyel P: p204 protein is a
novel modulator of ras activity. J Biol Chem. 283:5831–5848. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Luan Y, Lengyel P and Liu CJ: p204, a p200
family protein, as a multifunctional regulator of cell
proliferation and differentiation. Cytokine Growth Factor Rev.
19:357–369. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim EJ, Park JI and Nelkin BD: IFI16 is an
essential mediator of growth inhibition, but not differentiation,
induced by the leukemia inhibitory factor/JAK/STAT pathway in
medullary thyroid carcinoma cells. J Biol Chem. 280:4913–4920.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lengyel P and Liu CJ: The p200 family
protein p204 as a modulator of cell proliferation and
differentiation: A brief survey. Cell Mol Life Sci. 67:335–340.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kolc W: Coordinating ERK/MAPK signalling
through scaffolds and inhibitors. Nat Rev Mol Cell Biol. 6:827–837.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Brunet A, Roux D, Lenormand P, Dowd S,
Keyse S and Pouysségur J: Nuclear translocation of p42/p44
mitogen-activated protein kinase is required for growth
factor-induced gene expression and cell cycle entry. EMBO J.
18:664–674. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Su N, Peng L, Xia B, Zhao Y, Xu A, Wang J,
Wang X and Jiang B: Lyn is involved in CD24-induced ERK1/2
activation in colorectal cancer. Mol Cancer. 11:432012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Whitehurst AW, Robinson FL, Moore MS and
Cobb M: The death effector domain protein PEA-15 prevents nuclear
entry of ERK2 by inhibiting required interactions. J Biol Chem.
279:12840–12847. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Torii S, Kusakabe M, Yamamoto T, Maekawa M
and Nishida E: Sef is a spatial regulator forr Ras/MAP kinase
signaling. Dev Cell. 7:33–44. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gil-Jaramillo N, Rocha AP, Raiol T, Motta
FN, Favali C, Brigido MM, Bastos IMD and Santana JM: Trypanosoma
cruzi The First Contact of human Dendritic cells with reveals
response to virus as an unexplored central pathway. Front Immunol.
12:6380202021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lu N and Malemud CJ: Extracellular
signal-regulated Kinase: A regulator of cell growth, inflammation,
chondrocyte and bone cell receptor-mediated gene expression. Int J
Mol Sci. 20:37922019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kis-Toth K, Szanto A, Thai TH and Tsokos
GC: Cytosolic DNA-activated human dendritic cells are potent
activators of the adaptive immune response. J Immunol.
187:1222–1234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Qi Z, Yan F, Chen D, Xing W, Li Q, Zeng W,
Bi B and Xie J: Identification of prognostic biomarkers and
correlations with immune infiltrates among Cgas-STING in
hepatocellular carcinoma. Biosci Rep. 40:BSR202026032020.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Galon J, Costes A, Sanchez-Cabo F,
Kirilovsky A, Mlecnik B, Lagorce-pages C, Tosolini M, Camus M,
Berger A, Wind P, et al: Type, density, and location of immune
cells within human colorectal tumors predict clinical outcome.
Science. 313:1960–1964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mlecnik B, Tosolini M, Kirilovsky A,
Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman
WH, Pagès F and Galon J: Histopathologic-based prognostic factors
of colorectal cancers are associated with the state of the local
immune reaction. J Clin Oncol. 29:610–618. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Payandeh Z, Khalili S, Somi M,
Mard-Soltani M, Baghbanzadeh A, Hajiasgharzadeh K, Samadi N and
Baradaran B: PD-1/PD-L1-dependent immune response in colorectal
cancer. J Cell Physiol. 235:5461–5475. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Peng QH, Wang CH, Chen HM, Zhang RX, Pan
ZZ, Lu ZH, Wang GY, Yue X, Huang W and Liu RY: CMTM6 and PD-L1
coexpression is associated with an active immune microenvironment
and a favorable prognosis in colorectal cancer. J Immunother
Cancer. 9:e0016382021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Akiyoshi T, Gotoh O, Tanaka N, Kiyotani K,
Yamamoto N, Ueno M, Fukunaga Y and Mori S: T-cell complexity and
density are associated with sensitivity to neoadjuvant
chemoradiotherapy in patients with rectal cancer. Cancer Immunol
Immunother. 70:509–518. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kikuchi T, Mimura K, Okayama H, Nakayama
Y, Saito K, Yamada L, Endo E, Sakamoto W, Fujita S, Endo H, et al:
A subset of patients with MSS/MSI-low-colorectal cancer showed
increased CD8(+) TILs together with up-regulated IFN-γ. Oncol Lett.
18:5977–5985. 2019.PubMed/NCBI
|